Pilot Scale for Production and Purification of Lactic Acid from Ceratonia siliqua L. (Carob) Bagasse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Biomass Analysis
2.2. Larger-Scale Fermentation for Biomass
2.3. Sampling Analysis and Lactic Acid Optical Purity
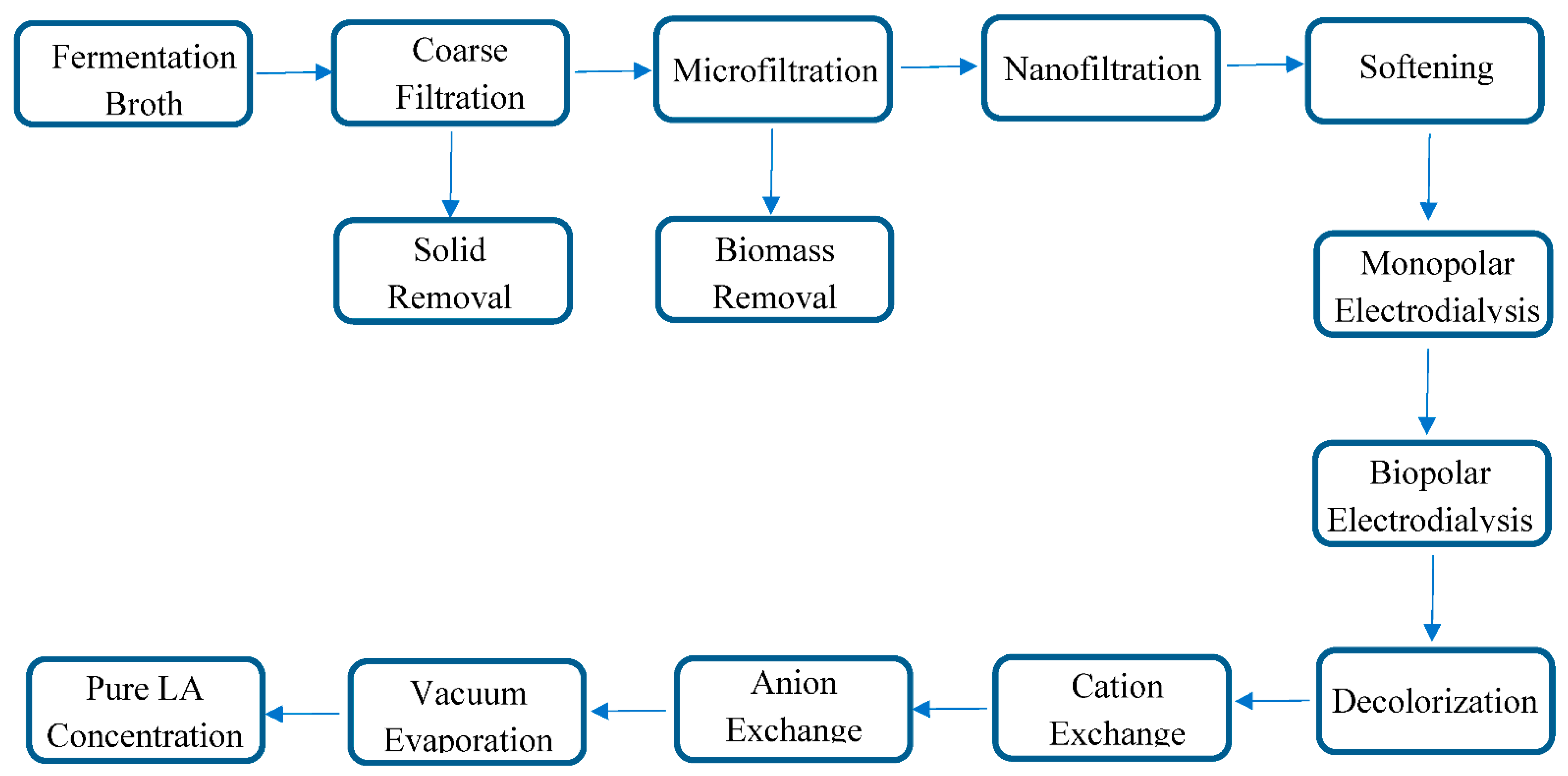
2.4. Downstream Process
2.5. Filtration and Softening
2.6. Electrodialysis
2.7. Decolorization, Cation and Anion Exchange
2.8. Distillation\Evaporation
3. Results and Discussion
3.1. Carob Content
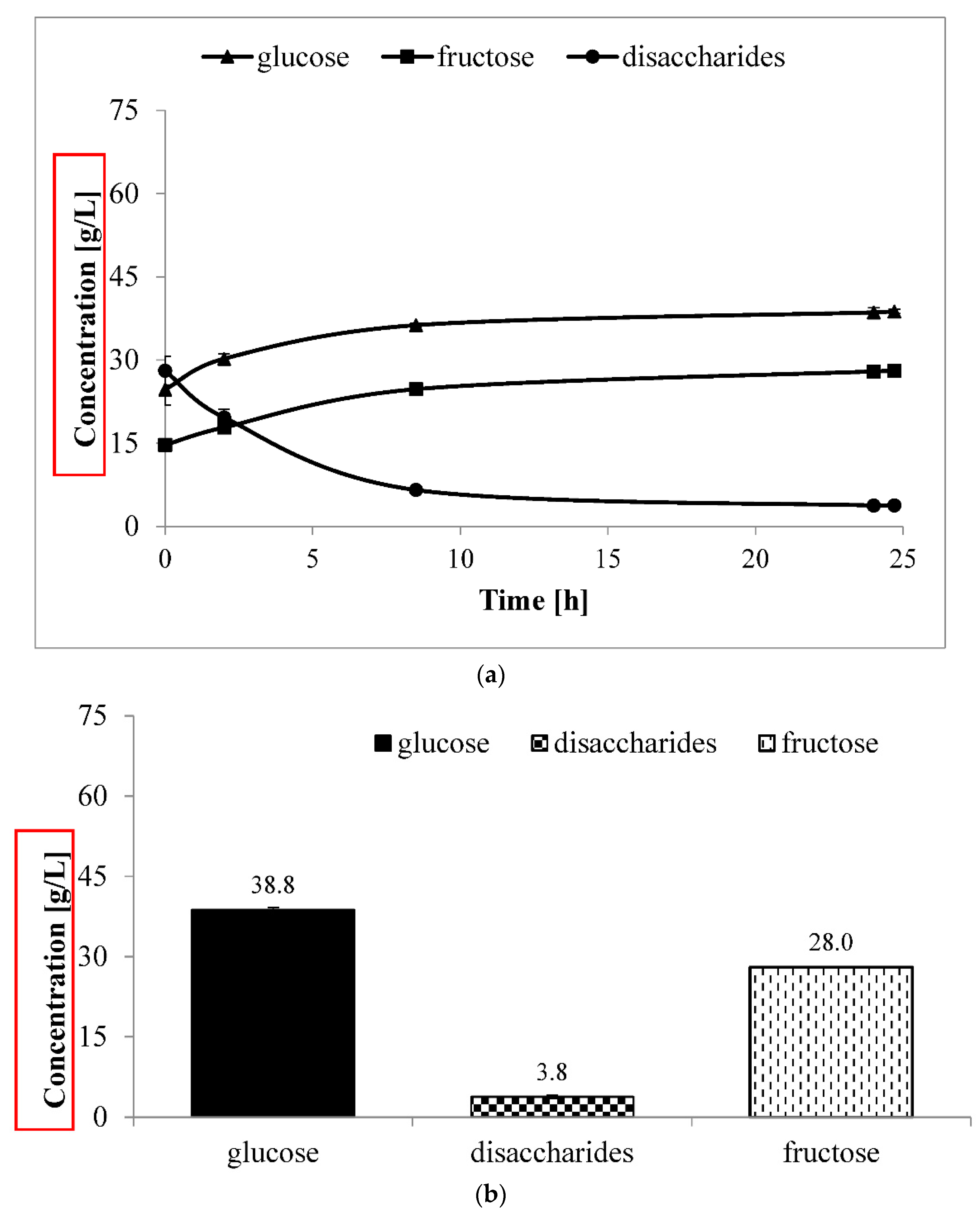
3.2. Large-Scale Fermentation
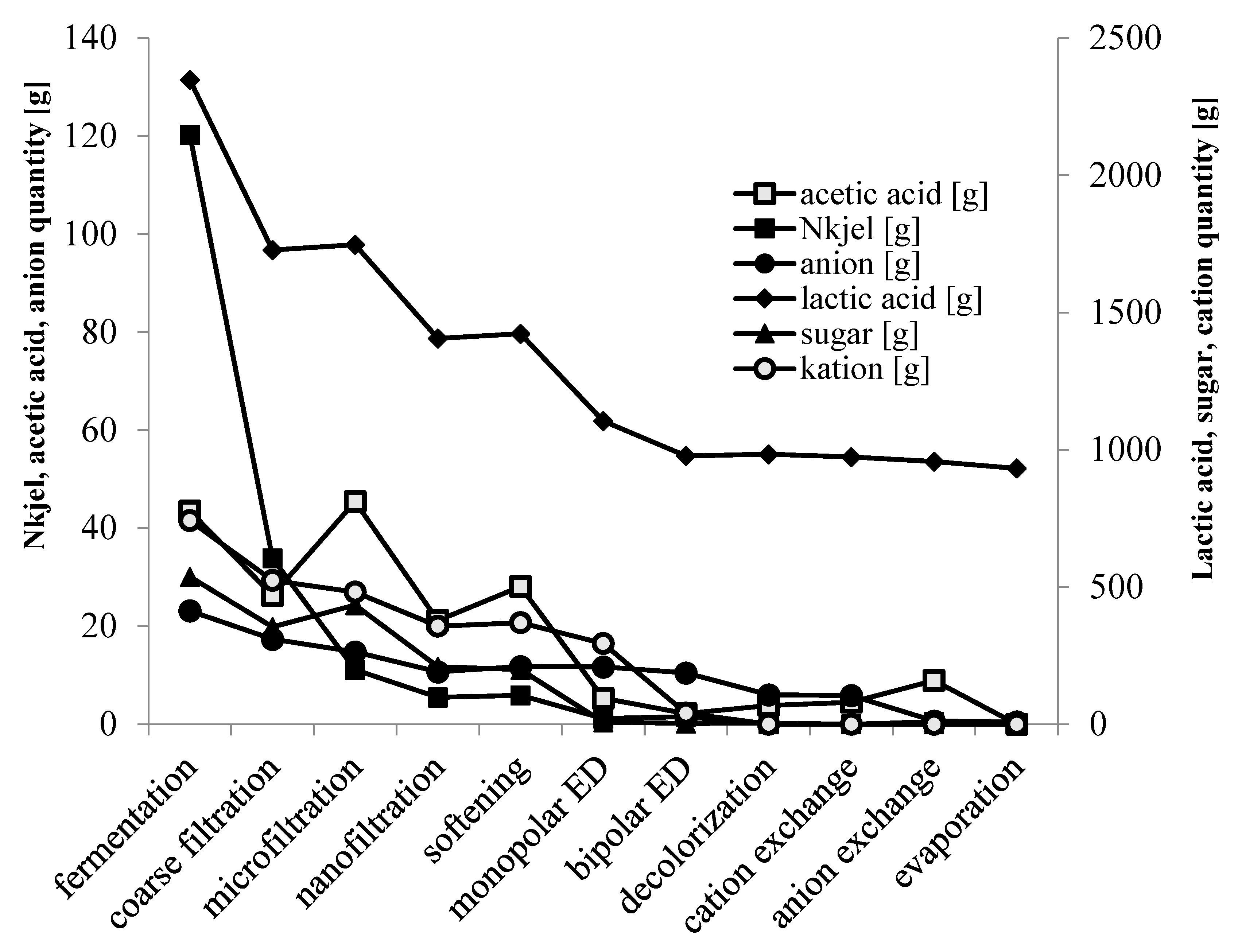
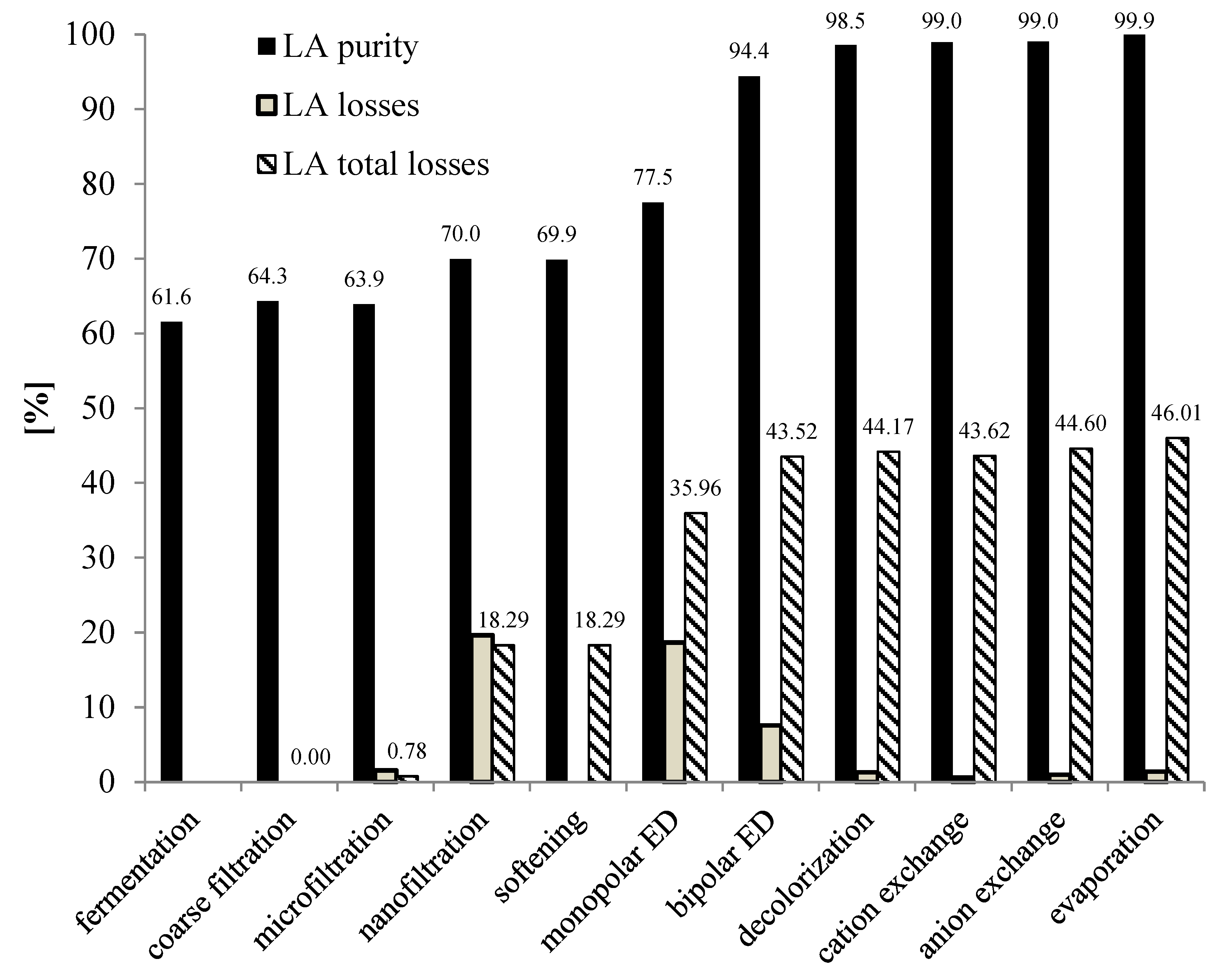
3.3. Downstream Processing of Lactic Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LA | Lactic acid |
| DSP | Downstream process |
| PLA | Polylactic acid |
| SHCF | Separate hydrolysis and co-fermentation |
| ED | Electrodialysis |
| LAP | Laboratory analytical protocol |
| NREL | National renewable energy laboratory |
| HPLC | High-Performance Liquid Chromatography |
| MF | Microfiltration |
| NF | Nanofiltration |
| UF | Ultrafiltration |
| CEM | Cation-exchange membrane |
| AEM | Anion exchange membrane |
| DM | Dry matter |
| Nkjel | Nitrogen Kjeldahl (g) |
| MED | Monopolar electrodialysis |
| BED | Bipolar electrodialysis |
References
- Othmen, B.K.; Elfalleh, W.; Lachiheb, B.; Haddad, M. Evolution of phytochemical and antioxidant activity of Tunisian carob (Ceratonia siliqua L.) pods during maturation. EuroBiotech J. 2019, 3, 135–142. [Google Scholar] [CrossRef]
