Guava Seed Oil: Potential Waste for the Rhamnolipids Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rhamnolipid Production
2.1.1. Microorganism
2.1.2. Culture Medium
2.1.3. Carbon Source—Guava Seed Oil
2.1.4. Cultivation Conditions
2.2. Optimization of Rhamnolipid Production
2.3. Sample Processing
2.3.1. Determination of Microbial Biomass
2.3.2. Determination of Consumption of Carbon Source
2.4. Extraction of Rhamnolipids
2.4.1. Determination of Rhamnolipid Concentration—HPLC
2.4.2. Determination of Composition of Homologues by LC-MS
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manga, E.B.; Celik, P.A.; Cabuk, A.; Banat, I.M. Biosurfactants: Opportunities for the development of a sustainable future. Adv. Colloid Interface 2021, 56, 101514. [Google Scholar] [CrossRef]
- Patel, S.; Kharawala, K. Biosurfactants and Their Biodegradability: A Review and Examination. IJEAT 2022, 11, 4–11. [Google Scholar] [CrossRef]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Costa, S.G.V.A.O. Biosurfactants in Food Industry. TIFS 2007, 18, 252–259. [Google Scholar] [CrossRef]
- Oliveira, F.J.S.; Vazquez, L.; Campos, N.P.; França, F.P. Production of rhamnolipids by a Pseudomonas alcaligenes strain. Process Biochem. 2009, 44, 383–389. [Google Scholar] [CrossRef]
- Lovaglio, R.B.; Costa, S.G.V.A.O.; Lima, C.J.B.; Cortezi, M.; Contiero, J. Effect of C/N ratio and physicochemical conditions on the production of rhamnolipids by Pseudomonas aeruginosa LBI. Res. J. Biotechnol. 2010, 5, 19–24. [Google Scholar]
- Henkel, M.; Müller, M.M.; Kügler, J.H.; Lovaglio, R.B.; Contiero, J.; Sydaltk, C.; Hausmann, R. Rhamnolipids as biosurfactants from renewable resources: Concepts for next-generation rhamnolipds production. Process Biochem. 2012, 47, 1207–1219. [Google Scholar] [CrossRef]
- Dobler, L.L.; Vilela, F.; Ameida, R.V.; Neves, B.C. Rhamnolipids in perspective: Gene regulatory pathways, metabolic engineering, production and technological forecasting. New Biotechnol. 2016, 33, 123–135. [Google Scholar] [CrossRef]
- Shao, B.; Liu, Z.; Zhong, H.; Zeng, G.; Liu, G.; Yu, M.; Liu, Y.; Zhang, X.; Li, Z.; Frang, Z.; et al. Effects of rhamnolipids on microorganism characteristics and applications in composting: A review. Microbiol. Res. 2017, 200, 33–44. [Google Scholar] [CrossRef]
- Biselli, A.; Willenbrink, A.L.; Leipnitz, M.; Jupke, A. Development, evaluation, and optimisation of downstream process concepts for rhamnolipids and 3-(3-hydroxyalkanoyloxy) alkanoic acids. Sep. Purif. Technol. 2020, 250, 117031. [Google Scholar] [CrossRef]
