Anaerobic Co-Digestion of Sugarcane Leaves, Cow Dung and Food Waste: Focus on Methane Yield and Synergistic Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. Batch Test Design
2.3. Physicochemical Analyses
2.4. Calculations
2.5. Kinetic Model-Based Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Feedstocks and Inoculum
3.2. Methanogenesis
3.2.1. Methane Yield Rate
3.2.2. Methane Content
3.2.3. Cumulative Methane Yield
3.3. Stability of Process and Degradability of Substrates
3.3.1. Variation of pH and Ammonia Nitrogen of the AD Process
3.3.2. Anaerobic Degradability and Removal of Organic Matter
3.4. Analysis of the Kinetics
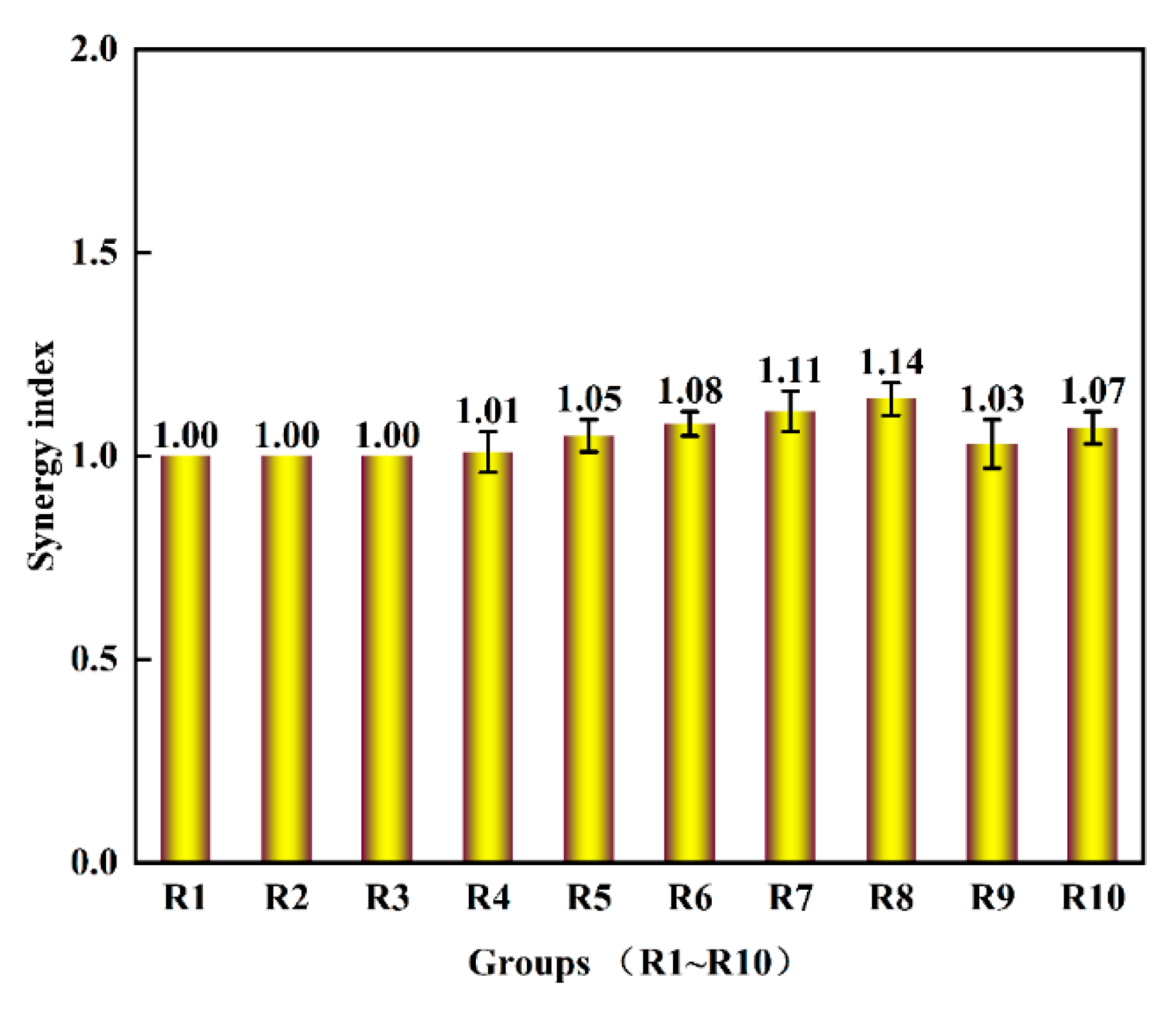
3.5. Synergy Impact of Anaerobic Co-Digestion
3.6. Significance of This Work
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shewa, W.A.; Hussain, A.; Chandra, R.; Lee, J.; Saha, S.; Lee, H.S. Valorization of food waste and economical treatment: Effect of inoculation methods. J. Clean. Prod. 2020, 261, 121170. [Google Scholar] [CrossRef]
- Hou, T.; Zhao, J.; Lei, Z.; Shimizu, K.; Zhang, Z. Synergistic effects of rice straw and rice bran on enhanced methane production and process stability of anaerobic digestion of food waste. Bioresour. Technol. 2020, 314, 123775. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, Y. Turning food waste to energy and resources towards a great environmental and economic sustainability: An innovative integrated biological approach. Biotechnol. Adv. 2019, 37, 107414. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, L.D.; Koch, K.; Bolzonella, D.; Drewes, J.E. Full scale co-digestion of wastewater sludge and food waste: Bottlenecks and possibilities. Renew. Sustain. Energy Rev. 2017, 72, 354–362. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.P.; Li, D.X.; Liu, C.; Lu, X.H. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustai. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Moretti, M.; Van Dael, M.; Malina, R.; Van Passel, S. Environmental assessment of waste feedstock mono-dimensional and bio-refinery systems: Combining manure co-digestion and municipal waste anaerobic digestion. J. Clean. Prod. 2018, 171, 954–961. [Google Scholar] [CrossRef]
- Yuan, T.G.; Shi, X.Y.; Sun, R.; Ko, J.H.; Xu, Q.Y. Simultaneous addition of biochar and zero-valent iron to improve food waste anaerobic digestion. J. Clean. Prod. 2021, 278, 123627. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, Y.; De Clercq, D. What is the true value of food waste? A case study of technology integration in urban food waste treatment in Suzhou City, China. J. Clean. Prod. 2016, 118, 88–96. [Google Scholar] [CrossRef]
