Production of Single-Cell Protein from Fruit Peel Wastes Using Palmyrah Toddy Yeast
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Physicochemical Properties of Fruit Peels
2.3. Culture Media and Inoculum Preparations
2.4. Production of SCP Using Liquid State Fermentation Process
2.5. Selection of the Best Substrate for SCP Production
2.6. Optimization of Fermentation Condition and Comparison with Control Medium
2.7. Effect of Nucleic Acid Reduction on SCP Production
2.8. Estimation of Amino Acid Content
2.9. Statistical Analysis
3. Results and Discussion
3.1. Compositional Analysis of Substrates
3.2. Selection of the Best Substrate for SCP Production
3.3. Optimization of Process Parameters in the SCP Production
3.3.1. Optimization of pH for SCP Production
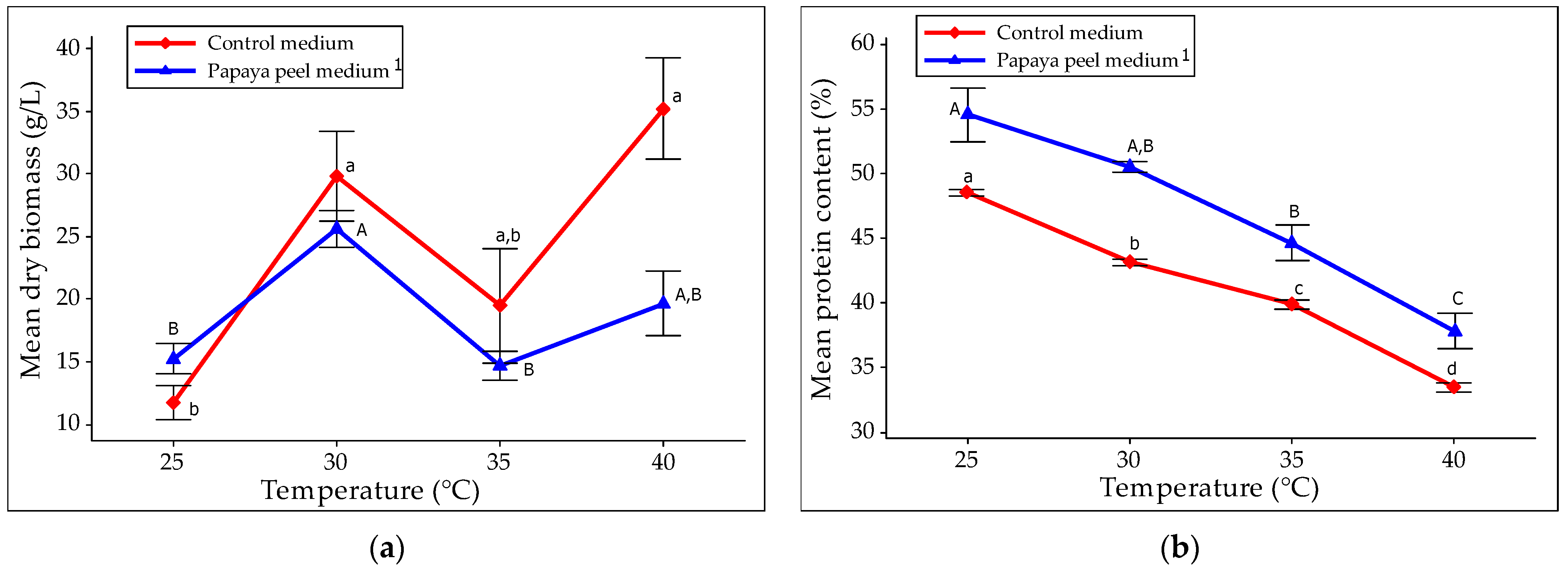
3.3.2. Optimization of Incubation Temperature for SCP Production
3.3.3. Optimization of Incubation Time for the SCP Production
3.3.4. Effect of Optimization on Biomass and Protein Content
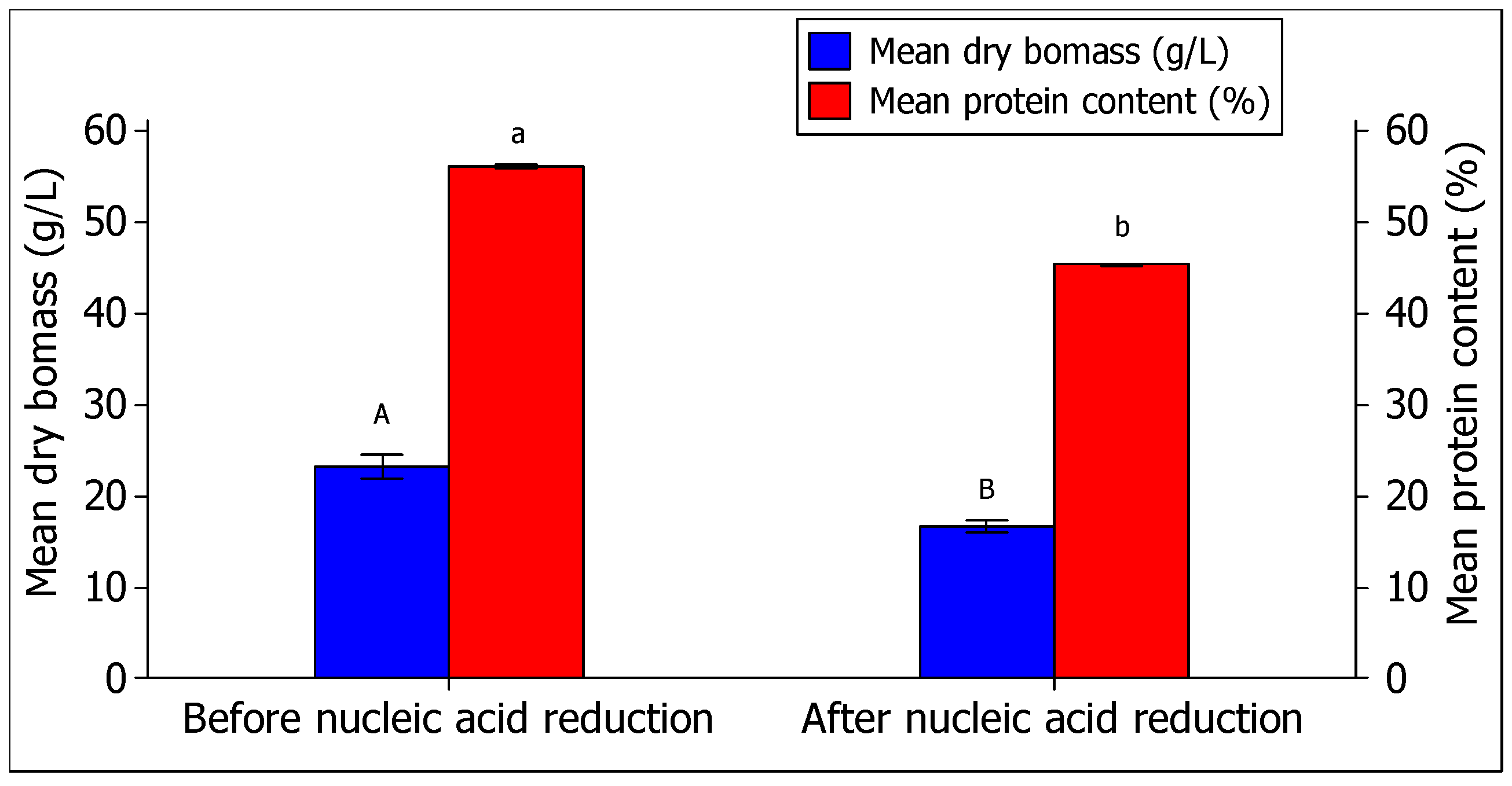
3.3.5. Effect of Nucleic Acid Reduction
3.3.6. Nutrient Analysis
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spalvins, K.; Zihare, L.; Blumberga, D. Single Cell Protein Production from Waste Biomass: Comparison of Various Industrial by-Products. Energy Procedia 2018, 147, 409–418. [Google Scholar] [CrossRef]
- Seviour, R.J.; Harvey, L.M.; Fazenda, M.; McNeil, B. 5—Production of Foods and Food Components by Microbial Fermentation: An Introduction. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; McNeil, B., Archer, D., Giavasis, I., Harvey, L., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2013; pp. 97–124. ISBN 978-0-85709-343-1. [Google Scholar]
- Abdel-Shafy, H.I.; Mansour, M.S.M. Solid Waste Issue: Sources, Composition, Disposal, Recycling, and Valorization. Egypt. J. Pet. 2018, 27, 1275–1290. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of Apple Processing Wastes as Low-Cost Substrates for Bioproduction of High Value Products: A Review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Panda, S.K.; Ray, R.C.; Mishra, S.S.; Kayitesi, E. Microbial Processing of Fruit and Vegetable Wastes into Potential Biocommodities: A Review. Crit. Rev. Biotechnol. 