- Moutinho, L.F.; Moura, F.R.; Silvestre, R.C.; Dumaresq, A.S.R. Microbial biosurfactants A broad analysis of properties, applications, biosynthesis, and techno-economical assessment of rhamnolipid production. Biotechnol. Progress 2021, 37, e3093. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Liu, G.; Jiang, Y.; Yang, J.; Liu, Y.; Yang, X.; Liu, Z.; Zeng, G. Transport of bacteria in porous media and its enhancement by surfactants for bioaugmentation: A review. Biotechnol. Adv. 2017, 35, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Pham, J.V.; Yilma, M.A.; Feliz, A.; Majid, M.T.; Maffetone, N.; Walker, J.R.; Kim, E.; Cho, H.J.; Reynolds, J.M.; Song, M.C.; et al. A Review of the Microbial Production of Bioactive Natural Products and Biologics. Front. Microbiol. 2019, 10, 1404. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, C.N. Environmental applications for biosurfactants. Environ. Pollut. 2005, 133, 183–198. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Q.; Zhang, Y.; Lei, L. Anaerobic biosynthesis of rhamnolipid by Pseudomonas aeruginosa: Performance, mechanism and its application potential for enhanced oil recovery. Microb. Cell Fact. 2021, 20, 103. [Google Scholar] [CrossRef]
- Huang, C.P.; Wang, H.W.; Chiu, P.C. Nitrate reduction by metallic iron. Water Res. 1998, 32, 2257–2264. [Google Scholar] [CrossRef]
- Choe, S.; Liljestrand, H.M.; Khim, J. Nitrate Reduction by Zero-Valent Iron under Different PH Regimes. Appl. Geochem. 2004, 19, 335–342. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zhang, T.C. Effects of Low PH on Nitrate Reduction by Iron Powder. Water Res. 2004, 38, 2631–2642. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, D.G.; Shin, H.S. Mechanism Study of Nitrate Reduction by Nano Zero Valent Iron. J. Hazard. Mater. 2011, 185, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Crane, R.A.; Scott, T.B. Nanoscale Zero-Valent Iron: Future Prospects for an Emerging Water Treatment Technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on Nano Zerovalent Iron (NZVI): From Synthesis to Environmental Applications. Chem. Eng. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Yirsaw, B.D.; Megharaj, M.; Chen, Z.; Naidu, R. Environmental Application and Ecological Significance of Nano-Zero Valent Iron. J. Environ. Sci. 2016, 44, 88–98. [Google Scholar] [CrossRef]