- Durazzo, A.; Turfani, V.; Narducci, V.; Azzini, E.; Maiani, G.; Carcea, M. Nutritional characterization and bioactive components of commercial carobs flours. Food Chem. 2014, 153, 109–113. [Google Scholar] [CrossRef] [PubMed]
- El Batal, H.; Hasib, A.; Dehbi, F.; Zaki, N.; Ouatmane, A.; Boulli, A. Assessment of nutritional composition of Carob pulp (Ceratonia siliqua L.) collected from various locations in Morocco. J. Mater. Environ. Sci. 2016, 7, 3278–3285. [Google Scholar]
- Markis, D.P.; Kefalas, P. Carob pods (Ceratonia siliqua L.) as a source of polyphenolic antioxydants. Food Technol. Biotechnol. 2004, 42, 105–108. [Google Scholar]
- Moreira, T.C.; Da Silva, Á.T.; Fagundes, C.; Ferreira, S.M.R.; Cândido, L.M.B.; Passos, M.; Magnani, M. Elaboration of yogurt with reduced level of lactose added of carob (Ceratonia siliqua L.). LWT Food Sci. Technol. 2017, 76, 326–329. [Google Scholar] [CrossRef]
- El Batal, H.; Hasib, A.; Jaouad, A.; Ouatmane, A. Contribution to the Promotion of the Moroccan Locust: Morphological and Physico-Chemical Characterization. Application of the Method of Experimental Design for Optimizing the Extraction of the Seed Gum and Production of the Pulp Syrup. Ph.D. Thesis, University of Sultan Moulay Slimane, Beni Mellal, Morocco, 2014. [Google Scholar]
- Azaizeh, H.; Abu Tayeh, H.; Schneider, R.; Klongklaew, A.; Venus, J. Production of lactic acid from carob, banana and sugarcane lignocellulose biomass. Mol. J. 2020, 25, 2956. [Google Scholar] [CrossRef] [PubMed]
- Ouis, N.; Hariri, A. Improving of lactic acid production by Lactobacillus plantarum from carob pods syrup. Wulfenia 2018, 27, 12–25. [Google Scholar]
