Abstract
Legume proteins have a promising future in the food industry due to their nutritional, environmental, and economic benefits. However, their application is still limited due to the presence of antinutritional and allergenic compounds, their poor technological properties, and their unpleasant sensory characteristics. Fermentation has been traditionally applied to counteract these inconveniences. At present, lactic acid fermentation of legumes is attracting the attention of researchers and industry in relation to the development of healthier, tasty, and technologically adapted products. Hence, we aimed to review the literature to shed light on the effect of lactic acid fermentation on legume protein composition and on their nutritional, functional, technological, and sensorial properties. The antimicrobial activity of lactic acid bacteria during legume fermentation was also considered. The heterogenicity of raw material composition (flour, concentrate, and isolate), the diversity of lactic acid bacteria (nutriment requirements, metabolic pathways, and enzyme production), and the numerous possible fermenting conditions (temperature, time, oxygen, and additional nutrients) offer an impressive range of possibilities with regard to fermented legume products. Systematic studies are required in order to determine the specific roles of the different factors. The optimal selection of these criteria will allow one to obtain high-quality fermented legume products. Fermentation is an attractive technology for the development of legume-based products that are able to satisfy consumers’ expectations from a nutritional, functional, technological, and sensory point of view.
1. Introduction
Fermentation is one of the oldest food processing methods, consisting of modifying food through the use of microorganisms (bacteria, molds, and yeasts). Microorganisms use a part of the substrate to grow and reproduce and enrich it with the products of their metabolism. Enzymes from microorganisms, particularly amylases, proteases, and lipases, hydrolyze polysaccharides, proteins, and lipids and produce compounds that prevent food spoilage and consequently modify the nutritional, technological, and sensory attributes of foods [1].
Different classifications have been proposed for food fermentation [2]. When lactic acid is produced, researchers refer to it as lactic acid fermentation. Lactic acid bacteria (LAB) are of great interest since they are considered safe, and they can offer particular technological, sensory, nutritional, and functional properties [3]. LAB are able to ferment a variety of food substrates, such as milk products, meat and fish, vegetables, and legumes. In a complete review of traditional fermented foods, Tamang et al. [4] studied the most common fermented legume products, indicating the kind of legume, the fermenting microorganism, and the final sensory features. The growing interest in the lactic acid fermentation of legumes is clear from the increasing number of scientific studies about the effects of lactic acid fermentation on the nutritional, physicochemical, and sensorial properties of various sources of legume protein ingredients, including soybeans, chickpeas, lupins, peas, faba beans, lentils, beans, etc. Lactic acid fermentation can be applied to legume seed-, flour-, or protein-enriched ingredients. However, the efficiency and functionality of lactic acid fermentation depends greatly on the type of LAB strain, the fermentation technique, the type of legume, the composition of the protein ingredient, and slightly on the genetic variety [5,6].
This study was conceived as a comprehensive compilation of the scientific knowledge about the effect of lactic acid fermentation on legume proteins. In this review, after a brief description of the characteristics of lactic acid bacteria and legumes, we describe the influence of fermentation techniques on legume products and the effects of lactic acid fermentation on protein composition, as well as on nutritional, functional, physicochemical, technological, sensory, and antimicrobial properties. Figure 1 summarizes the effects of lactic acid fermentation on legume protein.
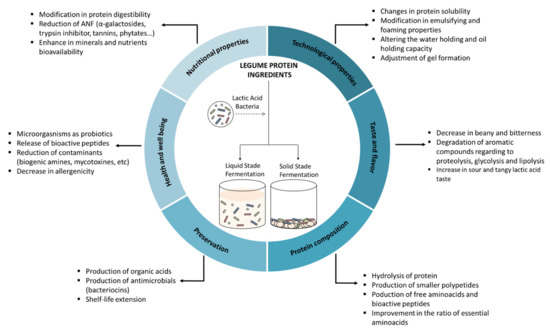
Figure 1.
Schematic depicting the effect of lactic acid fermentation on legume protein.
2. Lactic Acid Bacteria
LAB are acid-tolerant, aero-tolerant, and non-spore-forming bacteria that can adapt to different food matrices. LAB genera include Lactobacillus (Lb.), Lactococcus (L.), Leuconostoc (Lc.), Pediococcus, Streptococcus (S.), Aerococcus, Alloiococcus, Carnobacterium, Dolosigranulum, Enterococcus, Oenococcus, Tetragenococcus, Vagococcus, and Weissella (W.), with Lactobacillus being the largest genus. The genus Lactobacillus has been recently reclassified into 25 genera. The new genera are Lactobacillus and Paralactobacillus, and the 23 novel genera are Amylolactobacillus, Acetilactobacillus, Agrilactobacillus, Apilactobacillus, Bombilactobacillus, Companilactobacillus, Dellaglioa, Fructilactobacillus, Furfurilactobacillus, Holzapfelia, Lacticaseibacillus, Lactiplantibacillus, Lapidilactobacillus, Latilactobacillus, Lentilactobacillus, Levilactobacillus, Ligilactobacillus, Limosilactobacillus, Liquorilactobacillus, Loigolactobacilus, Paucilactobacillus, Schleiferilactobacillus, and Secundilactobacillus [7].
The main outcome of LAB is the production of lactic acid as the final product of fermentation, which leads to the acidification of the products. This is why LAB are largely used as starters in the food fermentation industry. The selection criteria for starter cultures are decisive in terms of the properties of the final product, as indicated in a prior review [8]. LAB can also contribute to the flavor, texture, and nutritional value of fermented foods through the production of aroma components or a reduction in off-flavors; the production or degradation of exopolysaccharides, lipids, and proteins; the production of nutritional components such as vitamins; and the promotion of health benefits when used as probiotics [9,10,11].
In fact, LAB require energy to grow, and they obtain this energy through the metabolism and consumption of different compounds, such as minerals, amino acids, fatty acids, and sugar. The different metabolic pathways of lactic acid fermentation lead to different metabolic conversions, specifically glycolysis (fermentation of sugars and acid formation), lipolysis (degradation of fat), and proteolysis (degradation of proteins) [12]. Glycolysis is the breakage of glucose into two molecules of three-carbon pyruvate, called pyruvic acid, which can alternatively be reduced to lactic acid. Lactic acid has two optical isomers, L-(+)-lactic acid and D-(−)-lactic acid [13]. The L-(+) isomer can be produced by LAB of the genera Streptococcus, Pediococcus, Lactococcus, and Lactobacillus, whereas the D-(–) isomer can be produced only by particular strains of the genus Lactobacillus [14]. When the unique product of glycolysis is lactic acid, fermentation is called homofermentation. This is the case for species such as Lactobacillus acidophilus, Lactobacillus amylophilus, Lactobacillus bulgaricus, and Lactobacillus helveticus. A mixture of two lactic acid stereoisomers is usually produced by homofermentative LAB that belong to the genera Pediococcus and Lactobacillus [14]. On the other hand, heterofermentation is the process in which bacteria produce several other metabolites, including acetic acid, ethanol, and carbon dioxide, along with lactate. The microorganisms using the latter mechanism for the consumption of glucose are called heterofermentative and include Levilactobacillus brevis, Limosilactobacillus fermentum, Limosilactobacillus reuteri, etc. [15]. There are hexoses other than glucose, such as fructose, mannose, and galactose, which can be consumed by LAB. LAB are also able to hydrolyze disaccharides such as maltose, sucrose, and lactose and more complex sugars such as α-galactosides, depending on their enzymatic capacity [16].
Another important metabolic activity of LAB is proteolysis. In fact, LAB are unable to synthesize many amino acids, vitamins, or nucleic acids, so they need to hydrolyze proteins and peptides in food matrices in order to free amino acids and subsequently utilize them. LAB also produce secondary metabolites, including exopolysaccharides, enzymes, and bacteriocins, which are used to increase the quality and shelf-life of fermented foods [12].
3. Legume Proteins
Legumes or pulses are not only cheap and consumed all around the world, but they also show nutritional benefits related to their protein, fiber, and mineral contents. They belong to the Fabaceae or Leguminosae families and represent the second most commonly produced food crop after cereals. Legumes comprise oil-seed legumes (i.e., soybean) and pulses such as lentils, chickpeas, peas, lupines, and beans [17]. They can be consumed either as whole grains, milled (flour and semolina), or as part of grain components such as protein concentrates and isolates. Protein concentrates exhibit protein contents of around 60–70% and are obtained from starch-rich legumes after pin-milling plus air classification processes. Protein isolates are richer in protein than concentrates (>85% protein content) and are obtained by means of wet processes such as alkaline solubilization/isoelectric precipitation, acid extraction, or salt-induced extraction [18]. The majority of the proteins found in legumes consist of globulins (60–70%) and albumins (15–20%) [19]. These two fractions can be purified according to their solubility. The albumin fraction consists of different water-soluble proteins with low molecular weight, belonging mainly to the 2S group (e.g., PA1 and PA2 in pea seeds) and also containing biologically active proteins such as enzymes and trypsin inhibitors. Globulins are storage proteins that are soluble in salt buffers and that contain different ratios of 7S, 11S, and 15S proteins [20]. Differences in composition and in the extraction process determine structural differences and, consequently, their use as food ingredients. Akharume et al. [21] detailed the physical, chemical, and biological modifications of plant proteins for improved functionality.
Legumes can be considered an alternative source of protein since they are available, cost-effective, and promising in regard to both human and environmental health. However, the consumption of legume protein is still limited due to their undesirable sensory attributes and to the presence of antinutritional factors (ANFs) that create digestive discomfort, prevent mineral availability, and decrease protein digestibility [22]. Lactic acid fermentation seems to be one of the possible solutions available to counteract these inconveniences.
4. The Effects of Applied Fermentation Techniques on Legume Products
The selection of the fermentation technique is one of the most important parameters defining the properties of fermented legumes. Solid- and liquid-state fermentation (SSF and LSF, respectively) are two common techniques that have been used for food preservation and for the improvement of the nutritional quality of legume ingredients. SSF is defined as fermentation involving solids in the absence or near-absence of free water [23]. In this case, the substrate must possess enough moisture to support the growth and metabolism of microorganisms. Hence, the microorganisms grow between inter-particle spaces that are surrounded by a gaseous phase [24]. The use of SSF is favorable from an environmental and economic point of view compared to LSF since it requires less energy and water. In LSF, also known as submerged fermentation (SmF), the fermenting microorganisms grow in liquid containing nutrients [25].