2018, 38, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, A.T.; Rasoul-Amini, S.; Morowvat, M.H.; Ghasemi, Y. Single Cell Protein: Production and Process. Am. J. Food Technol. 2011, 6, 103–116. [Google Scholar] [CrossRef]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single Cell Protein: Sources, Mechanism of Production, Nutritional Value and Its Uses in Aquaculture Nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Suman, G.; Nupur, M.; Anuradha, S.; Pradeep, B. Single Cell Protein Production: A Review. Int. J. Curr. Microbiol. App. Sci. 2015, 4, 251–262. [Google Scholar]
- Anupama; Ravindra, P. Value-Added Food: Single Cell Protein. Biotechnol. Adv. 2000, 18, 459–479. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M. Alternative Dietary Protein Sources for Farmed Tilapia, Oreochromis Spp. Aquaculture 1999, 179, 149–168. [Google Scholar] [CrossRef]
- García-Garibay, M.; Gómez-Ruiz, L.; Cruz-Guerrero, A.E.; Bárzana, E. SINGLE CELL PROTEIN | The Algae. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 425–430. ISBN 978-0-12-384733-1. [Google Scholar]
- Sadler, M.J. Fungal Protein. In New and Developing Sources of Food Proteins; Hudson, B.J.F., Ed.; Springer US: Boston, MA, USA, 1994; pp. 343–362. ISBN 978-1-4615-2652-0. [Google Scholar]
- Hezarjaribi, M.; Ardestani, F.; Ghorbani, H.R. Single Cell Protein Production by Saccharomyces Cerevisiae Using an Optimized Culture Medium Composition in a Batch Submerged Bioprocess. Appl. Biochem. Biotechnol. 2016, 179, 1336–1345. [Google Scholar] [CrossRef]
- Ugalde, U.O.; Castrillo, J.I. Single Cell Proteins from Fungi and Yeasts. In Applied Mycology and Biotechnology; Khachatourians, G.G., Arora, D.K., Eds.; Agriculture and Food Production; Elsevier: Amsterdam, The Netherlands, 2002; Volume 2, pp. 123–149. [Google Scholar]
- Wikandari, R.; Manikharda; Baldermann, S.; Ningrum, A.; Taherzadeh, M.J. Application of Cell Culture Technology and Genetic Engineering for Production of Future Foods and Crop Improvement to Strengthen Food Security. Bioengineered 2021, 12, 11305–11330. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.W.; Karpol, A.; Friedman, S.; Maru, B.T.; Tracy, B.P. Recent Advances in Single Cell Protein Use as a Feed Ingredient in Aquaculture. Curr. Opin. Biotechnol. 2020, 61, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Mensah, J.K.M.; Twumasi, P. Use of Pineapple Waste for Single Cell Protein (SCP) Production and the Effect of Substrate Concentration on the Yield. J. Food Process Eng. 2017, 40, e12478. [Google Scholar] [CrossRef]
