Determination of Phenolic Compounds in Blue Corn Flour (Zea mays L.) Produced and/or Metabolized by Colletotrichum gloeosporioides in a Fermentation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism, Culture Maintenance and Inoculum Preparation
2.2. Blue Corn Flour Preparation
2.3. Fermentation
2.4. Phenolic Compounds Extraction: Initial and during the Fermentation Process
2.5. Analysis by HPLC
2.6. Statistical Analysis
3. Results
3.1. Blue Corn (Zea mays L.) Flour and Colletotrichum gloeosporioides Fermentation
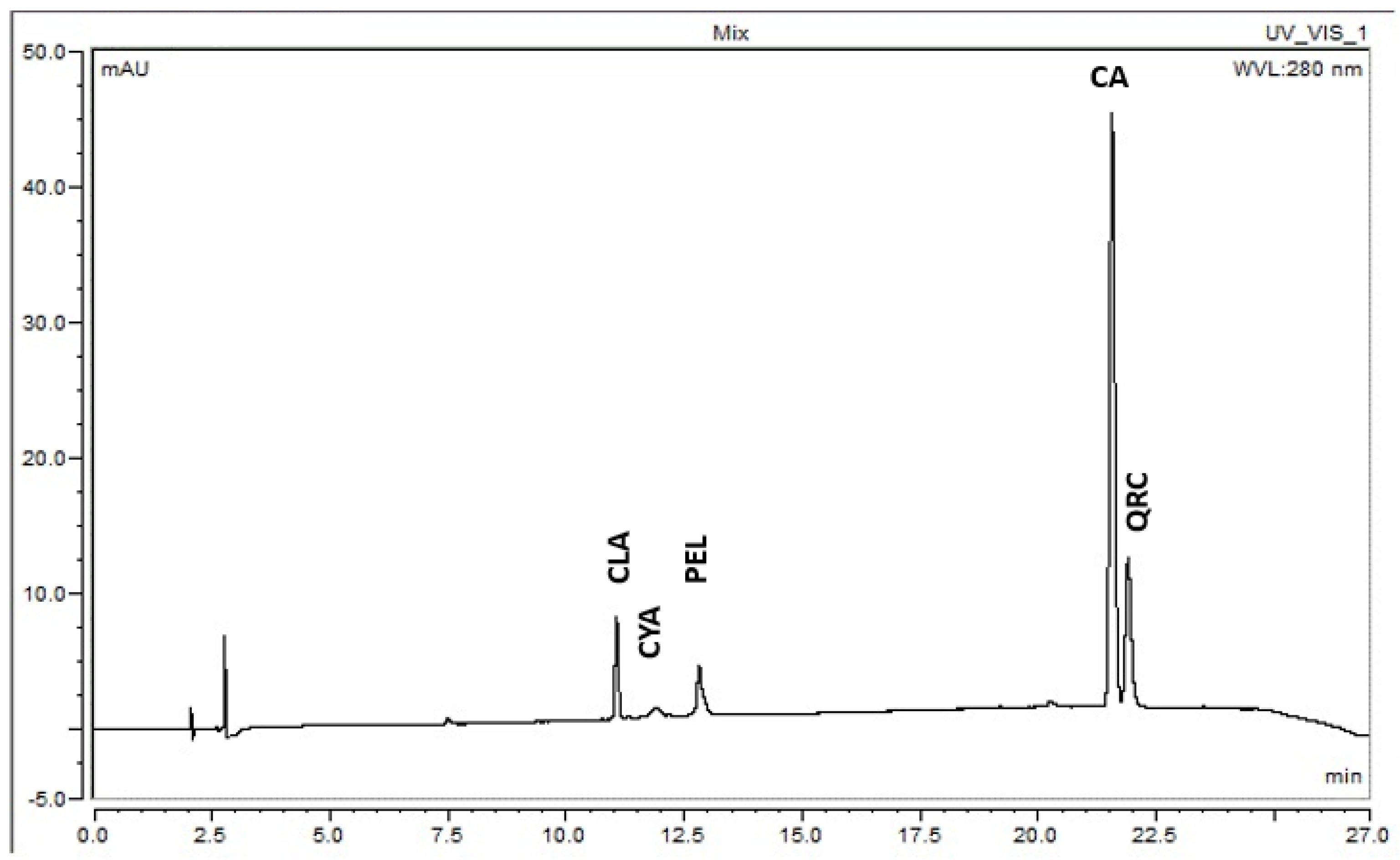
3.2. Analysis by High-Performance Liquid Chromatography (HPLC)
3.2.1. Pelargonidin 3-Glucoside (PEL)
3.2.2. Cyanidin 3-Glucoside (CYA)
3.2.3. Cinnamic Acid (CA)
3.2.4. Quercetin (QRC)
3.2.5. Chlorogenic Acid (CLA)
3.2.6. Analysis of Variance (ANOVA) of the Fermented Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Clydesdale, F.M.; Francis, F.J. Food Nutrition and Health; The AVI Publishing Company, Inc.: Westport, CT, USA, 1985. [Google Scholar]
- Oh, M.M.; Trick, H.N.; Rajashekar, C.B. Secondary Metabolism and Antioxidants are Involved in Environmental Adaptation and Stress Tolerance in Lettuce. J. Plant Physiol. 2009, 166, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Ming, Q.L.; Lin, B.; Rahman, K.; Zheng, C.J.; Han, T.; Qin, L.P. Medicinal Plant Cell Suspension Cultures: Pharmaceutical Applications and High-Yielding Strategies for the Desired Secondary Metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Valdés, L.; Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. The Relationship between Phenolic Compounds from Diet and Microbiota: Impact on Human Health. Food Funct. 2015, 6, 2424–2439. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; García-Salinas, C.; Ramírez-Díaz, J.L.; Alemán-de la Torre, I. Phenolic Compounds in Maize Grains and Its Nixtamalized Products. In Phenolic Compounds-Natural Sources, Importance and Applications; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, M.R., Eds.; Publisher Intech Open: London, UK, 2017; pp. 215–232. [Google Scholar]
