Valorization of Low-Cost Substrates for the Production of Odd Chain Fatty Acids by the Oleaginous Yeast Yarrowia lipolytica
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yarrowia Lipolytica Strain
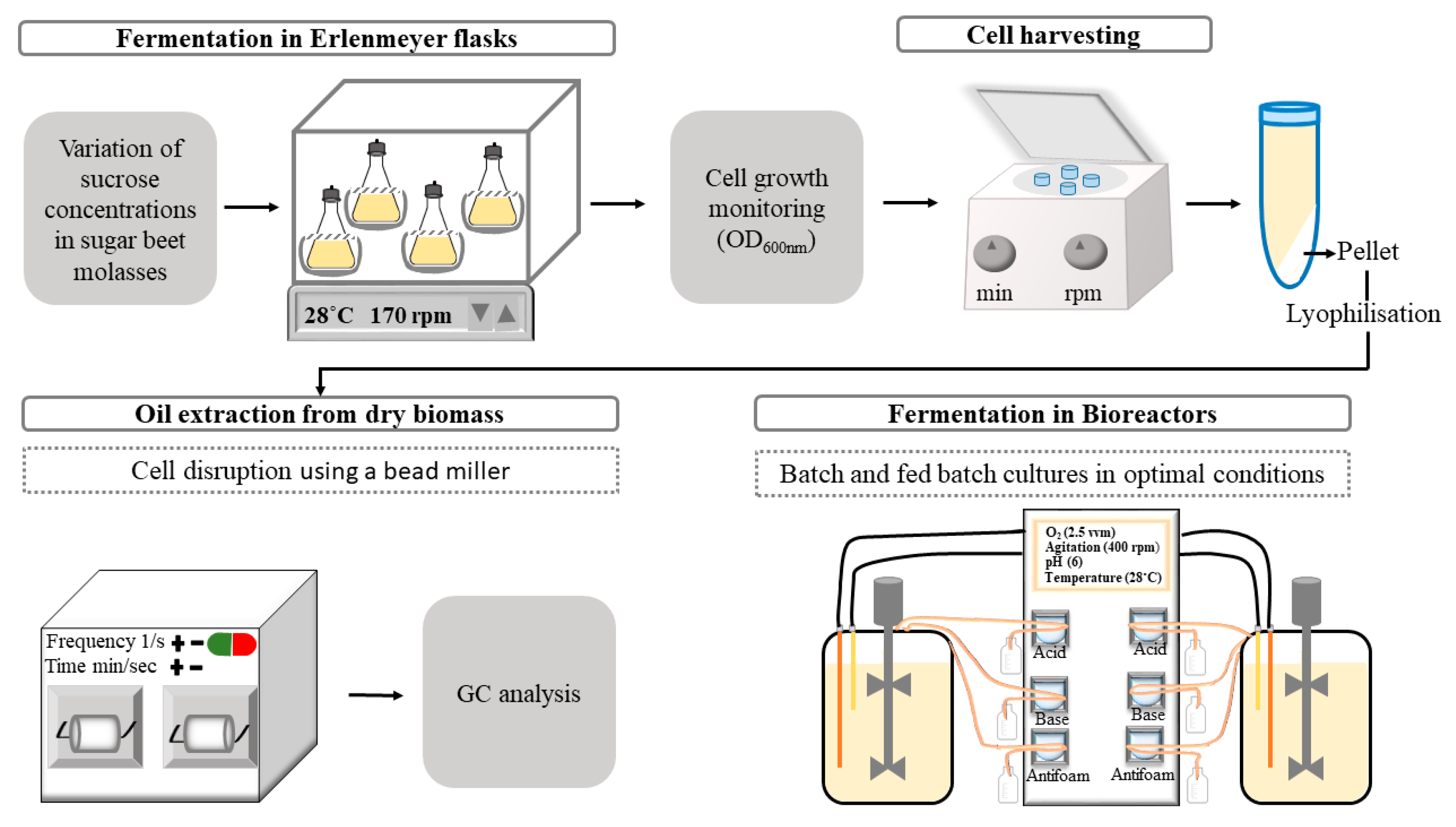
2.2. Fermentation Conditions
2.2.1. Shake Flask Experiments
2.2.2. Bioreactor Experiments
2.3. Substrates Consumption Analysis
2.4. Lipid Extraction and Fatty Acid Analysis
2.4.1. Lipid Extraction from Dry Biomass
2.4.2. Preparation of Fatty Acid Methyl Esters (FAMEs) for Gas Chromatography (GC) Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Impact of Sugar Beet Molasses and Crude Glycerol on Cell Growth
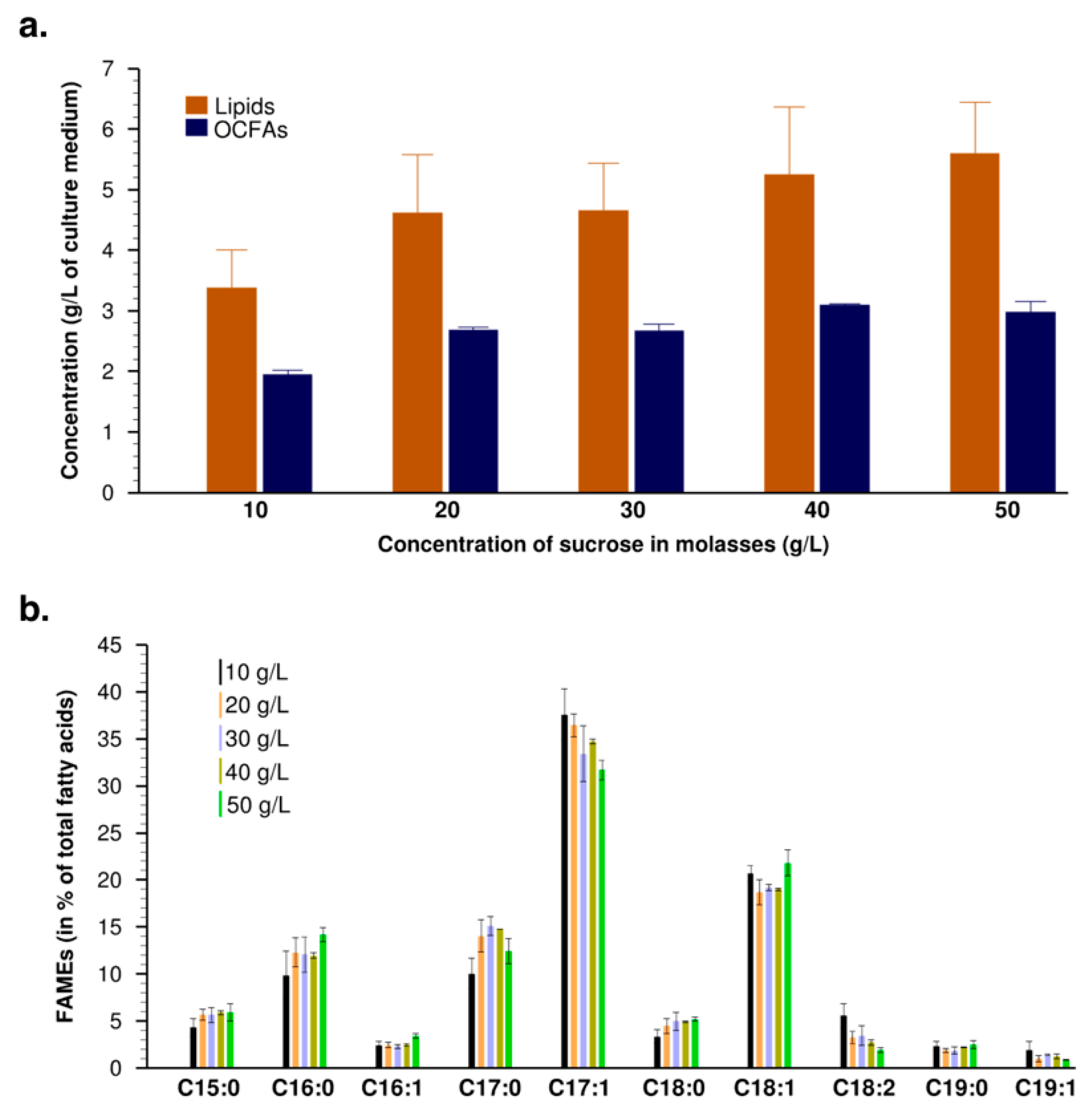
3.2. Evaluation of Lipid Production in Erlenmeyer Flasks
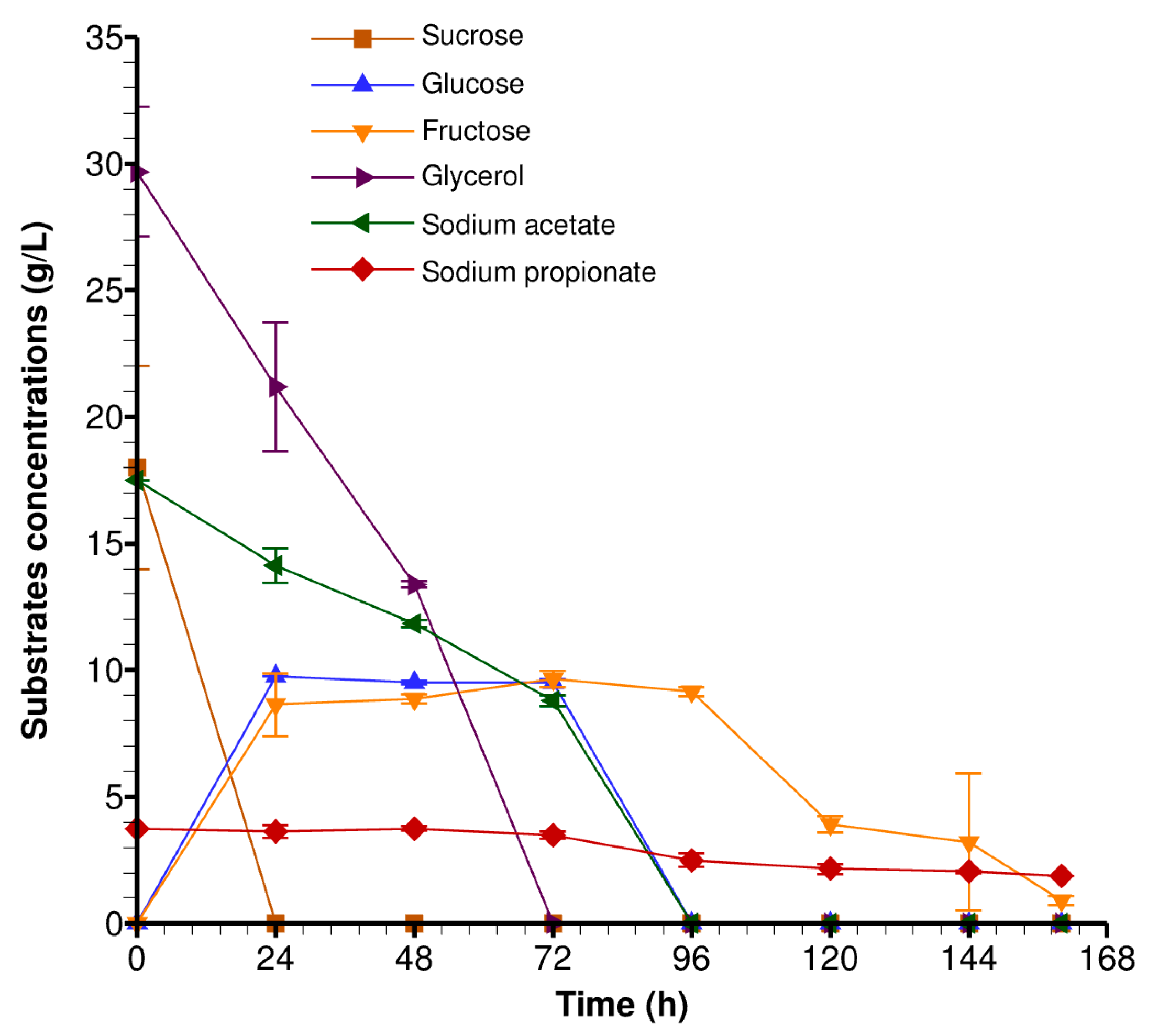
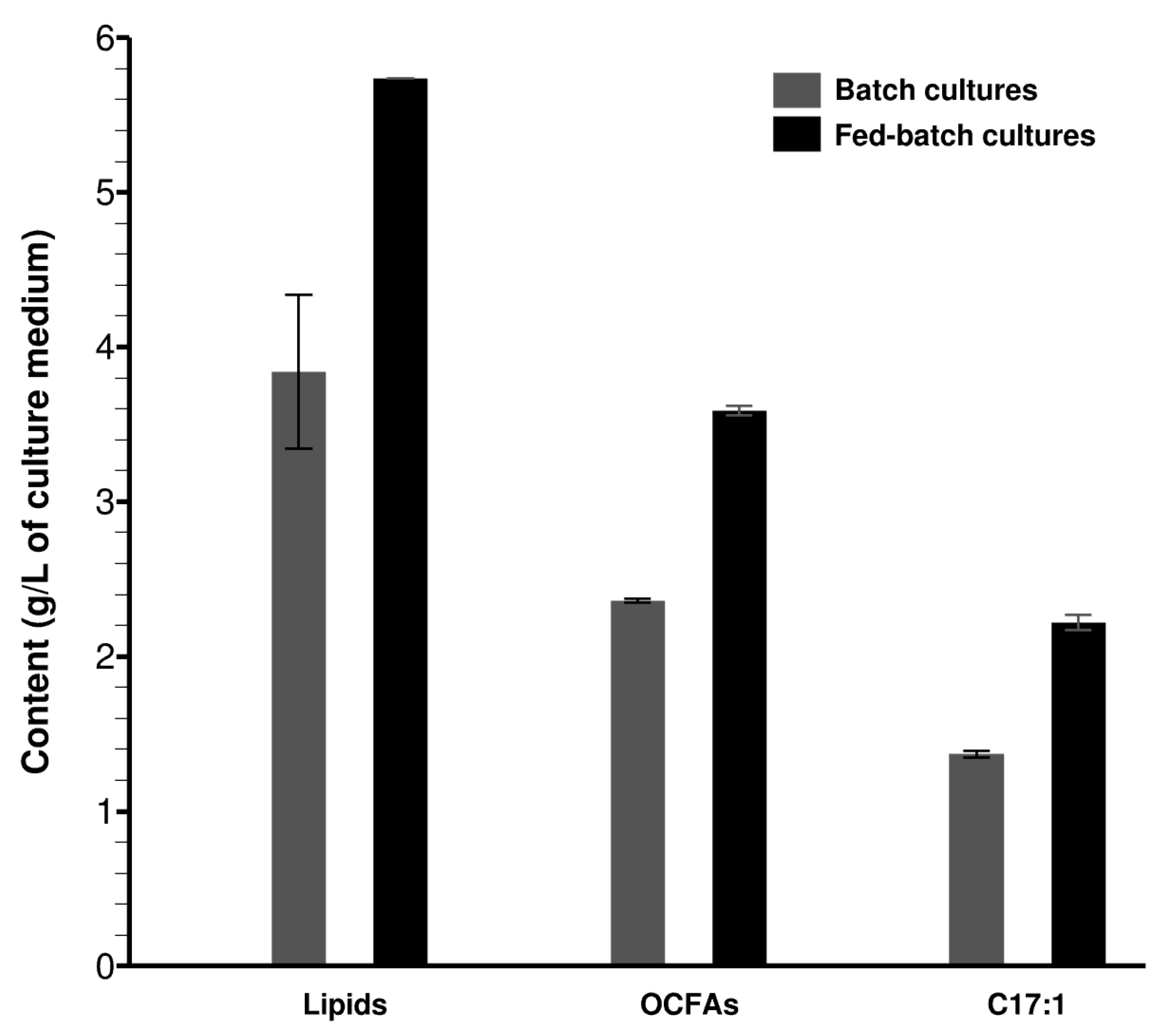
3.3. Evaluation of Lipid Production in Bioreactors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ledesma-Amaro, R.; Dulermo, T.; Nicaud, J.M. Engineering Yarrowia Lipolytica to Produce Biodiesel from Raw Starch. Biotechnol. Biofuels 2015, 8, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larroude, M.; Rossignol, T.; Nicaud, J.M.; Ledesma-Amaro, R. Synthetic Biology Tools for Engineering Yarrowia Lipolytica. Biotechnol. Adv. 2018, 36, 2150–2164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Liang, S.; Zong, M.H.; Yang, J.G.; Lou, W.Y. Microbial Synthesis of Functional Odd-Chain Fatty Acids: A Review. World J. Microbiol. Biotechnol. 2020, 36, 35. [Google Scholar] [CrossRef]