- Xue, W.; Huang, D.; Zeng, G.; Wan, J.; Zhang, C.; Xu, R.; Cheng, M.; Deng, R. Nanoscale Zer-Valent Iron Coated with Rhamnolipid as an Effective Stabilizer for Immobilization of Cd and Pb in River Sediments. J. Hazard. Mater. 2018, 341, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.C.; Salazar-Bryam, A.M.; Piazza, R.D.; Santo, C.C.; Jafelicci, M., Jr.; Marques, R.F.C.; Contiero, J. Rhamnolipids as Green Stabilizers of nZVI and Application in the Removal of Nitrate from Simulated Groundwater. Front. Bioeng. Biotechnol. 2022, 10, 794460. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, A.; Sharma, R.; Aurora, R.; Jain, S.K. Biosurfactants as a Novel Additive in Pharmaceutical Formulations: Current Trends and Future Implications. Curr. Drug Metab. 2020, 21, 885–901. [Google Scholar] [CrossRef]
- Bjerk, T.R.; Severino, P.; Jain, S.; Marques, C.; Silva, A.M.; Pashirova, T.; Souto, E.B. Biosurfactants: Properties and Applications in Drug Delivery, Biotechnology and Ecotoxicology. Bioengineering 2021, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Çelik, P.A.; Manga, E.B.; Çabuk, A.; Banat, I.M. Biosurfactants’ Potential Role in Combating COVID-19 and Similar Future Microbial Threats. Appl. Sci. 2021, 11, 334. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, H.; Yang, X.; Liu, Y.; Shao, B.; Liu, Z. Advances in applications of rhamnolipids biosurfactant in environmental remediation: A review. Biotechnol. Bioeng. 2017, 115, 796–814. [Google Scholar] [CrossRef]
- Lang, S.; Wagner, F. Structure and properties of biosurfactants. In Biosurfactants and Biotechnology, 1st ed.; Kosaric, N., Cairns, W.L., Gray, N.C.C., Eds.; Routledge: New York, NY, USA, 1987; 25p. [Google Scholar]
- El-Housseiny, G.S.; Aboshanab, K.M.; Aboulwafa, M.M.; Hassouna, N.A. Structural and Physicochemical Characterization of Rhamnolipids produced by Pseudomonas aeruginosa P6. AMB Express 2020, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipds: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biothecnol. 2011, 86, 1323–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, S.; Das, P.; Sen, R. Towards Commercial Production of Microbial Surfactants. Trends Biotechnol. 2006, 24, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.H.; Pirôllo, M.P.S.; Lovaglio, R.B.; Costa, S.G.V.B.O.; Pizzolitto, E.L.; Contiero, J. Factors affecting Rhamnolipids Production by Pseudomonas aeruginosa LBI. Res. J. Biotechnol. 2008, 3, 45–49. [Google Scholar]