- Alves de Oliveira, R.; Komesu, A.; Vaz Rossell, C.F.; Filho, R.M. Challenges and opportunities in lactic acid bioprocess design- From economic to production aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Mora-Villalobos, J.A.; Montero-Zamora, J.; Barboza, N.; Rojas-Garbanzo, C.; Usaga, J.; Redondo-Solano, M.; Schroedter, L.; Olszewska-Widdrat, A.; López-Gómez, J.P. Multi-product lactic acid bacteria fermentations: A Review. Fermentation 2020, 6, 23. [Google Scholar] [CrossRef]
- Hu, Y.; Kwan, T.H.; Daoud, W.A.; Sze, C.; Lin, K. Continuous ultrasonic-mediated solvent extraction of lactic acid from fermentation broths. J. Cleaner Prod. 2017, 145, 142–150. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Venus, J. A review on the current developments in continuous lactic acid fermentations and case studies utilizing inexpensive raw materials. Process Biochem. 2018, 79, 1–10. [Google Scholar] [CrossRef]
- Brock, S.; Kuenz, A.; Prüße, U. Impact of hydrolysis methods on the utilization of agricultural residues as nutrient source for D-lactic acid production by Sporolactobacillus inulinus. Fermentation 2019, 5, 12. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernandez, C.; Bellesteros, M.; Tomas-Pejo, E. Biotechnological advances in lactic acid production by lactic acid bacteria: Lignocellulose as novel substrate. Biofu. Bioprod. Biorefin. 2018, 12, 290–303. [Google Scholar] [CrossRef]
- Olszewska-Widdrat, A.; Alexandri, M.; Pablo López-Gómez, J.; Schneider, R.; Mandl, M.; Venus, J. Production and purification of l-lactic acid in lab and pilot scales using sweet sorghum juice. Fermentation 2019, 5, 36. [Google Scholar] [CrossRef]
