Probiotics, Prebiotics, Synbiotics, and Fermented Foods as Potential Biotics in Nutrition Improving Health via Microbiome-Gut-Brain Axis
Abstract
:1. Introduction
1.1. Nutrition and Health through Nutraceuticals
1.2. Biological, Social, and Psychological Practices Affecting Nutrition and Health
2. Biotics Related to Nutrition and Health
2.1. Probiotics
2.2. Postbiotics and Paraprobiotics
2.3. Prebiotics
3. Functioning of Prebiotics and Fibers Affecting Gut Microbiota
3.1. Fermentable Oligosaccharides
3.2. Preparation of OS-Prebiotics in Microbial Process
4. The Synbiotics Concept
4.1. Complementary Synbiotics
4.2. Synergistic Synbiotics
4.3. Functions of Synbiotics
5. Fermented Foods as Potential Biotics
6. Difference between Probiotics and Fermented Food
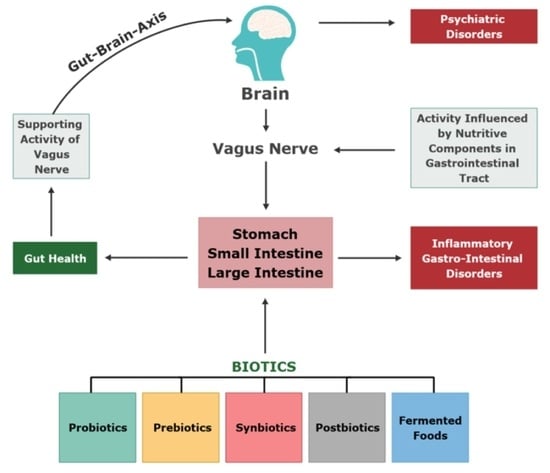
7. Microbiome-Gut-Brain Axis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Million, M.; Diallo, A.; Raoult, D. Gut microbiota and malnutrition. Microb. Pathog. 2017, 106, 127–138. [Google Scholar] [CrossRef]
- Boulangé, C.; Neves, A.; Chilloux, J.; Nicholson, J.; Dumas, M. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blandino, G.; Inturri, R.; Lazzara, F.; Di Rosa, M.; Malaguarnera, L. Impact of gut microbiota on diabetes mellitus. Diabetes Metab. 2016, 42, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Schneiderhan, J.; Master-Hunter, T.; Locke, A. Targeting gut flora to treat and prevent disease. J. Fam. Pract. 2016, 65, 34–38. [Google Scholar] [PubMed]
- Dahiya, D.; Nigam, P.S. The Gut Microbiota Influenced by the Intake of Probiotics and Functional Foods with Prebiotics Can Sustain Wellness and Alleviate Certain Ailments like Gut-Inflammation and Colon-Cancer. Microorganisms 2022, 10, 665. [Google Scholar] [CrossRef]
- Ganatsios, V.; Nigam, P.; Plessas, S.; Terpou, A. Kefir as a Functional Beverage Gaining Momentum towards Its Health-Promoting Attributes. Beverages 2021, 7, 48. [Google Scholar] [CrossRef]
- Plessas, S.; Pherson, L.; Bekatorou, A.; Nigam, P.; Koutinas, A.A. Breadmaking using kefir grains as baker’s yeast. Food Chem. 2005, 93, 585–589. [Google Scholar] [CrossRef]
- Harta, O.; Iconomopoulou, M.; Bekatorou, A.; Nigam, P.; Kontominas, M.; Koutinas, A.A. Effect of various carbohydrate substrates on the production of kefir grains for use as a novel baking starter. Food Chem. 2004, 88, 237–242. [Google Scholar] [CrossRef]
- Plessas, S.; Bekatorou, A.; Gallanagh, J.; Nigam, P.; Koutinas, A.A.; Psarianos, C. Evolution of aroma volatiles during storage of sourdough bread made by mixed cultures of Kluyveromyces marxianus and Lactobacillus delbrueckii ssp bulgaricus or Lactobacillus helveticus. Food Chem. 2008, 107, 883–889. [Google Scholar] [CrossRef]
- Plessas, S.; Fisher, A.; Koureta, K.; Psarianos, C.; Nigam, P.; Koutinas, A.A. Application of Kluyveromyces marxianus, Lactobacillus delbrueckii ssp bulgaricus and L. helveticus for sourdough bread making. Food Chem. 2008, 106, 985–990. [Google Scholar] [CrossRef]
- Plessas, S.; Trantallidi, M.; Bekatorou, A.; Kanellaki, M.; Nigam, P.; Koutinas, A.A. Immobilization of kefir and Lactobacillus casei on brewery spent grains for use in sourdough wheat bread making. Food Chem. 2007, 105, 187–194. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of Gut Microbiota, Probiotics and Prebiotics in the Cardiovascular Diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Langella, P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization; World Health Organization. Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation. In This Definition Was Adopted by the International Scientific Association for Probiotics and Prebiotics (ISAPP) in 2013; FAO: Rome, Italy, 2006. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Feord, J. Lactic acid bacteria in a changing legislative environment. Antonie Leeuwenhoek 2012, 82, 353–360. [Google Scholar] [CrossRef]