- Rodrigues, M.I.; Iemma, A.F. Planejamento de Experimentos e Otimização de Processos. Uma Estratégia Sequencial de Planejamento, 1st ed.; Casa do Pão: Campinas, Brazil, 2005; 326p. [Google Scholar]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.; Wong, J.W.C.; Bui, X.-T. A critical review on various feedstocks as sustainable substrates for biosurfactants production: A way towards cleaner production. Microb. Cell Fact. 2021, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Ogugua, V.; Okpashi, V.E.; Okechukwu, I.; Njoku, O.U. Production of Biosurfactants from submerged fermented fruit bagasse of yellow cashew (Anacardium occidentale) using Pseudomonas aeruginosa. Int. J. Sci. Eng. Res. 2015, 6, 1442–1453. [Google Scholar]
- Santos, B.F.; Simiqueli, A.P.R.; Pnezi, A.N.; Pastore, G.M.; Fileti, A.M.F. Monitoring of biosurfactant production by Bacillus subtilis using beet peel as culture medium via the development of a neural soft-sensor in an electronic spreadsheet. Braz. J. Chem. Eng. 2018, 35, 1355–1369. [Google Scholar] [CrossRef]
- Salazar-Bryam, A.M.; Lovaglio, R.B.; Contiero, J. Biodiesel byproduct bioconversion to rhamnolipids: Upstream aspects. Heliyon 2017, 3, e00337. [Google Scholar] [CrossRef] [Green Version]
- Panadare, D.; Rathod, V. Applications of Waste Cooking Oil Other Than Biodiesel: A Review. Iran. J. Chem. Eng. 2015, 12, 55–76. [Google Scholar]
- Reddy, K.S.; Khan, M.Y.; Archana, K.; Reddy, M.G.; Hameeda, B. Utilization of mango kernel oil for the rhamnolipid production by Pseudomonas aeruginosa DR1 towards its application as biocontrol agent. Bioresour. Technol. 2016, 221, 291–299. [Google Scholar] [CrossRef]
- Vitti, K.A.; Lima, L.M.; Martines Filho, J.G. Agricultural and economic characterization of guava product in Brazil. Rev. Bras. Frutic. 2020, 42, 1–11. [Google Scholar] [CrossRef] [Green Version]
- FAO—Food and Agriculture Organization of the United Nations. Area: All Countries. Production Items: Mangoes, Mangosteens, Guavas and Lemons and Limes. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 26 May 2020).
- Kobori, C.N.; Jorge, N. Caracterização dos Óleos de Algumas Sementes de Frutas como Aproveitamento de Resíduos Industriais. Ciênc. Agrotec. 2005, 29, 1008–1014. [Google Scholar] [CrossRef] [Green Version]
- Nicanor, A.B.; Moreno, A.O.; Martinez-Ayala, A.L.; Ortiz, G.D. Guava seed protein isolate: Function and nutritional characterization. J. Food Biochem. 2001, 25, 77–90. [Google Scholar] [CrossRef]