- Kurotani, K.; Sato, M.; Yasuda, K.; Kashima, K.; Tanaka, S.; Hayashi, T.; Shirouchi, B.; Akter, S.; Kashino, I.; Hayabuchi, H.; et al. Even-And Odd-Chain Saturated Fatty Acids in Serum Phospholipids Are Differentially Associated with Adipokines. PLoS ONE 2017, 12, e0178192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeuffer, M.; Jaudszus, A. Pentadecanoic and Heptadecanoic Acids: Multifaceted Odd-Chain Fatty Acids. Adv. Nutr. 2016, 7, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Mika, A.; Stepnowski, P.; Kaska, L.; Proczko, M.; Wisniewski, P.; Sledzinski, M.; Sledzinski, T. A Comprehensive Study of Serum Odd- and Branched-Chain Fatty Acids in Patients with Excess Weight. Obesity 2016, 24, 1669–1676. [Google Scholar] [CrossRef] [Green Version]
- Řezanka, T.; Kolouchová, I.; Sigler, K. Precursor Directed Biosynthesis of Odd-Numbered Fatty Acids by Different Yeasts. Folia Microbiol. 2015, 60, 457–464. [Google Scholar] [CrossRef]
- Avis, T.J.; Boulanger, R.R.; Bélanger, R.R. Synthesis and Biological Characterization of (Z)-9-Heptadecenoic and (Z)-6-Methyl-9-Heptadecenoic Acids: Fatty Acids with Antibiotic Activity Produced by Pseudozyma Flocculosa. J. Chem. Ecol. 2000, 26, 987–1000. [Google Scholar] [CrossRef]
- Park, Y.K.; Dulermo, T.; Ledesma-Amaro, R.; Nicaud, J.-M. Optimization of Odd Chain Fatty Acid Production by Yarrowia Lipolytica. Biotechnol. Biofuels 2018, 11, 158. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.-K.; Ledesma-Amaro, R.; Nicaud, J.-M. De Novo Biosynthesis of Odd-Chain Fatty Acids in Yarrowia Lipolytica Enabled by Modular Pathway Engineering. Front. Bioeng. Biotechnol. 2020, 7, 484. [Google Scholar] [CrossRef]