- Parte, A.; Sardà Carbasse, J.; Meier-Kolthoff, J.; Reimer, L.; Göker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int. J. Syst. Evol. Microbiol. 2020, 70, 5607–5612. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Amara, A.A.; Shibl, A. Role of Probiotics in Health Improvement, Infection Control and Disease Treatment and Management. Saudi Pharm. J. 2015, 23, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Chandra, A.; Dhar, A.; Shukla, P.; Baishya, D. Multi-Efficient Thermostable Endoxylanase from Bacillus Velezensis AG20 and Its Production of Xylooligosaccharides as Efficient Prebiotics with Anticancer Activity. Process Biochem. 2021, 109, 59–71. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, Gut Microbiota, and Their Influence on Host Health and Disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Collado, M.C.; Vinderola, G.; Salminen, S. Postbiotics: Facts and Open Questions. A Position Paper on the Need for a Consensus Definition. Benef. Microbes 2019, 10, 711–719. [Google Scholar] [CrossRef] [PubMed]
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on Their Ability to Modify Biological Responses, Inactivation Methods and Perspectives on Their Application in Foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and Paraprobiotics: From Concepts to Applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.P.; Guimarães, J.T.; Esmerino, E.A.; Duarte, M.C.K.; Silva, M.C.; Silva, R.; Ferreira, B.M.; Sant’Ana, A.S.; Freitas, M.Q.; Cruz, A.G. Paraprobiotics and Postbiotics: Concepts and Potential Applications in Dairy Products. Curr. Opin. Food Sci. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Wargo, J.A. Modulating Gut Microbes. Science 2020, 369, 1302–1303. [Google Scholar] [CrossRef]
- Markowiak, P.; Slizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 15, 1021. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Rasika, D.M.; Vidanarachchi, J.K.; Rocha, R.S.; Balthazar, C.F.; Cruz, A.G.; Sant’Ana, A.S.; Ranadheera, C.S. Plant-based milk substitutes as emerging probiotic carriers. Curr. Opin. Food Sci. 2021, 38, 8–20. [Google Scholar] [CrossRef]
- Lichtenstein, L.; Avni-Biron, I.; Ben-Bassat, O. Probiotics and Prebiotics in Crohn’s Disease Therapies. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 81–88. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M. Prebiotics: The concept revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [CrossRef] [Green Version]
- Guarino, M.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Updating the Concept of Prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Freitas, C.; Terrone, C.C.; Masarin, F.; Carmona, E.C.; Brienzo, M. In Vitro Study of the Effect of Xylooligosaccharides Obtained from Banana Pseudostem Xylan by Enzymatic Hydrolysis on Probiotic Bacteria. Biocatal. Agric. Biotechnol. 2021, 33, 101973. [Google Scholar] [CrossRef]
- Rashid, R.; Sohail, M. Xylanolytic Bacillus Species for Xylooligosaccharides Production: A Critical Review. Bioresour. Bioprocess. 2021, 8, 16. [Google Scholar] [CrossRef]
- Kanpiengjai, A.; Nuntikaew, P.; Wongsanittayarak, J.; Leangnim, N.; Khanongnuch, C. Isolation of Efficient Xylooligoligosaccharides-Fermenting Probiotic Lactic Acid Bacteria from Ethnic Pickled Bamboo Shoot Products. Biology 2022, 11, 638. [Google Scholar] [CrossRef]
- Roupar, D.; Colunga, A.; Martins, J.T.; Botelho, C.M.; Teixeira, J.A.; Nobre, C. Prebiotic potential of fructo-oligosaccharides produced by Aspergillus ibericus in a bacterial community representative of the gut microbiota. In Proceedings of the BioIberoAmerica 2022—3rd IberoAmerican Congress on Biotechnology, Braga, Portugal, 7–9 April 2022; pp. 148–149. Available online: http://hdl.handle.net/1822/77013 (accessed on 10 May 2022).