- Ye, M.; Liu, J.Y.; Ma, C.N.; Li, Y.Y.; Zou, L.P.; Qian, G.R.; Xu, Z.P. Improving the stability and efficiency of anaerobic digestion of food waste using additives: A critical review. J. Clean. Prod. 2018, 192, 316–326. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, B.; Li, A.; Zhang, L.; Li, R.; Yang, T.; Xing, W. Mechanism of process imbalance of long-term anaerobic digestion of food waste and role of trace elements in maintaining anaerobic process stability. Bioresour. Technol. 2019, 275, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Baldi, F.; Pecorini, I.; Lannelli, R. Comparison of single-stage and two-stage anaerobic co-digestion of food waste and activated sludge for hydrogen and methane production. Renew. Energy 2019, 143, 1755–1765. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. Optimization of methane production during anaerobic co-digestion of rice straw and hydrilla verticillata using response surface methodology. Fuel 2019, 235, 92–99. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Baek, G.; Lee, C. Anaerobic co-digestion of spent coffee grounds with different waste feedstocks for biogas production. Waste Manag. 2017, 60, 322–328. [Google Scholar] [CrossRef]
- Montoro, S.B.; Lucas, J.; Santos, D.F.L.; Costa, M.S.S.M. Anaerobic co-digestion of sweet potato and dairy cattle manure: A technical and economic evaluation for energy and biofertilizer production. J. Clean. Prod. 2019, 226, 1082–1091. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Song, K. Anaerobic co-digestion of waste activated sludge and fish waste: Methane production performance and mechanism analysis. J. Clean. Prod. 2021, 279. [Google Scholar] [CrossRef]
- Gao, M.; Yang, M.; Ma, X.; Xie, D.; Wu, C.; Wang, Q. Effect of co-digestion of tylosin fermentation dreg and food waste on anaerobic digestion performance. Bioresour. Technol. 2021, 325, 124693. [Google Scholar] [CrossRef]
- Cheng, J.H.; Zhu, C.; Zhu, J.; Jing, X.; Kong, F.; Zhang, C.Y. Effects of waste rusted iron shavings on enhancing anaerobic digestion of food wastes and municipal sludge. J. Clean. Prod. 2020, 242, 118195. [Google Scholar] [CrossRef]
- Helenas Perin, J.K.; Biesdorf Borth, P.L.; Torrecilhas, A.R.; Santana da Cunha, L.; Kuroda, E.K.; Fernandes, F. Optimization of methane production parameters during anaerobic co-digestion of food waste and garden waste. J. Clean. Prod. 2020, 272, 123130. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Luo, L.N.; Li, W.Z.; Wang, X.Y.; Sun, Y.; Sun, Y.M.; Gong, W.J. Optimization of mixing ratio of ammoniated rice straw and food waste co-digestion and impact of trace element supplementation on biogas production. J. Mater. Cycles Waste 2018, 20, 745–753. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Joshi, R.; Dhar, H.; Verma, S.; Awasthi, M.K.; Varjani, S.; Sarsaiya, S.; Zhang, Z.; Kumar, S. Improving methane yield and quality via co-digestion of cow dung mixed with food waste. Bioresour. Technol. 2018, 251, 259–263. [Google Scholar] [CrossRef]