- Tarraran, L.; Mazzoli, R. Alternative strategies for lignocellulose fermentation through lactic acid bacteria: The state of the art and perspectives. FEMS Microbiol. Lett. 2018, 365, fny126. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Alexandri, M.; Komesu, A.; Venus, J.; Eduardo Vaz Rossell, C.; Maciel Filho, R. Current Advances in Separation and Purification of Second-Generation Lactic Acid. Sep. Purif. Rev. 2019, 49, 159–175. [Google Scholar] [CrossRef]
- Jantasee, S.; Kienberger, M.; Mungma, N.; Siebenhofer, M. Potential and assessment of lactic acid production and isolation—A review. J. Chem. Technol. Biotechnol. 2017, 92, 2885–2893. [Google Scholar] [CrossRef]
- Komesu, A.; Wolf Maciel, M.R.; Maciel Filho, R. Separation and purification technologies for lactic acid—A brief review. BioResources 2017, 12, 6885–6901. [Google Scholar] [CrossRef]
- Garrett, B.G.; Srinivas, K.; Ahring, B.K. Performance and stability of AmberliteTM IRA-67 ion exchange resin for product extraction and pH control during homolactic fermentation of corn stover sugars. Biochem. Eng. J. 2015, 94, 1–8. [Google Scholar] [CrossRef]
- Alexandri, M.; Schneider, R.; Venus, J. Membrane technologies for lactic acid separation from fermentation broths derived from renewable resources. Membranes 2018, 8, 94. [Google Scholar] [CrossRef]
- Oonkhanond, B.; Jonglertjunya, W.; Srimarut, N.; Bunpachart, P.; Tantinukul, S.; Nasongkla, N.; Sakdaronnarong, C. Lactic acid production from sugarcane bagasse by an integrated system of lignocellulose fractionation, saccharification, fermentation, and ex-situ nanofiltration. J. Environ. Chem. Eng. 2017, 5, 2533–2541. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.C.; Hu, Y.; Ngo, H.H.; Li, Y. Dynamic membrane-assisted fermentation of food wastes for enhancing lactic acid production. Bioresour. Technol. 2017, 234, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Structure Carbohydrates and Lignin in Biomass. Available online: http://www.nrel.gov/biomass/pdfs/lap_carbslignin_2007.pdf (accessed on 11 February 2008).
- Lee, H.D.; Lee, M.Y.; Hwang, Y.S.; Cho, Y.H.; Kim, H.W.; Park, H.B. Separation and Purification of Lactic Acid from Fermentation Broth Using Membrane-Integrated Separation Processes. Ind. Eng. Chem. Res. 2017, 56, 8301–8310. [Google Scholar] [CrossRef]
- Neu, A.K.; Pleissner, D.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative utilization of coffee mucilage using Bacillus coagulans and investigation of down-stream processing of fermentation broth for optically pure l(+)-lactic acid production. Bioresour. Technol. 2016, 211, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.; Ye, J.; Hahn, H.H. Recent advances in application of electrodialysis with bipolar membranes for organic acid recovery from fermentation broth. Curr. Org. Chem. 2016, 20, 2753–2761. [Google Scholar] [CrossRef]
- Lech, M.; Trusek, A. Batch Electrodialysis of Lactic Acid Obtained from Lab Fermentation. Polish J. Chem. Technol. 2018, 20, 81–86. [Google Scholar] [CrossRef]
- Chen, G.Q.; Eschbach, F.I.I.; Weeks, M.; Gras, S.L.; Kentish, S.E. Removal of lactic acid from acid whey using electrodialysis. Sep. Purif. Technol. 2016, 158, 230–237. [Google Scholar] [CrossRef]
