Succession of Bacterial and Fungal Communities during Fermentation of Medicinal Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Plants
2.2. Fermentation According to the GHP Standard
2.3. Sampling
2.4. DNA/RNA Extraction
2.5. Sequencing
2.6. Bioinformatics
2.7. Statistical Analysis
3. Results
3.1. Richness and Diversity
3.2. Community Composition and Succession
3.3. Fermentation of Achillea in 2016 and 2018
3.4. Co-Occurrence of Bacteria and Fungi during Fermentation
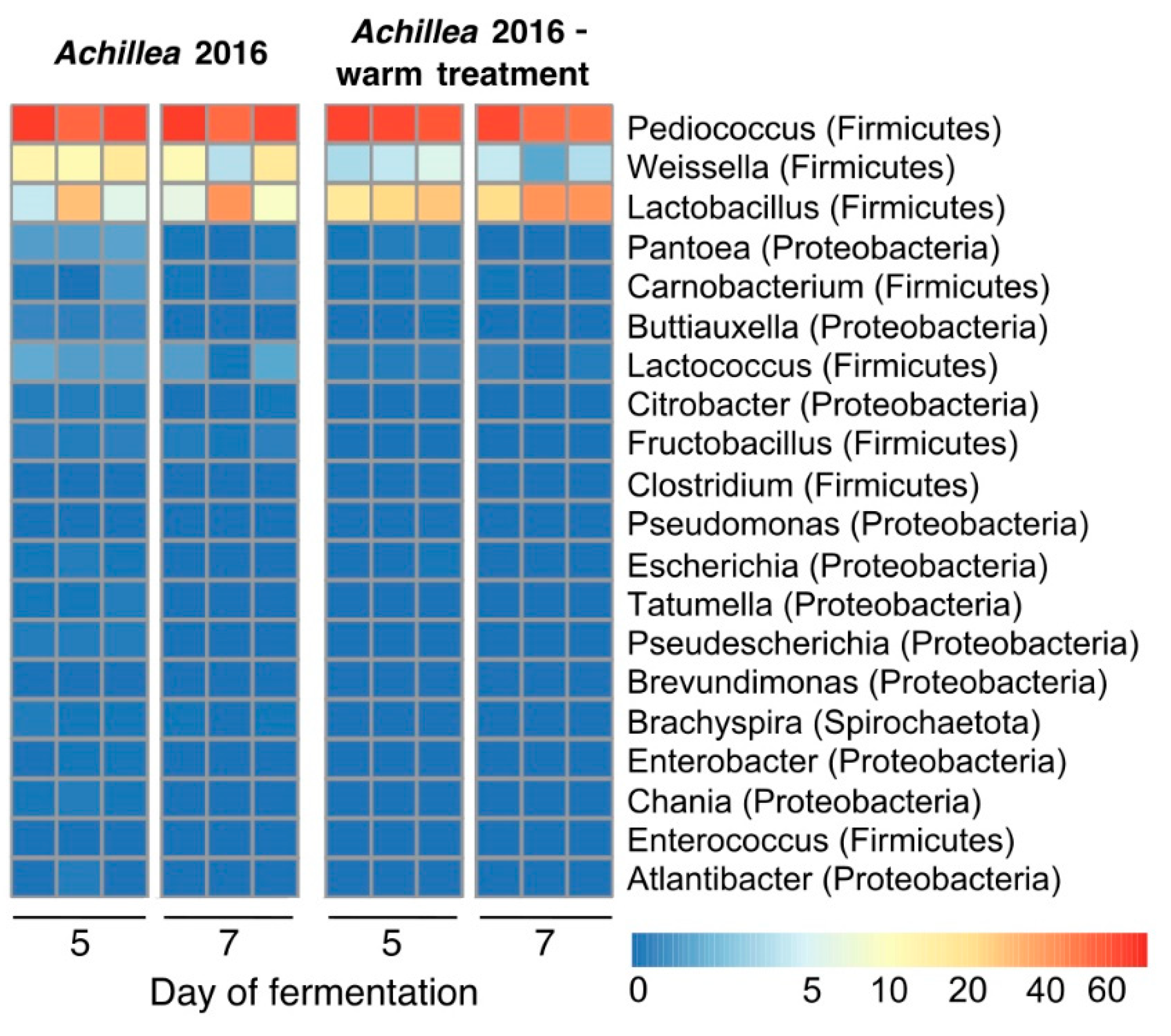
3.5. Effect of Cooling in the Fermentation of Achillea
4. Discussion
4.1. Early Fermentation Phase
4.2. Intermediate Fermentation Phase
4.3. Late Fermentation Phase
4.4. Co-Occurrence of Bacteria and Fungi
4.5. Recurring Cooling and Its Significance for the Fermenting Microbial Community
5. Summary and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiferaw Terefe, N.; Augustin, M.A. Fermentation for tailoring the technological and health related functionality of food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 2887–2913. [Google Scholar] [CrossRef]
- Subramaniyam, R.; Vimala, R. Solid state and submerged fermentation for the production of bioactive substances: A comparative study. Int. J. Sci. Nat. 2012, 3, 480–486. [Google Scholar]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.X.; Gao, W.Y.; Wang, H.Y. Review of traditional Chinese medicine processed by fermentation. China J. Chin. Mater. Medica 2012, 37, 3695–3700. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Fan, W.; Jiang, Y.; Zhang, C.; Li, J.; Peng, W.; Wu, C. The application of fermentation technology in Traditional Chinese Medicine: A review. Am. J. Chin. Med. 2020, 48, 899–921. [Google Scholar] [CrossRef]
- Naveen Chandra, D.; Preethidan, D.S.; Sabu, A.; Haridas, M. Traditional fermentation of Ayurvedic medicine yields higher proinflammatory enzyme inhibition compared to wine-model product. Front. Life Sci. 2015, 8, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Okutsu, K.; Futagami, T.; Yoshizaki, Y.; Tamaki, H.; Maruyama, T.; Toume, K.; Komatsu, K.; Hashimoto, F.; Takamine, K. Microbial community structure and chemical constituents in Shinkiku, a fermented crude drug used in Kampo medicine. Front. Nutr. 2020, 7, 115. [Google Scholar] [CrossRef]
- Millet, A.; Stintzing, F.; Merfort, I. Flavonol quantification and stability of phenolics in fermented extracts from fresh Betula pendula leaves. J. Pharm. Biomed. Anal. 2010, 53, 137–144. [Google Scholar] [CrossRef]
- Schwarzenberger, M.; Stintzing, F.; Meyer, U.; Lindequist, U. Biochemical, microbiological and phytochemical studies on aqueous-fermented extracts from Atropa belladonna L. Part 1—Biochemistry and microbiology. Pharmazie 2012, 67, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Duckstein, S.M.; Lorenz, P.; Stintzing, F.C. Conversion of phenolic constituents in aqueous Hamamelis virginiana leaf extracts during fermentation. Phytochem. Anal. 2012, 23, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, P.; Bunse, M.; Sauer, S.; Conrad, J.; Stintzing, F.C.; Kammerer, D.R. Conversion of plant secondary metabolites upon fermentation of Mercurialis perennis L. extracts with two lactobacteria strains. Fermentation 2019, 5, 42. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Bose, S.; Wang, J.H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Kim, H.Y.; Bae, W.Y.; Yu, H.S.; Chang, K.H.; Hong, Y.H.; Lee, N.K.; Paik, H.D. Inula britannica fermented with probiotic Weissella cibaria D30 exhibited anti-inflammatory effect and increased viability in RAW 264.7 cells. Food Sci. Biotechnol. 2020, 29, 569–578. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Cao, G.; Ma, F.; Xu, J.; Zhang, Y. Microbial community succession and toxic alkaloids change during fermentation of Huafeng Dan Yaomu. Lett. Appl. Microbiol. 2020, 70, 318–325. [Google Scholar] [CrossRef]
- Sanchez, S.; Demain, A.L. Bioactive products from fungi. In Food Bioactives; Puri, M., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2017; pp. 59–87. ISBN 978-3-319-51637-0. [Google Scholar]
- Pham, J.V.; Yilma, M.A.; Feliz, A.; Majid, M.T.; Maffetone, N.; Walker, J.R.; Kim, E.; Cho, H.J.; Reynolds, J.M.; Song, M.C.; et al. A review of the microbial production of bioactive natural products and biologics. Front. Microbiol. 2019, 10, 1404. [Google Scholar] [CrossRef] [Green Version]
- Dowarah, R.; Verma, A.K.; Agarwal, N. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: A review. Anim. Nutr. 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Nayak, B.S.; Marshall, J.R.; Isitor, G.; Adogwa, A. Hypoglycemic and hepatoprotective activity of fermented fruit juice of Morinda citrifolia (noni) in diabetic rats. Evidence-Based Complement. Altern. Med. 2011, 2011, 875293. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Akanda, M.R.; Islam, A.; Yang, H.D.; Lee, S.C.; Lee, J.H.; Kim, S.K.; Choi, Y.J.; Im, S.Y.; Park, B.Y. The anti-stress effect of Mentha arvensis in immobilized rats. Int. J. Mol. Sci. 2018, 19, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.; Bin Jeon, S.; Lee, Y.; Lee, H.; Kim, J.; Kwon, B.R.; Yu, K.Y.; Cha, J.D.; Hwang, S.M.; Choi, K.M.; et al. Fermented red ginseng extract inhibits cancer cell proliferation and viability. J. Med. Food 2015, 18, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Sugimoto, S.; Noda, M.; Yokooji, T.; Danshiitsoodol, N.; Higashikawa, F.; Sugiyama, M. Interleukin-8 release inhibitors generated by fermentation of Artemisia princeps Pampanini herb extract with Lactobacillus plantarum SN13T. Front. Microbiol. 2020, 11, 1159. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.L. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef]
- Wakefield, J.; Hassan, H.M.; Jaspars, M.; Ebel, R.; Rateb, M.E. Dual induction of new microbial secondary metabolites by fungal bacterial co-cultivation. Front. Microbiol. 2017, 8, 1284. [Google Scholar] [CrossRef] [Green Version]
- Maglangit, F.; Fang, Q.; Kyeremeh, K.; Sternberg, J.M.; Ebel, R.; Deng, H. A co-culturing approach enables discovery and biosynthesis of a bioactive indole alkaloid metabolite. Molecules 2020, 25, 256. [Google Scholar] [CrossRef] [Green Version]