- Nobre, C.; Gonçalves, D.A.; Teixeira, J.A.; Rodrigues, L.R. One-Step Co-Culture Fermentation Strategy to Produce High-Content Fructo-Oligosaccharides. Carbohydr. Polym. 2018, 201, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Bangotra, R.; Sharma, S.; Vaid, S.; Kapoor, N.; Dutt, H.C.; Bajaj, B.K. Bioprocess Development for Production of Xylooligosaccharides Prebiotics from Sugarcane Bagasse with High Bioactivity Potential. Ind. Crops Prod. 2022, 178, 114591. [Google Scholar] [CrossRef]
- Depeint, F.; Tzortzis, G.; Vulevic, J.; I’Anson, K.; Gibson, G.R. Prebiotic evaluation of a novel galacto-oligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: A randomized, double-blind, crossover, placebo-controlled intervention study. Am. J. Clin. Nutr. 2008, 87, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented Foods in a Global Age: East Meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loo, J.; Coussement, P.; De Leenheer, L.; Hoebregs, H.; Smits, G. On the Presence of Inulin and Oligofructose as Natural Ingredients in the Western Diet. Crit. Rev. Food Sci. Nutr. 1995, 35, 525–552. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Macfarlane, S.; Gibson, G.R. Validation of a Three-Stage Compound Continuous Culture System for Investigating the Effect of Retention Time on the Ecology and Metabolism of Bacteria in the Human Colon. Microb. Ecol. 1998, 35, 180–187. [Google Scholar] [CrossRef]
- Zaman, S.A.; Sarbini, S.R. The Potential of Resistant Starch as a Prebiotic. Crit. Rev. Biotechnol. 2015, 36, 578–584. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [Green Version]
- Goffin, D.; Delzenne, N.; Blecker, C.; Hanon, E.; Deroanne, C.; Paquot, M. Will Isomalto-Oligosaccharides, a Well-Established Functional Food in Asia, Break through the European and American Market? The Status of Knowledge on These Prebiotics. Crit. Rev. Food Sci. Nutr. 2011, 51, 394–409. [Google Scholar] [CrossRef]
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and Functional Characteristics of Dietary Fibre in Beans, Lentils, Peas, and Chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Pathania, S.; Kaur, N. Utilization of fruits and vegetable by-products for isolation of dietary fibres and its potential application as functional ingredients. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100295. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Utilization of mango, apple and banana fruit peels as prebiotics and functional ingredients. Agriculture 2021, 11, 584. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic Inulin-Type Fructans Induce Specific Changes in the Human Gut Microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of Inulin on the Human Gut Microbiota: Stimulation of Bifidobacterium Adolescentis and Faecalibacterium Prausnitzii. Br. J. Nutr. 2008, 101, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Kaulpiboon, J.; Rudeekulthamrong, P.; Watanasatitarpa, S.; Ito, K.; Pongsawasdi, P. Synthesis of Long-Chain Isomaltooligosaccharides from Tapioca Starch and an in Vitro Investigation of Their Prebiotic Properties. J. Mol. Catal. B Enzym. 2015, 120, 127–135. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Kandylis, P.; Panas, P.; Dooley, J.S.G.; Nigam, P.; Koutinas, A.A. Evaluation of Freeze-Dried Kefir Coculture as Starter in Feta-Type Cheese Production. Appl. Environ. Microbiol. 2006, 72, 6124–6135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosnea, L.A.; Moschakis, T.; Nigam, P.S.; Biliaderis, C.G. Growth Adaptation of Probiotics in Biopolymer-Based Coacervate Structures to Enhance Cell Viability. LWT 2017, 77, 282–289. [Google Scholar] [CrossRef]