- Thiviya, P.; Gamage, A.; Kapilan, R.; Merah, O.; Madhujith, T. Single Cell Protein Production Using Different Fruit Waste: A Review. Separations 2022, 9, 178. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture—Statistical Yearbook 2021; FAO Statistical Yearbook—World Food and Agriculture; FAO: Rome, Italy, 2021; ISBN 978-92-5-134332-6. [Google Scholar]
- Weerahewa, J.; Rajapakse, C.; Pushpakumara, G. An Analysis of Consumer Demand for Fruits in Sri Lanka. 1981–2010. Appetite 2013, 60, 252–258. [Google Scholar] [CrossRef]
- Romelle, F.D.; Rani, A.; Manohar, R.S. Chemical Composition of Some Selected Fruit Peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Abdullah; Mat, H.B. The Characteristic of Pineapple Waste from Canning Industry. Adv. Sci. Lett. 2017, 23, 5691–5693. [Google Scholar] [CrossRef]
- Rayavarapu, B.; Tallapragada, P.; Usha, M.S. Statistical Optimization of γ-Aminobutyric Acid Production by Response Surface Methodology and Artificial Neural Network Models Using Lactobacillus Fermentum Isolated from Palm Wine. Biocatal. Agric. Biotechnol. 2019, 22, 101362. [Google Scholar] [CrossRef]
- Vengaiah, P.C.; Murthy, G.N.; Sattiraju, M.; Maheswarappa, H.P. Value Added Food Products from Palmyrah Palm (Borassus flabellifer L). J. Nutr. Health Sci. 2017, 4, 105. [Google Scholar] [CrossRef][Green Version]
- Theivendirarajah, K.; Chrystopher, R.K. Microflora and Microbial Activity in Palmyrah (Borassus Flabellifer) Palm Wine in Sri Lanka. Mircen J. 1987, 3, 23–31. [Google Scholar] [CrossRef]
- Limtong, S.; Am-In, S.; Kaewwichian, R.; Kaewkrajay, C.; Jindamorakot, S. Exploration of Yeast Communities in Fresh Coconut, Palmyra, and Nipa Palm Saps and Ethanol-Fermenting Ability of Isolated Yeasts. Antonie Van Leeuwenhoek 2020, 113, 2077–2095. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.S.; Bezawada, J.; Ajila, C.M.