- Fontanille, P.; Kumar, V.; Christophe, G.; Nouaille, R.; Larroche, C. Bioconversion of Volatile Fatty Acids into Lipids by the Oleaginous Yeast Yarrowia Lipolytica. Bioresour. Technol. 2012, 114, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and Techno-Economic Evaluation of Microbial Oil Production as a Renewable Resource for Biodiesel and Oleochemical Production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Cavallo, E.; Nobile, M.; Cerrutti, P.; Foresti, M.L. Exploring the Production of Citric Acid with Yarrowia Lipolytica Using Corn Wet Milling Products as Alternative Low-Cost Fermentation Media. Biochem. Eng. J. 2020, 155, 107463. [Google Scholar] [CrossRef]
- Gao, R.; Li, Z.; Zhou, X.; Cheng, S.; Zheng, L. Oleaginous Yeast Yarrowia Lipolytica Culture with Synthetic and Food Waste-Derived Volatile Fatty Acids for Lipid Production. Biotechnol. Biofuels 2017, 10, 247. [Google Scholar] [CrossRef]
- Gao, R.; Li, Z.; Zhou, X.; Bao, W.; Cheng, S.; Zheng, L. Enhanced Lipid Production by Yarrowia Lipolytica Cultured with Synthetic and Waste-Derived High-Content Volatile Fatty Acids under Alkaline Conditions. Biotechnol. Biofuels 2020, 13, 3. [Google Scholar] [CrossRef]
- Louhasakul, Y.; Cheirsilp, B.; Intasit, R.; Maneerat, S.; Saimmai, A. Enhanced Valorization of Industrial Wastes for Biodiesel Feedstocks and Biocatalyst by Lipolytic Oleaginous Yeast and Biosurfactant-Producing Bacteria. Int. Biodeterior. Biodegrad. 2020, 148, 104911. [Google Scholar] [CrossRef]