- Urias-Lugo, D.A.; Heredia, J.B.; Muy-Rangel, M.D.; Valdez-Torres, J.B.; Serna Saldívar, S.O.; Gutiérrez-Uribe, J.A. Anthocyanins and phenolic acids of hybrid and native blue maize (Zea mays L.) extracts and their antiproliferative activity in mammary 95 (MCF7), liver (HepG2), colon (Caco2 and HT29) and prostate (PC3) Cancer Cells. Plant Foods Hum. Nutr. 2015, 70, 193–199. [Google Scholar] [CrossRef]
- Mesfin, T.; Wassie, H.; Kassa, S. Effects of Nitrogen and Blended Fertilizers on Yield and Yield Components of tef (Eragrostis tef Trotter) in Central Highlands of Ethiopia. Int. J. Adv. Agric. Sci. Tech. 2019, 6, 15–64. [Google Scholar]
- Dulf, F.V. Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fraction of plum fruit (Prunus domestica L.) by-products. Food Chem. 2016, 209, 27–36. [Google Scholar] [CrossRef]
- Alarcón-Saenz, M.A. Aislamiento e Identificación de un Hongo Endófito del Fruto de Chagalapoli (Ardisia Compressa) y Su Relación en la Composición Química. Master’s Thesis, Universidad Autónoma de Chihuahua, Chihuahua, Mexico, 2017. [Google Scholar]
- Colomé, J.; Cano, R.; Kubinski, A.; Grady, D. Laboratory Exercises in Microbiology; West Publishing Company: St. Paul, MN, USA, 1996; pp. 283–295. [Google Scholar]
- Aguilar, C.N. Fungal culture systems for production of antioxidant phenolics using pecan nut shells as sole carbon source. Am. J. Agric. Biol. Sci. 2010, 5, 397–402. [Google Scholar]
- Dewanto, V.; Wu, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; García-Salinas, C.; Coutiño-Estrada, B.; Vidal-Martínez, V. Content and type variability of anthocyanins in blue/purple colored grains of Mexican maize populations. Rev. Fitotec. Mex. 2010, 36, 285–294. [Google Scholar]
- Salinas-Moreno, Y.; Pérez-Alonso, J.; Vázquez-Carrillo, G.; Aragón-Cuevas, F.; Velázquez-Cardelas, G.A. Antocianinas y actividad antioxidante en maíces (Zea mays L.) de las razas Chalqueño, Elotes Cónicos y Bolita. Agrociencia 2012, 47, 815–825. [Google Scholar]
- Herrera-Sotero, M.; Cruz-Hernández, C.E.; Trujillo-Carretero, C.; Rodríguez-Dorantes, M. Antioxidant and antiproliferative activity of blue corn and tortilla from native maize. Chem. Cent. J. 2017, 11, 110. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Ponce-García, N.; Ramírez-Wong, B.; Torres-Chávez, P.I.; Figueroa-Cárdenas, J.; Gutiérrez-Dorado, R. Specific Anthocyanin Contents of Whole Blue Maize Second-Generation Snacks: An Evaluation Using Response Surface Methodology and Lime Cooking Extrusion. J. Chem. 2016, 2016, 5491693. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.; Young, C.; Rabalski, I. Anthocyanin Composition in Black, Blue, Pink, Purple and Red Cereal Grains. J. Agric. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Lewers, K.S. Antioxidant Capacity and Flavonoid Content in Wild Strawberries. J. Am. Soc. Hort. Sci. 2007, 132, 629–637. [Google Scholar] [CrossRef]
- Kumar-Singh, P.; Singh, R.; Singh, S. Cinnamic acid induced changes in reactive oxygen species scavenging enzymes and protein profile in maize (Zea mays L.) plants grown under salt stress. Physiol. Mol. Biol. Plants 2013, 19, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and other cinnamates, nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Lakhanpal, P.; Rai, D.K. Quercetin: A Versatile Flavonoid. Internet J. Med. Update 2007, 2, 22–37. [Google Scholar] [CrossRef]
- Álvarez-Castro, E.; Orallo-Cambeiro, F. Actividad biológica de los flavonoides (I) Acción frente al cáncer. Ámbito farmacéutico. Bioquímica 2003, 22, 130–140. [Google Scholar]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Quintero, J.D.; Rosales-Nolasco, A.; Molina-Macedo, A.; Miranda Piliado, A.; Willcox, M.; Hernández-Casillas, J.M.; Palacios-Rojas, N. Cuantificación De Antocianinas Mediante Espectroscopía De Infrarrojo Cercano Y Cromatografía Líquida En Maíces Pigmentados. Rev. Fitotec. Mex. 2017, 40, 219–225. [Google Scholar] [CrossRef][Green Version]
- Awas, R.; Hamad, H.; Dayyih, W.A.; Mallah, E.; Hamad, M. Quality Control of Cinnamic Acid in Traditional Medicinal Plants In Jordan Using High Performance Liquid Chromatography. Int. J. Pharm. Pharm. Sci. 2014, 6, 411–418. [Google Scholar]
- Muñoz-Muñoz, J.A.; Morgan Machado, J.E.; Trujillo González, M. Validation of an HPLC method for quantification of total quercetin in Calendula officinalis extracts. Rev. Cuba. Farm. 2015, 49, 91–102. [Google Scholar]
- Türköz-Acar, E.; Celep, M.E.; Charehzaz, M.; Akyüz, G.S.; Yesilada, E. Development and Validation of a High-performance Liquid Chromatography–Diode-array Detection Method for the Determination of Eight Phenolic Constituents in Extracts of Different Wine Species. Turk J. Pharm. Sci. 2018, 15, 22–28. [Google Scholar]