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Mixed Culture of Kluyveromyces Marxianus and Candida Krusei for Single-Cell Protein Production and Organic Load Removal from Whey. Bioresour. Technol. 2014, 164, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, S.; Arasaratnam, V. Comparison of Industrial Scale Ethanol Production from a Palmyrah-Based Carbon Source by Commercial Yeast and a Mixed Culture from Palmyrah Toddy. J. Inst. Brew. 2009, 115, 105–110. [Google Scholar] [CrossRef]
- Rajendran, S.; Kapilan, R.; Vasantharuba, S. Papaw Fruit Juice as Source for Single Cell Protein Production Using Natural Palmyrah Toddy Yeast. Ceylon J. Sci. 2018, 47, 379–386. [Google Scholar] [CrossRef]
- Reihani, S.F.S.; Khosravi-Darani, K. Influencing Factors on Single-Cell Protein Production by Submerged Fermentation: A Review. Electron. J. Biotechnol. 2019, 37, 34–40. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- BeMiller, J.N. Carbohydrate Analysis. In Food Analysis; Nielsen, S.S., Ed.; Food Analysis; Springer: Boston, MA, USA, 2010; pp. 147–177. ISBN 978-1-4419-1478-1. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Dharumadurai, D.; Subramaniyan, L.; Subhasish, S.; Nooruddin, T.; Annamalai, P. Production of Single Cell Protein from Pineapple Waste Using Yeast. Innov. Rom. Food Biotechnol. 2011, 8, 26–32. [Google Scholar]
- Kheiralla, Z.H.; El-Gendy, N.S.; Ahmed, H.A.; Shaltout, T.H.; Hussein, M.M.D. One-Factor-at-a-Time (OFAT) Optimization of Hemicellulases Production from Fusarium Moniliforme in Submerged Fermentation. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 1877–1885. [Google Scholar] [CrossRef]
- Abou-Zeid, A.-Z.A.; Khan, J.A.; Abulnaja, K.O. On Methods for Reduction of Nucleic Acids Content in a Single-Cell Protein from Gas Oil. Bioresour. Technol. 1995, 52, 21–24. [Google Scholar] [CrossRef]
- Herbert, D.; Phipps, P.J.; Strange, R.E. Chapter III Chemical Analysis of Microbial Cells. In Methods in Microbiology; Norris, J.R., Ribbons, D.W., Eds.; Academic Press: London, UK, 1971; Volume 5, pp. 209–344. [Google Scholar]
- Fountoulakis, M.; Lahm, H.-W. Hydrolysis and Amino Acid Composition Analysis of Proteins. J. Chromatogr. A 1998, 826, 109–134. [Google Scholar] [CrossRef]
- Joint FAO/WHO/UNU. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2007; ISBN 978-92-4-120935-9.
