Simultaneous Saccharification and Fermentation of Empty Fruit Bunches of Palm for Bioethanol Production Using a Microbial Consortium of S. cerevisiae and T. harzianum
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Chemicals and Microorganisms
Microorganisms Cultivation
2.3. Pretreatments of EFBs
2.4. EFBs Analysis
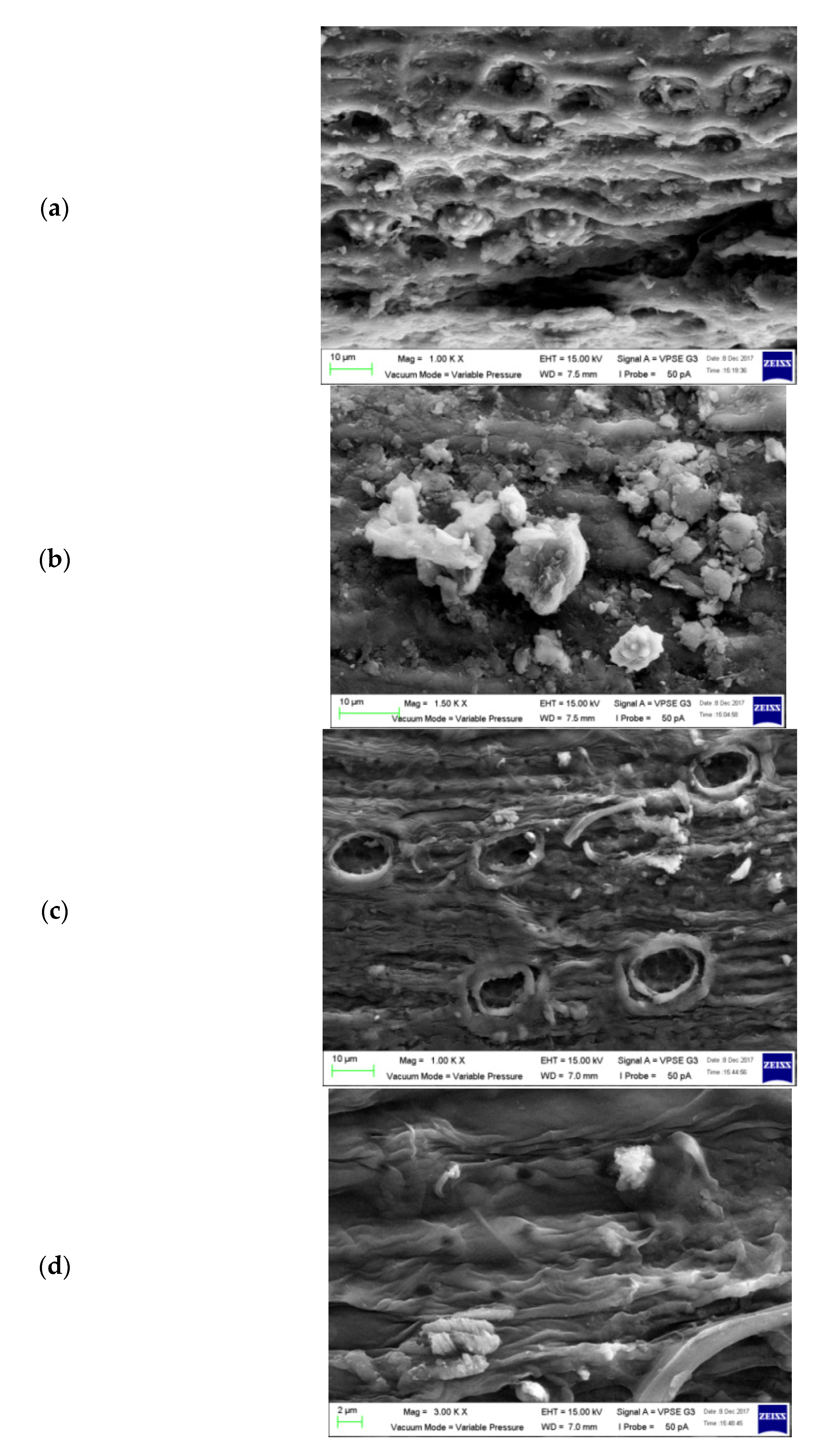
2.4.1. Scanning Electron Microscope (SEM) Analysis of EFBs
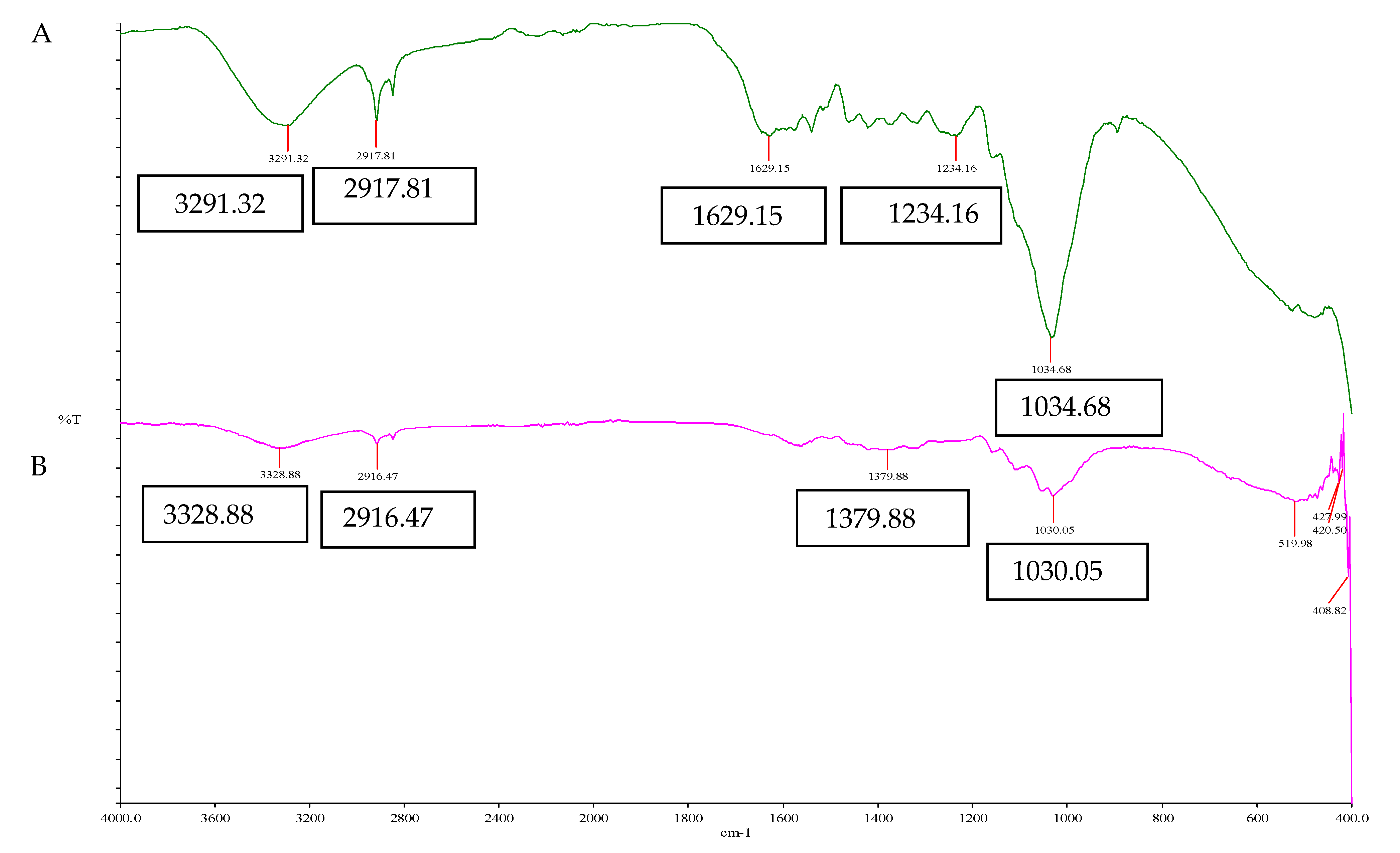
2.4.2. Fourier Transform Infrared (FTIR) Analysis of EFBs
2.5. Enzymatic Saccharification of EFBs
2.6. Selection of Microorganisms and Enzyme Combinations
2.7. Optimization of Simultaneous Saccharification and Fermentation
Central Composite Design (CCD) for Optimization
2.8. Simultaneous Saccharification and Fermentation
2.9. Analytical Methods
2.9.1. Bioethanol Determination
2.9.2. Statistical Analysis of the Experiment
3. Results and Discussion
3.1. Pretreatment of EFBs
| EFB Components | Content (%) | |||||
|---|---|---|---|---|---|---|
| Untreated | Cellulose | 25.71 | 42.2 | 41.8 | 32.26 | 36.59 |
| Hemicellulose | 17.37 | 29.4 | 35.6 | 17.62 | 24.97 | |
| Lignin | 34.02 | 13.8 | 18.8 | 33.02 | 26.53 | |
| Ash | - | - | - | 1.82 | 1.79 | |
| Treated | Cellulose | 90.5 | 62.6 | 85.4 | 65.91 | 75.05 |
| Hemicellulose | 0.00 | 5.6 | 3,5 | 15.55 | 10.19 | |
| Lignin | 9.13 | 24.3 | 5.3 | 11.70 | 8.11 | |
| Ash | - | - | 0.62 | 2.22 | ||
| References | [46] a | [47] a | [48] a | [52] b | [53] b | |
3.1.1. Physical Analysis of EFBs
3.1.2. Scanning Electron Microscope (SEM) Analysis of EFBs
3.1.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of EFBs
3.2. Enzymatic Saccharification of Pretreated EFBs
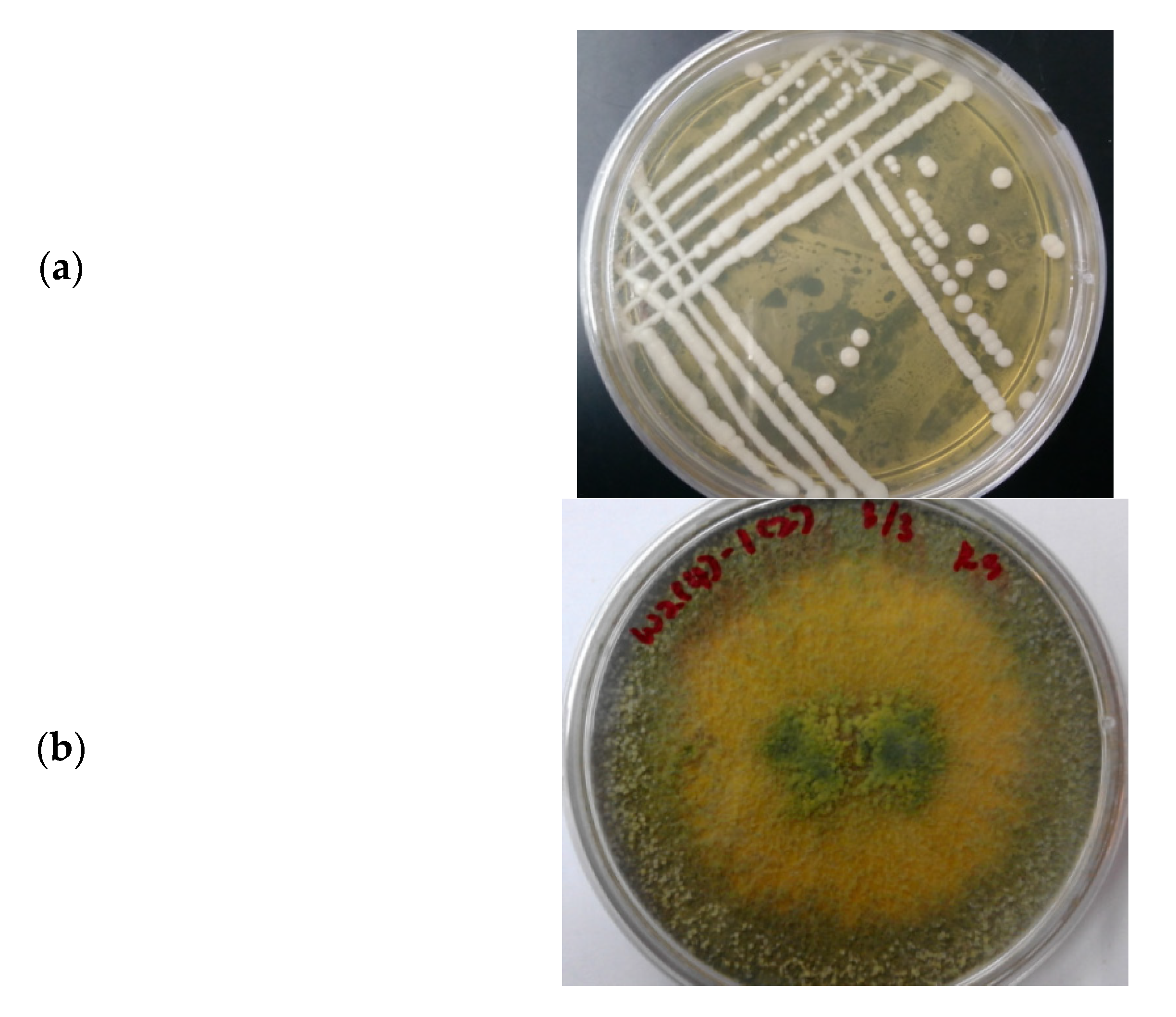
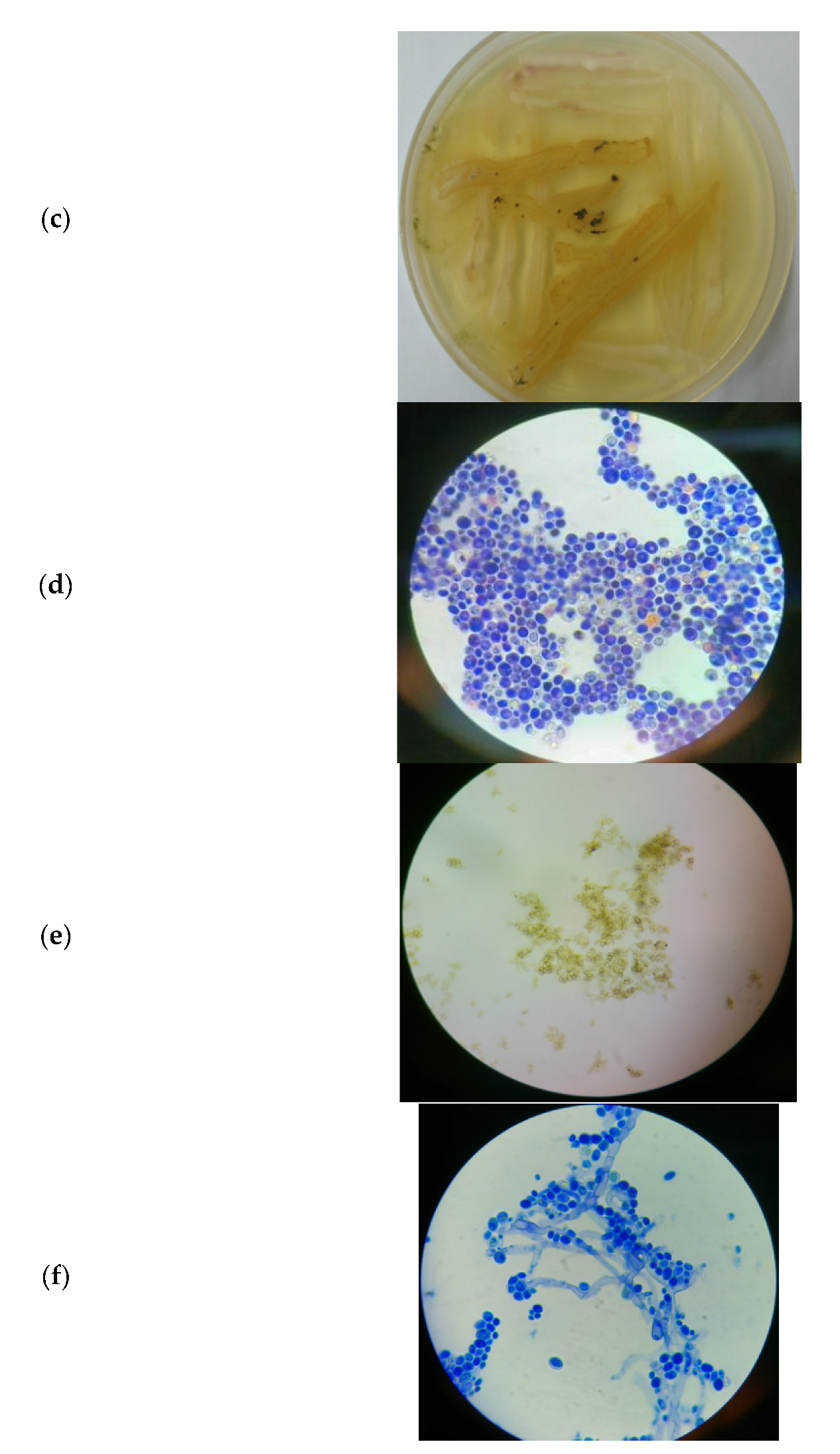
3.3. Microbial Consortium of S. cerevisiae and T. harzianum
3.3.1. Morphology of the Microbial Consortium of S. cerevisiae and T. harzianum
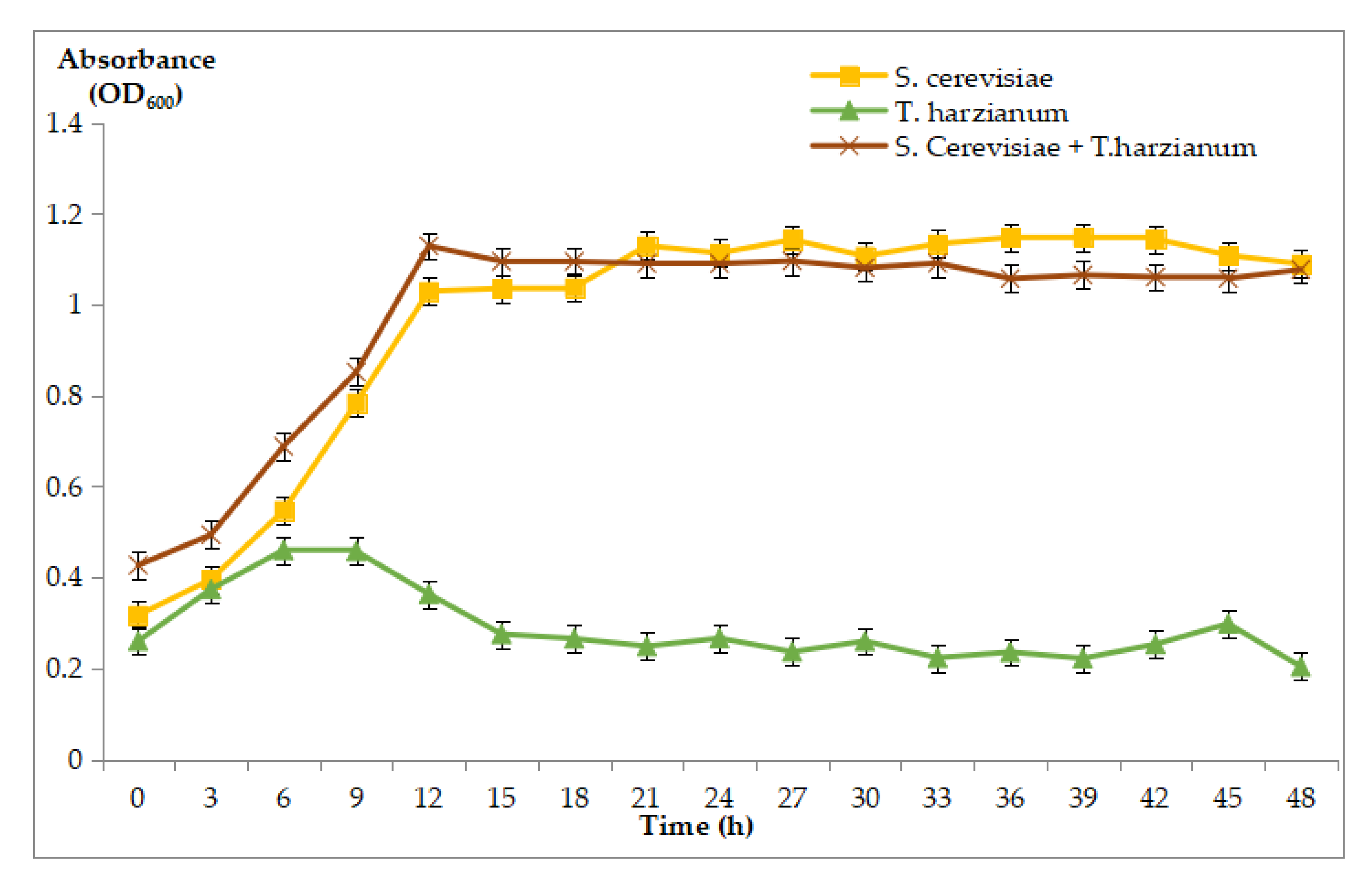
3.3.2. Growth Curve of S. cerevisiae, T. harzianum, and the Co-Culture of S. cerevisiae and T. harzianum
3.4. Selection of Microorganisms and Enzyme Combinations
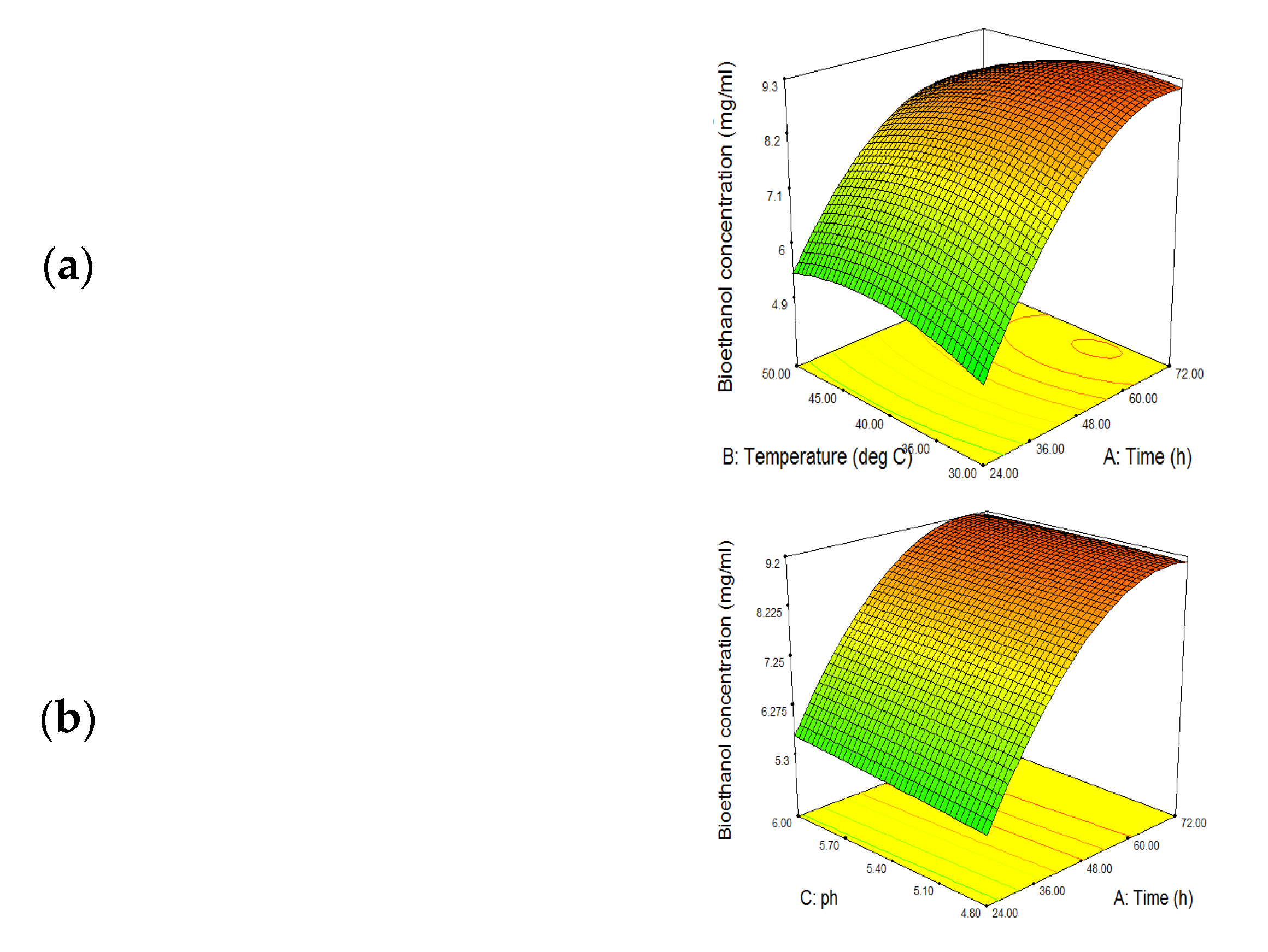
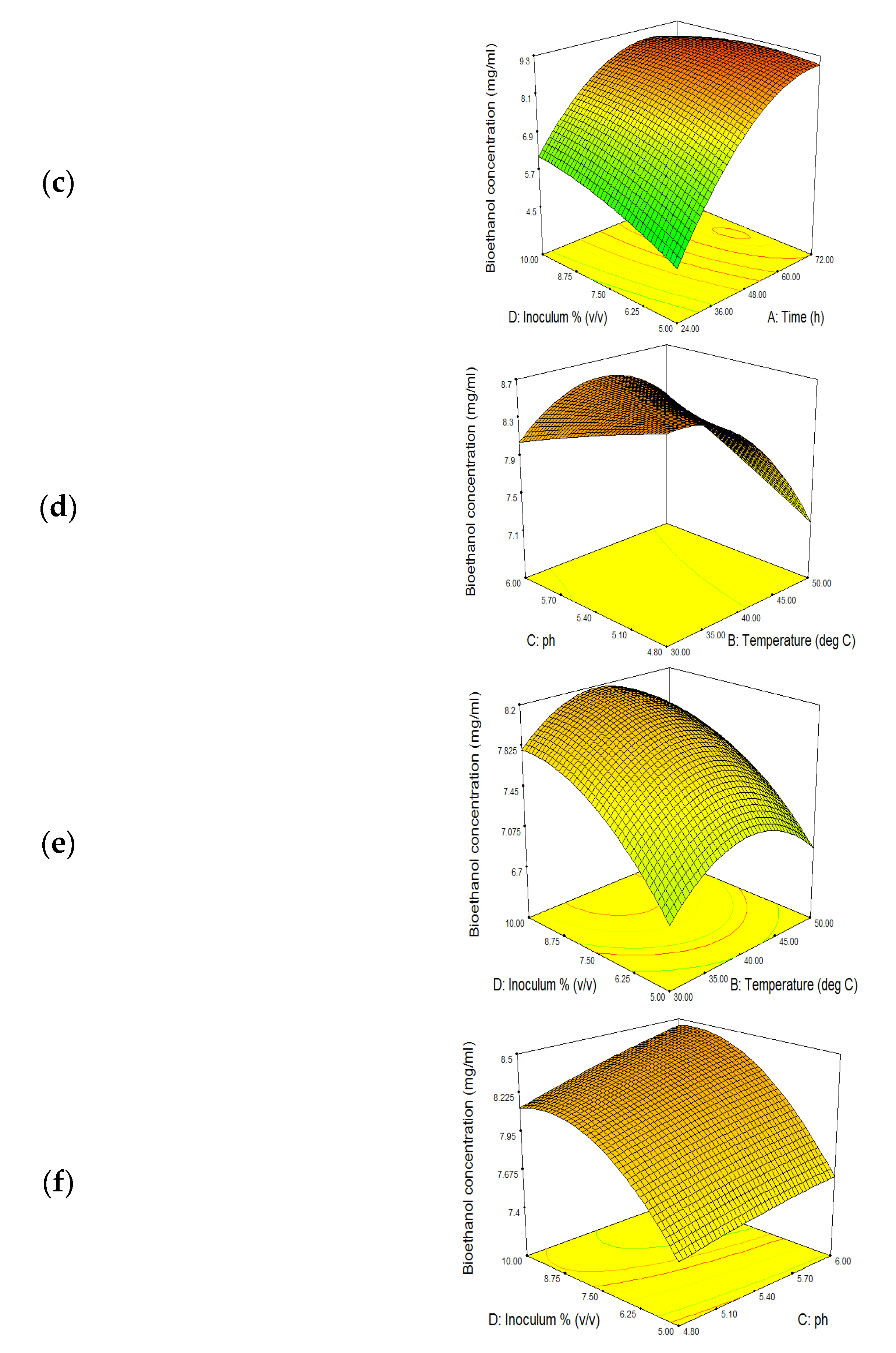
3.5. SSF Optimization for Bioethanol Production
0.38 (BC) − 0.043 (BD) + 0.041 (CD) − 1.14 (A2) − 0.42 (B2) − 0.021 (C2) − O.26 (D2)
3.5.1. Effect of Fermentation Time
3.5.2. Effect of Temperature
3.5.3. Effect of pH
3.5.4. Effect of Inoculum Concentration
3.6. Bioethanol Production Using Optimized Conditions of Fermentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Zhang, B.; Luo, L.; Zhang, F.; Yi, Y.; Shan, Y.; Bianfang, L.; Yuan, Z.; Xin, W.; Xin, L. A review on recycling techniques for bioethanol production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2021, 149, 111370. [Google Scholar] [CrossRef]
- Hosseinzadeh-bandbafha, H.; Nazemi, F.; Khounani, Z.; Ghanavati, H. Safflower-based biorefinery producing a broad spectrum of biofuels and biochemicals: A life cycle assessment perspective. Sci. Total Environ. 2022, 802, 149842. [Google Scholar] [CrossRef]
- Hanif, M.; Mahlia, T.M.I.; Aditiya, H.B.; Chong, W.T. Techno-economic and environmental assessment of bioethanol production from high starch and root yield Sri Kanji 1 cassava in Malaysia. Energy Rep. 2016, 2, 246–253. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kaviti, A.K.; Mehra, R.; Mer, K.K.S. Progress in performance analysis of ethanol-gasoline blends on SI engine. Renew. Sustain. Energy Rev. 2017, 69, 324–340. [Google Scholar] [CrossRef]
- Masum, B.M.; Masjuki, H.H.; Kalam, M.A.; Fattah, I.M.R.; Palash, S.M.; Abedin, M.J. Effect of ethanol—Gasoline blend on NOx emission in SI engine. Renew. Sustain. Energy Rev. 2013, 24, 209–222. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Ahmad, A.; Buang, A.; Bhat, A.H. Renewable and sustainable bioenergy production from microalgal co-cultivation with palm oil mill effluent ( POME ): A review. Renew. Sustain. Energy Rev. 2016, 65, 214–234. [Google Scholar] [CrossRef]
- Derman, E.; Abdulla, R.; Marbawi, H.; Sabullah, M.K. Oil palm empty fruit bunches as a promising feedstock for bioethanol production in Malaysia. Renew. Energy 2018, 129, 285–298. [Google Scholar] [CrossRef]