Studies have shown that both SSF and LSF can lead to diverse modifications in protein functional properties and nutritional qualities. Limón et al. [26] studied the effect of both LSF and SSF using Lactiplantibacillus plantarum on bioactive compounds of kidney bean seeds, and the results showed that both methods were suitable for obtaining a water-soluble protein fraction. However, it seems that the amount of peptide released during LSF of kidney bean flour was higher compared to SSF of cracked kidney beans. The same researchers showed that some protein bands disappeared in the electrophoresis pattern of SSF compared to LFS. However, in their study, SSF presented a higher content of soluble phenolic compounds and higher antioxidant activity compared to LSF. In fact, increasing the content of total phenolic compounds and decreasing the content of antinutritional compounds in the SSF of cracked grains compared to the LSF of flour has been discussed in many studies. To name but a few, Bartkiene et al. [6] studied the fermentation of lupine seed and soy beans with Lactobacillus sakei, Pediococcus acidilactici, and Pediococcus pentosaceus; Fernandez-Orozco et al. [27] studied the fermentation of soybeans with Lb. plantarum; and Xing et al. [10] studied the fermentation of chickpea-protein-enriched fractions with P. pentosaceus and P. acidilactici. On the contrary, Torino et al. [28] conducted a study of both LSF and SSF of lentil seeds using Lb. plantarum and showed that the water-soluble fraction of lentil protein possessed higher antioxidant activity in the case of LSF compared to SSF. They also showed that the SSF protein had slightly fewer free amino groups compared to LSF, which means that the SSF group showed higher proteolytic activity compared to LSF. In this review, the fermentation type is only mentioned in Table 1.

Table 1.
The effects of lactic acid fermentation on nutritional properties of legume protein.
5. Effect of Fermentation on Legume Protein Composition
Lactic acid fermentation modifies the content and the composition of proteins due to the presence of enzymes and due to the presence of other components such as acids. Microbial proteases are able to break peptide bonds and produce new polypeptides or even free amino acids [3]. LAB have a complex proteinase system, which is composed of extracellular protease, which initiates the degradation of protein into peptides; the peptide transporter; and intracellular proteases, which degrade peptides into shorter oligopeptides and free amino acids. Some of these extracellular proteases have been characterized, such as PrtS from S. thermophylus, PrtP from L. lactis, PrtH from L. helveticus, and PrtR from L. rhamnosus [51]. The effect of some extracellular proteases on the technological characteristics of soy protein has been studied [52]. Organic acids such as lactic acid and acetic acid may disrupt the ionic interaction between protein side chains that stabilize the secondary structure, and thus, the presence of acid may lead to the loss of the secondary and tertiary structures of the protein [53]. It has been observed [54] that fermented pea flours with a mixed culture containing Streptococcus thermophiles, Lb. bulgaricus, and Lb. acidophilus exhibited severe damage in the legume cell wall structure compared to non-fermented flours, as determined through the use of scanning electron microscopy. These changes led to smaller peaks in the differential scanning calorimetry (DSC) thermograms of fermented samples, indicating less energy for the breakage of intermolecular bonds within protein bodies to achieve protein denaturation. Native soy protein is more resistant to fermentation with different species of Lactobacillus and P. pentosaceus than denatured protein [34]. When hydrolysis conditions are adequate, fermentation may even modify the free amino acid profile. Changes in the amino acid profile seem to be dependent on the kind of legume and microorganism used. In the fermentation of pea-protein-enriched flours with Lb. plantarum 43], all amino acid mass fractions (mg aa/100 g sample) increased with fermentation time, except for arginine and tryptophan, which remained similar. Studies have also shown [37,55] an improvement in the ratio of essential amino acids in faba beans after fermentation.
The hydrolysis of legume protein differs between LAB strains [56]. Aguirre et al. [34] observed that enzymes from twelve different lactic acid bacteria from Lacticaseibacillus paracasei, Lb. fermentum, Lactobacillus lactis, Lb. plantarum, Lb. helveticus, Lb. reuteri, and P. pentosaceus hydrolyzed soy protein fractions (7S β-conglycinin, 11S glycinin) from protein isolates to different extents. In a study on soy milk fermentation by Lb. plantarum [57], it was observed that both α and β subunits of conglycinin were likely to be degraded by almost all of the strains. However, glycinin was less preferable for almost all of the strains. Emkani et al. [58] observed differences in the polypeptide profiles of pea globulin and albumin fractions when using a novel pea protein extraction method, assisted by lactic acid fermentation, compared with a traditional extraction method. These differences, probably related to the proteolytic activity of bacteria, induced changes in the thermal denaturation properties measured by DSC through modification of the polypeptide composition and conformation. Moreover, differences in the polypeptide profiles of the initial legume proteins may determine their sensitivity during fermentation. This could be the reason that differences were observed in the proteolytic effects of lactic acid fermentation with Leuconostoc mesenteroides, Lb. plantarum, and Lb. brevis co-cultures when comparing flours from different lupine cultivars [59]. The degree of proteolysis also depended on the legume type, as observed in the fermentation of different legume flours (yellow and red lentils, white and black beans, chickpeas, and peas) with nine selected LAB strains of different species, i.e., Lb. plantarum, P. acidilactici, Lc. mesenteroides, Lactobacillus rossiae, and Lb. brevis [48]. The highest proteolysis in their study corresponded to white beans, followed by chickpeas and black beans. Comparing the concentrations of free amino acid in various legumes, the authors also showed that, except for red and yellow lentils, which had low amino acid contents before and after fermentation, the highest and lowest increase in the total free amino acids corresponded to peas and chickpeas, respectively. Microorganisms were able to decompose medium-molecular-weight and low-molecular-weight polypeptides in studies of lactic acid fermentation of pea [42], lupine [60], and mung bean [61] flours with S. thermophilus and different species of Lactobacillus, respectively.
Studies on the effect of fermentation on protein content have shown different results, depending on the legume substrate and the lactic acid bacteria used for fermentation. Changes in the protein structure and hydrolysis of proteins up to the formation of amino acids may decrease the extraction yield of proteins, as indicated in a study of faba bean flour fermentation with different LAB strains [62] and in a study of lupine flour fermentation with a mixture of Lc. mesenteroides, Lb. plantarum, and Lb. brevis [59]. Other authors, however, did not observe a diminution in protein content after fermentation, indicating that fermentation only changes the molecular size of proteins, such as in the fermentation of lyophilized chickpea and faba bean flours with a yogurt starter (Lb. bulgaricus and S. thermophilus) [63]. Other authors have indicated an increase in the protein content after fermentation. In a study of the fermentation of pea-protein-enriched flour using Lb. plantarum [43], the authors observed an increase in the percentage of protein, fat, and ashes, presumably due to an increase in the bacterial biomass and the loss of carbohydrates during the fermentation process.
6. Effect of Lactic Acid Fermentation on Nutritional Properties of Legume Protein
One of the most widely studied aspects of the lactic acid fermentation of legumes is the impact on their nutritional properties. Aspects such as protein digestibility, antinutritional factors, antioxidant capacity, and allergenicity have been specifically considered (Table 1).
6.1. Protein Digestibility
Protein digestibility can be defined as how well protein is hydrolyzed by humans [55]. Considering the fact that fermentation favors the release of protein components (see Section 5), an increase in in vitro protein digestibility after fermentation is expected. Certain authors have also suggested that the reduction of antinutritional compounds (e.g., phenolics and tannins) due to fermentation would limit protein crosslinking, making proteins more susceptible to proteolytic attack [44,63]. The improvement of protein digestibility after lactic acid fermentation has been studied, as in the case of yellow field pea flour with a lactic acid mixed culture [44]; grass pea flour fermented using Lb. plantarum [42]; and lupine and soy flours fermented using different species of lactic acid bacteria, i.e., Lb. sakei, P. acidilactici, and P. pentosaceus [64]. However, other authors have not observed an obvious trend in regard to in vitro protein digestibility after fermentation, such as in the fermentation of pea protein with Lb. plantarum [43]. Different behavior has been observed regarding protein digestibility depending on the kind of legume [63] and even the legume cultivar [64].
6.2. Antinutritional Compounds
Legume seeds contain certain components, classified as antinutritional factors or non-nutritive compounds, that negatively affect the nutritional quality of legume ingredients [65,66,67]. Examples of these antinutritional factors (ANFs) are α-galactosides; phenolic compounds including tannins, trypsin, and chymotrypsin inhibitors; phytic acid; saponins; isoflavones; and biogenic amines [10].
Lactic acid fermentation is able to degrade α-galactosides [16]. The α-galactosides of sucrose, also known as the raffinose family of oligosaccharides (RFOs) (raffinose, stachyose, and verbascose), are responsible for digestive discomfort and flatulence due to their fermentation by gut bacteria in the large intestine. However, moderate doses of α-galactosides favor the metabolism of beneficial intestinal microorganisms such as Bifidobacteria [68]. Certain fermenting microorganisms, such as Streptococcus sp., Leuconostoc sp., and Lactobacillus sp., show α-galactosidase activity, which gives them the ability to transform α-galactosides into absorbable mono- and disaccharides [9,69,70]. Sourdough fermentation of legumes such as chickpeas fermented with Pediococcus strains [10]; yellow and red lentil, white and black bean, chickpea, and pea flours fermented with Lb. plantarum and Lb. brevis [48]; faba beans and field peas fermented with Lb. reuteri [53]; and faba beans fermented with P. pentosaceus [71] were found to exhibit decreased concentrations of raffinose in the legumes. However, the diminution depended on the kind of legume, as indicated in a study of fermentation of different varieties of chickpeas, lentils, and peas using Lb. plantarum and Lb. brevis [72].
Phenolic compounds are able to crosslink proteins, making them less susceptible to enzyme action during digestion and thus decreasing protein digestibility [73]. However, phenolic compounds have also shown interesting properties, such as antiallergenic, antiatherogenic, anti-inflammatory, antimicrobial, antioxidant, antithrombotic, cardioprotective, and vasodilatory effects. The main groups of phenolic compounds found in legumes are phenolic acids, flavonoids, and condensed tannins [74]. In general, lactic acid fermentation has been found to increase the total phenolic compounds (TPC) of legumes, with differences depending on the kind of legume, the type of LAB bacteria, and the fermentation process. Indeed, the increase in TPC related to the release of these components from the cell wall of the plant tissue could occur due to structural degradation or due to enzymatic conversion during fermentation [10].
De Pasquale et al. [48] compared the TPC values of four different legume flours—yellow and red lentils, white and black beans, chickpeas, and peas—fermented by Lb. plantarum, P. acidilactici, Lc. mesenteroides, Lb. rossiae, and Lb. brevis. Their results showed that the TPC content was higher for fermented samples compared to the unfermented ones. However, the amount of TPC differed between different fermented legumes. The highest and lowest TPC values belonged to chickpeas and red lentils, respectively. The enhancement of total phenolic content in soymilk fermented using S. thermophilus and Bifidobacterium infantis [75] has also been observed. Total phenolic compounds also increased after the fermentation of pea protein concentrate with Lb. plantarum [43], cowpeas with Lb. plantarum [76], kidney beans with Lb. plantarum, and natural fermentation [26]. During fermentation, it is likely that polymeric phenolic compounds are hydrolyzed by microbial enzymes, and simpler and/or biologically more active phenolic compounds are released [76,77]. Furthermore, fermentation degrades the lignocellulosic matrix of legumes and thus liberates phenolic compounds from an inaccessible state. These authors indicated that polyphenol oxidases would break polyphenols into low-molecular-weight condensed polyphenols.