- Saejung, C.; Salasook, P. Recycling of Sugar Industry Wastewater for Single-Cell Protein Production with Supplemental Carotenoids. Environ. Technol. 2020, 41, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Saheed, O.K.; Jamal, P.; Karim, M.I.A.; Alam, M.Z.; Muyibi, S.A. Utilization of Fruit Peels as Carbon Source for White Rot Fungi Biomass Production under Submerged State Bioconversion. J. King Saud Univ. Sci. 2016, 28, 143–151. [Google Scholar] [CrossRef]
- Dias, P.G.I.; Sajiwanie, J.W.A.; Rathnayaka, R.M.U.S.K. Chemical Composition, Physicochemical and Technological Properties of Selected Fruit Peels as a Potential Food Source. Int. J. Fruit Sci. 2020, 20, S240–S251. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate Composition, Mineral Contents and Fatty Acid Composition of the Different Parts and Dried Peels of Tropical Fruits Cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.d.P.; Gutiérrez, L.-F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Valorisation of Mango Peel: Proximate Composition, Supercritical Fluid Extraction of Carotenoids, and Application as an Antioxidant Additive for an Edible Oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Ajila, C.M.; Bhat, S.G.; Prasada Rao, U.J.S. Valuable Components of Raw and Ripe Peels from Two Indian Mango Varieties. Food Chem. 2007, 102, 1006–1011. [Google Scholar] [CrossRef]
- Wongkaew, M.; Kittiwachana, S.; Phuangsaijai, N.; Tinpovong, B.; Tiyayon, C.; Pusadee, T.; Chuttong, B.; Sringarm, K.; Bhat, F.M.; Sommano, S.R.; et al. Fruit Characteristics, Peel Nutritional Compositions, and Their Relationships with Mango Peel Pectin Quality. Plants 2021, 10, 1148. [Google Scholar] [CrossRef]
- Garcia-Amezquita, L.E.; Tejada-Ortigoza, V.; Campanella, O.H.; Welti-Chanes, J. Influence of Drying Method on the Composition, Physicochemical Properties, and Prebiotic Potential of Dietary Fibre Concentrates from Fruit Peels. J. Food Qual. 2018, 2018, e9105237. [Google Scholar] [CrossRef]
- Banerjee, S.; Ranganathan, V.; Patti, A.; Arora, A. Valorisation of Pineapple Wastes for Food and Therapeutic Applications. Trends Food Sci. Technol. 2018, 82, 60–70. [Google Scholar] [CrossRef]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Waste to Wealth: A Case Study of Papaya Peel. Waste and Biomass Valorization 2019, 10, 1755–1766. [Google Scholar] [CrossRef]
- Mondal, A.K.; Sengupta, S.; Bhowal, J.; Bhattacharya, D.K. Utilization of Fruit Wastes in Producing Single Cell Protein. Int. J. Sci. Environ. Technol. 2012, 1, 430–438. [Google Scholar]
- Mahnaaz, K.; Khan, S.S.; Zafar, A.; Arshiya, T. Production of Fungal Single Cell Protein Using Rhizopus Oligosporus Grown on Fruit Wastes. In Biological Forum; Satya Prakashan: Delhi, India, 2009; Volume 1, pp. 26–28. [Google Scholar]