- Khairil Anwar, N.A.K.; Hassan, N.; Mohd Yusof, N.; Idris, A. High-titer bio-succinic acid production from sequential alkalic and metal salt pretreated empty fruit bunch via simultaneous saccharification and fermentation. Ind. Crop Prod. 2021, 166, 113478. [Google Scholar] [CrossRef]
- Anita, S.H.; Fitria; Solihat, N.N.; Sari, F.P.; Risanto, L.; Fatriasari, W.; Risanto, L.; Fatriasari, W.; Hermiati, E. Optimization of Microwave-Assisted Oxalic Acid Pretreatment of Oil Palm Empty Fruit Bunch for Production of Fermentable Sugars. Waste Biomass Valorization 2019, 11, 2673–2687. [Google Scholar] [CrossRef]
- Chang, S.H. An overview of empty fruit bunch from oil palm as feedstock for bio-oil production. Biomass Bioenergy 2014, 62, 174–181. [Google Scholar] [CrossRef]
- Tan, L.; Wang, M.; Li, X.; Li, H.; Zhao, J.; Qu, Y.; Choo, Y.M.; Loh, S.K. Fractionation of oil palm empty fruit bunch by bisulfite pretreatment for the production of bioethanol and high value products. Bioresour. Technol. 2016, 200, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Aditiya, H.B.; Chong, W.T.; Mahlia, T.M.I.; Sebayang, A.H.; Berawi, M.A.; Nur, H. Second generation bioethanol potential from selected Malaysia ’s biodiversity biomasses: A review. Waste Manag. 2016, 47, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Piarpuzan, D.; Quintero, J.A.; Cardona, C.A. Empty fruit bunches from oil palm as a potential raw material for fuel ethanol production. Biomass Bioenergy 2011, 35, 1130–1137. [Google Scholar] [CrossRef]
- Aguilar-reynosa, A.; Romaní, A.; Rodríguez-jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Comparison of microwave and conduction-convection heating autohydrolysis pretreatment for bioethanol production. Bioresour Technol. 2017, 243, 273–283. [Google Scholar] [CrossRef]
- Abo-state, M.A.; Ragab, A.M.E.; El-gendy, N.S.; Farahat, L.A. Bioethanol Production from Rice Straw Enzymatically Saccharified by Fungal Isolates, Trichoderma viride F94 and Aspergillus terreus F98. Soft 2014, 3, 19–29. [Google Scholar] [CrossRef][Green Version]
- Javed, M.R.; Noman, M.; Shahid, M.; Ahmed, T.; Khurshid, M.; Rashid, M.H.; Ismail, M.; Sadaf, M.; Khan, F. Current situation of biofuel production and its enhancement by CRISPR /Cas9-mediated genome engineering of microbial cells. Microbiol. Res. 2019, 219, 1–11. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, V.; Kuila, A. Simultaneous Saccharification and Fermentation of Corn Husk by Co-Culture Strategy. J. Pet. Environ. Biotechnol. 2019, 9, 360. [Google Scholar] [CrossRef]
- Dahnum, D.; Octavia, S.; Triwahyuni, E.; Nurdin, M. Comparison of SHF and SSF processes using enzyme and dry yeast for optimization of bioethanol production from empty fruit bunch. Energy Procedia 2015, 68, 107–116. [Google Scholar] [CrossRef]
- Park, E.Y.; Naruse, K.; Kato, T. One-pot bioethanol production from cellulose by co-culture of Acremonium cellulolyticus and Saccharomyces cerevisiae. Biotechnol. Biofuels 2012, 5, 64. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. A review of the production of ethanol from softwood. Appl. Microbiol. Biotechnol. 2002, 59, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, X.; Zeng, J.; Loh, S.K.; Choo, Y.M.; Liu, D. Robust enzymatic hydrolysis of Formiline-pretreated oil palm empty fruit bunches (EFB) for efficient conversion of cellulose to sugars and. Bioresour. Technol. 2014, 166, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, C.H. Bioethanol production using the sequential acid/alkali-pretreated empty palm fruit bunch fiber. Renew. Energy 2013, 54, 150–155. [Google Scholar] [CrossRef]
- Jung, S.; Shetti, N.P.; Reddy, K.R.; Nadagouda, M.N.; Park, Y.; Aminabhavi, T.M.; Kwon, E.E. Synthesis of different biofuels from livestock waste materials and their potential as sustainable feedstocks—A review. Energy Convers. Manag. 2021, 236, 114038. [Google Scholar] [CrossRef]
- Polprasert, S.; Choopakar, O.; Elefsiniotis, P. Bioethanol production from pretreated palm empty fruit bunch (PEFB) using sequential enzymatic hydrolysis and yeast fermentation. Biomass Bioenergy 2021, 149, 106088. [Google Scholar] [CrossRef]