- Tsigie, Y.A.; Wang, C.Y.; Truong, C.T.; Ju, Y.H. Lipid Production from Yarrowia Lipolytica Po1g Grown in Sugarcane Bagasse Hydrolysate. Bioresour. Technol. 2011, 102, 9216–9222. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Louhasakul, Y. Industrial Wastes as a Promising Renewable Source for Production of Microbial Lipid and Direct Transesterification of the Lipid into Biodiesel. Bioresour. Technol. 2013, 142, 329–337. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, Y.; Yu, S. Optimisation of Ultrasonic-Assisted Extraction of Phenolic Compounds, Antioxidants, and Anthocyanins from Sugar Beet Molasses. Food Chem. 2015, 172, 543–550. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Nicaud, J.M. Metabolic Engineering for Expanding the Substrate Range of Yarrowia Lipolytica. Trends Biotechnol. 2016, 34, 798–809. [Google Scholar] [CrossRef]
- Saisriyoot, M.; Thanapimmetha, A.; Suwaleerat, T.; Chisti, Y.; Srinophakun, P. Biomass and Lipid Production by Rhodococcus Opacus PD630 in Molasses-Based Media with and without Osmotic-Stress. J. Biotechnol. 2019, 297, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Palmonari, A.; Cavallini, D.; Sniffen, C.J.; Fernandes, L.; Holder, P.; Fagioli, L.; Fusaro, I.; Biagi, G.; Formigoni, A.; Mammi, L. Short Communication: Characterization of Molasses Chemical Composition. J. Dairy Sci. 2020, 103, 6244–6249. [Google Scholar] [CrossRef] [PubMed]
- Valli, V.; Gómez-Caravaca, A.M.; Di Nunzio, M.; Danesi, F.; Caboni, M.F.; Bordoni, A. Sugar Cane and Sugar Beet Molasses, Antioxidant-Rich Alternatives to Refined Sugar. J. Agric. Food Chem. 2012, 60, 12508–12515. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, A.; Mituła, P.; Rymowicz, W.; Mirończuk, A.M. Efficient Conversion of Crude Glycerol from Various Industrial Wastes into Single Cell Oil by Yeast Yarrowia Lipolytica. Bioresour. Technol. 