The degradation of tannins due to fermentation has been indicated in pea flour with a mixed lactic acid bacteria culture containing S. themophilus, Lb. bulgaricus, and Lb. acidophilus [44]; in red and yellow lentil, white and black bean, chickpea, and pea flours with Lb. plantarum [48]; and in faba beans with Lb. plantarum [35]. An increase in the total tannin levels has been observed in the first five hours of fermentation of pea protein concentrate with Lb. plantarum [43], but it decreases afterwards. The initial increase could be caused by the same factors that affected the increase in the phenolic content. The hydrolysis of condensed tannins follows different pathways, which involve enzymes such as decarboxylases and oxygenases [72]. In consequence, the tannase activity present in fermenting microorganisms was responsible for the degradation of tannins [78,79].
In general, trypsin inhibitor activity was also decreased in fermented yellow pea flour by S. thermophilus, Lb. bulgaricus, and Lb. acidophilus [44]; in pea protein concentrate by Lb. plantarum [43]; in red and yellow lentil, white and black bean, chickpea, and pea flours by Lb. plantarum, Lb. rossiae, Lb. brevis, P. acidilactici, and Lc. mesenteroides [48]; in grass pea flour by Lb. plantarum [42]; in faba bean grains by natural fermentation [80]; and in faba bean flour by Lb. plantarum [35]. However, Chandra-Hioe et al. [63] did not observe significant differences due to fermentation in chickpea (desi/kabuli) and faba bean flour after 16 h of fermentation with a freeze-dried yogurt culture. The enzymatic hydrolysis of trypsin inhibitors by microbial proteases during fermentation permitted the reduction in trypsin inhibitor activity. Chymotrypsin inhibitory activity continuously decreased up to 11 h of fermentation of pea concentrate with Lb. plantarum [43].
Phytic acid decreases protein digestibility because it binds with enzymes such as proteases and amylases [81,82]. Additionally, phytic acid forms complexes with certain minerals, such as calcium, copper, magnesium, iron, manganese, zinc, and amino group derivatives in protein moieties, and thus decreases their absorption in the gastrointestinal tract. For people with high daily pulse consumption, this can result in anemia due to iron deficiency [82]. Some LAB are able to degrade phytic acid by producing a phytase enzyme [83,84]. For instance, phytic acid decreased during the fermentation of soymilk with S. thermophilus [75], Lb. fermentum, Lb. plantarum, Lacticaseibacillus casei, Lb. bulgaricus, and Lb. acidophilus [85]. Fermentation of faba, chickpea, lentil, and pea flours with Lb. plantarum and a mix of Lb. fermentum and Lactobacillus pontis also reduced the phytic acid content as a result of bacterial phytases [48,72,86]. Lupine flour also showed lower phytic acid content after fermentation with lactic acid bacteria [87]. Microorganism-inherent phytases dissociate non-soluble organic complexes with minerals [88,89]. Studies have indicated that the effect on phytates was closely dependent on the microbial strain [90]. It has also been observed that phytic acid degradation is pH-dependent [91,92]. The optimal pH for most phytases ranges between 4.0 and 6.0 [93].
Saponins are a class of glucosides found mainly in plants. They are generally characterized by their bitter taste and by their ability to affect membrane integrity [94]. This ability has been associated with both deleterious and beneficial effects on human health. Low levels of saponins are not dangerous but could become toxic at high concentrations. Saponins reduce nutrient absorption due to the complexation of vitamins or the inhibition of digestive enzymes [81]. However, soybean saponins show health-promoting benefits, such as the prevention of hypercholesterolemia [95], the suppression of colon cancer cell proliferation [96], and the anti-peroxidation of lipids [97]. It seems that lactic acid fermentation leads to the reduction in saponin from fermented legumes, as indicated in a study of soymilk fermented with S. thermophilus [75] and in a study of the fermentation of soy flour with Lb. plantarum. The authors in [5] also observed a reduction in the saponin content of soy flour, and Hubert et al. [98] observed a reduction in glycosylated soya saponins during LAB fermentation of soybean germs for 48 h, which could be due to the transformation of DDMP (2,3-dihydroxy-2,5-dihydroxy-6-methyl-4H-pyran-4-one) to non-DDMP forms.
Isoflavones, including genistein and daidzein, are present in all plants, but their quantity is only important in legumes [99]. They show the ability to chelate ferric and ferrous ions and are therefore considered antinutritional factors [100]. They also have estrogenic activity (phytoestrogen) and can thus be considered disrupters of hormonal development [101]. However, they have shown beneficial effects on health. For example, isoflavones are considered ideal antioxidants because they possess the quality of reducing agents in addition to their metal-ion-chelating properties [102]. Isoflavone glucosides are hydrolyzed into their corresponding aglycones during the fermentation of some Asian fermented soybean foods, such as sufu and douchi in China [103], miso and natto in Japan [104], chungkokjang and doenjang in Korea [105], and tempeh in Indonesia [106]. The fermentation of soybean meal and soy beverages with Lb. paracasei and Lacticaseibacillus rhamnosus [107,108] also favors the release of aglycones from their corresponding glycosides, increasing isoflavone bioavailability. It has also been observed that lactic acid fermentation of legume seed sprouts clearly increased the content of isoflavones, especially in chickpeas [109].
The action of microorganisms on certain amino acids can produce biogenic amines [69]. The most important biogenic amines in foods, both quantitatively and qualitatively, are histamine, tyramine, putrescine, cadaverine, and β-phenylethylamine [110]. However, the formation of biogenic amines depends not only on the presence of decarboxylase-positive bacteria but also on the environmental conditions (raw materials, pH, ionic strength, and temperature) [111]. At low concentrations, biogenic amines are essential for many functions in the human body due to their physiological effects and biological activity. However, at high concentrations, biogenic amines may cause headache, nausea, rash, giddiness, and hypo- or hypertension [112]. The occurrence of biogenic amines in traditional fermented foods has been reported previously [4,113]. Some strategies are available to avoid the formation of biogenic amines, such as the choice of microbial starters that are unable to produce them or the oxidation of biogenic amines via the action of amino oxidase enzymes [110]. Furthermore, a number of bacteria associated with these fermentations exhibit the potential for degrading histamine and tyramine through the production of mono- and di-amino-oxidases [114]. In a study of the ability of P. acidilactici and Lb. sakei to degrade biogenic amines during solid-state and submerged fermentation of lupine whole meal flour [64], it was observed that solid-state fermentation produced a lower quantity of biogenic amines than submerged fermentation.
Finally, in the particular case of faba beans, the presence of the pyrimidine glycosides vicine and convicine can cause favism, an acute form of hemolytic anemia, in humans who have an X-chromosome-inherited glucose-6-phosphate dehydrogenase (G6PD) deficiency [115]. It also causes a significant reduction in the efficiency of production systems for broiler chickens, laying hens, and pigs. Vicine and convicine are thermostable; however, the application of lactic acid fermentation can significantly decrease the quantity of these compounds [116]. The degradation of vicine and convince in faba bean flour with Lb. plantarum has been already demonstrated [35,37,40].
To conclude, the majority of studies have shown that lactic acid fermentation decreases the content of some antinutritional compounds, such as α-galactosides, tannins, trypsin, phytic acid, saponin, vicine and convicine, and biogenic amines. Lactic acid fermentation increases the content of total phenolic compounds and isoflavone.
6.3. Antioxidant Activity
The antioxidant capacity of legumes is also modified by fermentation because of changes in antioxidant components such as vitamin C, tocopherol, and glutathione. Furthermore, some of the active peptides and amino acids formed during proteolysis have antioxidant activity [45]. Antioxidant capacity is measured by means of different methods, such as tests of peroxyl-radical-trapping capacity (PRTC), superoxide dismutase (SOD)-like activity, Trolox-equivalent antioxidant capacity (TEAC), the diminution in 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis-(3-ethylbenzthiazolin-6-sulfonic acid (ABTS) radicals, and lipid peroxidation inhibition.
Studies have shown an increase in PRTC in fermented soybean grains and flour with Lb. plantarum compared to non-fermented samples [27]. These authors observed that the LSF of soybean flour resulted in higher PRTC values than the SSF of cracked grains. On the contrary, a decrease in PRTC after the fermentation of cowpea flours with Lb. plantarum or naturally has also been observed [117].
When SOD-like activity was measured, it was observed that fermentation caused a drastic decrease in SOD-like activity in soybean grains and flour [27] and in cowpea flour [117]. However, Wang et al. [118] observed that the fermentation of soybean milk with Lb. acidophilus, S. thermophilus, and Bifidobacterium longum increased SOD activity, and the increase was dependent on the bacteria used for fermentation. These results suggest that, during fermentation, microorganisms involved in the process present different proteolytic activities that are responsible for the SOD-like activity of the final fermented products [27].
The TEAC of soybean grains and flour decreased after fermentation with Lb. plantarum [27]. It seems that some microorganisms develop oxidative stress protection mechanisms when they are exposed to reactive oxygen substances [119]. The kind of substrate and the fermentation process also determine TEAC.
Fermentation enhanced the capacity to scavenge DPPH and ABTS radicals, as indicated by different studies: in the fermentation of cowpea flour with Lb. plantarum [117]; in the fermentation of lentil, bean, chickpea, and pea flours by Lb. plantarum, P. acidilactici, Lc. mesenteroides, Lb. rossiae, and Lb. brevis [48]; in the fermentation of pea protein isolate by Lb. rhamnosus [45]; and in the fermentation of faba bean flour with Lb. sakei, Lactococcus lactis, Lb. mesenteroides, P. pentosaceus, and Weissella cibaria [36]. The antioxidant activity seems to increase with the time of fermentation [120]. These results indicate that the antioxidant compounds formed during fermentation could inhibit the formation of radicals or transform them into less harmless products. Little information about the inhibition of lipid peroxidation in fermented samples has been published, with different results depending on the type of legume, the fermentation process, and the microorganisms involved [27,117,121].
Antioxidant activity during fermentation has also been studied by observing changes in antioxidant compounds. Vitamin C is an effective antioxidant that acts both directly via a reaction with aqueous peroxyl radicals and indirectly by restoring the antioxidant properties of fat-soluble vitamin E [122]. Studies have shown that fermentation reduces the content of vitamin C in cowpea flour [117] and lupine seeds [121] and flour [123]. Other authors, however, have not detected vitamin C in raw or fermented legumes such as soybeans [27] and pigeon peas [124].
Fermentation has also been shown to affect tocopherol content and, consequently, vitamin E content. Vitamin E is the most important lipophilic radical-scavenging antioxidant ever studied [125]. The effect of fermentation on tocopherol and vitamin E depends on the kind of legume, the kind of microorganism, and even the kind of fermentation process used [27]. In the fermentation of soybean seeds and flour with Lb. plantarum, vitamin E activity decreased as a result of a sharp diminution in α-tocopherol and a slight diminution in β- and γ-tocopherol. δ-Tocopherol, however, increased sharply [27]. In lupine seed fermentation, the authors in [121] indicated that α-tocopherol increased, whereas γ and δ decreased, during fermentation with Lb. plantarum or with autochthonous microflora. In consequence, vitamin E levels notably decreased. Fermentation of cowpea flour with Lb. plantarum or autochthonous microflora produced a sharp diminution in γ- and δ-tocopherol content but an increase in β-tocopherol [117]. Vitamin E levels therefore notably increased in Lb. plantarum fermentation.