- Ali, I.W.; Aziz, K.K.; Syahadah, A.A.H. Bioethanol Production from Acid Hydrolysates of Date Palm Fronds Using a Co-culture of Saccharomyces cerevisiae and Pichia stipitis. Int. J. Enhanc. Res. Sci. Technol. Eng. 2014, 3, 35–44. [Google Scholar]
- Mishra, A.; Ghosh, S. Saccharification of kans grass biomass by a novel fractional hydrolysis method followed by co-culture fermentation for bioethanol production. Renew. Energy 2020, 146, 750–759. [Google Scholar] [CrossRef]
- Izmirlioglu, G.; Demirci, A. Simultaneous saccharification and fermentation of ethanol from potato waste by co-cultures of Aspergillus niger and Saccharomyces cerevisiae in biofilm reactors. Fuel 2017, 202, 260–270. [Google Scholar] [CrossRef]
- Kabbashi, N.A.; Alam, M.Z.; Tompang, M.F. Direct Bioconversion of Oil Palm Empty Fruit Bunches for Bioethanol Production by Solid State Bioconversion. IIUM Eng. J. 2007, 8, 25–36. [Google Scholar] [CrossRef]
- Adela, N.; Nasrin, A.B.; Loh, S.K.; Choo, Y.M. Bioethanol Production by Fermentation of Oil Palm Empty Fruit Bunches Pretreated with Combined Chemicals. J. Appl. Environ. Biol. Sci. 2014, 4, 234–242. [Google Scholar]
- Swain, M.R.; Mishra, J.; Thatoi, H. Bioethanol Production from Sweet Potato (Ipomoea batatas L.) Flour using Co-Culture of Trichoderma sp. and Saccharomyces cerevisiae in Solid-State Fermentation. Braz. Arch. Biol. Technol. 2013, 56, 171–179. [Google Scholar] [CrossRef]
- Bhadwal, A.S.; Le, A.; Le, T.T.; Shrivastava, S.; Bera, T. Trichoderma koningii assisted biogenic synthesis of silver nanoparticles and evaluation of their antibacterial activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 35005. [Google Scholar]
- Bergman, L.W. Growth and Maintenance of Yeast. In Methods in Molecular Biology, Two Hybrid Systems: Methods and Protocols; MacDonald, P.N., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2001; Volume 177, pp. 9–39. [Google Scholar]
- Rodrigues, B.; Lima-Costa, M.E.; Constantino, A.; Raposo, S.; Felizardo, C.; Gonçalves, D.; Peinado, J.M. Growth kinetics and physiological behavior of co-cultures of Saccharomyces cerevisiae and Kluyveromyces lactis, fermenting carob sugars extracted with whey. Enzym. Microb. Technol. 2016, 92, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Paschos, T.; Xiros, C.; Christakopoulos, P. Simultaneous saccharification and fermentation by co-cultures of Fusarium oxysporum and Saccharomyces cerevisiae enhances ethanol production from liquefied wheat straw at high solid content. Ind. Crop Prod. 2015, 76, 793–802. [Google Scholar] [CrossRef]
- Sasikumar, E.; Viruthagiri, T. Optimization of Process Conditions Using Response Surface Methodology (RSM) for Ethanol Production from Pretreated Sugarcane Bagasse: Kinetics and Modeling. Bioenergy Resour. 2008, 1, 239–247. [Google Scholar] [CrossRef]
- Duangwang, S.; Sangwichien, C. Utilization of Oil Palm Empty Fruit Bunch Hydrolysate for Ethanol Production by Baker’s Yeast and Loog-Pang. Energy Procedia 2015, 79, 157–162. [Google Scholar] [CrossRef]
- Sudiyani, Y.; Styarini, D.; Triwahyuni, E.S.; Sembiring, K.C.; Aristiawan, Y.; Han, M.H. Utilization of biomass waste empty fruit bunch fiber of palm oil for bioethanol production using pilot—Scale unit. Energy Procedia 2013, 32, 31–38. [Google Scholar] [CrossRef]
- Baharuddin, A.S.; Nor, A.A.R.; Umi, K.M.S.; Mohd, A.H.; Minato, W.; Yoshihito, S. Evaluation of pressed shredded empty fruit bunch (EFB)-palm oil mill effluent (POME) anaerobic sludge based compost using Fourier transform infrared (FTIR) and nuclear magnetic resonance (NMR) analysis. Afr. J. Biotechnol. 2011, 10, 8082–8089. [Google Scholar]
- Sindhu, R.; Kuttiraja, M.; Prabisha, T.P.; Binod, P.; Sukumaran, R.K.; Pandey, A. Development of a combined pretreatment and hydrolysis strategy of rice straw for the production of bioethanol and biopolymer. Bioresour. Technol. 2016, 215, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, H.I.; Nassar, H.N.; Madian, H.R.; Amr, S.S.A.; El-Gendy, N.S. Response Surface Optimization of Bioethanol Production from Sugarcane Molasses by Pichia veronae Strain HSC-22. Biotechnol. Res. Int. 2015, 10, 905792. [Google Scholar] [CrossRef] [PubMed]