- Grosser, A.; Grobelak, A.; Rorat, A.; Courtois, P.; Vandenbulcke, F.; Lemiere, S.; Guyoneaud, R.; Attard, E.; Celary, P. Effects of silver nanoparticles on performance of anaerobic digestion of sewage sludge and associated microbial communities. Renew. Energy 2021, 171, 1014–1025. [Google Scholar] [CrossRef]
- Li, P.C.; Liu, Z.Y.; Zhao, M.X.; Dai, X.H.; Ruan, W.Q. Evaluation of Biogas Performance and Process Stability from Food, Kitchen, and Fruit/Vegetable Waste by Mono-, Co-, and Tridigestion. Energy Fuel 2020, 34, 12734–12742. [Google Scholar] [CrossRef]
- Ma, X.X.; Yu, M.; Yang, M.; Gao, M.; Wu, C.F.; Wang, Q.H. Synergistic effect from anaerobic co-digestion of food waste and Sophora flavescens residues at different co-substrate ratios. Environ. Sci. Pollut. R 2019, 26, 37114–37124. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 2002, 28, 350–356. [Google Scholar] [CrossRef]
- Prabhu, A.V.; Raja, S.A.; Avinash, A.; Pugazhendhi, A. Parametric optimization of biogas potential in anaerobic co-digestion of biomass wastes. Fuel 2021, 288. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Lee, C. Anaerobic co-digestion of food waste, human feces, and toilet paper: Methane potential and synergistic effect. Fuel 2019, 248, 189–195. [Google Scholar] [CrossRef]
- Kafle, G.K.; Kim, S.H.; Sung, K.I. Ensiling of fish industry waste for biogas production: A lab scale evaluation of biochemical methane potential (BMP) and kinetics. Bioresour. Technol. 2013, 127, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, E.S.; Abomohra, A.F.; Xu, C.; Elsayed, M.; Abdelkader, T.K.; Lin, J.C.; Yuan, Q.X. Enhancement of biogas production from rape straw using different co-pretreatment techniques and anaerobic co-digestion with cattle manure. Bioresour. Technol. 2020, 309, 123311. [Google Scholar] [CrossRef]
- Forster-Carneiro, T.; Perez, M.; Romero, L.I. Anaerobic digestion of municipal solid wastes: Dry thermophilic performance. Bioresour. Technol. 2008, 99, 8180–8184. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, C.; Guarino, G.; D’Amelia, L.I.; Morrone, B.; Minale, M. The peculiar role of C/N and initial pH in anaerobic digestion of lactating and non-lactating water buffalo manure. Waste Manag. 2020, 103, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.Y.; Lu, X.Q.; Kobayashi, T.; Kumar, G.; Xu, K.Q. Anaerobic co-digestion on improving methane production from mixed microalgae (Scenedesmus sp., Chlorella sp.) and food waste: Kinetic modeling and synergistic impact evaluation. Chem. Eng. J. 2016, 299, 332–341. [Google Scholar] [CrossRef]