Reduced and oxidized glutathione have also been found to be modified by fermentation. Glutathione acts as an important thiol redox buffer for cells. Oxidized glutathione is converted into reduced glutathione, which protects cells by destroying compounds that cause oxidative stress, such as superoxide radicals, hydrogen peroxide, hydroxyl radicals, nitric oxide radicals, peroxynitrite, and lipid peroxyl radicals [126]. Studies have shown that fermentation decreases the amount of reduced glutathione but increases the amount of oxidized glutathione in soybean seeds and cowpea flour fermented with Lb. plantarum [27,117]. However, glutathione seems to be affected by the kind of microorganism and the particle size of the substrate [27].
6.4. Other Vitamins and Bioactive Compounds
Apart from antinutritional compounds and antioxidant vitamins, fermentation may also affect the levels of other bioactive compounds. Results have indicated that, in general, natural fermentation reduces the pigment content, such as the anthocyanin content, of indigenous Nigerian legumes seeds (cowpea, Bambara nut, red bean, pigeon pea, Qgrica breadfruit, African yam bean seed, African oil bean seed, and groundnut) [127]. This decrease could be related to the adsorption mechanism between the fermenting flora and anthocyanins [128]. Decreases in carotenoid and flavonoid contents during fermentation have also been observed [127].
It has also been demonstrated that the fermentation of legume flours such as faba bean, soybean, and lupine flours with Levilactobacillus brevis (formerly Lb. brevis) noticeably increased the amount of vitamin B12 [129]. The scientific use of the term “vitamin B12” is usually confined to cyanocobalamin. B12 is naturally present in foods of animal origin. In foods of vegetal origin, B12 can be found only after fermentation or fortification [130].
6.5. Allergenicity
Legume allergies are some of the most common food-related allergies [131]. A food allergy is defined as an adverse reaction of the human immune system to an otherwise harmless food component [132]. The main allergens in legumes are proteins [133], due to the presence of certain proteins responsible for adverse reactions, or due to the poor absorption of legume proteins in the gut [134]. In consequence, modifications in protein structure and content could reduce legume allergenicity. As previously indicated (see Section 6.1), legume fermentation partially hydrolyzes proteins and improves digestibility, and thus, fermentation would decrease the allergenicity of legumes. The fermentation of soybean meal with a mixture of Lb. casei, yeast, and B. subtilis degraded major protein allergens due to hydrolysis during fermentation [135]. These authors also observed a reduction in allergenicity measured via the diminution in in vitro immunoglobulin E (IGE)-binding capacity and via milder damage to rat intestines. Aguirre et al. [34] showed that the fermentation of soybean protein isolate using different LAB bacteria led to the degradation of allergens such as glycin glycinin and β-conglycinin. In consequence, fermentation offers an interesting opportunity to produce hypoallergenic food products from legumes [136].
7. Effect of Fermentation on the Functional Properties of Legume Protein
A food can be considered functional if it is satisfactorily demonstrated to beneficially affect one or more target functions in the body beyond its adequate nutritional effects, improve well-being and health, or reduce the risk of a disease [137]. According to this definition, fermented legumes could be considered functional foods as a result of their effects relating to the diminution in antinutritional compounds and to increases in antioxidant activity and protein digestibility. Furthermore, certain fermenting microorganisms are considered probiotics, that is, live microorganisms that, when administrated in adequate amounts, confer their own health benefits to the host [138]. Tamang et al. [139] published an interesting review of the functional properties of microorganisms in traditional fermented foods. The consumption of fermented legumes has been associated with health benefits [140,141,142,143] such as anti-obesity, antihypertensive, antiallergic, antimicrobial, and antioxidant effects, as well as the prevention of heart disease, cancer, gastrointestinal disorders, diabetes, and osteoporosis. Sanjukta and Rai et al. [144], in their study of the potential health benefits of bioactive peptides produced during soybean fermentation, indicated that bioactive peptides are either produced by protein hydrolysis during fermentation or released by fermenting microorganisms. The characteristics of these bioactive peptides depend on the specific microbial strains and the initial proteins. A study of the fermentation of soymilk with S. thermophilus and B. infantis [75] indicated that enhanced antitumor activity occurred due to the combined action of the original antitumor cell components of legumes, the starter organisms, and the antitumor bioactive properties of cells formed during fermentation. Comparing the bioactive peptides in kidney bean protein obtained via LSF and SSF with Lb. plantarum [26] showed higher potential antihypertensive activity due to the presence of high angiotensin l-converting enzyme (ACE)-inhibitory activity and the content of γ-aminobutyric acid (GABA). That study suggested that Lb. plantarum had a higher capacity for the production of bioactive peptides compared to B. subtilis. This indicates that the effect was tightly related to the kind of microorganism [145]. In consequence, the increase in the consumption of fermented legumes may increase the health and the quality of life in a significant proportion of the population [146].
8. Effect of Fermentation on the Physicochemical and Technological Properties of Legume Protein
The use of legumes and/or their ingredients in food products requires the optimization of their technological properties. Legume proteins possess valuable technological properties for the food industry, such as solubility; water and oil retention capacity; and emulsifying, foaming, and gelling properties. Lactic acid fermentation may modify these properties, creating a wide range of possibilities depending on the kind of legume or legume ingredient used, the fermentation conditions, and the characteristics of the final product (bread-making, dairy, etc.) (Table 2).
8.1. Protein Solubility
Protein solubility is the key property in terms of the technological characteristics of proteins since it defines most of their other properties, such as their emulsifying, foaming, and gelling properties. The literature has shown contradictory results regarding the effect of fermentation on legume protein solubility. Different authors have indicated the diminution in protein solubility of soybean protein isolate and soybean meal after fermentation with Lb. helveticus and Lb. plantarum [53] and of lupine protein isolate after fermentation with different bacteria (Lb. reuteri, Lb. amylolyticus, Lactobacillus parabucchneri, Lactobacillus carnosus, Lb. helveticus, Lb. brevis, and Lactobacillus delbruekii) [60]. The acid produced by certain fermenting microorganisms might induce the irreversible coagulation of proteins and thus reduced solubility, although pH was observed to have increased far beyond pI after fermentation [60]. Furthermore, changes in the protein surface could induce the exposure of hydrophobic groups and favor protein–protein interactions [147]. However, Emkani et al. [58] studied a new extraction method of pea proteins assisted by lactic acid fermentation and observed an increase in the solubility of globulins at low pH, probably as a result of proteolysis. The solubility of proteins increased after the fermentation of lupine flour with P. pentosaceus [3]; with a mixture of Lc. mesenteroides, Lb. plantarum, and Lb. brevis [59]; or with Lb. sakei, P. acidilactici, or P. pentosaceus strains [148]. These authors concluded that the diminution in the protein size and the changes in conformation induced by microbial hydrolysis and acid-induced hydrolysis were probably responsible for the increase in the protein’s solubility. Taking into account the fact that phytic acid is able to bind proteins [149], its diminution during fermentation (see Section 6.2) may also facilitate protein solubility. Furthermore, the degradation of starch would reduce the interaction with proteins and thus facilitate protein solubility [16]. Protein solubility also depends on the pH and the microorganism used for pea protein isolate fermentation [147].
8.2. Charge of Proteins and Hydrophobicity
Low changes in the net charge of pea proteins were observed after the fermentation of pea-protein-enriched flours with Lb. plantarum [150], which would indicate limited hydrolysis or limited changes in the exposure of both positively and negatively charged groups. In the study of the surface hydrophobicity of fermented proteins, these authors observed different behavior depending on pH. At pH 4, protein hydrophobicity increased after 1 h of fermentation, indicating the progressive unraveling of the protein and the release of peptides, which exposed buried reactive charged and hydrophobic sites [151]. At pH 7, the hydrophobicity of fermented pea protein decreased compared to the non-fermented substrate. Conformation changes at pH 7 hide previously exposed hydrophobic sites. It is known that hydrophobicity is affected by protein structure and environmental conditions [152]. A diminution in hydrophobicity (measured at pH 7) was also observed in lupine proteins fermented with a mixture of Lc. mesenteroides, Lb. plantarum, and Lb. brevis [59]. These authors indicated that changes were dependent on other factors, such as the cultivar or the presence of hulls.
8.3. Water-Holding Capacity and Oil-Holding Capacity
Water-holding capacity appears to be increased in fermented legume proteins compared to non-fermented ones, as observed after the fermentation of pea-protein-enriched flour with Lb. plantarum [150]; lupine flour with a blend of Lc. mesenteroides, Lb. plantarum, and Lb. brevis [59]; and chickpea protein concentrate with P. pentosaceus and P. acidilactici [10]. This increase in the water-holding capacity of legume protein is attributed to the proteolytic activity of bacteria, causing changes in the protein structure and configuration, leading to the exposure of more hydrophilic sites [10]. Changes in protein structure and conformation caused by fermentation, such as hydrolysis and unfolding, would expose previously buried hydrophilic sites that could interact with more water. There are also reports indicating increases in the oil-holding capacity after fermentation of pea-protein-enriched flour with Lb. plantarum [150] and the fermentation of chickpea and faba bean flours with a mixture of Lb. bulgaricus and S. thermophilus [63].
8.4. Emulsifying Properties
The properties of emulsions are closely related to the protein characteristics, to environmental conditions, and to the conditions of the emulsification process [153]. Since fermentation is able to modify the properties of proteins (such as their structure, molecular size, conformation, flexibility, solubility, and surface hydrophobicity) along with the environmental conditions (pH or mineral content), the emulsifying properties of legume proteins are expected to be modified during fermentation.
The fermentation of a soy protein isolate by Lb. helveticus resulted in a decrease in the emulsifying capacity [53]. The fermentation of pea-protein-enriched flour with Lb. plantarum [150] and pea protein isolate with six different LAB (Lb. plantarum, Lb. casei, Lactobacillus perolens, Lb. fermentum, Lc. mesenteroides subsp. cremoris, and P. pensosaceus) [147] also decreased the emulsifying capacity. The low emulsifying capacity observed for fermented samples might be related to the aggregation of proteins and the interaction with by-products, which can prevent hydrophobic interactions between protein and oil molecules and reduce the amphiphilic character of the proteins [147]. Emulsifying stability, however, depends on pH [150]. These authors indicated that the soluble protein concentration, hydrophobicity, and structural flexibility of proteins clearly affected the obtained results. In the case of lupine, the literature offers variable data regarding the emulsifying capacity after fermentation. For example, lactic acid fermentation of lupine protein using a mixture of Lc. mesenteroides, Lb. plantarum, and Lb. brevis decreased the emulsifying capacity of protein [59]. Fermentation with P. pentosaceus produced a significant increase [3], whereas fermentation with Lb. reuteri, Lb. amylolyticus, Lb. helveticus, Lb. brevis, and Lb. delbrueckii did not show any uniform behavior [60]. The influences of different fermenting microorganisms and fermenting conditions are thus responsible for discrepant results.