- Nicanor, A.B.; Scilingo, A.A.; Añón, M.C.; Ortíz, G.D. Guava seed storage protein: Fractionation and characterization. LWT 2006, 39, 902–910. [Google Scholar] [CrossRef]
- Kapoor, D.; Sharma, P.; Sharma, M.M.; Kumari, A.; Kumar, R. Microbes in pharmaceutical industry. In Microbial Diversity, Interventions and Scope, 1st ed.; Sharma, S., Sharma, N., Sharma, M., Eds.; Springer: Singapore, 2020; pp. 259–299. [Google Scholar]
- Lovaglio, R.B.; Silva, V.L.; Capelini, T.D.L.; Eberlin, M.N.; Hausmann, R.; Henkel, M.; Contiero, J. Rhamnolipids production by a Pseudomonas aeruginosa LBI mutant: Solutions and homologues characterization. Tenside Surfact. Det. 2014, 51, 397–405. [Google Scholar] [CrossRef]
- Müller, M.M.; Hörmann, B.; Syldatk, C.; Hausmann, R. Pseudomonas aeruginosa PAO1 as a model for rhamnolipid production in bioreactor systems. Appl. Micriobiol. Biotechnol. 2010, 87, 167–174. [Google Scholar] [CrossRef]
- Müller, M.M.; Hörmann, B.; Kugel, M.; Syldatk, C.; Hausmann, R. Evaluation of rhamnolipids production capacity of Pseudomonas aeruginosa PAO1 in comparison to the rhamnolipids over-producer strains DSM 7108 and DSM 2874. Appl. Microbiol. Biotechnol. 2011, 89, 585–592. [Google Scholar] [CrossRef]
- Wittgens, A.; Kovacic, F.; Müller, M.M.; Gerlitzki, M.; Santiago-Schübel, B.; Hofmann, D.; Tiso, T.; Blank, L.M.; Henkel, M.; Hausmann, R.; et al. Novel insights into biosynthesis and uptake of rhamnolipids and their precursors. Appl. Microbiol. Biotechnol. 2017, 101, 2865–2878. [Google Scholar] [CrossRef] [Green Version]
- Schenk, T.; Schuphan, I.; Schmidt, B. High Performance liquid chromatographic determination of the rhamnolipids produced by Pseudomonas aeruginosa. J. Chromatogr. 1995, 693, 7–13. [Google Scholar] [CrossRef]
- Cheng, T.; Liang, J.; He, J.; Hu, X.; Ge, Z.; Liu, J. A novel rhamnolipid-producing Pseudomonas aeruginosa ZS1 isolate derived from petroleum sludge suitable for bioremediation. AMB Express 2017, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Raza, Z.A.; Khalid, Z.M.; Banat, I.M. Characterization of rhamnolipids produced by a Pseudomonas aeruginosa mutant strain grown on waste oils. J. Environ. Sci. Health A 2009, 44, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Mohammad, A.W.; Jahim, J.M.; Anuar, N. A holistic approach to managing palm oil mill effluent (POME): Biotechnological advances in the sustainable reuse of POME. Biotechnol. Adv. 2007, 27, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Santos, L.; KäppelI, O.; Fiechter, A. Pseudomonas aeruginosa biosurfactant production in continuous culture with glucose as carbon source. Appl. Environ. Microbiol. 