- Gu, J.; Liu, R.; Cheng, Y.; Stanisavljevic, N.; Li, L.; Djatkov, D.; Peng, X.; Wang, X. Anaerobic co-digestion of food waste and sewage sludge under mesophilic and thermophilic conditions: Focusing on synergistic effects on methane production. Bioresour. Technol. 2020, 301, 122765. [Google Scholar] [CrossRef]
- Hashemi, B.; Sarker, S.; Lamb, J.J.; Lien, K.M. Yield improvements in anaerobic digestion of lignocellulosic feedstocks. J. Clean. Prod. 2021, 288, 125447. [Google Scholar] [CrossRef]
- Herout, M.; Malaťák, J.; Kučera, L.; Dlabaja, T. Biogas composition depending on the type of plant biomass used. Res. Agric. Eng. 2011, 57, 137–143. [Google Scholar] [CrossRef]
- Lyytimaki, J. Renewable energy in the news: Environmental, economic, policy and technology discussion of biogas. Sustain. Prod. Consump. 2018, 15, 65–73. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, L.; Zhu, K.; Ma, J.; Ifran, M.; Li, A. Anaerobic co-digestion of sewage sludge, food waste and yard waste: Synergistic enhancement on process stability and biogas production. Sci. Total Environ. 2020, 704, 135429. [Google Scholar] [CrossRef]
- Tasnim, F.; Iqbal, S.A.; Chowdhury, A.R. Biogas production from anaerobic co-digestion of cow manure with kitchen waste and Water Hyacinth. Renew. Energy 2017, 109, 434–439. [Google Scholar] [CrossRef]
- Dima, A.D.; Parvulescu, O.C.; Mateescu, C.; Dobre, T. Optimization of substrate composition in anaerobic co -digestion of agricultural waste using central composite design. Biomass Bioenerg. 2020, 138, 105602. [Google Scholar] [CrossRef]
- Ma, X.; Yu, M.; Song, N.; Xu, B.; Gao, M.; Wu, C.; Wang, Q. Effect of ethanol pre-fermentation on organic load rate and stability of semi-continuous anaerobic digestion of food waste. Bioresour. Technol. 2020, 299, 122587. [Google Scholar] [CrossRef] [PubMed]
- Taboada-Santos, A.; Braz, G.H.R.; Fernandez-Gonzalez, N.; Carballa, M.; Lema, J.M. Thermal hydrolysis of sewage sludge partially removes organic micropollutants but does not enhance their anaerobic biotransformation. Sci. Total Environ. 2019, 690, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, R.; Masse, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Y.; Yun, S.N.; Wang, K.J.; Shah, F.A.; Xing, T.; Li, B.J. Static-magnetic-field coupled with fly-ash accelerant: A powerful strategy to significantly enhance the mesophilic anaerobic-co-digestion. Bioresour. Technol. 2021, 327, 124793. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Hong, X.; Wang, T.; Huang, X.; Tong, Y.; Yang, H. Reutilization of high COD leachate via recirculation strategy for methane production in anaerobic digestion of municipal solid waste: Performance and dynamic of methanogen community. Bioresour. Technol. 2019, 288, 121509. [Google Scholar] [CrossRef]