8.5. Foaming Properties
Similarly to emulsion-forming properties, foaming properties depend on the protein attributes (the ability to migrate to the air–water interface, to lower surface tension, and to realign its hydrophobic groups towards the apolar phase and the hydrophilic groups towards the polar phase); on the environmental conditions (the protein concentration, pH, temperature, and the presence of other components); and on the conditions of the foam-forming process. Contrasting results have been obtained regarding the effect of lactic acid fermentation on the foaming properties of legume protein. Briefly, foaming capacity was improved but foam stability was decreased after the fermentation of a soy protein isolate with Lb. helveticus [53]; of pea-protein-enriched flour with Lb. plantarum [150]; and of lupine with Lb. reuteri, Lactobacillus amylolyticus, Lb. helveticus, Lb. brevis, and Lb. delbrueckii [43] and with P. pentosaceus [3]. On the contrary, other authors [10] have reported that the foaming capacity of chickpea protein concentrate decreased after lactic acid fermentation with P. pentosaceus and P. acidilactici compared to the non-fermented samples. It is worth noting that the foaming capacity is highly related to the pH and fermentation time [154]. In the fermentation of pea-protein-enriched flour with Lb. plantarum [150], different behavior was observed depending on the pH. The authors noted that, at pH 4, the foaming capacity increased in the first 5 h of fermentation as the protein unfolded and exposed its hydrophobic groups. After 5 h of fermentation, the foaming capacity decreased, probably due to an overabundance of hydrophobic groups, reducing the ability of the protein to migrate to the interface. At pH 7, the foaming capacity and the foam stability were relatively similar during all fermentation processes, which was most likely due to the relatively constant surface properties at this pH. Foam properties also depended on the kind of microorganism used during lupine protein isolate fermentation [60]. The authors observed an increase in the foam activity after fermentation, but it was more noticeable with Lb. reuteri, Lb. amylolyticus, and Lb. helveticus compared to Lb. brevis and Lb. delbrueckii.
8.6. Sourdough Preparation and Bread-Making Properties
The high amount of starch and proteins found in legumes has awakened the interest of scientists aiming to include legumes in bread-making. Various studies have been conducted to analyze the behavior of fermented legume flours in bread-making. The addition of lupine protein fermented with P. pentosaceus to dough improved bread quality [3]. Bartkiene et al. [155] observed that the use of lupine sourdough (obtained via the fermentation of lupine flour with P. acidilactici) increased the water absorbance capacity of dough, strengthened the gluten network, and resulted in higher resistance to extension during dough fermentation. These modifications resulted in a more porous bread crumb, higher specific volume, higher springiness and resilience, and lower hardness and chewiness of bread. The fermentation of chickpea dough with Weissella paramesenteroides and Lb. plantarum improved the oil-holding, gelation, and emulsifying capacities, and Lb. plantarum improved the oil-holding capacity, as well as gelation and emulsifying properties [31]. The fermentation of lentil/wheat composite bread, together with in situ dextran formation, considerably modified all of the textural attributes of the composite breads, resulting in higher volume, softer crumbs, and higher springiness and cohesiveness [156].
8.7. Fermented Plant-Based Products
The effect of legume fermentation has also been studied in the elaboration of non-dairy yogurt-like gels prepared from pulse ingredients. Indeed, pulses are the ideal yogurt ingredient when fermenting with LAB due to their high protein content, improved gelling behavior, and promising nutritional properties [157]. In the case of pea protein gels with different Streptococcus and Lactobacillus strains, an increase in the pea protein concentration resulted in products with higher acidity, greater syneresis, and lower firmness than the reference samples [158]. Zare et al. [159] showed that lactic acid fermentation of pea flour with probiotic strains, i.e., Lb. rhamnosus, resulted in faster pH reduction, improved gel stability with lower syneresis, and improved viscoelastic properties. Pea protein fermented with yogurt culture containing Lb. delbrueckii ssp. bulgaricus and S. thermophilus could provide an alternative to legume-based yogurt since it offers high gelling stability and increased viscous properties [160]. In the case of fermenting broad beans and chickpeas with yogurt starter cultures containing S. thermophilus; Lb. delbrueckii subsp. Bulgaricus; and a mixture of Lb. casei, Lb. lactis subsp. cremoris, Lc. lactis subsp. lactis, Leuconostoc spp, and Lc. lactis subsp. lactis biovar, broad bean fermentation registered higher titratable acidity, lower syneresis, and significant decolorization compared to that of chickpeas [161]. However, chickpea based fermented product was associated with a creamier structure of the gel. The fermentation of soybean milk with S. thermophilus and Lb. casei [162] resulted in the firm texture of soy yogurt. Soy yogurt prepared using Lb. plantarum [57] at low pH exhibited high values in terms of hardness and gumminess, which is representative of a strong and compact structure of the gel.

Table 2.
The effects of lactic acid fermentation on techno-functional properties of legume protein.
Table 2.
The effects of lactic acid fermentation on techno-functional properties of legume protein.
| Legume Type | Protein Ingredients Treated | LAB Strains | Techno-Functional Properties | References | |||
|---|---|---|---|---|---|---|---|
| Protein Solubility | Emulsifying Properties | Foaming Properties | Surface and Bulk Properties | ||||
| Chickpea | Flour | W. paramesenteroides Lb. plantarum | _ | EC increased | _ | WHC and OHC both increased Gel formation increased | [31] |
| Protein concentrate | P. pentosaceus P. acidilactici | _ | _ | FC decreased | _ | [10] | |
| Soybean | Flour | Lb. plantarum Lb. rhamnosus Lactobacillus nantensis Lb. fermentum Lb. reuteri P. acidilactici Lb. brevis | _ | EC increased | _ | WHC decreased OHC increased Gelation capacity decreased | [163] |
| Protein isolate | Lb. helveticus | Decreased at pH 7 Increased at pH 4 | EA decreased | FA increased FD decreased FS increased | WHC and OHC both increased | [53] | |
| Protein isolate | Lb. plantarum | Decreased at pH 7 | _ | _ | SH increased | [33] | |
| Pea | Pea protein-enriched | Lb. plantarum | _ | EA decreased Lower EA at pH 7 ES decreased at pH 4 | FC increased at pH 4 FC not changed at pH 7 | Surface charge decreased SH increased at pH 4 and decreased at pH 7 WHC and OHC changed with time but not pH | [150] |
| Protein isolate | Lb. plantarum Lb. fermentum Lb. casei Lc. mesenteroides P. pentosaceus Lb. perolens | Increased at pH 5 but decreased at pH 3, 7, and 8 | EC decreased The highest EC for Lb. plantarum The lowest EC for Lb. perolens EC increased for Lb. casei and Lc. cremoris after 48 h | Unable to form foam | _ | [147] | |
| Protein isolate | Lb. plantarum | Decreased | No differences | FS decreased No differences in FC | OHC increased WHC decreased | [154] | |
| Lupine | Protein concentrate | co-culture of Lc. Mesenteroides Lb. plantarum Lb. brevi | _ | Small effect on EA Decrease in the emulsifying properties | _ | Higher SH for samples with hulls SH decreased WHC increased | [59] |
| Protein isolate | P. pentosaceus | _ | EA increased with time and pH 8 ES increased with pH but was not affected by time | FC increased at pH 8 and 48 h FS increased at pH 8 compared to pH 6 | _ | [3] | |
| Protein isolate | Lb. Reuteri Lb. brevis Lb. amylolyticus Lb. parabuchneri Lb. sakei Lb. helveticus Lb. delbrueckii | Decreased at pH 7 No difference at pH 4 | Highest EC for Lb. parabuchneri and lowest for Lb. parabuchneri, EC decreased | FA increased FS increased in all strains except for Lb. parabuchneri and Lb. helveticus | _ | [60] | |
FC: foaming capacity; EC: emulsifying capacity; WHC: water-holding capacity; OHC: oil-holding capacity; SH: surface hydrophobicity.
9. Effect of Lactic Acid Fermentation on the Sensory Attributes of Legumes
Numerous studies have confirmed improvements in the sensory profiles of legumes after lactic acid fermentation (Table 3). The flavor of legumes is mainly characterized by beany and green notes, which are perceived as undesirable by consumers [164]. Trindler et al. [165] conducted a review in which they discussed the origin of the aroma molecules responsible for the green notes associated with pea protein, along with possible strategies to reduce these off-flavors. Fischer et al. [166] also published a review on the volatile compounds responsible for the “beany” off-flavor of pea protein and the potential use of microorganisms in their reduction. Lactic acid fermentation reduces the perception of these off-flavors as a result of the diminution in the precursors of undesirable volatile compounds or as a result of the formation of new compounds that mask the undesirable ones [167,168]. Modifications are closely related to the kind of legume, the fermenting microorganisms, and the fermentation metabolism. Indeed, the main pathways for the improvement of flavor in fermented legume products are the fermentation of sugar, the degradation of fat, and the degradation of protein [169] (Figure 2). Lactate is the main product generated from glucose metabolism, and the intermediate fraction, pyruvate, can alternatively be converted by α-acetolactate to diacetyl, acetoin, acetaldehyde, or acetic acid, which are considered important molecules in determining the flavor of legume products. Diacetyl is a volatile compound, identified as belonging to the group of ketones, that positively contributes to the perception of buttery and creamy flavors in butter and some fermented milk products [170]. It is a product of citrate metabolism, which is considered an important metabolite for LAB used in the dairy industry. Ben-Harb et al. [171] conducted a study comparing the sensory attributes of pea protein isolate and a mixture of this isolate with milk and found that volatile compounds, including butane-2,3-dione (=diacetyl), pentan-2-one, 2-butan-2-one and 3-methylbutan-2-one, and nonan-3-one, were only observed for fermented samples obtained by LAB and not for those fermented by the other bacteria and yeasts.
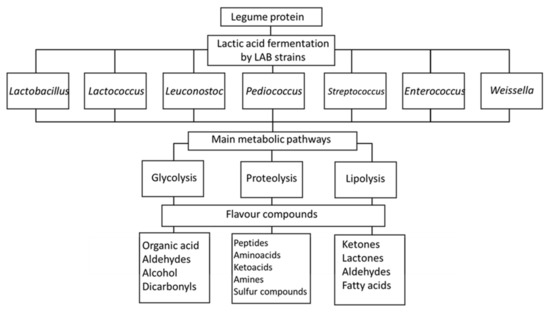
Figure 2.
Schematic of the main pathway in lactic acid fermentation leading to the production of aromatic compounds.
There are other important aromatic compounds in legume protein, including hexanal, heptanal, nonanal, and octanal compounds, which contribute to the undesirable “green notes” of vegetables and belong to the family of aliphatic aldehydes. These could be derived from either the enzymatic or auto-oxidation of lipids [172]. It has been shown that the non-fermented pea protein isolate is mainly associated with aliphatic green aldehyde compounds (hexanal, nonanal, and octanal) [171]. However, after fermentation with a mixture of LAB, the amounts of other aldehydes, such as (2E,4E)-hepta-2,4-dienal, 3-methybutanal, and 2-methylpropanal, increased. These compounds are responsible for malted, grilled, and roasted notes. Decreases in the contents of aldehydes and ketones and an increase in the content of alcohol in the fermentation of pea protein isolates using Lb. plantarum have also been shown [154].