- Roullier-Gall, C.; David, V.; Hemmler, D.; Schmitt-Kopplin, P.; Alexandre, H. Exploring yeast interactions through metabolic profiling. Sci. Rep. 2020, 10, 6073. [Google Scholar] [CrossRef] [Green Version]
- Canon, F.; Nidelet, T.; Guédon, E.; Thierry, A.; Gagnaire, V. Understanding the mechanisms of positive microbial interactions that benefit lactic acid bacteria co-cultures. Front. Microbiol. 2020, 11, 2088. [Google Scholar] [CrossRef]
- Pontonio, E.; Di Cagno, R.; Tarraf, W.; Filannino, P.; De Mastro, G.; Gobbetti, M. Dynamic and assembly of epiphyte and endophyte lactic acid bacteria during the life cycle of Origanum vulgare L. Front. Microbiol. 2018, 9, 1372. [Google Scholar] [CrossRef]
- Sauer, S.; Dlugosch, L.; Kammerer, D.R.; Stintzing, F.C.; Simon, M. The microbiome of the medicinal plants Achillea millefolium L. and Hamamelis virginiana L. Front. Microbiol. 2021, 12, 696398. [Google Scholar] [CrossRef]
- EMA/HMPC Addendum to Assessment Report on Achillea millefolium L., flos. 2018. Available online: www.ema.europa.eu/contact (accessed on 28 February 2022).
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: A review. Phyther. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, P.; Beckmann, C.; Felenda, J.; Meyer, U.; Stintzing, F.C. Das Waldbingelkraut (Mercurialis perennis L.)—Pharmakognosie einer alten Arzneipflanze. Zeitschrift für Phyther. 2013, 34, 40–46. [Google Scholar] [CrossRef]
- Blanco-Salas, J.; Vazquez, F.M.; Hortigón-Vinagre, M.P.; Ruiz-Tellez, T. Bioactive phytochemicals from Mercurialis spp. used in traditional Spanish medicine. Plants 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vodnar, D.C.; Pop, O.L.; Socaciu, C. Monitoring lactic acid fermentation in media containing dandelion (Taraxacum officinale) by FTIR spectroscopy. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, M.E.M.; Díaz Peralta, K.; Jorquera Martínez, L.; Chamy Maggi, R. Taraxacum genus: Potential antibacterial and antifungal activity. In Herbal Medicine; IntechOpen: London, UK, 2019; pp. 247–270. [Google Scholar]
- Kenny, O.; Brunton, N.P.; Walsh, D.; Hewage, C.M.; McLoughlin, P.; Smyth, T.J. Characterisation of antimicrobial extracts from dandelion root (Taraxacum officinale) using LC-SPE-NMR. Phyther. Res. 2015, 29, 526–532. [Google Scholar] [CrossRef]
- Di Napoli, A.; Zucchetti, P. A comprehensive review of the benefits of Taraxacum officinale on human health. Bull. Nat. Res. Cent. 2021, 45, 110. [Google Scholar] [CrossRef]
- GHP. German Homoeopathic Pharmacopoeia (GHP 2020); Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2020; ISBN 978-3-8047-5086-9. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Parks, D.H.; Chuvochina, M.; Waite, D.W.; Rinke, C.; Skarshewski, A.; Chaumeil, P.A.; Hugenholtz, P. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 2018, 36, 996–1004. [Google Scholar] [CrossRef]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, R.H.; Kõljalg, U. UNITE general FASTA release for eukaryotes 2. UNITE Community 2020. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.4-3. 2017. Available online: https://cran.r-project.org/package=vegan (accessed on 28 February 2022).