2016, 207, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Cui, Y.; Trushenski, J.; Blackburn, J.W. Converting Crude Glycerol Derived from Yellow Grease to Lipids through Yeast Fermentation. Bioresour. Technol. 2010, 101, 7581–7586. [Google Scholar] [CrossRef]
- Kumar, L.R.; Yellapu, S.K.; Tyagi, R.D.; Zhang, X. A Review on Variation in Crude Glycerol Composition, Bio-Valorization of Crude and Purified Glycerol as Carbon Source for Lipid Production. Bioresour. Technol. 2019, 293, 122155. [Google Scholar] [CrossRef]
- Park, Y.-K.; Bordes, F.; Letisse, F.; Nicaud, J.-M. Engineering Precursor Pools for Increasing Production of Odd-Chain Fatty Acids in Yarrowia Lipolytica. Metab. Eng. Commun. 2021, 12, e00158. [Google Scholar] [CrossRef]
- Al Sahyouni, W.; El Kantar, S.; Khelfa, A.; Park, Y.-K.; Nicaud, J.-M.; Louka, N.; Koubaa, M. Optimization of Cis-9-Heptadecenoic Acid Production from the Oleaginous Yeast Yarrowia Lipolytica. Fermentation 2022, 8, 245. [Google Scholar] [CrossRef]
- Back, A.; Rossignol, T.; Krier, F.; Nicaud, J.-M.; Dhulster, P. High-Throughput Fermentation Screening for the Yeast Yarrowia Lipolytica with Real-Time Monitoring of Biomass and Lipid Production. Microb. Cell Factories 2016, 15, 147. [Google Scholar] [CrossRef]
- Gajdoš, P.; Ledesma-Amaro, R.; Nicaud, J.-M.; Čertík, M.; Rossignol, T. Overexpression of Diacylglycerol Acyltransferase in Yarrowia Lipolytica Affects Lipid Body Size, Number and Distribution. FEMS Yeast Res. 2016, 16, fow062. [Google Scholar] [CrossRef] [Green Version]