Fermentation reduces all of the negative sensory attributes of soybeans, such as beany, bitter, mouthcoating, and astringent effects [53]. Blagden and Gilliland et al. [173] indicated that fermentation with Lactobacillus and Streptococcus strains decreased the beany flavor of soymilk due to the partial or total elimination of hexanal. Soymilk fermentation converted n-hexanal into n-hexanoic acid [174]. The improvement of the sensory attributes of soymilk after fermentation with Lactobacillus harbinensis was related not only to the reduction in the hexanal content but also to the formation of desirable compounds such as acetoin and 2,3-butanedione [175]. The presence of other beany compounds, such as methanol and acetaldehyde, considerably depended on the culture used for fermentation [173]. Twenty-four-hour fermentation of a pea protein isolate with six different bacteria (Lb. plantarum, Lb. casei, Lb. perolens, Lb. fermentum, Lc. mesenteroides subsp. cremoris, or P. pensosaceus) decreased the typical aroma attributes, such as pea-like, green, and earthy aromas, and decreased the bitter taste of pea protein isolates [147]. Flavor clearly depended on the kind of fermenting microorganisms used. Specifically, hexanal diminution was observed in the fermentation of peas with P. pentosaceus and Lb. plantarum [176] and in the fermentation of pea protein emulsions [168] and pea and pea-milk mixed gels [171]. These authors indicated that the adequate selection of microbial consortia permitted the development of plant-based food products with diversified sensory characteristics. In a study of lupine fermentation with eight different microorganisms, the use of Lb. reuteri, Lb. amylolyticus, Lb. helveticus, Lb. brevis, and Lb. delbruekii noticeably decreased the “green notes“ of a lupine protein isolate [39]; this effect was closely related to the microorganism used. For example, Lb. brevis noticeably decreased the green aroma compounds, whereas Lb. parabuchneri did not show a clear effect, nor was a diminution observed in green aroma compounds when fermenting lupine with a commercial yogurt culture [161]. Lower n-hexanal values in lupine protein fermented with Lb. plantarum compared to non-fermented protein were also obtained [177]. The authors also indicated that fermentation ensured lower n-hexanal values for more than two months of storage. Depending on the microorganism used for lupine fermentation, new pleasant aroma notes were perceived, such as cheesy aromas (using Lb. reuteri), oat-milk-like and fatty aromas (using Lb. brevis, Lb. delbrueckii), and popcorn-like and roasted aromas (using Lb. amilolyticus, Lb. helveticus) [60]. All of these authors agreed on the positive effect of fermentation on the overall aroma characteristics of lupine and its potential improvement of acceptability to consumers. Furthermore, the incorporation of fermented lupine and soybean into muffins increased the odor-active volatiles and improved the muffins’ aroma [12].
The degradation of proteins due to the activity of cell-membrane proteinase yields small peptides and free amino acids, which can be further converted into various alcohols, aldehydes, acids, esters, and sulfur compounds for specific flavor development in dairy products [12]. Amino acids released during proteolysis are not only an important source of energy for the growth of lactic acid bacteria but also the precursors of flavor compounds via oxidative deamination and/or transamination reactions. These are called aminotransferase and/or carboxylase reactions, during which a number of volatile compounds identified in fermented products, including 3-methylbutanal and benzaldehyde, can be recognized as products of amino acid catabolism. The conversion of leucine, isoleucine, and valine takes place via transamination of the amino acids to the corresponding α-keto acids and, subsequently, via a chemical or enzymatic decarboxylation step, to 3-methylbutanal, 2-methylbutanal, and 2-methylpropanal, respectively [178,179]. These volatile molecules are in the other aldehyde class. Bitterness is also reduced during soy fermentation as a result of the diminution of the hydrophobic peptide content, although the extent of this diminution depends on the kind of microorganism used [53]. The generation of hydrophilic peptides during the fermentation of soy protein with 12 different lactic acid bacteria strains from Lb. paracasei, Lb. fermentum, Lc. lactis, Lb. plantarum, Lb. helveticus, Lb. reuteri, and P. pentosaceus has been observed [34]. Hydrophilic peptides are normally correlated with desirable fermented soy flavors [180]. Studies have also shown that the presence of roasted and grilled notes in a lactic-fermented pea protein isolate alone and in its mixture with milk could be related to the proteolysis of pea vicilin by LAB [168]. It seems that hydrophobic free amino acids and hydrophilic peptides produced during the hydrolysis of vicilin by microorganisms are responsible for these specific aroma compounds [113,181].

Table 3.
The effects of lactic acid fermentation on sensorial properties of legume protein.
Table 3.
The effects of lactic acid fermentation on sensorial properties of legume protein.
| Legume Type | Protein Ingredients Treated | LAB Strains (Addition of Sugar) | Sensorial Profile | References | |||
|---|---|---|---|---|---|---|---|
| Sensorial Attributes | Aromatic Related to Proteolysis Compounds | Aromatic Related to Glycolysis Compounds | Aromatic Related to Fatty Acid Compounds | ||||
| Soybean | Protein isolate | Lb. helveticus (No sugar) | Decrease in beany, bitter, mouthcoating, and astringent properties Increase in sour, tangy lactic acid taste and bitterness Better sensory results after 24 h rather than 48 h | Degradation of peptides rich in proline and leucine Degradation of bitter peptides | _ | Degradation of isopentanol, n-hexanal, and hexanol | [53] |
| Milk | Lb. pentosus Lb. plantarum (No sugar) | Slight sweet taste and good texture properties | _ | _ | _ | [182] | |
| Milk | Lb. acidophilus Lb. casei S. thermophilus Lb. delbrueckii (No sugar) | Reduction in beany flavor | _ | Decrease in methanol, acetaldehyde, and ethanol | Decrease in hexanal | [173] | |
| Juice | Leuconostoc Lactobacillus Lactococcous Streptococcous (No sugar) | Nuts, soy, fresh, caramel, and hay descriptors for S. thermophilus Acid, sour, floral, pineapple, spicy, cheesy, kefir, and sorrel descriptors for Lb. plantarum Lb. pentosus was described as “plastic” Soy sauce, black bread, cabbage, salty, and broth descriptors for Lc. lactis Lb. acidophilus had a “goat” odor Lb. lactis had a “cabbage” and/or a “broth” odor Lb. lactis, Lb. plantarum, had “floral” odors | _ | _ | Increase in aldehydes, carbonyl, and alcohol for S. thermophilus and L. delbrueckii Increase in pentane-2,3-dione, heptane-2,3-dione, methyl acetate, and ethyl acetate for S. thermophilus and Lb. delbrueckii Increase in 2,4-dimethylbenzaldehyde for S. thermophilus Lb. plantarum, Lb. pentosus, Lb. coryniformis, and Lb. lactis produced four acids (acetic, butanoic, pentanoic, and hexanoic acids), two carbonyl compounds (1-hydroxypropan-2-one and 3-hydroxybutan-2-one), and two alcohols (2-methylpropan-1-ol and ethanol) | [9] | |
| Faba bean | Flour | Lb. plantarum (No sugar) | Increase in pungent odor and flavor | _ | _ | _ | [39] |
| Flour | Lb. plantarum | Crumb flavor | _ | _ | _ | [55] | |
| Pea | Protein isolate | Lb. plantarum Lb. perolens Lb. fermentum, Lactobacillus Lb. casei L. mesenteroides Pediococcus P. pentosaceus (0.5% Glucose) | Better aroma after 48 h compared to 24 h Decrease in bitter and astringent attributes Lowest pea-like aroma after 24 h Lb. plantarum for 24 h also masked green and earthy notes Increase in buttery aroma for Lb. perolens for 24 h Increase in floury attribute for P. pentosaceus Fecal aroma for Lb. fermentum after 48 h Intense cheesy aroma for Lc. cremoris after 48 h Decrease in bitter intensity for Lb. plantarum and Lc. cremoris after 24 h Increase in bitter and acid tastes for Lb. perolens | Increase in undesirable compounds such as p-cresol, indole, and skatole for Lb. fermentum after 48 h | Increase in diacetyl for Lb. perolens | _ | [147] |
| Protein isolate | Lb. plantarum | Lower color intensity, beany aroma, beany flavor, and lower amount of bitterness | _ | _ | Decrease in aldehydes and ketones Increase in alcohol | [154] | |
| Protein isolate | Co-culture Lb. acidophilus, S. thermophilus Lb. delbrueckii B. lactis (3% Sucrose) | Decrease “beer/yeast” notes | _ | _ | Presence of ester Increase in alcohol Decrease in aldehydes, ketones, and furans Presence of (E)-2-heptenal, 6-methyl-5-hepten-2-one, and trans-2-methyl-2-butenal Decrease in 2-pentyl-furan and 2-ethyl-furan | [167] | |
| Protein isolate | Lb. plantarum P. pentosaceus (No sugar) | Pleasant odor with weak milky attributes | 1-Pyrroline with a sperm-like odor produced from the degradation or oxidation of proline, spermine, spermidine, or putrescine | _ | No difference in the content of n-hexanal between strainsN-hexanal concentration reduced during fermentation and no negative effect on storage stability The pungent/cheese-like and floral/rose-like attributes in fermented samples were identified as butan-2-one and as β-damascenone, respectively During storage, slight increase in n-hexanal Presence of β-damascenone and butan-2-one, n-hexanal, n-botanal, and dimethyl trisulfid | [176] | |
| Lupine | Flour | P. pentosaceus (No sugar) | Intensive taste and acidity | _ | _ | _ | [3] |
| Protein isolate | Co-culture Lb. casei Lb. plantarum Lb. paracasei Lc. mesenteroides Lc. lactis S. thermophilus Lb. delbrueckii Streptococcus thermophilus Lb. delbruecki Lb. Acidophilus Bifidobacterium animalis Lc. lactis Leuconostoc pseudomesenteroides P. pentosaceus Lc. lactis Lb. plantarum (No sugar) | Increase in 3-methyl-1-butanol) Increase in alcohol compounds such as 3-methyl-1-butanol | Increase in 1-Nonen-2-ol | Increase in hexanal Increase in the content of acetic acid and hexanoic acid, responsible for sour and sweat odors Increase in 1-octen-3-ol | [183] | ||
| Protein isolate | Lb. reuteri Lb. brevis Lb. amylolyticus Lb. parabuchneri Lb. sakei Lb. helveticus Lb. delbrueckii (0.5% Glucose) | Increase in aroma perception of cheesy, roasty, and popcorn-like notes for Lb. brevis and Lb. amyloslyticus Lb. reuteri for cheesy, fatty, and oatmeal-like Lb. brevis, cheesy and oatmeal-like Lb. amylolyticus popcorn-like and roasty Lb. parabuchneri pea-like, green bell pepper, and cheesy Lb. sakei popcorn-like and roasty Lb. helvticus roasty and popcorn-like Lb. delbrukei oatmeal-like and fatty | _ | _ | Reduction of n-hexanal | [60] | |