- Ziberna, L.; Lunder, M.; Moze, S.; Vanzo, A.; Tramer, F.; Passamonti, S.; Drevensek, G. Acute Cardioprotective and Cardiotoxic Effects of Bilberry Anthocyanins in Ischemia–Reperfusion Injury: Beyond Concentration-Dependent Antioxidant Activity. Cardiovasc. Toxicol. 2010, 10, 283–294. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Moonsan, P.; Yibchok-Anun, S. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J. Agric. Food Chem. 2008, 56, 7838–7844. [Google Scholar] [CrossRef]
- Li, N.; Sun, C.; Zhou, B.; Xing, H.; Ma, D.; Chen, D.; Weng, D. Low Concentration of Quercetin Antagonizes the Cytotoxic Effects of Anti-Neoplastic Drugs in Ovarian Cancer. PLoS ONE 2014, 9, e100314. [Google Scholar] [CrossRef]
- Karthikesan, K.; Pari, L.; Menon, V.P. Antihyperlipidemic effect of chlorogenic acid and tetrahydrocurcumin in rats subjected to diabetogenic agents. Chem.-Biol. Interact. 2010, 188, 643–650. [Google Scholar] [CrossRef]
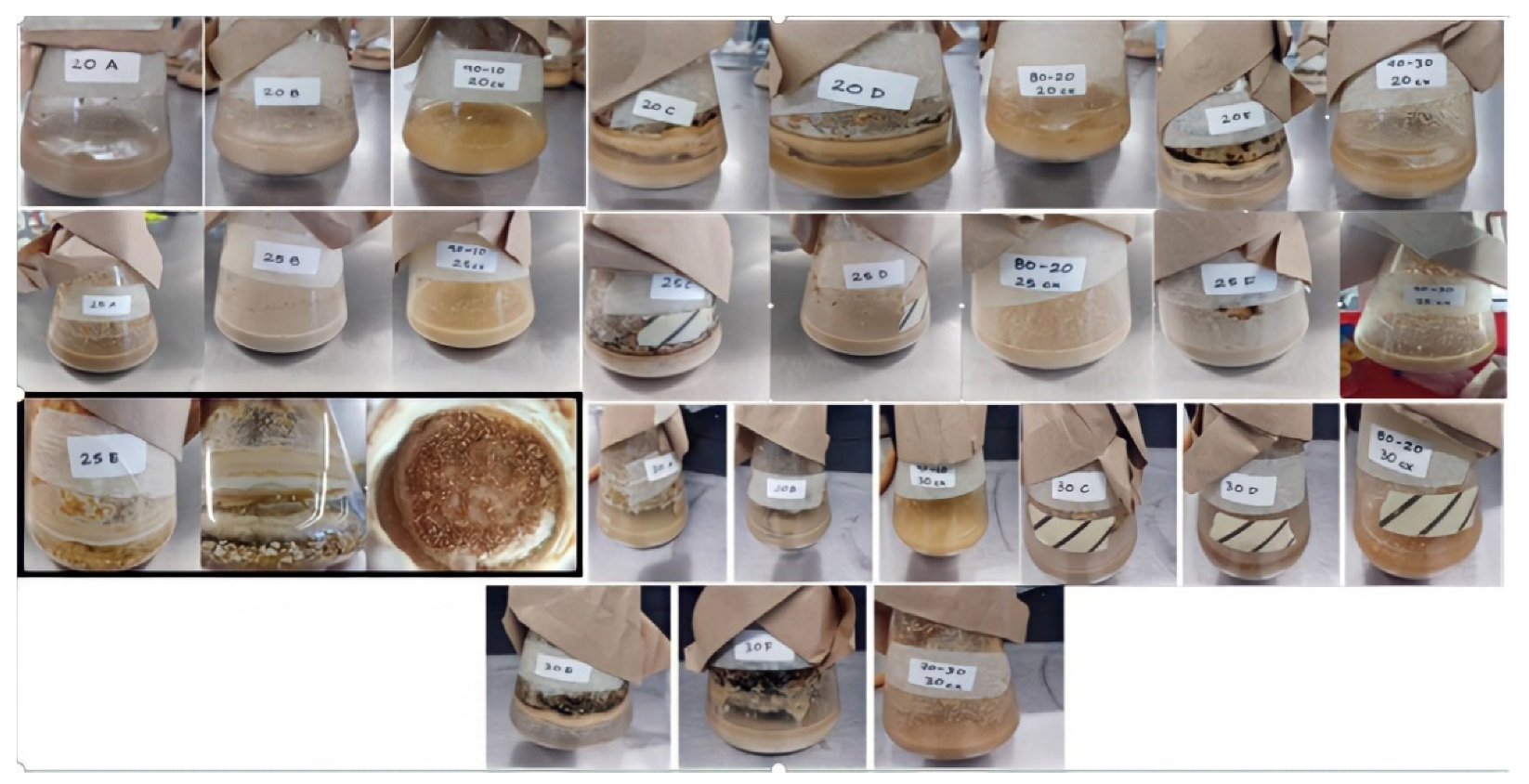

| Sample | CYA (mg kg−1) | PEL (mg kg−1) | CA (mg kg−1) | QRC (mg kg−1) | CLA (mg kg−1) |
|---|---|---|---|---|---|
| 20 A | 99.82 | 80.23 | 113.32 | 23.49 | 267.47 |
| 20 B | 90.32 | 87.24 | 116.12 | 28.52 | 266.18 |
| 20 C | 86.26 | 77.24 | 108.27 | 25.65 | 275.92 |
| 20 D | 93.21 | 88.98 | 114.96 | 28.93 | 260.27 |
| 20 E | 86.10 | 83.15 | 110.69 | 24.80 | 273.33 |
| 20 F | 91.32 | 94.11 | 115.92 | 28.15 | 307.47 |
| 25 A | 110.82 | 108.83 | 136.88 | 34.26 | 325.40 |
| 25 B | 145.43 | 143.15 | 188.27 | 58.39 | 404.71 |
| 25 C | 92.04 | 101.90 | 112.02 | 28.61 | 254.15 |
| 25 D | 98.10 | 110.82 | 128.76 | 35.76 | 291.65 |
| 25 E | 99.98 | 85.48 | 111.11 | 25.32 | 260.31 |
| 25 F | 314.32 | 7514 | 123.64 | 29.29 | 303.20 |
| 30 A | 76.10 | 89.97 | 149.13 | 22.06 | 258.20 |
| 30 B | 72.93 | 62.11 | 145.34 | 22.27 | 274.93 |
| 30 C | 175.43 | 98.05 | 207.92 | 42.87 | 507.30 |
| 30 D | 113.54 | 73.15 | 139.36 | 23.80 | 349.71 |
| 30 E | 170.37 | 59.04 | 128.28 | 20.76 | 342.47 |
| 30 F | 142.10 | 66.65 | 136.62 | 21.52 | 339.15 |
| Phenolic Compound | Reference | Reference Concentration (mg/L) | Biological Activity | Maximum Concentration in Fermented Samples (mg/L) | Average Concentration of Fermented Samples (mg/L) |