- Drévillon, L.; Koubaa, M.; Nicaud, J.-M.; Vorobiev, E. Cell Disruption Pre-Treatments towards an Effective Recovery of Oil from Yarrowia Lipolytica Oleaginous Yeast. Biomass Bioenergy 2019, 128, 105320. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B. Process Optimization of Biological Hydrogen Production from Molasses by a Newly Isolated Clostridium Butyricum W5. J. Biosci. Bioeng. 2009, 107, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Karatay, S.E.; Dönmez, G. Improving the Lipid Accumulation Properties of the Yeast Cells for Biodiesel Production Using Molasses. Bioresour. Technol. 2010, 101, 7988–7990. [Google Scholar] [CrossRef] [PubMed]
- Taskin, M.; Ortucu, S.; Aydogan, M.N.; Arslan, N.P. Lipid Production from Sugar Beet Molasses under Non-Aseptic Culture Conditions Using the Oleaginous Yeast Rhodotorula Glutinis TR29. Renew. Energy 2016, 99, 198–204. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Erten, H. Lipids by Yarrowia Lipolytica Strains Cultivated on Glucose in Batch Cultures. Microorganisms 2020, 8, 1054. [Google Scholar] [CrossRef]
- Kolouchová, I.; Schreiberová, O.; Sigler, K.; Masák, J.; Řezanka, T. Biotransformation of Volatile Fatty Acids by Oleaginous and Non-Oleaginous Yeast Species. FEMS Yeast Res. 2015, 15, fov076. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Han, Y.H.; Park, Y.L.; Jung, H.R.; Yang, S.Y.; Song, H.S.; Yang, Y.H. A Clean and Green Approach for Odd Chain Fatty Acids Production in Rhodococcus Sp. YHY01 by Medium Engineering. Bioresour. Technol. 2019, 286, 121383. [Google Scholar] [CrossRef]