| Protein isolate | Lb. Plantarum P. pentosaceus (No sugar) | Sweet, solvent, and fungal but also musty, earthy, burnt, dusty, or cereal-like odor | Presence of 1-pyrroline, which is known as the Strecker degradation product of proline | _ | Presence of hexanal Decrease in alcohol and aldehydes such as n-hexanal Decrease in lipid degradation compounds such as n-pentanal, n-heptanal, 1-octen-3-ol, and 2-pentylfuran Presence of 1-Octen-3-ol, which is the product of fatty acid degradation | [177] | |
| Protein isolate | Lb. sakei Lb. amylolyticus Lb. helveticus (0.5% Glucose) | Increase in intensity and aroma perception (cocoa-like and malty) Increase in bitter intensity Higher intensity of saltiness for Lb. helveticus | _ | _ | _ | [184] | |
| Mung beans | Seed | Lb. plantarum (No sugar) | More fragrant odor Stronger odor of grass and fat, related to the high content of aldehydes | Disappearance of nonanal, 5-methyl-2-formylthiophene, and phenylacetaldehyde | Increase in 2,3-butanediol Increase in ester Increase in isoamyl acetate and ethyl acetate | Decrease in alcohols (hexanol, 3-methyl-3-buten-1-ol, and (E)-2-hexen-1-ol) and aldehydes (nonanal, octanal, 2-furfural, and 3-methylbutanal) Decrease in hexanal, hexanol, and 1-octen-3-ol Increase in the content of acids Increase in the content of ketones Increase in 2-propanone and 3-hydroxy-2-butanone Majority of volatile flavors were ethyl hexanoate, heptanal, and butanal | [185] |
| Pea + cow protein | Protein isolate | Lb. delbrueckii S. thermophilous Lb. acidophilus Lb. helveticus Lb. casei Lb. rhamnosus Lb. fermentum (No sugar) | Lb. delbrueckii + Lb. fermentum, S. thermophilus + Lb. rhamnosus, Lb. rhamnosus have higher intensities for positive descriptors such as creamy, dairy, and sweet and lower intensities for negative descriptors such as vegetal, earth, and vinegar. Lb. delbrueckii + Lb. helveticus, Lb. delbrueckii + Lb. rhamnosus, S. thermophilus + Lb. helveticus: higher intensities for negative descriptors such as acid and astringent but rather low intensities for the negative descriptors pea and earth. | _ | _ | _ | [158] |
| Pea (P), Pea + milk (PM) | Protein isolate + milk protein | Lb. casei Lb. plantarum Lb. rhamnosus Lactococcus lactis, Leuconostoc lactis W. cibaria (No sugar) | Increase in fruity and flowery notes related to the presence of Lb. plantarum Increase in sweety and creamy descriptors | Proteolysis of pea vicilin by LAB strains, leading to roasted/grilled notes | Decrease in hexanal and heptanal Production of 3-methyl-1-butanol in the mixed emulsion and 2-methylpropanal and 2-butanone in the pea protein isolate emulsion | [168] | |
| Protein isolate | Microbial communities including some bacteria, yeast, and Lc. lactis Leuconostoc lactis Lb. rhamnosus (No sugar) | _ | Formation of 3-methylbutanal and benzaldehyde, which are responsible for chocolate and roasted coffee notes | Formation of butane-2,3-dione (=diacetyl) (for PM), pentan-2-one, 2-butan-2one, and 3-methylbutan-2-one (for P), which are responsible for buttery and creamy flavors | Elimination of aldehydes responsible for green notes, i.e., hexanal, heptanal, nonanal, octanal, and (E)-2-ethylbut-2-enal Increase in aldehydes responsible for grilled and roasted note, i.e., (2E,4E)-hepta-2,4-dienal, 3-methybutanal, and 2-methylpropanal Formation of other aromatic hydrocarbons, including toluene, benzene, and 2pentylfuran | [171] | |
10. Antimicrobial Activity of Lactic Acid Bacteria on Legume Protein
Lactic fermentation has been traditionally used to improve the shelf-life of legumes, protecting against spoilage or pathogenic microbiota such as fungi and spore-forming bacteria. In consequence, and following the definition of Su et al. [186], lactic acid fermentation can be considered a method of legume bio-preservation. At present, the increasing demand for natural and fresh legume-based ingredients and foods has renewed the interest in fermentation as a preservation method. Licandro et al. [133] published a review on the use of LAB fermentation to improve safety issues in legume food products. Fermentation decreases pH and may produce antimicrobial compounds that inhibit spoiling microflora by destabilizing cell membranes and thus interfering with the proton gradient, inhibiting enzyme activity, and/or creating reactive oxygen species [187]. The pH diminution observed during LAB fermentation is mainly the result of lactic acid formation. However, other organic acids can also be formed, such as butyric acid in the fermentation of soybeans by Lactobacillus spp. [186] and acetic acid in the fermentation of chickpeas by Lb. plantarum and Lb. brevis [48]. Furthermore, LAB strains are able to produce various antimicrobial substances, such as low-molecular-weight metabolites (reuterin, reutericyclin, diacetyl, and fatty acids), hydrogen peroxide, antifungal compounds (propionate, phenyl-lactate, hydroxyphenyl-lactate, and 3-hydroxy fatty acids), and/or bacteriocins [188]. The action of these compounds has demonstrated a protective effect against undesirable microorganisms; examples include soybeans fermented with Lb. plantarum [2], faba beans fermented with Lb. casei and Lb. plantarum [189], lentils fermented with Lb. plantarum [28] or chickpea, and lentil sprouts fermented with Lb. casei [109]. Furthermore, in addition to the presence of all of these protective substances, undesirable microorganisms will not be able to compete against a LAB culture that is already adapted to the substrate [2].
11. Conclusions and Future Prospects
All of the studies conducted to date on legume fermentation agree on the fact that lactic acid fermentation modifies the composition and the nutritional, functional, technological, and sensory characteristics of legumes. Lactic acid fermentation allows modifications in the conformation and structure of proteins, carbohydrates, and fats due to the presence of microbial enzymes and the presence of other compounds, such as acids. Changes in composition determine all of the other properties of legumes. It is clear that lactic acid fermentation is a viable method of reducing the levels of antinutritional compounds and improving the protein digestibility of legumes. Furthermore, lactic acid fermentation reduces the allergenicity of legumes. The beneficial effects of fermented legumes on health due to changes in composition or due to the presence of probiotics can be used to convert fermented legumes into functional foods.
From the technological point of view, fermentation modifies protein solubility; charge; hydrophobicity; water- and oil-holding capacities; and emulsifying, foaming, gelling, and bread-making properties. However, the extent of these changes depends on the kind of legume, the fermenting microorganism, and the conditions of the process. Studies of lactic acid fermentation have been mainly applied to soybeans, probably due to the large variety of traditional soy-fermented products existing worldwide. Peas and lupine have also been considered, but to a lesser extent. The effect of lactic acid fermentation has been studied on grains, flours, and legume ingredients such as protein concentrates and isolates. The characteristics of different substrates affect the results obtained.
In future works, it is important to carefully select the fermenting substrate, the fermenting microorganisms, and the fermenting conditions. The importance of the substrate’s composition in regard to the fermentation result requires the careful selection of the kind of legume and the type of ingredient used (flour, concentrate, and isolate). Even the kind of cultivar should be considered. The presence/absence of certain compounds should be studied to determine the exact role of these compounds during fermentation. The addition of other ingredients, such as additional sugar, should also be analyzed since their presence can lead to changes in bacterial metabolism. Considering the microorganisms used in fermentation, several past and recent studies on legume substrates have been conducted with a great variety of microorganisms, not only lactic acid bacteria but also other bacteria, molds, and yeasts. It is necessary to select the adapted reference strains or to screen the natural strains (naturally present on the matrices) regarding their enzymatic or metabolic activities (e.g., protease, tannase, phytase, and galactosidase activity). Their enzymatic action could favor mineral bioavailability, could improve the digestibility of legumes, or may decrease antinutritional factors. However, bacteria could also consume certain bioactive compounds that are considered health-promoting compounds (such as phenolic compounds), which could be considered a disadvantage. The careful selection of bacterial strains is required in order to attain the desired effect in the final product. LAB strains can also be used in co-cultures, offering synergistic effects during the fermentation of legume ingredients. A greater understanding is required to control the interactions between microorganisms in fermented plant substrates. Successive cultures with different microorganisms, combining solid- and liquid-state fermentation processes, are also expected to expand the applications and enhance the final quality of legume proteins. Other fermentation conditions (temperature, time, the presence of O2, and added carbon sources) require optimization and clearly depend on the fermenting microorganisms and the substrate used, as well as on the desired characteristics of the final product. Finally, additional uses of legume fermentation should be explored, such as in bread-making (sourdough elaboration) and dairy products (cheese and fermented milks). The implementation of fermentation during protein extraction should also be considered. Thus, the diversity of LAB strains and process conditions has created an impressive number of different nutritional, functional, technological, and sensory characteristics of fermented legumes and legume-based ingredients. The conscientious selection of legume substrates, microorganisms (alone or in consortia), and fermentation conditions will allow researchers to obtain tailored solutions for the development of new legume-based foods. Studies with a global approach are required in order to predict fermentation behavior in its totality.
Fermenting legume proteins and legume-based ingredients is a promising area in food technology relating to the development of novel foods to fulfill the needs of consumers today and in the future. In response to current sociological and environmental challenges worldwide, fermentation allows the consumption of legume proteins and the development of novel, healthy, and flavorful foods. The current topic requires a multidisciplinary approach that demands knowledge not only in relation to microbiology and biotechnology but also to protein physical chemistry, extraction technology, and the chemistry of secondary metabolites in legumes.