1984, 48, 301–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramana, K.V.; Karanth, N.G. Factors Affecting Biosurfactant Production Using Pseudomonas aeruginosa CFTR-6 under Submerged Conditions. J. Chem. Technol. Biotechnol. 1989, 45, 249–257. [Google Scholar] [CrossRef]
- Radzuan, M.N.; Winterburn, J.; Banat, I. Bioreactor Rhamnolipid Production Using Palm Oil Agricultural Refinery By-Products. Processes 2021, 9, 2037. [Google Scholar] [CrossRef]
- Sodagari, M.; Ju, L.-K. Addressing the critical challenge for rhamnolipid production: Discontinued synthesis in extended stationary phase. Process Biochem. 2020, 91, 83–89. [Google Scholar] [CrossRef]
- Francy, D.S.; Thomas, J.M.; Raymond, R.L.; Ward, C.H. Emulsification of hydrocarbons by subsurface bacteria. J. Ind. Microbiol. 1991, 8, 237–245. [Google Scholar] [CrossRef]
- Käppeli, O.; Fiechter, A. Component from the cell surface of the hydrocarbon-utilizing yeast Candida tropicalis with possible relation to hydrocarbon transport. J. Bacteriol. 1977, 131, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Déziel, E.; Lépine, F.; Milot, S.; Villemur, R. rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy) alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology 2003, 149, 2005–2013. [Google Scholar] [CrossRef] [Green Version]
- İkizler, B.; Arslan, G.; Kipcak, E.; Dirik, C.; Çelenk, D.; Aktuğlu, T.; Helvac, Ş.Ş.; Peker, S. Surface adsorption and spontaneous aggregation of rhamnolipid mixtures in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2017, 519, 125–136. [Google Scholar] [CrossRef]
- Nicolò, M.S.; Cambria, M.G.; Impallomeni, G.; Rizzo, M.G.; Pellicorio, C.; Ballistreri, A.; Guglielmino, S.P.P. Carbon source effecst on the mono/dirhamnolipids ratio prodction by Pseudomonas aeruginosa L05, a new human respiratory isolate. New Biothec. 2017, 39, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, J.; Zhang, H.; Gao, M.; Liu, L.; Zhan, X. Recycling of cooking oil fume condensate for the production of rhamnolipids by Pseudomonas aeruginosa WB505. Bioprocess Biosyst. Eng. 2019, 42, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Costa, S.G.V.A.; Contiero, J. Rhamnolipids and PHAs: Recent reports on Pseudomonas-derived molecules of increasing industrial interest. Proc. Biochem. 2011, 46, 621–630. [Google Scholar] [CrossRef]