- Madzingaidzo, L.; Danner, H.; Braun, R. Process development and optimization of lactic acid purification using electrodialysis. J. Biotechnol. 2002, 96, 223–239. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhang, X.; Feng, H.; Xu, T. In-situ combination of fermentation and electrodialysis with bipolar membranes for the production of lactic acid: Continuous operation. Bioresour. Technol. 2013, 147, 442–448. [Google Scholar] [CrossRef]
- Joglekar, H.G.; Rahman, I.; Babu, S.; Kulkarni, B.D.; Joshi, A. Comparative assessment of downstream processing options for lactic acid. Sep. Purif. Technol. 2006, 52, 1–17. [Google Scholar] [CrossRef]
- Msuya, N.; Katima, J.H.Y.; Masanja, E.; Temu, A.K. Poly(lactic-acid) Production-From Monomer to Polymer: A review. Sci. Fed. J. Polym. 2017, 1, 1–15. [Google Scholar]

| V | LA | PO43+ | Cl- | SO42+ | NO3- | Na+ | K+ | Mg+ | Ca2+ | NH4+ | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [L] | [g·L−1] | [mg·L−1] | |||||||||
| End fermentation | 50.6 | 46.4 | 26.6 | 273 | 118 | 3.89 | 12,762 | 1627 | 152 | 427 | 3.07 |
| Filtrate 150 µm | 36.2 | 50.9 | 66.3 | 267 | 119 | 1.30 | 12,081 | 1640 | 149 | 436 | 5.34 |
| Permeate microfiltration | 48.25 | 37.6 | 47.4 | 171 | 69.6 | 1.41 | 8765 | 1147 | 101 | 258 | 8.81 |
| Permeate nanofiltration | 64.5 | 21.9 | 15.7 | 124 | 12.8 | 1.39 | 4858 | 622 | 16.7 | 50.7 | 5.37 |
| Softening | 72.1 | 19.8 | 18.0 | 115 | 21.2 | 1.92 | 4701 | 450 | 0.42 | 5.24 | 2.3 |
| Monopolar electrodialysis concentrate | 12.3 | 94.0 | 84.7 | 698 | 90.1 | 6.37 | 22,529 | 2082 | <0.1 | 3.85 | 2 |