- Wang, Z.; Yun, S.; Xu, H.; Wang, C.; Zhang, Y.; Chen, J.; Jia, B. Mesophilic anaerobic co-digestion of acorn slag waste with dairy manure in a batch digester: Focusing on mixing ratios and bio-based carbon accelerants. Bioresour. Technol. 2019, 286, 121394. [Google Scholar] [CrossRef]
- Yu, M.; Wu, C.; Wang, Q.; Sun, X.; Ren, Y.; Li, Y.Y. Ethanol prefermentation of food waste in sequencing batch methane fermentation for improved buffering capacity and microbial community analysis. Bioresour. Technol. 2018, 248, 187–193. [Google Scholar] [CrossRef]
- Himanshu, H.; Murphy, J.D.; Grant, J.; O’Kiely, P. Antagonistic effects on biogas and methane output when co-digesting cattle and pig slurries with grass silage in in vitro batch anaerobic digestion. Biomass Bioenerg. 2018, 109, 190–198. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, F.; Yu, J.; Cai, Y.; Luo, X.; Cui, Z.; Hu, Y.; Wang, X. Co-digestion of oat straw and cow manure during anaerobic digestion: Stimulative and inhibitory effects on fermentation. Bioresour. Technol. 2018, 269, 143–152. [Google Scholar] [CrossRef]




| Ratio (FW:SL:CD) a | FW (g) b | SL (g) b | CD (g) b | Inoculum (g) b |
|---|---|---|---|---|
| R1 (100:0:0) | 43.50 | - | - | 408.06 |
| R2 (0:100:0) | - | 57.36 | - | 408.06 |
| R3 (0:0:100) | - | - | 55.89 | 408.06 |
| R4 (75:25:0) | 32.62 | 14.34 | - | 408.06 |
| R5 (75:0:25) | 32.62 | - | 13.97 | 408.06 |
| R6 (15:63.75:21.25) | 6.52 | 36.56 | 11.88 | 408.06 |
| R7 (25:56.25:18.75) | 10.87 | 32.26 | 10.48 | 408.06 |
| R8 (50:37.5:12.5) | 21.75 | 21.51 | 6.99 | 408.06 |
| R9 (75:18.75:6.25) | 32.62 | 10.75 | 3.49 | 408.06 |
| R10 (85:11.25:3.75) | 36.97 | 6.45 | 2.10 | 408.06 |
| Parameter | FW | SL | CD | Inoculum |
|---|---|---|---|---|
| pH | - | - | - | 7.87 ± 0.01 |
| TS a% | 25.84 ± 0.17 | 21.41 ± 0.22 | 20.27 ± 0.24 | 4.41 ± 0.01 |
| VS b% | 94.30 ± 0.02 | 86.34 ± 3.19 | 93.56 ± 0.67 | 58.85 ± 2.36 |
| C b% | 49.27 ± 0.82 | 43.59 ± 0.05 | 44.03 ± 0.02 | - |
| H b% | 6.47 ± 0.20 | 6.91 ± 0.01 | 6.82 ± 0.01 | - |
| O b% | 36.04 ± 0.10 | 41.49 ± 0.08 | 40.79 ± 0.35 | - |
| N b% | 2.75 ± 0.22 | 1.19 ± 0.10 | 1.69 ± 0.19 | - |
| S b% | 0.24 ± 1.01 | 0.27 ± 0.05 | 0.36 ± 0.01 | - |
| C/N ratio | 17.89 | 36.51 | 26.10 | - |
| Soluble Carbohydrates b% | 20.88 ± 0.49 | 3.69 ± 0.23 | 0.44 ± 0.03 | - |
| Lipids b% | 22.49 ± 0.21 | 4.58 ± 0.26 | 2.97 ± 0.10 | - |
| Soluble Proteins b% | 16.33 ± 012 | 5.75 ± 0.05 | 6.16 ± 0.03 | - |
| pH | - | - | - | 7.87 ± 0.01 |
| NH4+-N (mg/L) | - | - | - | 1244.33 ± 4.04 |
| SCOD (mg/L) | - | - | - | 536 ± 3.46 |