- Mansa, R.F.; Chen, W.F.; Yeo, S.J.; Farm, Y.Y.; Bakar, H.A.; Sipaut, C.S. Chapter 13: Fermentation Study on Macroalgae Eucheuma cottonii for Bioethanol Production via Varying Acid Hydrolysis. In Advanced Biofuels; Sarbatly, R.P.H., Ed.; Springer Science and Business Media: New York, NY, USA, 2013; pp. 219–240. [Google Scholar]
- Tan, I.S.; Lee, K.T. Enzymatic hydrolysis and fermentation of seaweed solid wastes for bioethanol production: An optimization study. Energy 2014, 78, 53–62. [Google Scholar] [CrossRef]
- Burhani, D.; Putri, A.M.H.; Waluyo, J.; Nofiana, Y.; Sudiyani, Y. The effect of two-stage pretreatment on the physical and chemical characteristic of oil palm empty fruit bunch for bioethanol production. AIP Conf. Proc. 2017, 1904, 20016. [Google Scholar]
- Campioni, T.S.; Soccol, C.R.; Libardi Junior, N.; Rodrigues, C.; Woiciechowski, A.L.; Letti, L.A.J.; Vandenberghe, L.P.D.S. Sequential chemical and enzymatic pretreatment of palm empty fruit bunches for Candida pelliculosa bioethanol production. Biotechnol. Appl. Biochem. 2019, 67, 723–731. [Google Scholar] [CrossRef]
- Akhtar, J.; Teo, C.L.; Lai, L.W.; Hassan, N.; Idris, A.; Aziz, R.A. Factors affecting delignification of oil palm empty fruit bunch by microwave-assisted dilute acid/alkali pretreatment. BioResources 2015, 10, 588–596. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Villaflores, O.B.; Ordono, E.E.; Caparanga, A.R. Effects of acidified aqueous glycerol and glycerol carbonate pretreatment of rice husk on the enzymatic digestibility, structural characteristics, and bioethanol production. Bioresour. Technol. 2017, 228, 264–271. [Google Scholar] [CrossRef]
- Abdul, P.M.; Jahim, M.J.; Harun, S.; Markom, M.; Lutpi, N.A.; Hassan, O.; Mohd Nor, M.T. Effects of changes in chemical and structural characteristic of ammonia fibre expansion (AFEX) pretreated oil palm empty fruit bunch fibre on enzymatic saccharification and fermentability for biohydrogen. Bioresour. Technol. 2016, 211, 200–208. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Sari, A.A.; Ariani, N.; Muryanto; Kristiani, A.; Utomo, T.B.; Sudarno. Potential of Oil Palm Empty Fruit Bunches for Bioethanol Production and Application of Chemical Methods in Bioethanol Wastewater Treatment OPEFB for Bioethanol and Its Wastewater Treatment. In Proceedings of the International Conference on Sustainable and Renewable Energy Engineering, Hiroshima, Japan, 10–12 May 2017; pp. 1–4. [Google Scholar]
- Triwahyuni, E.; Muryanto; Sudiyani, Y.; Abimanyu, H. The effect of substrate loading on simultaneous saccharification and fermentation process for bioethanol production from oil palm empty fruit bunches. Energy Procedia 2015, 68, 138–146. [Google Scholar] [CrossRef]
- Fatriasari, W.; Ulwan, W.; Aminingsih, T.; Puspita, F.; Suryanegara, L.; Heri, A.; Ghozali, M.; Kholida, L.N.; Hussin, M.H. Optimization of maleic acid pretreatment of oil palm empty fruit bunches (OPEFB) using response surface methodology to produce reducing sugars. Ind. Crop Prod. 2021, 171, 113971. [Google Scholar] [CrossRef]
- Baharuddin, A.S.; Sulaiman, A.; Kim, D.H.; Mokhtar, M.N.; Hassan, M.A.; Wakisaka, M.; Shirai, Y.; Nishida, H. Selective component degradation of oil palm empty fruit bunches (OPEFB) using high-pressure steam. Biomass Bioenergy 2013, 55, 268–275. [Google Scholar] [CrossRef][Green Version]
- Paramasivan, S.; Sankar, S.; Velavan, R.S.; Krishnakumar, T.; Sithara, R.; Batcha, I.; Muthuvelu, K.S. Assessing the potential of lignocellulosic energy crops as an alternative resource for bioethanol production using ultrasound assisted dilute acid pretreatment. Mater. Today Proc. 2021, 45, 3279–3285. [Google Scholar] [CrossRef]
- Musatto, S.I.; Dragone, G.; Fernandes, M.; Milagres, A.M.F.; Roberto, I.C. The effect of agitation speed, enzyme loading and substrate concentration on enzymatic hydrolysis of cellulose from brewer’s spent grain. Cellulose 2008, 15, 711–721. [Google Scholar] [CrossRef]
- Ariffin, H.; Hassan, M.; Umi Kalsom, M.; Abdullah, N.; Shirai, Y.; Ariffin, H. Effect of physical, chemical and thermal pretreatments on the enzymatic hydrolysis of oil palm empty fruit bunch (OPEFB). J. Trop. Agric. Food Sci. 2008, 36, 1–10. [Google Scholar]