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2018, 35, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. InterJournal Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Friedman, J.; Alm, E.J. Inferring correlation networks from genomic survey data. PLoS Comput. Biol. 2012, 8, e1002687. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Huang, C.; Zhao, H.; Deng, M. CCLasso: Correlation inference for compositional data through Lasso. Bioinformatics 2015, 31, 3172–3180. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Liang, H.; Yin, L.; Zhang, Y.; Chang, C.; Zhang, W. Dynamics and diversity of a microbial community during the fermentation of industrialized Qingcai paocai, a traditional Chinese fermented vegetable food, as assessed by Illumina MiSeq sequencing, DGGE and qPCR assay. Ann. Microbiol. 2018, 68, 111–122. [Google Scholar] [CrossRef]
- Cason, E.D.; Mahlomaholo, B.J.; Taole, M.M.; Abong, G.O.; Vermeulen, J.G.; de Smidt, O.; Vermeulen, M.; Steyn, L.; Valverde, A.; Viljoen, B. Bacterial and fungal dynamics during the fermentation process of sesotho, a traditional beer of Southern Africa. Front. Microbiol. 2020, 11, 1451. [Google Scholar] [CrossRef] [PubMed]
- Khanongnuch, C.; Unban, K.; Kanpiengjai, A.; Saenjum, C. Recent research advances and ethno-botanical history of miang, a traditional fermented tea (Camellia sinensis var. assamica) of northern Thailand. J. Ethn. Foods 2017, 4, 135–144. [Google Scholar] [CrossRef]
- Unban, K.; Kodchasee, P.; Shetty, K.; Khanongnuch, C. Tannin-tolerant and extracellular tannase producing Bacillus isolated from traditional fermented tea leaves and their probiotic functional properties. Foods 2020, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Köberl, M.; Erschen, S.; Etemadi, M.; White, R.A.; El-Arabi, T.F.; Berg, G. Deciphering the microbiome shift during fermentation of medicinal plants. Sci. Rep. 2019, 9, 13461. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.B.; Biango-Daniels, M.N.; Hodge, K.T.; Worobo, R.W. Nature abhors a vacuum: Highly diverse mechanisms enable spoilage fungi to disperse, survive, and propagate in commercially processed and preserved foods. Compr. Rev. Food Sci. Food Saf. 2019, 18, 286–304. [Google Scholar] [CrossRef] [PubMed]
- Cuvas-Limon, R.B.; Nobre, C.; Cruz, M.; Rodriguez-Jasso, R.M.; Ruíz, H.A.; Loredo-Treviño, A.; Texeira, J.A.; Belmares, R. Spontaneously fermented traditional beverages as a source of bioactive compounds: An overview. Crit. Rev. Food Sci. Nutr. 2021, 61, 2984–3006. [Google Scholar] [CrossRef]
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Li, Q.; Huang, J.; Li, Y.; Zhang, Y.; Luo, Y.; Chen, Y.; Lin, H.; Wang, K.; Liu, Z. Fungal community succession and major components change during manufacturing process of Fu brick tea. Sci. Rep. 2017, 7, 6947. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, J.; Dan, T.; Zhang, W.; Zhang, H. Phylogenesis and evolution of lactic acid bacteria. In Lactic Acid Bacteria: Fundamentals and Practice; Springer: Dordrecht, The Netherlands, 2014; pp. 1–101. ISBN 9789401788410. [Google Scholar]
- Ruiz Rodríguez, L.G.; Mohamed, F.; Bleckwedel, J.; Medina, R.; De Vuyst, L.; Hebert, E.M.; Mozzi, F. Diversity and functional properties of lactic acid bacteria isolated from wild fruits and flowers present in northern Argentina. Front. Microbiol. 2019, 10, 1091. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Dong, M.; Li, Q.; Xu, F.; Wang, S.; Chen, J.; Li, W. Effects of microbial inoculants on the fermentation characteristics and microbial communities of sweet sorghum bagasse silage. Sci. Rep. 2020, 10, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassim, M.A.; Baijnath, H.; Odhav, B. Effect of traditional leafy vegetables on the growth of lactobacilli and bifidobacteria. Int. J. Food Sci. Nutr. 2014, 65, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Fusco, V.; Quero, G.M.; Cho, G.S.; Kabisch, J.; Meske, D.; Neve, H.; Bockelmann, W.; Franz, C.M.A.P. The genus Weissella: Taxonomy, ecology and biotechnological potential. Front. Microbiol. 2015, 6, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Saren, G.; Ji, Y.; Guan, Y.; Feng, K. Microbial community dynamics and metabolome changes during spontaneous fermentation of Northeast sauerkraut from different households. Front. Microbiol. 2020, 11, 1878. [Google Scholar] [CrossRef]
- Lakra, A.K.; Domdi, L.; Hanjon, G.; Tilwani, Y.M.; Arul, V. Some probiotic potential of Weissella confusa MD1 and Weissella cibaria MD2 isolated from fermented batter. LWT 2020, 125, 109261. [Google Scholar] [CrossRef]
- Fessard, A.; Remize, F. Why are Weissella spp. not used as commercial starter cultures for food fermentation? Fermentation 2017, 3, 38. [Google Scholar] [CrossRef] [Green Version]
- Hampson, D.J.; Lugsomya, K.; La, T.; Phillips, N.D.; Trott, D.J.; Abraham, S. Antimicrobial resistance in Brachyspira—An increasing problem for disease control. Vet. Microbiol. 2019, 229, 59–71. [Google Scholar] [CrossRef]
- Yu, A.O.; Leveau, J.H.J.; Marco, M.L. Abundance, diversity and plant-specific adaptations of plant-associated lactic acid bacteria. Environ. Microbiol. Rep. 2020, 12, 16–29. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Chai, S.; Li, Y.; Huang, J.; Luo, Y.; Xiao, L.; Liu, Z. Biochemical components associated with microbial community shift during the pile-fermentation of primary dark tea. Front. Microbiol. 2018, 9, 1509. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhao, Q.S.; Chang, S.; Yang, J.G. Analysis of the microbial community structure during brewing of Sichuan Xiaoqu Baijiu. J. Am. Soc. Brew. Chem. 2019, 77, 210–219. [Google Scholar] [CrossRef]
- Wuyts, S.; Van Beeck, W.; Oerlemans, E.F.M.; Wittouck, S.; Claes, I.J.J.; De Boeck, I.; Weckx, S.; Lievens, B.; De Vuyst, L.; Lebeer, S. Carrot juice fermentations as man-made microbial ecosystems dominated by lactic acid bacteria. Appl Environ. Microbiol 2018, 84, e00134-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivey, M.; Massel, M.; Phister, T.G. Microbial interactions in food fermentations. Annu. Rev. Food Sci. Technol. 2013, 4, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Legras, J.L.; Zhang, P.; Chen, D.; Howell, K. Diversity and dynamics of fungi during spontaneous fermentations and association with unique aroma profiles in wine. Int. J. Food Microbiol. 2021, 338, 108983. [Google Scholar] [CrossRef]
- Wang, S.; Xiong, W.; Wang, Y.; Nie, Y.; Wu, Q.; Xu, Y.; Geisen, S. Temperature-induced annual variation in microbial community changes and resulting metabolome shifts in a controlled fermentation system. mSystems 2020, 5, e00555-20. [Google Scholar] [CrossRef]
- Vylkova, S.; Carman, A.J.; Danhof, H.A.; Collette, J.R.; Zhou, H.; Lorenz, M.C. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. MBio 2011, 2, e00055-11. [Google Scholar] [CrossRef] [Green Version]
- Michalak, M.; Gustaw, K.; Waśko, A.; Polak-Berecka, M. Composition of lactic acid bacteria during spontaneous curly kale (Brassica oleracea var. sabellica) fermentation. Microbiol. Res. 2018, 206, 121–130. [Google Scholar] [CrossRef]
- De Roos, J.; Vandamme, P.; De Vuyst, L. Wort substrate consumption and metabolite production during lambic beer fermentation and maturation explain the successive growth of specific bacterial and yeast species. Front. Microbiol. 2018, 9, 2763. [Google Scholar] [CrossRef]