| Groups | TMY (mL/g VS) | CMY (mL/g VS) | ABD% | VS Reduction (%) | SCOD Reduction (%) |
|---|---|---|---|---|---|
| R1 | 535.35 | 307.36 ± 6.78 | 57.41 ± 1.27 | 60.96 ± 0.68 | 63.27 ± 0.72 |
| R2 | 476.41 | 141.20 ± 14.36 | 29.64 ± 3.02 | 38.27 ± 0.24 | 37.10 ± 1.39 |
| R3 | 476.27 | 37.74 ± 9.11 | 7.92 ± 1.91 | 28.89 ± 0.19 | 13.71 ± 0.15 |
| R4 | 520.61 | 269.44 ± 11.96 | 51.76 ± 2.30 | 41.01 ± 0.11 | 42.26 ± 0.09 |
| R5 | 520.58 | 251.53 ± 8.64 | 48.32 ± 1.66 | 39.79 ± 0.40 | 40.68 ± 0.51 |
| R6 | 485.22 | 155.65 ± 4.81 | 32.08 ± 0.99 | 37.06 ± 2.58 | 43.87 ± 0.35 |
| R7 | 491.12 | 180.63 ± 7.54 | 36.78 ± 1.54 | 36.08 ± 0.72 | 40.90 ± 0.34 |
| R8 | 505.86 | 239.89 ± 8.70 | 47.42 ± 1.72 | 41.50 ± 0.27 | 45.37 ± 0.24 |
| R9 | 520.60 | 268.27 ± 16.66 | 51.53 ± 3.20 | 43.95 ± 0.99 | 52.65 ± 0.08 |
| R10 | 520.50 | 297.16 ± 11.88 | 56.44 ± 2.26 | 44.35 ± 1.46 | 55.38 ± 0.33 |
| Parameter | Y (mL/g VS) | Rm (mL/g-VS/day) | λ (day) | R2 |
|---|---|---|---|---|
| R1 | 310.99 ± 3.70 | 48.93 ± 3.28 | 0.29 ± 0.22 | 0.9855 |
| R2 | 138.18 ± 1.04 | 28.53 ± 1.52 | 0.13 ± 0.14 | 0.9913 |
| R3 | 36.29 ± 0.45 | 5.62 ± 0.38 | 0.78 ± 0.23 | 0.9872 |
| R4 | 270.88 ± 2.63 | 48.92 ± 3.02 | 0.22 ± 0.18 | 0.9880 |
| R5 | 252.64 ± 1.99 | 50.88 ± 2.77 | 0.28 ± 0.14 | 0.9912 |
| R6 | 152.77 ± 0.92 | 44.70 ± 1.89 | 0.23 ± 0.08 | 0.9955 |
| R7 | 178.02 ± 0.61 | 55.24 ± 1.77 | 0.33 ± 0.06 | 0.9976 |
| R8 | 238.43 ± 0.55 | 67.98 ± 1.38 | 0.38 ± 0.04 | 0.9990 |
| R9 | 267.75 ± 0.76 | 62.54 ± 1.37 | 0.32 ± 0.05 | 0.9987 |
| R10 | 297.67 ± 1.14 | 65.83 ± 1.86 | 0.36 ± 0.07 | 0.9978 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Bi, G.; Liu, X.; Yu, Q.; Li, D.; Yuan, H.; Chen, Y.; Xie, J. Anaerobic Co-Digestion of Sugarcane Leaves, Cow Dung and Food Waste: Focus on Methane Yield and Synergistic Effects. Fermentation 2022, 8, 399. https://doi.org/10.3390/fermentation8080399
Xu S, Bi G, Liu X, Yu Q, Li D, Yuan H, Chen Y, Xie J. Anaerobic Co-Digestion of Sugarcane Leaves, Cow Dung and Food Waste: Focus on Methane Yield and Synergistic Effects. Fermentation. 2022; 8(8):399. https://doi.org/10.3390/fermentation8080399
Chicago/Turabian StyleXu, Sunqiang, Guican Bi, Xiangmeng Liu, Qiang Yu, Dong Li, Haoran Yuan, Yong Chen, and Jun Xie. 2022. "Anaerobic Co-Digestion of Sugarcane Leaves, Cow Dung and Food Waste: Focus on Methane Yield and Synergistic Effects" Fermentation 8, no. 8: 399. https://doi.org/10.3390/fermentation8080399
APA StyleXu, S., Bi, G., Liu, X., Yu, Q., Li, D., Yuan, H., Chen, Y., & Xie, J. (2022). Anaerobic Co-Digestion of Sugarcane Leaves, Cow Dung and Food Waste: Focus on Methane Yield and Synergistic Effects. Fermentation, 8(8), 399. https://doi.org/10.3390/fermentation8080399