- Kim, D.Y.; Kim, Y.; Kim, T.; Oh, K. Two-stage, acetic acid-aqueous ammonia, fractionation of empty fruit bunches for increased lignocellulosic biomass utilization. Bioresour. Technol. 2016, 199, 121–127. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Santosh, I.; Ashtavinayak, P.; Amol, D.; Sanjay, P. Enhanced bioethanol production from different sugarcane bagasse cultivars using co-culture of Saccharomyces cerevisiae and Scheffersomyces (Pichia) stipitis. J. Environ. Chem. Eng. 2017, 5, 2861–2868. [Google Scholar] [CrossRef]
- Liong, Y.Y.; Halis, R.; Lai, O.M.; Mohamed, R. Conversion Of Lignocellulosic Biomass From Grass To Bioethanol Using Materials Pretreated With Alkali And The White Rot Fungus, Phanerochaete Chrysosporium. BioResources 2012, 7, 5500–5513. [Google Scholar] [CrossRef]
- Thanapimmetha, A.; Saisriyoot, M.; Khomlaem, C.; Chisti, Y.; Srinophakun, P. A comparison of methods of ethanol production from sweet sorghum bagasse. Biochem. Eng. J. 2019, 151, 107352. [Google Scholar] [CrossRef]
- Coral Medina, J.D. Woiciechowski, A.; Zandona Filho, A.; Noseda, M.D.; Kaur, B.S.; Soccol, C.R. Lignin preparation from oil palm empty fruit bunches by sequential acid/alkaline treatment—A biorefinery approach. Bioresour. Technol. 2015, 194, 172–178. [Google Scholar] [CrossRef]
- Norul Izani, M.A.; Paridah, M.T.; Anwar, U.M.K.; Mohd Nor, M.Y.; Ng, P.S. Effects of fiber treatment on morphology, tensile and thermogravimetric analysis of oil palm empty fruit bunches fibers. Compos. B Eng. 2013, 45, 1251–1257. [Google Scholar] [CrossRef]
- Chin, S.X.; Chia, H.C.; Zakaria, S.; Fang, Z.; Ahmad, S. Ball milling pretreatment and diluted acid hydrolysis of oil palm empty fruit bunch (EFB) fibres for the production of levulinic acid. J. Taiwan Inst. Chem. Eng. 2015, 52, 85–92. [Google Scholar] [CrossRef]
- Tye, Y.Y.; Leh, C.P.; Wan Abdullah, W.N. Total glucose yield as the single response in optimizing pretreatments for Elaeis guineensis fibre enzymatic hydrolysis and its relationship with chemical composition of fibre. Renew. Energy 2017, 114, 383–393. [Google Scholar] [CrossRef]
- Shamsudin, S.; Md Shah, U.K.; Zainudin, H.; Abd-Aziz, S.; Mustapa Kamal, S.M. Shirai, Y.; Hassan, M.A. Effect of steam pretreatment on oil palm empty fruit bunch for the production of sugars. Biomass Bioenergy 2012, 36, 280–288. [Google Scholar] [CrossRef]
- Isroi; Cifriadi, A.; Panji, T.; Wibowo, N.A.; Syamsu, K. Bioplastic production from cellulose of oil palm empty fruit bunch. IOP Conf. Ser. Earth Environ. Sci. 2017, 65, 12011. [Google Scholar] [CrossRef]
- Nurul Hazirah, C.H.; Masturah, M.; Osman, H.; Jamaliah, M.J.; Shuhaida, H. Preliminary Study on Analysis of the Chemical Compositions and Characterization of Empty Fruit Bunch (EFB) in Malaysia. Adv. Mater. Res. 2014, 970, 204–208. [Google Scholar] [CrossRef]
- Eliza, M.Y.; Shahruddin, M.; Noormaziah, J.; Rosli, W.D.W. Carboxymethyl Cellulose (CMC) from oil palm empty fruit bunch (OPEFB) in the new solvent dimethyl sulfoxide (DMSO)/tetrabutylammonium fluoride (TBAF). J. Phys. Conf. Ser. 2015, 622, 12026. [Google Scholar] [CrossRef]
- Zulkefli, S.; Abdulmalek, E.; Abdul Rahman, M.B. Pretreatment of oil palm trunk in deep eutectic solvent and optimization of enzymatic hydrolysis of pretreated oil palm trunk. Renew. Energy 2017, 107, 36–41. [Google Scholar] [CrossRef]
- Hamzah, F.; Idris, A.; Shuan, T.K. Preliminary study on enzymatic hydrolysis of treated oil palm (Elaeis) empty fruit bunches fibre (EFB) by using combination of cellulase and β, 1-4 glucosidase. Biomass Bioenergy 2011, 35, 1055–1059. [Google Scholar] [CrossRef]
- Abu Bakar, N.K.; Zanirun, Z.; Abd-Aziz, S.; Ghazali, F.M.; Hassan, M.A. Production of fermentable sugars from oil palm empty fruit bunch using crude cellulase cocktails with Trichoderma asperellum UPM1 and Aspergillus fumigatus UPM2 for bioethanol production. BioResources 2012, 7, 3627–3639. [Google Scholar]
- Hossain, M.N.B.; Basu, J.K.; Mamun, M. The Production of Ethanol from Micro-Algae Spirulina. Procedia Eng. 2015, 105, 733–738. [Google Scholar] [CrossRef]