- Spalvins, K.; Ivanovs, K.; Blumberga, D. Single Cell Protein Production from Waste Biomass: Review of Various Agricultural by-Products. Agron. Res. 2018, 16, 14931508. [Google Scholar] [CrossRef]
- Bratosin, B.C.; Darjan, S.; Vodnar, D.C. Single Cell Protein: A Potential Substitute in Human and Animal Nutrition. Sustainability 2021, 13, 9284. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.S.; Ahmed, Z.; Tanveer, A. Production of Single Cell Protein from Saccharomyces Cerevisiae by Utilizing Fruit Wastes. Nanobiotech. Univers. 2010, 1, 127–132. [Google Scholar]
- Yabaya, A.; Ado, S.A. Mycelial Protein Production by Aspergillus Niger Using Banana Peels. Sci. World J. 2008, 3, 9–12. [Google Scholar] [CrossRef]
- Tropea, A.; Ferracane, A.; Albergamo, A.; Potortì, A.G.; Lo Turco, V.; Di Bella, G. Single Cell Protein Production through Multi Food-Waste Substrate Fermentation. Fermentation 2022, 8, 91. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef]
- Thiviya, P.; Kapilan, R.; Madhujith, T. Bioconversion of Fruit Wastes of Papaya, Watermelon, and Banana into Single Cell Protein Production. Trop. Agric. Res. 2021, 32, 503–514. [Google Scholar] [CrossRef]
- Yousufi, M.K. Impact of PH on the Single Cell Protein Produced on Okara-Wheat Grit Substrates Using Rhizopus Oligosporus and Aspergillus Oryzae. IOSR J. Environ. Sci. Toxicol. Food Technol. 2012, 1, 32–35. [Google Scholar] [CrossRef]
- Umesh, M.; Thazeem, B.; Preethi, K. Valorization of Pineapple Peels through Single Cell Protein Production Using Saccharomyces Cerevisiae NCDC 364. Appl. Food Biotechnol. 2019, 6, 255–263. [Google Scholar] [CrossRef]
- Jaganmohan, P.; Daas, B.P.; Prasad, S.V. Production of Single Cell Protein (SCP) with Aspergillus Terreus Using Solid State Fermentation. Eur. J. Biol. Sci. 2013, 5, 38–43. [Google Scholar] [CrossRef]
- Rages, A.A.; Haider, M.M. Alkaline Hydrolysis of Olive Fruits Wastes for the Production of Single Cell Protein by Candida Lipolytica. Biocatal. Agric. Biotechnol. 2021, 33, 101999. [Google Scholar] [CrossRef]
- Milala, M.A.; Yakubu, M.; Burah, B.; Laminu, H.H.; Bashir, H. Production and Optimization of Single Cell Protein from Orange Peels by Saccharomyces Cerevisiae. J. Biosci. Biotechnol. Discov. 2018, 3, 99–104. [Google Scholar] [CrossRef]
- Nedwell, D.B. Effect of Low Temperature on Microbial Growth: Lowered Affinity for Substrates Limits Growth at Low Temperature. FEMS Microbiol. Ecol. 1999, 30, 101–111. [Google Scholar] [CrossRef]
- Munawar, R.; Irfan, M.; Nadeem, M.; Syed, Q.; Siddique, Z. Biosynthesis of Single Cell Biomass of Candida Utilis by Submerged Fermentation. Pak. J. Sci. 2010, 62, 1–5. [Google Scholar]
- Mahan, K.M.; Le, R.K.; Wells, T., Jr.; Anderson, S.; Yuan, J.S.; Stoklosa, R.J.; Bhalla, A.; Hodge, D.B.; Ragauskas, A.J. Production of Single Cell Protein from Agro-Waste Using Rhodococcus Opacus. J. Ind. Microbiol. Biotechnol. 2018, 45, 795–801. [Google Scholar] [CrossRef]