- Mata-Sandoval, J.C.; Karns, J.; Torrents, A. High-performance liquid chromatography method for the characterization of rhamnolipid mixtures produced by pseudomonas aeruginosa UG2 on corn oil. J. Chromatogr. 1999, 864, 211–220. [Google Scholar] [CrossRef]
- Perfumo, A.; Smyth, T.J.P.; Marchant, R.; Banat, I.M. Production and Roles of Biosurfactants and Bioemulsifiers in Accessing Hydrophobic Substrates. In Handbook of Hydrocarbon and Lipid Microbiology, 1st ed.; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1501–1514. [Google Scholar]
- Déziel, E.; Lepine, F.; Dennie, D.; Boismenu, D.; Mamer, O.A.; Villemur, R. Liquid chromatography/mass spectrometry analysis of mixtures of rhamnolipids produced by Pseudomonas aeruginosa strain 57RP grown on mannitol or naphthalene. Biochim. Biophys. Acta 1999, 1440, 244–252. [Google Scholar] [CrossRef]

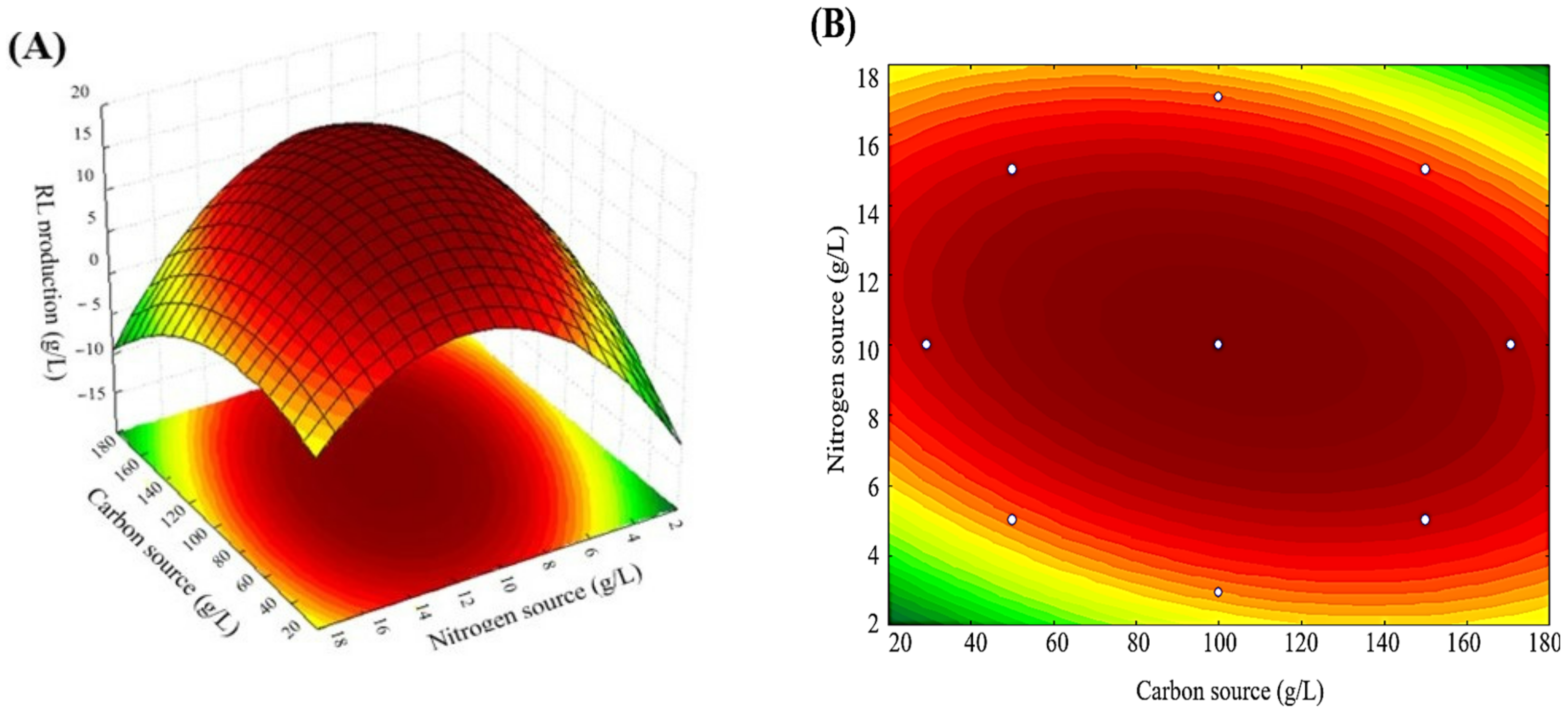
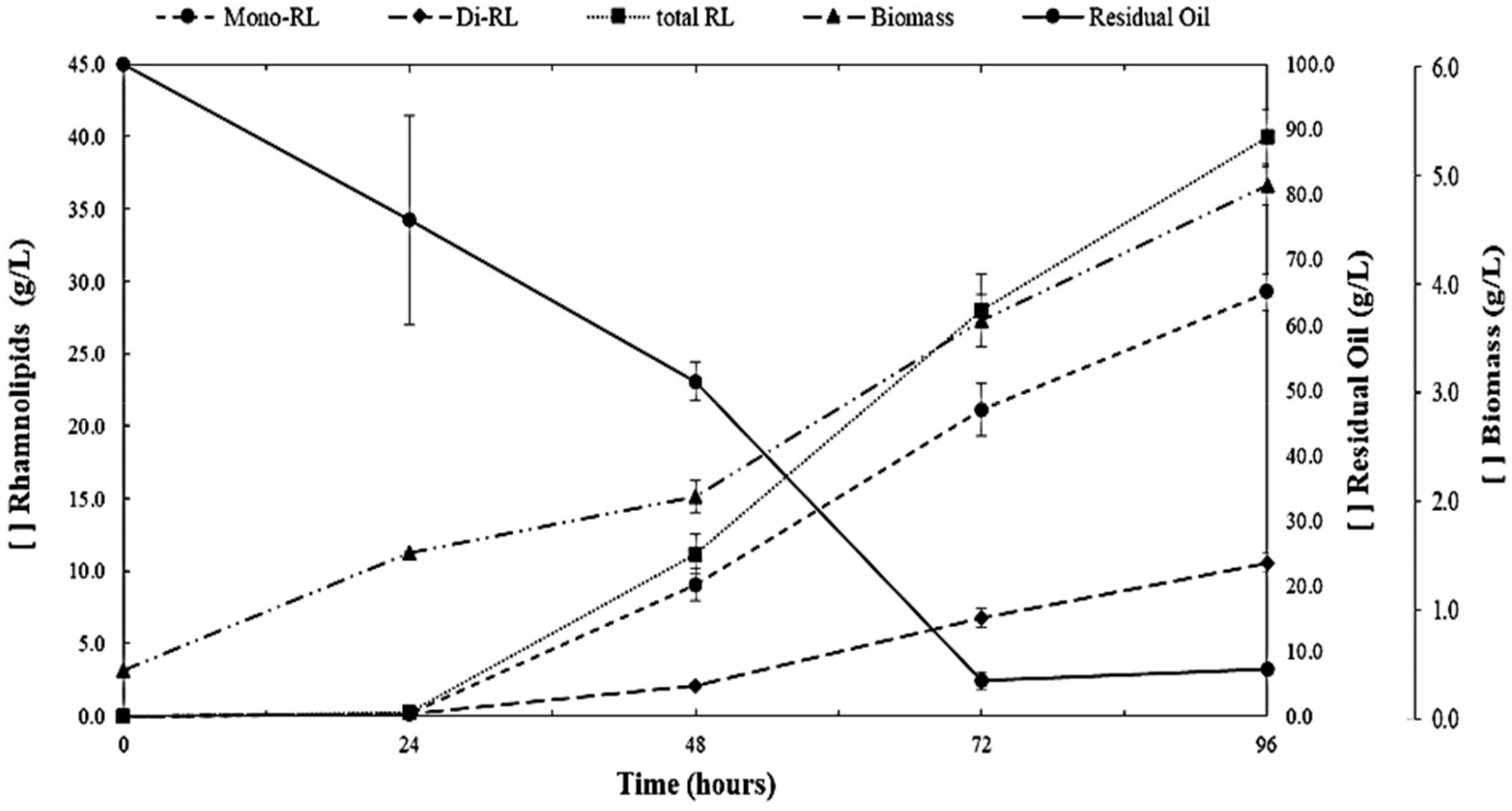
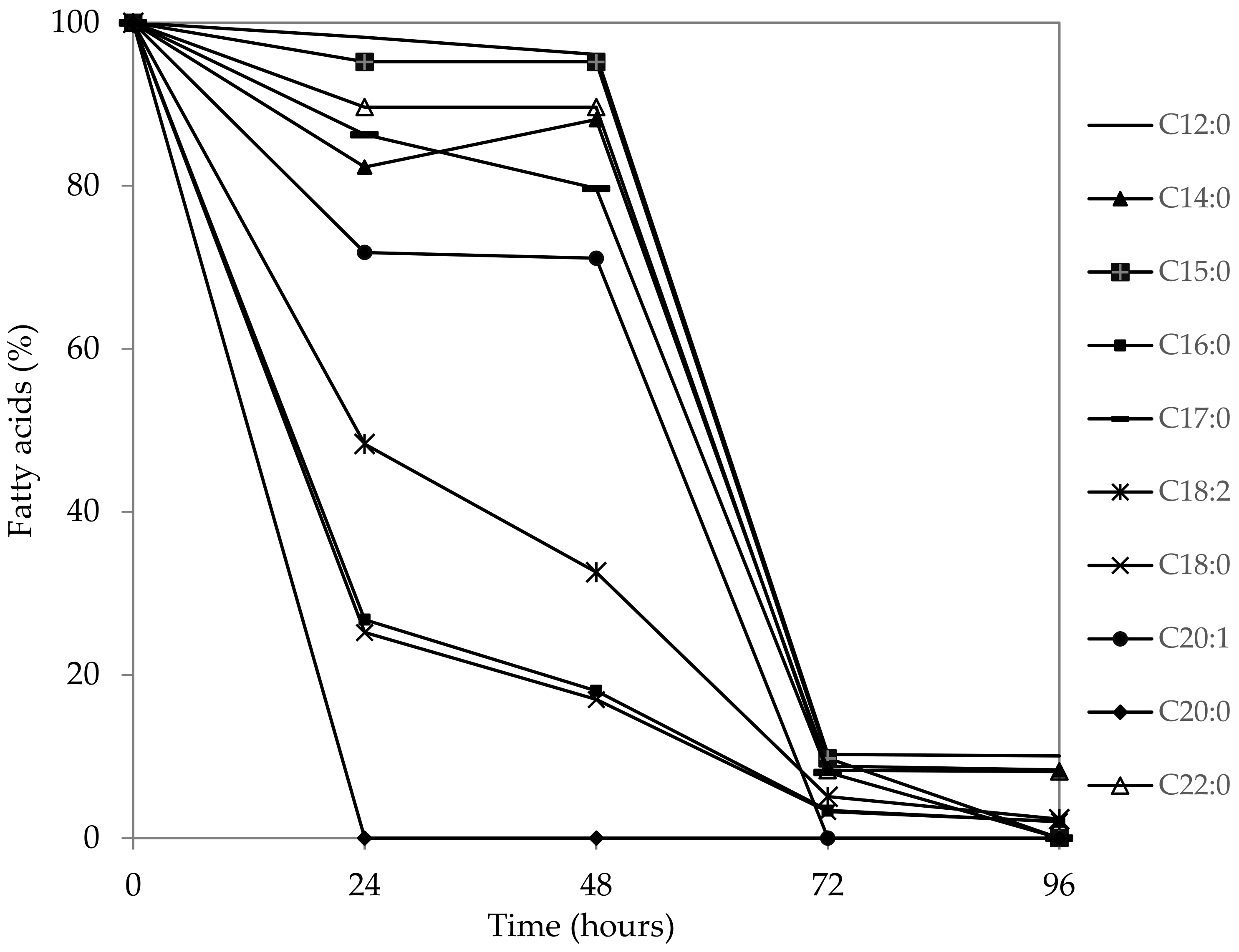
| Essay | Carbon Source (X1) | Nitrogen Source (X2) | ||
|---|---|---|---|---|
| Coded Level | Real Values (g/L) | Coded Level | Real Values (g/L) | |
| E1 | −1 | 50 | −1 | 5 |
| E2 | −1 | 50 | 1 | 15 |
| E3 | 1 | 150 | −1 | 5 |
| E4 | 1 | 150 | 1 | 15 |
| E5 | −1.414 | 29.3 | 0 | 10 |
| E6 | 1.414 | 170.7 | 0 | 10 |
| E7 | 0 | 100 | −1.414 | 2.93 |
| E8 | 0 | 100 | 1.414 | 17.07 |
| E9 | 0 | 100 | 0 | 10 |
| E10 | 0 | 100 | 0 | 10 |
| E11 | 0 | 100 | 0 | 10 |
| E12 | 0 | 100 | 0 | 10 |
| Essays | Coded Levels | Real Levels (g/L) | RL (Y) (g/L) | Biomass (g/L) | Conversion Factor Product/Biomass (g/g) | Proportion C/N | ||
|---|---|---|---|---|---|---|---|---|
| C (X1) | N (X2) | C (X1) | N (X2) | |||||
| E1 | −1 | −1 | 50.00 | 5.00 | 11.05 | 2.09 | 5.29 | 10.00 |
| E2 | −1 | 1 | 50.00 | 15.00 | 26.66 | 4.83 | 5.52 | 3.30 |
| E3 | 1 | −1 | 150.00 | 5.00 | 19.55 | 5.15 | 3.80 | 30.00 |
| E4 | 1 | 1 | 150.00 | 15.00 | 12.97 | 3.39 | 3.83 | 10.00 |
| E5 | −1.41 | 0 | 29.30 | 10.00 | 24.33 | 4.54 | 5.36 | 3.00 |
| E6 | +1.41 | 0 | 170.70 | 10.00 | 33.73 | 4.75 | 7.10 | 17.10 |
| E7 | 0 | −1.41 | 100.00 | 2.93 | 13.33 | 5.06 | 2.63 | 33.90 |
| E8 | 0 | +1.41 | 100.00 | 17.70 | 12.98 | 4.80 | 2.70 | 5.90 |
| E9 | 0 | 0 | 100.00 | 10.00 | 40.71 | 5.32 | 7.65 | 10.00 |
| E10 | 0 | 0 | 100.00 | 10.00 | 37.64 | 4.40 | 8.55 | 10.00 |