| Bipolar electrodialysis acid | 10.51 | 102 | 83.0 | 815 | 100 | 7.14 | 3047 | 305 | <0.1 | 1.12 | 1.5 |
| Decolorization | 19.6 | 53.6 | 41.9 | 247 | 48.1 | 3.95 | 0.87 | 0.16 | <0.01 | 0.15 | 0.05 |
| Cation exchanger | 21.2 | 48.9 | 42.1 | 216 | 44.9 | 3.49 | 0.67 | 0.15 | 0.01 | 0.14 | 0.13 |
| Anion exchanger | 22.0 | 46.3 | 14.1 | 2.39 | 16.3 | 0.03 | 0.67 | 0.24 | 0.01 | 0.14 | 0.12 |
| Concentrate vacuum distillation | 1.14 | 864 | 223 | 69.1 | 200 | 0.00 | 133 | 6.63 | 0.25 | 4.01 | 1.64 |
| D-LA (g/L) | L-LA (g/L) | Total LA (g/L) | L/D (%) |
|---|---|---|---|
| 3.2 ± 3.1 | 859.25 ± 1.34 | 862.4 ± 1.7 | 99.65 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azaizeh, H.; Abu Tayeh, H.N.; Schneider, R.; Venus, J. Pilot Scale for Production and Purification of Lactic Acid from Ceratonia siliqua L. (Carob) Bagasse. Fermentation 2022, 8, 424. https://doi.org/10.3390/fermentation8090424
Azaizeh H, Abu Tayeh HN, Schneider R, Venus J. Pilot Scale for Production and Purification of Lactic Acid from Ceratonia siliqua L. (Carob) Bagasse. Fermentation. 2022; 8(9):424. https://doi.org/10.3390/fermentation8090424
Chicago/Turabian StyleAzaizeh, Hassan, Hiba Nazmi Abu Tayeh, Roland Schneider, and Joachim Venus. 2022. "Pilot Scale for Production and Purification of Lactic Acid from Ceratonia siliqua L. (Carob) Bagasse" Fermentation 8, no. 9: 424. https://doi.org/10.3390/fermentation8090424
APA StyleAzaizeh, H., Abu Tayeh, H. N., Schneider, R., & Venus, J. (2022). Pilot Scale for Production and Purification of Lactic Acid from Ceratonia siliqua L. (Carob) Bagasse. Fermentation, 8(9), 424. https://doi.org/10.3390/fermentation8090424