- Terpou, A.; Nigam, P.S.; Bosnea, L.; Kanellaki, M. Evaluation of Chios Mastic Gum as Antimicrobial Agent and Matrix Forming Material Targeting Probiotic Cell Encapsulation for Functional Fermented Milk Production. LWT 2018, 97, 109–116. [Google Scholar] [CrossRef]
- Vassiliki, S.; Terpou, A.; Bosnea, L.; Kanellaki, M.; Nigam, P.S. Entrapment of Lactobacillus Casei ATCC393 in the Viscus Matrix of Pistacia Terebinthus Resin for Functional Myzithra Cheese Manufacture. LWT 2018, 89, 441–448. [Google Scholar] [CrossRef]
- Terpou, A.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A.; Nigam, P. Enhanced Probiotic Viability and Aromatic Profile of Yogurts Produced Using Wheat Bran (Triticum aestivum) as Cell Immobilization Carrier. Process Biochem. 2017, 55, 115–127. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic acid bacteria: Their applications in foods. J. Bacteriol. Mycol. 2018, 6, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Bell, V.; Ferrão, J.; Fernandes, T. Nutritional guidelines and fermented food frameworks. Foods 2017, 6, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beganović, J.; Kos, B.; Pavunc, A.L.; Uroić, K.; Jokić, M.; Šušković, J. Traditionally produced sauerkraut as source of autochthonous functional starter cultures. Microbiol. Res. 2014, 169, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Beganović, J.; Pavunc, A.L.; Gjuračić, K.; Špoljarec, M.; Šušković, J.; Kos, B. Improved sauerkraut production with probiotic strain Lactobacillus plantarum L4 and Leuconostoc mesenteroides LMG 7954. J. Food Sci. 2011, 76, M124–M129. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.R.; Park, G.S.; Lee, Y.H.; Im, S.; Jeong, D.Y.; Kang, J. Whole genome analysis of Lactobacillus plantarum strains isolated from kimchi and determination of probiotic properties to treat mucosal infections by Candida albicans and Gardnerella vaginalis. Front. Microbiol. 2019, 10, 433. [Google Scholar] [CrossRef]
- Chelule, P.K.; Mokoena, M.P.; Gqaleni, N. Advantages of traditional lactic acid bacteria fermentation of food in Africa. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 1160–1167. [Google Scholar]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Lee, Y.; Salminen, S. Handbook of Probiotics and Prebiotics, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Lee, C.; Sim, J.H.; Kim, J.H.; Song, Y.-J.; Son, E.-I.; Kim, Y.-M.; Yu, A.D. Optimization of synbiotics of lactic acid bacteria derived from kelp kimchi using the response surface methodology. J. Korean Soc. Food Sci. Nutr. 2021, 50, 1385–1391. [Google Scholar] [CrossRef]
- Moon, C.; Heo, M. Characteristics of probiotics isolated from Korean traditional foods and antibacterial activity of synbiotics. Microbiol. Biotechnol. Lett. 2021, 49, 552–558. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Méndez-Trujillo, V.; Hernández-Delgado, N.C.; Bermúdez-Humarán, L.G.; Reyes-Pavón, D. Looking inside mexican traditional food as sources of synbiotics for developing novel functional products. Fermentation 2022, 8, 123. [Google Scholar] [CrossRef]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef]
- Ibrahem, A.A.; Al-Shawi, S.G.; Al-Temimi, W.K.A. The antagonistic activity of the synbiotic containing Lactobacillus acidophilus and pineapple residue FOS against pathogenic bacteria. Braz. J. Biol. 2024, 84, e258277. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; van Hylckama Vlieg, J.E.T. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015, 23, 354–366. [Google Scholar] [CrossRef] [Green Version]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, probiotics, synbiotics, paraprobiotics and postbiotic compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef]