- Yang, J.; Kim, J.E.; Kim, J.K.; Lee, S.H.; Yu, J.H.; Kim, K.H. Evaluation of commercial cellulase preparations for the efficient hydrolysis of hydrothermally pretreated empty fruit bunches. BioResources 2017, 12, 7834–7840. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Choi, Y.K.; Kan, E.; Kim, Y.G.; Yang, Y.H. Biotechnological potential of microbial consortia and future perspectives. Crit. Rev. Biotechnol. 2018, 38, 1209–1229. [Google Scholar] [CrossRef]
- Kusfanto, H.F.; Maggadani, B.P.; Suryadi, H. Fermentation of Bioethanol from the Biomass Hydrolyzate of Oil Palm Empty Fruit Bunch using selected yeast isolates. Int. J. Appl. Pharm. 2017, 9, 49–53. [Google Scholar] [CrossRef][Green Version]
- Nguyen, K.; Murray, S.; Lewis, J.A.; Kumar, P. Morphology, cell division, and viability of Saccharomyces cerevisiae at high hydrostatic pressure. arXiv 2017, arXiv:170300547. [Google Scholar]
- Evcan, E.; Tari, C. Production of bioethanol from apple pomace by using cocultures: Conversion of agro-industrial waste to value added product. Energy 2015, 88, 775–782. [Google Scholar] [CrossRef]
- Ab Majid, A.H.; Zahran, Z.; Abd Rahim, A.H.; Ismail, N.A.; Abdul Rahman, W. Mohammad Zubairi, K.S.; Satho, T. Morphological and molecular characterization of fungus isolated from tropical bed bugs in Northern Peninsular Malaysia, Cimex hemipterus (Hemiptera: Cimicidae). Asian Pac. J. Trop. Biomed. 2015, 5, 707–713. [Google Scholar] [CrossRef]
- Kannangara, S.; Dharmarathna, R.M.G.C.S. Jayarathna, D. Isolation, Identification and Characterization of Trichoderma Species as a Potential Biocontrol Agent against Ceratocystis paradoxa. J. Agric. Sci. 2017, 12, 51–62. [Google Scholar]
- Shah, S.; Nasreen, S.; Sheikh, P. Cultural and Morphological Characterization of Trichoderma spp. Associated with Green Mold Disease of Pleurotus spp. in Kashmir. Res. J. Microbiol. 2012, 7, 139–144. [Google Scholar] [CrossRef][Green Version]
- Mustafa, A.; Khan, M.A.; Inam-ul-Haq, M.; Pervez, M.A.; Umar, U.D. Usefulness of Different culture media for in-vitro evaluation of Trichoderma spp. against seed borne fungi of economic importance. J. Phytopathol. 2009, 21, 83–88. [Google Scholar]
- Prajankate, P.; Siwarasak, P. Co-culture of Trichoderma reesei RT-P1 with Saccharomyces cerevisiae RT-P2: Morphological Study. Sci. Technol. 2011, 4, 75–78. [Google Scholar]
- De Azevedo, A.M.C.; De Marco, J.L.; Felix, C.R. Characterization of an amylase produced by a Trichoderma harzianum isolate with antagonistic activity against Crinipellis perniciosa, the causal agent of witches’ broom of cocoa. FEMS Microbiol. Lett. 2000, 188, 171–175. [Google Scholar] [CrossRef]
- Kumar, D.; Surya, K.; Verma, R. Bioethanol production from apple pomace using co-cultures with Saccharomyces cerevisiae in solid-state fermentation. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 742–745. [Google Scholar] [CrossRef]
- Aunsbjerg, S.D.; Andersen, K.R.; Knøchel, S. Real-time monitoring of fungal inhibition and morphological changes. J. Microbiol. Methods 2015, 119, 196–202. [Google Scholar] [CrossRef]
- Alsuhaim, H.; Vojisavljevic, V.; Pirogova, E. Effects of Non-thermal Microwave Exposures on the Proliferation Rate of Saccharomyces Cerevisiae yeast. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Beijing, China, 26–31 May 2012; pp. 1–5. [Google Scholar]
- Jung, Y.H.; Kim, I.J.; Kim, H.K.; Kim, K.H. Dilute acid pretreatment of lignocellulose for whole slurry ethanol fermentation. Bioresour. Technol. 2013, 132, 109–114. [Google Scholar] [CrossRef]
- Raman, J.K.; Gnansounou, E. Ethanol and lignin production from Brazilian empty fruit bunch biomass. Bioresour. Technol. 2014, 172, 241–248. [Google Scholar] [CrossRef]
- Pandey, A.K.; Edgard, G.; Negi, S. Optimization of concomitant production of cellulase and xylanase from Rhizopus oryzae SN5 through EVOP-factorial design technique and application in Sorghum Stover based bioethanol production. Renew Energy 2016, 98, 51–56. [Google Scholar] [CrossRef]
- Rajnish, K.N.; Samuel, M.S.; John, A.J.; Datta, S.; Chandrasekar, N.; Balaji, R.; Jose, S.; Selvarajan, E. Immobilization of cellulase enzymes on nano and micro-materials for breakdown of cellulose for biofuel production-a narrative review. Int. J. Biol. Macromol. 2021, 182, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Shokrkar, H.; Ebrahimi, S. Synergism of cellulases and amylolytic enzymes in the hydrolysis of microalgal carbohydrates. Biofuels. Bioprod. Biorefining 2018, 12, 749–755. [Google Scholar] [CrossRef]