- Oshoma, C.E.; Eguakun-Owie, S.O. Conversion of Food Waste to Single Cell Protein Using Aspergillus Niger. J. Appl. Sci. Environ. Manag. 2018, 22, 350–355. [Google Scholar] [CrossRef]
- Gervasi, T.; Pellizzeri, V.; Calabrese, G.; Di Bella, G.; Cicero, N.; Dugo, G. Production of Single Cell Protein (SCP) from Food and Agricultural Waste by Using Saccharomyces Cerevisiae. Nat. Prod. Res. 2018, 32, 648–653. [Google Scholar] [CrossRef]
- Ojokoh, A.O.; Uzeh, R.E. Production of Saccharomyces Cerevisiae Biomass in Papaya Extract Medium. Afr. J. Biotechnol. 2005, 4, 1281–1284. [Google Scholar] [CrossRef]
- Zayed, G.; Mostafa, N. Studies on the Production and Kinetic Aspects of Single Cell Protein from Sugar Cane Bagasse Saccharified by Aspergillus Niger. Biomass Bioenergy 1992, 3, 363–367. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein—State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. Single Cell Protein Production from Lignocellulosic Biomass; Bajpai, P., Ed.; SpringerBriefs in Molecular Science; Springer: Singapore, 2017; ISBN 978-981-10-5873-8. [Google Scholar]
- Linder, T. Making the Case for Edible Microorganisms as an Integral Part of a More Sustainable and Resilient Food Production System. Food Sec. 2019, 11, 265–278. [Google Scholar] [CrossRef]
- Hedenskog, G.; Ebbinghaus, L. Reduction of the Nucleic Acid Content of Single-Cell Protein Concentrates. Biotechnol. Bioeng. 1972, 14, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Parajó, J.C.; Santos, V.; Domínguez, H.; Vázquez, M. NH4OH-Based Pretreatment for Improving the Nutritional Quality of Single-Cell Protein (SCP). Appl Biochem. Biotechnol. 1995, 55, 133–149. [Google Scholar] [CrossRef]
- Adedayo, M.R.; Ajiboye, E.A.; Akintunde, J.K.; Odaibo, A. Single Cell Proteins: As Nutritional Enhancer. Adv. Appl. Sci. Res. 2011, 2, 396–409. [Google Scholar]
- Oshoma, C.E.; Eguakun-Owie, S.O.; Obuekwe, I.S. Utilization of Banana Peel as a Substrate for Single Cell Protein and Amylase Production by Aspergillus Niger. Afr. Sci. 2019, 18, 143–150. [Google Scholar]
- Bajpai, P. Use of Mixed Cultures. In Single Cell Protein Production from Lignocellulosic Biomass; Bajpai, P., Ed.; SpringerBriefs in Molecular Science; Springer: Singapore, 2017; pp. 37–40. ISBN 978-981-10-5873-8. [Google Scholar]




| Fruit Peel | Yield (%) | pH | TSS (%) | Reducing Sugar (%) | Moisture (%) | Ash (%) 1 | Fat (%) 1 | Protein (%) 1 | Total Carbohydrate (%) 1 |
|---|---|---|---|---|---|---|---|---|---|
| Pineapple | 15.3 ± 0.9 b | 3.7 ± 0.0 e | 10.8 ± 0.0 c | 2.6 ± 0.1 b | 84.7 ± 0.9 b | 4.5 ± 0.3 c | 0.9 ± 0.1 c | 6.9 ± 0.1 c,d | 87.7 ± 0.4 b |