- Dulermo, T.; Lazar, Z.; Dulermo, R.; Rakicka, M.; Haddouche, R.; Nicaud, J.M. Analysis of ATP-Citrate Lyase and Malic Enzyme Mutants of Yarrowia Lipolytica Points out the Importance of Mannitol Metabolism in Fatty Acid Synthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Sagnak, R.; Cochot, S.; Molina-Jouve, C.; Nicaud, J.M.; Guillouet, S.E. Modulation of the Glycerol Phosphate Availability Led to Concomitant Reduction in the Citric Acid Excretion and Increase in Lipid Content and Yield in Yarrowia Lipolytica. J. Biotechnol. 2018, 265, 40–45. [Google Scholar] [CrossRef]
- Unrean, P.; Champreda, V. High-Throughput Screening and Dual Feeding Fed-Batch Strategy for Enhanced Single-Cell Oil Accumulation in Yarrowia Lipolytica. Bioenergy Res. 2017, 10, 1057–1065. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Kantar, S.; Koubaa, M. Valorization of Low-Cost Substrates for the Production of Odd Chain Fatty Acids by the Oleaginous Yeast Yarrowia lipolytica. Fermentation 2022, 8, 284. https://doi.org/10.3390/fermentation8060284
El Kantar S, Koubaa M. Valorization of Low-Cost Substrates for the Production of Odd Chain Fatty Acids by the Oleaginous Yeast Yarrowia lipolytica. Fermentation. 2022; 8(6):284. https://doi.org/10.3390/fermentation8060284
Chicago/Turabian StyleEl Kantar, Sally, and Mohamed Koubaa. 2022. "Valorization of Low-Cost Substrates for the Production of Odd Chain Fatty Acids by the Oleaginous Yeast Yarrowia lipolytica" Fermentation 8, no. 6: 284. https://doi.org/10.3390/fermentation8060284
APA StyleEl Kantar, S., & Koubaa, M. (2022). Valorization of Low-Cost Substrates for the Production of Odd Chain Fatty Acids by the Oleaginous Yeast Yarrowia lipolytica. Fermentation, 8(6), 284. https://doi.org/10.3390/fermentation8060284