- Goderska, K.; Kozłowski, P. Evaluation of microencapsulated synbiotic preparations containing lactobionic acid. Appl. Biochem. Biotechnol. 2021, 193, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.; Chan, B.D.; Leung, T.; Chen, M.; Tai, W.C. Beneficial and anti-inflammatory effects of formulated prebiotics, probiotics, and synbiotics in normal and acute colitis mice. J. Funct. Foods. 2022, 88, 104871. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahiya, D.; Manuel, V.; Nigam, P.S. An Overview of Bioprocesses Employing Specifically Selected Microbial Catalysts for γ-Aminobutyric Acid Production. Microorganisms 2021, 9, 2457. [Google Scholar] [CrossRef]
- Khan, M.F.; Palukuri, M.V.; Shivakumar, S.; Rengaswamy, R.; Sahoo, S. A Computational Framework for Studying Gut-Brain Axis in Autism Spectrum Disorder. Front. Physiol. 2022, 13, 760753. [Google Scholar] [CrossRef]
- Lalitsuradej, E.; Sirilunm, S.; Sittiprapaporn, P.; Sivamaruthi, B.S.; Pintha, K.; Tantipaiboonwong, P.; Khongtan, S.; Fukngoen, P.; Peerajan, S.; Chaiyasut, C. The effects of synbiotics administration on stress-related parameters in thai Subjects-A preliminary study. Foods 2022, 11, 759. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Term | Description | Elements |
|---|---|---|
| Probiotics | Live microorganisms, intact whole cells of probiotic microbes | Selected probiotic strains Lactobacillus casei, L. acidophilus L. bulgaricus, Lactococcus lactis Bifidobacterium bifidum, B. lactis (Bifidus actiregularis (R) ** |
| Postbiotics | Components of lysed probiotic cells. Microbial cell wall fragmented compounds, microbial primary and secondary metabolites secreted by probiotics | Metabiotics, biogenics, metabolites, cell-free supernatants, and extracted fractions e.g., Saccharomyces boulardii (a variety of S. cerevisiae) |
| Paraprobiotics | Dead, nonviable, intact (non-lysed) cells | Inactivated cells of probiotics, or nonprobiotic cells |
| Prebiotics | Fruits, legumes, root vegetables, whole grains, seeds, and nuts | Fermentable Oligosaccharides (OS), dietary fibers |
| Potential Biotics | Fermented foods and fermented beverages | Products containing fermented substrates, microbes and their metabolites |
| Components Used for Fermentation | Fermented Substrates * Sustaining Growth and Activity of Cultures |
|---|---|
| Milk/cheese + Cultures ** | Fermented dairy products |
| Cereals + Cultures | Fermented Cereal Foods |
| Vegetable + Cultures | Fermented Vegetable Products |
| Legumes + Cultures | Fermented Legume Foods |
| Root vegetables + Cultures | Fermented Root Crop Foods |
| Meat + Cultures | Fermented Meat Foods |
| Fish + Cultures | Fermented Fish Products |
| Fruits + Cultures | Fermented undistilled Beverages |
| Group 1 Substrates Cereals/Grains | Group 1 Fermented Foods | Group 2 Substrates Vegetables | Group 2 Fermented Foods | Group 3 Substrates Beans, Seeds | Group 3 Fermented Foods |
|---|---|---|---|---|---|
| Wheat and Rye flour | Sourdough | Cabbage | Kimchi; Sauerkraut; Pao-cai | Soybean | Tempe; Bekang; Chungkokjang |
| Cereals | Boza | Leafy vegetables, Boiled rice | Pak-gard-dong | Locust bean | Dawadawa; Iru |
| Pearl millet | Ben-saalga | Leafy vegetable | Gundruk | Soybean | Douch; Doenjang |
| Rice and Black Gram | Dosa; Idli | Mustard | Burong mustala; Fu-tsai; Suan-tsai | Leaves of legume Cassia sp. | Kawal |
| Sorghum | Hussuwa; Kisra; | Bamboo shoot | Ekung; Eup; Jiang-sun; Naw-mai-dong; Mesu; Soibum; Soidon | Soybean | Meju; Miso; Natto |