| E11 | 0 | 0 | 100.00 | 10.00 | 42.15 | 4.64 | 9.08 | 10.00 |
| E12 | 0 | 0 | 100.00 | 10.00 | 39.38 | 5.16 | 7.63 | 10.00 |
| ANOVA | |||||
|---|---|---|---|---|---|
| Variable | Quadratic Sum | Degrees of Freedom | Mean Square | F | p |
| (1) Carbon (L) | 8.239 | 1 | 8.239 | 2.2351 | 0.231785 |
| Carbon (Q) | 258.699 | 1 | 258.699 | 70.1796 | 0.003567 |
| (2) Nitrogen (L) | 9.095 | 1 | 9.095 | 2.4673 | 0.214267 |
| Nitrogen (Q) | 1307.878 | 1 | 1307.878 | 354.7991 | 0.000327 |
| 1L by 2L | 122.933 | 1 | 122.933 | 33.3490 | 0.010324 |
| Lack of Adjustment | 78.904 | 3 | 26.301 | 7.1350 | 0.070406 |
| pure error | 11.059 | 3 | 3.686 | ||
| Total Quadratic Sum | 1619.716 | 11 | |||
| No. | Elementary Composition | Molecular Structure | [M-H]− | Retention Time [min] | Relative Abundance (%) |
|---|---|---|---|---|---|
| m/z | |||||
| I | C24H44O9 | RhaC8C10 | 475.6 | 10.12 | 11.84 |
| II | C26H48O9 | RhaC10C10 | 503.4 | 12.09 | 100.00 |
| III | C28H50O9 | RhaC10C12:1 | 529.2 | 13.27 | 43.44 |
| IV | C28H52O9 | RhaC10C12 | 530.9 | 14.29 | 48.63 |
| V | C30H51O9 | RhaC10C14:2 | 555.6 | 14.13 | 9.30 |
| VI | C30H54O13 | RhaRhaC10C8; RhaRhaC8C10 | 621.4 | 9.22; 9.33 | 7.59 |
| VII | C32H58O13 | RhaRhaC10C10 | 649.1 | 11.12 | 28.37 |
| VIII | C34H60O13 | RhaRhaC10C12:1 | 675.6 | 12.28 | 9.41 |
| IX | C34H62O13 | RhaRhaC12C10; RhaRhaC10C12 | 677.6 | 13.13 | 57.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimura, I.; Salazar-Bryam, A.M.; Faria, A.U.d.; Leite, L.P.; Lovaglio, R.B.; Contiero, J. Guava Seed Oil: Potential Waste for the Rhamnolipids Production. Fermentation 2022, 8, 379. https://doi.org/10.3390/fermentation8080379
Yoshimura I, Salazar-Bryam AM, Faria AUd, Leite LP, Lovaglio RB, Contiero J. Guava Seed Oil: Potential Waste for the Rhamnolipids Production. Fermentation. 2022; 8(8):379. https://doi.org/10.3390/fermentation8080379
Chicago/Turabian StyleYoshimura, Ingrid, Ana Maria Salazar-Bryam, Adriano Uemura de Faria, Lucas Prado Leite, Roberta Barros Lovaglio, and Jonas Contiero. 2022. "Guava Seed Oil: Potential Waste for the Rhamnolipids Production" Fermentation 8, no. 8: 379. https://doi.org/10.3390/fermentation8080379
APA StyleYoshimura, I., Salazar-Bryam, A. M., Faria, A. U. d., Leite, L. P., Lovaglio, R. B., & Contiero, J. (2022). Guava Seed Oil: Potential Waste for the Rhamnolipids Production. Fermentation, 8(8), 379. https://doi.org/10.3390/fermentation8080379