- Pardali, E.; Paramithiotis, S.; Papadelli, M.; Mataragas, M.; Drosinos, E.H. Lactic acid bacteria population dynamics during spontaneous fermentation of radish (Raphanus sativus L.) roots in brine. World J. Microbiol. Biotechnol. 2017, 33, 110. [Google Scholar] [CrossRef]
- Ercolini, D.; Pontonio, E.; De Filippis, F.; Minervini, F.; La Storia, A.; Gobbetti, M.; Di Cagno, R. Microbial ecology dynamics during rye and wheat sourdough preparation. Appl. Environ. Microbiol. 2013, 79, 7827–7836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco-Montealegre, M.E.; Dávila-Mora, L.L.; Botero-Rute, L.M.; Reyes, A.; Caro-Quintero, A. Fine resolution analysis of microbial communities provides insights into the variability of Cocoa bean fermentation. Front. Microbiol. 2020, 11, 650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartle, L.; Sumby, K.; Sundstrom, J.; Jiranek, V. The microbial challenge of winemaking: Yeast-bacteria compatibility. FEMS Yeast Res. 2019, 19, foz040. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhu, Y.; Fang, C.; Wijffels, R.H.; Xu, Y. Can we control microbiota in spontaneous food fermentation?—Chinese liquor as a case example. Trends Food Sci. Technol. 2021, 110, 321–331. [Google Scholar] [CrossRef]
- Cruz-O’Byrne, R.; Piraneque-Gambasica, N.; Aguirre-Forero, S. Microbial diversity associated with spontaneous coffee bean fermentation process and specialty coffee production in northern Colombia. Int. J. Food Microbiol. 2021, 354, 109282. [Google Scholar] [CrossRef]
- Zhang, Q.; Geng, Z.; Li, D.; Ding, Z. Characterization and discrimination of microbial community and co-occurrence patterns in fresh and strong flavor style flue-cured tobacco leaves. Microbiologyopen 2020, 9, e965. [Google Scholar] [CrossRef]
- Sanders, J.W.; Venema, G.; Kok, J. Environmental stress responses in Lactococcus lactis. FEMS Microbiol. Rev. 1999, 23, 483–501. [Google Scholar] [CrossRef]









| Month | 1 | 2–4 | 5–7 | 8–11 | ≥12 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | 1 | 2 | 4 | 6 | 8 | 12 | 14 | 20 | 21 | 24 | 28 | 33 | 42 | 43 | 46 | 49 | 50 | 53 | 58 | 61 | 100 | ||||||||
| Achillea 2016 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 13 | 56 | 140 | 291 | 424 | ||||||||||||||||
| Achillea 2018 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 14 | 28 | 42 | 56 | 84 | 168 | 294 | 366 | |||||||||||||
| Taraxacum | 0 | 1 | 3 | 5 | 7 | 27 | 166 | 322 | |||||||||||||||||||||
| Mercurialis | 0 | 0.5 | 1 | 3 | 7 | 39 | 192 | 226 | 301 | 352 | |||||||||||||||||||
| Euphrasia | 0 | 1 | 3 | 7 | 97 | 226 | 342 | 406 | |||||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauer, S.; Dlugosch, L.; Milke, F.; Brinkhoff, T.; Kammerer, D.R.; Stintzing, F.C.; Simon, M. Succession of Bacterial and Fungal Communities during Fermentation of Medicinal Plants. Fermentation 2022, 8, 383. https://doi.org/10.3390/fermentation8080383
Sauer S, Dlugosch L, Milke F, Brinkhoff T, Kammerer DR, Stintzing FC, Simon M. Succession of Bacterial and Fungal Communities during Fermentation of Medicinal Plants. Fermentation. 2022; 8(8):383. https://doi.org/10.3390/fermentation8080383
Chicago/Turabian StyleSauer, Simon, Leon Dlugosch, Felix Milke, Thorsten Brinkhoff, Dietmar R. Kammerer, Florian C. Stintzing, and Meinhard Simon. 2022. "Succession of Bacterial and Fungal Communities during Fermentation of Medicinal Plants" Fermentation 8, no. 8: 383. https://doi.org/10.3390/fermentation8080383
APA StyleSauer, S., Dlugosch, L., Milke, F., Brinkhoff, T., Kammerer, D. R., Stintzing, F. C., & Simon, M. (2022). Succession of Bacterial and Fungal Communities during Fermentation of Medicinal Plants. Fermentation, 8(8), 383. https://doi.org/10.3390/fermentation8080383