| Maize, Sorghum, Millet | Busa; Kunu-zaki; Mbege; Ogi | Cupers | Cupers (fermented) | Peanut press cake, Tapioca, soybean curd starter | Oncom-Hitam (Black Oncom) |
| Glutinous Rice | Khamak (Kao-mak) | Cucumbers | Jiang-gua; Cucumbers (fermented); Khalpi; Oiji | Soybean | Thua nao; Tungrymbai |
| Rice | Lao-chao; Puto | Wild vegetable | Goyang | African oil bean (Pentaclethra macrophylla) | Ugba |
| Maize | Gowé; Kenkey; Koko; Mawè; Poto poto; Pozol; | Mustard and Beetroot, eggplant (Aubergine) | Dha muoi | Melon-Seeds, castor oil seeds, pumpkin, sesame | Ogiri/Ogili |
| Maize, Sorghum | Pito | Bamboo shoot tips | Hirring; Tuaithur | Soybean | Yandou |
| Maize, sorghum, millet, Cassava flour | Uji | Olive | (fermented Olives) | Peanut press-cake, Tapioca, soybean curd | Oncom-Merah (Orange Oncom) |
| Cassava, Maize, Sorghum, Millet | Togwa | Red onion | Hom-dong | Locust bean | Soumbala |
| Wheat–Sheep milk | Tarhana | Leaves of Gynandropis pentaphylla | Pak-sian-dong | Soybean | Hawaijar |
| Rice–wheat flour–milk | Selroti | Vegetables | Suan-cai | Black Gram | Vari/Bari |
| Red rice | Ang-kak | Mustard leaves, cabbage, salt, coconut | Sayur asin | ||
| Glutinous rice, Ragi | Tape Ketan | Turnip | Sunki |
| Fermented Foods | Probiotic Supplements and Food |
|---|---|
| Fermented products are prepared using microorganisms without their characterization; mixed strains mostly involved in natural fermentation process | Products are prepared with characterized specifically selected strains of micro-organisms |
| Some products are fermented with known cultures; however, they could become inactivated during the preparation stages. Several fermented products contain no live or nonviable microbial cells as these are removed from the product | The product at the time of consumption contains viable cells of microbial strains in an adequate number. Commercial probiotic supplements of several types and brands are available on the market |
| Fermentations are purposely performed for the preservation, or long-term storage of seasonal fruits, cereals, and vegetables. In some cases, fermentation is used to prepare products of delicacies, and condiments | Commercial probiotic formulations have been designed with a combination of selected probiotic strains and their complementary prebiotic materials |
| No specific health benefits are clinically proven for fermented food products | Probiotic food or supplements are designed to target specific needs and proven clinical benefits for consumers |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahiya, D.; Nigam, P.S. Probiotics, Prebiotics, Synbiotics, and Fermented Foods as Potential Biotics in Nutrition Improving Health via Microbiome-Gut-Brain Axis. Fermentation 2022, 8, 303. https://doi.org/10.3390/fermentation8070303
Dahiya D, Nigam PS. Probiotics, Prebiotics, Synbiotics, and Fermented Foods as Potential Biotics in Nutrition Improving Health via Microbiome-Gut-Brain Axis. Fermentation. 2022; 8(7):303. https://doi.org/10.3390/fermentation8070303
Chicago/Turabian StyleDahiya, Divakar, and Poonam Singh Nigam. 2022. "Probiotics, Prebiotics, Synbiotics, and Fermented Foods as Potential Biotics in Nutrition Improving Health via Microbiome-Gut-Brain Axis" Fermentation 8, no. 7: 303. https://doi.org/10.3390/fermentation8070303
APA StyleDahiya, D., & Nigam, P. S. (2022). Probiotics, Prebiotics, Synbiotics, and Fermented Foods as Potential Biotics in Nutrition Improving Health via Microbiome-Gut-Brain Axis. Fermentation, 8(7), 303. https://doi.org/10.3390/fermentation8070303