|---|---|---|---|---|---|
| CYA PEL | [31] | Low: 0.01–1 High: 5–50 | cardioprotective activity cardiotoxic activity | 314 143 | 180 88 |
| CA | [32] | 4 | Insulin secretion stimulation | 207 | 132 |
| QRC | [33] | 0.5–1 | Suppression of injury induced by reactive oxygen species (ROS) | 58 | 29 |
| CLA | [34] | 5 | Improvement of lipid abnormalities due to type 2 diabetes | 507 | 308 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villarreal-Rodríguez, G.; Escajeda-García, J.; Amaya-Olivas, N.; Chávez-Flores, D.; Neder-Suárez, D.; Ayala Soto, J.G.; Quintero-Ramos, A.; Ruíz-Anchondo, T.; Hernández-Ochoa, L. Determination of Phenolic Compounds in Blue Corn Flour (Zea mays L.) Produced and/or Metabolized by Colletotrichum gloeosporioides in a Fermentation Process. Fermentation 2022, 8, 243. https://doi.org/10.3390/fermentation8060243
Villarreal-Rodríguez G, Escajeda-García J, Amaya-Olivas N, Chávez-Flores D, Neder-Suárez D, Ayala Soto JG, Quintero-Ramos A, Ruíz-Anchondo T, Hernández-Ochoa L. Determination of Phenolic Compounds in Blue Corn Flour (Zea mays L.) Produced and/or Metabolized by Colletotrichum gloeosporioides in a Fermentation Process. Fermentation. 2022; 8(6):243. https://doi.org/10.3390/fermentation8060243
Chicago/Turabian StyleVillarreal-Rodríguez, Guadalupe, Jesús Escajeda-García, Nubia Amaya-Olivas, David Chávez-Flores, David Neder-Suárez, Juan G. Ayala Soto, Armando Quintero-Ramos, Teresita Ruíz-Anchondo, and León Hernández-Ochoa. 2022. "Determination of Phenolic Compounds in Blue Corn Flour (Zea mays L.) Produced and/or Metabolized by Colletotrichum gloeosporioides in a Fermentation Process" Fermentation 8, no. 6: 243. https://doi.org/10.3390/fermentation8060243
APA StyleVillarreal-Rodríguez, G., Escajeda-García, J., Amaya-Olivas, N., Chávez-Flores, D., Neder-Suárez, D., Ayala Soto, J. G., Quintero-Ramos, A., Ruíz-Anchondo, T., & Hernández-Ochoa, L. (2022). Determination of Phenolic Compounds in Blue Corn Flour (Zea mays L.) Produced and/or Metabolized by Colletotrichum gloeosporioides in a Fermentation Process. Fermentation, 8(6), 243. https://doi.org/10.3390/fermentation8060243