- Poornejad, N.; Karimi, K.; Behzad, T. Ionic Liquid Pretreatment of Rice Straw to Enhance Saccharification and Bioethanol Production. J. Biomass Biofuel 2014, 1, 8–15. [Google Scholar] [CrossRef]
- Alam, M.Z.; Kabbashi, N.A.; Tompang, M.F. Development of single-step bioconversion process for bioethanol production by fungi and yeast using oil palm empty fruit bunches. In Proceedings of the 20th Symposium Malaysian Chememical Engineering, Kuala Lumpur, Malaysia, 19 December 2006; pp. 1–6. [Google Scholar]
- Ferreira Filho, J.A.; Horta, M.A.C.; Beloti, L.L.; Dos Santos, C.A.; de Souza, A.P. Carbohydrate-active enzymes in Trichoderma harzianum: A bioinformatic analysis bioprospecting for key enzymes for the biofuels industry. BMC Genom. 2017, 18, 779. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhai, L.; Geng, A. Enhanced cellulase and reducing sugar production by a new mutant strain Trichoderma harzianum EUA20. J. Biosci. Bioeng. 2020, 129, 242–249. [Google Scholar] [CrossRef]
- Qi, G.; Xiong, L.; Luo, M.; Huang, Q.; Huang, C.; Li, H.; Xuefang, C.; Xinde, C. Solvents production from cassava by co-culture of Clostridium acetobutylicum and Saccharomyces cerevisiae. J. Environ. Chem. Eng. 2018, 6, 128–133. [Google Scholar] [CrossRef]
- Verma, G.; Nigam, P.; Singh, D.; Chaudhary, K. Bioconversion of starch to ethanol in a single-step process by coculture of amylolytic yeasts and Saccharomyces cerevisiae 21. Bioresour. Technol. 2000, 72, 261–266. [Google Scholar] [CrossRef]
- Dey, P.; Rangarajan, V.; Nayak, J.; Bhusan, D.; Branden, S. An improved enzymatic pre-hydrolysis strategy for efficient bioconversion of industrial pulp and paper sludge waste to bioethanol using a semi-simultaneous saccharification and fermentation process. Fuel 2021, 294, 120581. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Xu, J.; Sun, Y.; Yuan, Z.; Xie, J. Consolidated bioprocess for bioethanol production with alkali-pretreated sugarcane bagasse. Appl. Energy 2015, 157, 517–522. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Kim, J.S.; Kim, S. Optimization of dilute acid and enzymatic hydrolysis for dark fermentative hydrogen production from the empty fruit bunch of oil palm. Int. J. Hydrog. Energy 2019, 44, 2191–2202. [Google Scholar] [CrossRef]
- Zainan, N.H.; Alam, Z.; Al-khatib, M.F. Production of sugar by hydrolysis of empty fruit bunches using palm oil mill effluent (POME) based cellulases: Optimization study. Afr. J. Biotechnol. 2011, 10, 18722–18727. [Google Scholar]
- Kamoldeen, A.A.; Keong, C.; Nadiah, W.; Abdullah, W.; Peng, C. Enhanced ethanol production from mild alkali-treated oil-palm empty fruit bunches via co-fermentation of glucose and xylose. Renew. Energy 2017, 107, 113–123. [Google Scholar] [CrossRef]
- Syadiah, E.A.; Haditjaroko, L.; Syamsu, K. Bioprocess engineering of bioethanol production based on sweet sorghum bagasse by co-culture technique using Trichoderma reesei and Saccharomyces cerevisiae. IOP Conf. Ser. Earth Environ. Sci. 2018, 209, 12018. [Google Scholar] [CrossRef]
- Jambo, S.A.; Abdulla, R.; Marbawi, H.; Gansau, J.A. Response surface optimization of bioethanol production from third generation feedstock—Eucheuma cottonii. Renew. Energy 2018, 132, 1–10. [Google Scholar] [CrossRef]
- Khoja, A.H.; Ehsan, A.; Kashaf, Z.; Ansari, A.A.; Azra, N.; Muneeb, Q. Comparative study of bioethanol production from sugarcane molasses by using Zymomonas mobilis and Saccharomyces cerevisiae. Afr. J. Biotechnol. 2015, 14, 2455–2462. [Google Scholar]
- Park, J.; Oh, B.; Seo, J.; Hong, W.K.; Yu, A.; Sohn, J.H.; Kim, C.H. Efficient Production of Ethanol from Empty Palm Fruit Bunch Fibers by Fed-Batch Simultaneous Saccharification and Fermentation Using Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2013, 170, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.U.; Bukar, A.; Usman, B. Production of bioethanol from rice husk using Aspergillus niger and Trichoderma harzianum. Bayero. J. Pure Appl. Sci. 2017, 10, 280. [Google Scholar]
- Kassim, M.A.; Loh, S.K.; Bakar, N.A.; Aziz, A.A.; Mat Som, R. Bioethanol production from Enzymatically Saccharified Empty Fruit Bunches Hydrolysate using Saccharomyces cerevisiae. Res. J. Env. Sci. 2011, 5, 573. [Google Scholar]