| Watermelon | 5.0 ± 0.4 c | 5.4 ± 0.0 b | 3.2 ± 0.0 f | 1.8 ± 0.1 c,d | 95.0 ± 0.4 a | 5.5 ± 0.2 b,c | 1.5 ± 0.1b | 10.3 ± 0.3 b | 82.6 ± 0.5 c |
| Papaya | 8.4 ± 0.2 c | 5.5 ± 0.0 a | 6.5 ± 0.0 e | 5.8 ± 0.1 a | 91.6 ± 0.2 a | 6.4 ± 0.4 a,b | 1.1 ± 0.1 b,c | 11.3 ± 0.6 a | 81.2 ± 0.9 c |
| Sour orange | 25.2 ± 2.5 a | 4.1 ± 0.0 d | 12.3 ± 0.0 b | 1.2 ± 0.1 d | 74.8 ± 2.5 c | 6.1 ± 1.0 a,b | 1.4 ± 0.1 b,c | 7.2 ± 0.1 c | 85.4 ± 0.9 a |
| Banana | 26.1 ± 2.1 a | 4.8 ± 0.0 c | 7.1 ± 0.0 d | 3.1 ± 0.4 b | 73.9 ± 2.1 c | 7.4 ± 0.3 a | 2.6 ± 0.4 a | 6.4 ± 0.2 d | 83.6 ± 0.7 a |
| Mango | 23.8 ± 0.2 a | 4.1 ± 0.0 d | 16.8 ± 0.0 a | 2.4 ± 0.3 b,c | 76.2 ± 0.2 c | 4.2 ± 0.5 c | 2.5 ± 0.3 a | 6.2 ± 0.2 d | 87.1 ± 0.8 a |
| Fruit Peel | Dry Biomass (g/L) | Crude Protein Content (%) |
|---|---|---|
| Pineapple | 9.40 ± 0.53 a,b | 49.7 ± 1.3 b |
| Watermelon | 5.33 ± 0.61 c | 45.2 ± 0.7 c |
| Papaya | 11.73 ± 0.81 a | 52.4 ± 0.4 a |
| Sour orange | 9.13 ± 0.64 a,b | 29.5 ± 1.2 d |
| Banana | 7.77 ± 1.88 b,c | 30.4 ± 0.6 d |
| Mango | 8.61 ± 0.90 b | 24.6 ± 0.2 e |
| Condition | Dry Biomass (g/L) | Crude Protein Content (%) |
|---|---|---|
| Control medium | 27.75 ± 0.93 A | 54.3 ± 0.6 b |
| Papaya peel medium | 23.15 ± 2.31 B | 56.1 ± 0.4 a |
| Condition | Dry Biomass (g/L) | Crude Protein Content (%) |
|---|---|---|
| Before nucleic acid reduction treatment | 23.2 ± 2.3 A | 56.1 ± 0.4 a |
| After nucleic acid reduction treatment | 16.7 ± 1.2 B | 45.3 ± 0.3 b |
| Description | Amino Acid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| His 1 | Ile 1 | Leu 1 | Lys 1 | Met + Cys 1,2 | Phe + Tyr 1,3 | Thr 1 | Trp 1 | Val 1 | Glu | Arg | |
| SCP from papaya peel based medium | 8.5 | 14.6 | 29.2 | 8.2 | 14.4 | 6.5 | 5.5 | 9.2 | 14.7 | 12.6 | 22.5 |
| Amino acid requirement (FAO/WHO/UNU, 2007 [39]) | 15 | 30 | 59 | 45 | 22 | 38 | 23 | 6 | 39 | NR | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiviya, P.; Gamage, A.; Kapilan, R.; Merah, O.; Madhujith, T. Production of Single-Cell Protein from Fruit Peel Wastes Using Palmyrah Toddy Yeast. Fermentation 2022, 8, 355. https://doi.org/10.3390/fermentation8080355
Thiviya P, Gamage A, Kapilan R, Merah O, Madhujith T. Production of Single-Cell Protein from Fruit Peel Wastes Using Palmyrah Toddy Yeast. Fermentation. 2022; 8(8):355. https://doi.org/10.3390/fermentation8080355
Chicago/Turabian StyleThiviya, Punniamoorthy, Ashoka Gamage, Ranganathan Kapilan, Othmane Merah, and Terrence Madhujith. 2022. "Production of Single-Cell Protein from Fruit Peel Wastes Using Palmyrah Toddy Yeast" Fermentation 8, no. 8: 355. https://doi.org/10.3390/fermentation8080355
APA StyleThiviya, P., Gamage, A., Kapilan, R., Merah, O., & Madhujith, T. (2022). Production of Single-Cell Protein from Fruit Peel Wastes Using Palmyrah Toddy Yeast. Fermentation, 8(8), 355. https://doi.org/10.3390/fermentation8080355