- Sahu, O. Appropriateness of rose (Rosa hybrida) for bioethanol conversion with enzymatic hydrolysis: Sustainable development on green fuel production. Energy 2021, 232, 120922. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abd-aziz, S.; Hassan, M.A. Oil Palm Empty Fruit Bunch as Alternative Substrate for Acetone—Butanol—Ethanol Production by Clostridium butyricum EB6. Appl. Biochem. Biotechnol. 2012, 166, 1615–1625. [Google Scholar] [CrossRef]
- Alam, M.Z.; Al khatib, N.H.; Rashid, M.F. Optimization of bioethanol production from empty fruit bunches by co-culture of Saccharomyces cerevisae and Aspergillus niger using statistical experimental design. J. Pure Appl. Microbiol. 2014, 8, 731–740. [Google Scholar]
- Anu; Singh, B.; Kumar, A. Process development for sodium carbonate pretreatment and enzymatic saccharification of rice straw for bioethanol production. Biomass Bioenergy 2020, 138, 105574. [Google Scholar] [CrossRef]
- Chohan, N.A.; Aruwajoye, G.S.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Valorisation of potato peel wastes for bioethanol production using simultaneous saccharification and fermentation: Process optimization and kinetic assessment. Renew. Energy 2019, 146, 1031–1040. [Google Scholar] [CrossRef]
- Ansar; Nazaruddin; Azis, A.D.; Fudholi, A. Enhancement of bioethanol production from palm sap (Arenga pinnata (Wurmb) Merr) through optimization of Saccharomyces cerevisiae as an inoculum. J. Mater. Res. Technol. 2021, 14, 548–554. [Google Scholar] [CrossRef]
- de Albuquerque Wanderley, A.C.; Soares, M.L.; Gouveia, E.R. Selection of inoculum size and Saccharomyces cerevisiae strain for ethanol production in simultaneous saccharification and fermentation (SSF) of sugar cane bagasse. Afr. J Biotechnol 2014, 13, 2762–2765. [Google Scholar]
- Neelakandan, T.; Usharani, G.; Nagar, A. Optimization and Production of Bioethanol from Cashew Apple Juice Using Immobilized Yeast Cells by Saccharomyces cerevisiae. Am. J. Sci. Res. 2009, 4, 85–88. [Google Scholar]

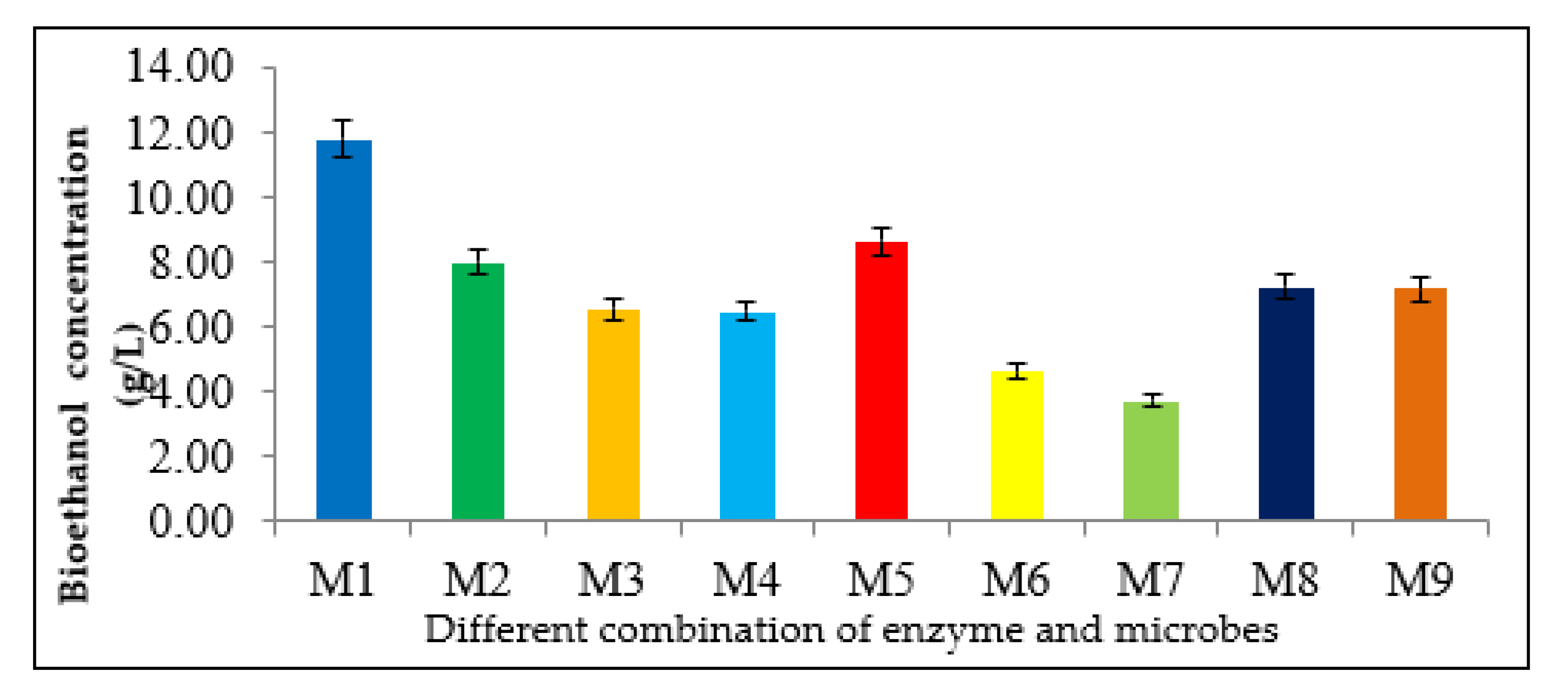
| Combination | Microorganisms and Enzymes | |||
|---|---|---|---|---|
| S. cerevisiae | T. harzianum | Cellulase | β-glucosidase | |
| M1 | ✓ | ✓ | ✓ | ✓ |
| M2 | ✓ | ✓ | ✓ | |
| M3 | ✓ | ✓ | ✓ | |
| M4 | ✓ | ✓ | ✓ | |
| M5 | ✓ | ✓ | ||
| M6 | ✓ | ✓ | ✓ | |
| M7 | ✓ | ✓ | ✓ | |
| M8 | ✓ | ✓ | ||
| M9 | ✓ | ✓ | ||
| Parameters | Levels | |||||
|---|---|---|---|---|---|---|
| −2 (α) | −1 | 0 | +1 | +2 (α) | ||
| A | Fermentation Time, (h) | 0.0 | 24.0 | 48.0 | 72.0 | 96.0 |
| B | Temperature, (°C) | 20.0 | 30.0 | 40.0 | 50.0 | 60.0 |
| C | pH | 4.2 | 4.8 | 5.4 | 6.0 | 6.6 |
| D | Inoculum concentration, % (v/v) | 2.5 | 5.0 | 7.5 | 10.0 | 12.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derman, E.; Abdulla, R.; Marbawi, H.; Sabullah, M.K.; Gansau, J.A.; Ravindra, P. Simultaneous Saccharification and Fermentation of Empty Fruit Bunches of Palm for Bioethanol Production Using a Microbial Consortium of S. cerevisiae and T. harzianum. Fermentation 2022, 8, 295. https://doi.org/10.3390/fermentation8070295
Derman E, Abdulla R, Marbawi H, Sabullah MK, Gansau JA, Ravindra P. Simultaneous Saccharification and Fermentation of Empty Fruit Bunches of Palm for Bioethanol Production Using a Microbial Consortium of S. cerevisiae and T. harzianum. Fermentation. 2022; 8(7):295. https://doi.org/10.3390/fermentation8070295
Chicago/Turabian StyleDerman, Eryati, Rahmath Abdulla, Hartinie Marbawi, Mohd Khalizan Sabullah, Jualang Azlan Gansau, and Pogaku Ravindra. 2022. "Simultaneous Saccharification and Fermentation of Empty Fruit Bunches of Palm for Bioethanol Production Using a Microbial Consortium of S. cerevisiae and T. harzianum" Fermentation 8, no. 7: 295. https://doi.org/10.3390/fermentation8070295
APA StyleDerman, E., Abdulla, R., Marbawi, H., Sabullah, M. K., Gansau, J. A., & Ravindra, P. (2022). Simultaneous Saccharification and Fermentation of Empty Fruit Bunches of Palm for Bioethanol Production Using a Microbial Consortium of S. cerevisiae and T. harzianum. Fermentation, 8(7), 295. https://doi.org/10.3390/fermentation8070295