Author Contributions
M.E.: writing—original draft, writing—review and editing, conceptualization, and visualization; B.O.: supervision, writing—original draft, writing—review and editing, conceptualization, and visualization; R.S.: supervision, writing—review and editing, conceptualization, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the French Agence Nationale de la Recherche (ANR PEAVALUE project, grant ANR-19-CE21-0008-03) and by the European Regional Development Fund (FEDER-FSE Bourgogne 2014/2020, grant N_ BG0025913).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steinkraus, K.H. Handbook of Indigenous Fermented Foods, Revised and Expanded, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Steinkraus, K.H. Fermentations in world food processing. Compr. Rev. Food Sci. Food Saf. 2002, 1, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Klupsaite, D.; Juodeikiene, G.; Zadeike, D.; Bartkiene, E.; Maknickiene, Z.; Liutkute, G. The influence of lactic acid fermentation on functional properties of narrow-leaved lupine protein as functional additive for higher value wheat bread. LWT 2017, 75, 180–186. [Google Scholar] [CrossRef]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Wang, M.; Zhang, Y.; Chen, X.; Li, L.; Liu, Y.; Dong, M. Optimization of soy solid-state fermentation with selected lactic acid bacteria and the effect on the anti-nutritional components. J. Food Process. Preserv. 2017, 41, e13290. [Google Scholar] [CrossRef]
- Bartkiene, E.; Krungleviciute, V.; Juodeikiene, G.; Vidmantiene, D.; Maknickiene, Z. Solid state fermentation with lactic acid bacteria to improve the nutritional quality of lupin and soya bean. J. Sci. Food Agric. 2015, 95, 1336–1342. [Google Scholar] [CrossRef]
- Ayivi, R.; Gyawali, R.; Krastanov, A.; Aljaloud, S.; Worku, M.; Tahergorabi, R.; Da Silva, R.; Ibrahim, S. Lactic acid bacteria: Food safety and human health applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; De Carvalho Neto, D.P.; Junqueira, A.C.D.O.; Karp, S.G.; Letti, L.A.; Magalhães Júnior, A.I.; Soccol, C.R. A review of selection criteria for starter culture development in the food fermentation industry. Food Rev. Int. 2020, 36, 135–167. [Google Scholar] [CrossRef]
- Harlé, O.; Falentin, H.; Niay, J.; Valence, F.; Courselaud, C.; Chuat, V.; Maillard, M.-B.; Guédon, É.; Deutsch, S.-M.; Thierry, A. Diversity of the metabolic profiles of a broad range of lactic acid bacteria in soy juice fermentation. Food Microbiol. 2020, 89, 103410. [Google Scholar] [CrossRef]
- Xing, Q.; Dekker, S.; Kyriakopoulou, K.; Boom, R.M.; Smid, E.J.; Schutyser, M.A. Enhanced nutritional value of chickpea protein concentrate by dry separation and solid state fermentation. Innov. Food Sci. Emerg. Technol. 2020, 59, 102269. [Google Scholar] [CrossRef]
- Reuben, R.; Roy, P.; Sarkar, S.; Alam, A.R.U.; Jahid, I. Characterization and evaluation of lactic acid bacteria from indigenous raw milk for potential probiotic properties. J. Dairy Sci. 2020, 103, 1223–1237. [Google Scholar] [CrossRef]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS microbiol. 2018, 4, 665. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Tsai, S.-P.; Bonsignore, P.; Moon, S.-H.; Frank, J.R. Technological and economic potential of poly (lactic acid) and lactic acid derivatives. FEMS Microbiol. Rev. 1995, 16, 221–231. [Google Scholar] [CrossRef]
- Trontel, A.; Batušić, A.; Gusić, I.; Slavica, A.; Šantek, B.; Novak, S. Production of D-and L-lactic acid by mono-and mixed cultures of Lactobacillus sp. Food Technol. Biotechnol. 2011, 49, 75–82. [Google Scholar]
- Müller, V. Bacterial fermentation. In eLS; John Wiley & Sons Ltd.: Chichester, UK, 2001. [Google Scholar]
- Gänzle, M.G.; Follador, R. Metabolism of oligosaccharides and starch in lactobacilli: A review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Ramsing, R.; Rahman, N.; Kraemer, K.; Bloem, M.W. Legumes as a sustainable source of protein in human diets. Global Food Security 2021, 28, 100520. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Crévieu, I.; Berot, S.; Guéguen, J. Large scale procedure for fractionation of albumins and globulins from pea seeds. Food/Nahrung 1996, 40, 237–244. [Google Scholar] [CrossRef]
- Boye, J.; Aksay, S.; Roufik, S.; Ribéreau, S.; Mondor, M.; Farnworth, E.; Rajamohamed, S. Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Res. Int. 2010, 43, 537–546. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Díaz-Batalla, L.; Widholm, J.M.; Fahey, G.C.; Castaño-Tostado, E.; Paredes-López, O. Chemical components with health implications in wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.). J. Agric. Food Chem. 2006, 54, 2045–2052. [Google Scholar] [CrossRef]
- Garrido-Galand, S.; Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. The potential of fermentation on nutritional and technological improvement of cereal and legume flours: A review. Food Res. Int. 2021, 145, 110398. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Liu, X.; Kokare, C. Microbial Enzymes of Use in Industry. In Biotechnology of Microbial Enzymes; Elsevier: Amsterdam, The Netherlands, 2017; pp. 267–298. [Google Scholar]
- Limón, R.I.; Peñas, E.; Torino, M.I.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J. Fermentation enhances the content of bioactive compounds in kidney bean extracts. Food Chem. 2015, 172, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Orozco, R.; Frias, J.; Muñoz, R.; Zielinski, H.; Piskula, M.K.; Kozlowska, H.; Vidal-Valverde, C. Fermentation as a bio-process to obtain functional soybean flours. J. Agric. Food Chem. 2007, 55, 8972–8979. [Google Scholar] [CrossRef]
- Torino, M.I.; Limón, R.I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef]
- Skrzypczak, K.; Jabłońska-Ryś, E.; Gustaw, K.; Sławińska, A.; Waśko, A.; Radzki, W.; Michalak-Majewska, M.; Gustaw, W. Reinforcement of the Antioxidative Properties of Chickpea Beverages Through Fermentation Carried Out by Probiotic Strain Lactobacillus plantarum 299v. J. Pure. Appl. Microbiol. 2019, 13, 01–12. [Google Scholar] [CrossRef]
- Adeyemo, S.; Onilude, A. Enzymatic reduction of anti-nutritional factors in fermenting soybeans by Lactobacillus plantarum isolates from fermenting cereals. Niger. Food J. 2013, 31, 84–90. [Google Scholar] [CrossRef]
- Sáez, G.D.; Sabater, C.; Fara, A.; Zárate, G. Fermentation of chickpea flour with selected lactic acid bacteria for improving its nutritional and functional properties. J. Appl. Microbiol. 2021. [Google Scholar] [CrossRef]
- Niyibituronasa, M.; Onyango, A.; Gaidashova, S.; Imathiu, S.; De Boevre, M.; Leenknecht, D.; Neyrinck, E.; De Saeger, S.; Vermeir, P.; Raes, K. The growth of different probiotic microorganisms in soymilk from different soybean varieties and their effects on antioxidant activity and oligosaccharide content. J. Food Res. 2019, 8, 41–51. [Google Scholar] [CrossRef]
- Rui, X.; Huang, J.; Xing, G.; Zhang, Q.; Li, W.; Dong, M. Changes in soy protein immunoglobulin E reactivity, protein degradation, and conformation through fermentation with Lactobacillus plantarum strains. LWT 2019, 99, 156–165. [Google Scholar] [CrossRef]
- Aguirre, L.; Garro, M.S.; De Giori, G.S. Enzymatic hydrolysis of soybean protein using lactic acid bacteria. Food Chem. 2008, 111, 976–982. [Google Scholar] [CrossRef]
- Coda, R.; Melama, L.; Rizzello, C.G.; Curiel, J.A.; Sibakov, J.; Holopainen, U.; Pulkkinen, M.; Sozer, N. Effect of air classification and fermentation by Lactobacillus plantarum VTT E-133328 on faba bean (Vicia faba L.) flour nutritional properties. Int. J. Food Microbiol. 2015, 193, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Wang, C.; Montemurro, M.; De Angelis, M.; Katina, K.; Rizzello, C.G.; Coda, R. Exploring the Microbiota of Faba Bean: Functional Characterization of Lactic Acid Bacteria. Front. Microbiol. 2017, 8, 2461. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; De Mastro, G.; De Cillis, F.; Gobbetti, M.; Rizzello, C.G. Lactic acid bacteria fermentation to exploit the nutritional potential of Mediterranean faba bean local biotypes. Food Res. Int. 2019, 125, 108571. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Re, M.; Karsma, A.; Laitila, A.; Nordlund, E. Phytic Acid Reduction by Bioprocessing as a Tool To Improve the In Vitro Digestibility of Faba Bean Protein. J. Agric. Food Chem. 2018, 66, 10394–10399. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Verni, M.; Koivula, H.; Montemurro, M.; Seppa, L.; Kemell, M.; Katina, K.; Coda, R.; Gobbetti, M. Influence of fermented faba bean flour on the nutritional, technological and sensory quality of fortified pasta. Food Funct. 2017, 8, 860–871. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Losito, I.; Facchini, L.; Katina, K.; Palmisano, F.; Gobbetti, M.; Coda, R. Degradation of vicine, convicine and their aglycones during fermentation of faba bean flour. Sci. Rep. 2016, 6, 32452. [Google Scholar] [CrossRef]
- Pulkkinen, M.; Coda, R.; Lampi, A.-M.; Varis, J.; Katina, K.; Piironen, V. Possibilities of reducing amounts of vicine and convicine in faba bean suspensions and sourdoughs. Eur. Food Res. Technol. 2019, 245, 1507–1518. [Google Scholar] [CrossRef]
- Starzyńska-Janiszewska, A.; Stodolak, B. Effect of inoculated lactic acid fermentation on antinutritional and antiradical properties of grass pea (Lathyrus sativus ‘Krab’) flour. Pol. J. Food Nutr. Sci. 2011, 61, 245–249. [Google Scholar] [CrossRef]
- Cabuk, B.; Nosworthy, M.G.; Stone, A.K.; Korber, D.R.; Tanaka, T.; House, J.D.; Nickerson, M.T. Effect of Fermentation on the Protein Digestibility and Levels of Non-Nutritive Compounds of Pea Protein Concentrate. Food Technol. Biotechnol. 2018, 56, 257–264. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Hu, X. Nutritional quality and techno-functional changes in raw, germinated and fermented yellow field pea (Pisum sativum L.) upon pasteurization. LWT 2018, 92, 147–154. [Google Scholar] [CrossRef]
- Stanisavljevic, N.; Vukotic, G.; Pastor, F.; Suznjevic, D.; Jovanovic, Z.; Strahinic, I.; Fira, D.; Radovic, S. Antioxidant activity of pea protein hydrolysates produced by batch fermentation with lactic acid bacteria. Arch. Biol. Sci. 2015, 67, 1033–1042. [Google Scholar] [CrossRef]
- Czarnecka, M.; Czarnecki, Z.; Nowak, J.; Roszyk, H. Effect of lactic fermentation and extrusion of bean and pea seeds on nutritional and functional properties. Food Nahr. 1998, 42, 7–11. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Van Camp, J.; Decroos, K.; Van Wijmelbeke, L.; Verstraete, W. The impact of fermentation and in vitro digestion on the formation of angiotensin-I-converting enzyme inhibitory activity from pea and whey protein. J. Dairy Sci. 2003, 86, 429–438. [Google Scholar] [CrossRef]
- De Pasquale, I.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Nutritional and functional effects of the lactic acid bacteria fermentation on gelatinized legume flours. Int. J. Food Microbiol. 2020, 316, 108426. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, M.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Investigation of the nutritional, functional and technological effects of the sourdough fermentation of sprouted flours. Int. J. Food Microbiol. 2019, 302, 47–58. [Google Scholar] [CrossRef]
- Fritsch, C.; Vogel, R.F.; Toelstede, S. Fermentation performance of lactic acid bacteria in different lupin substrates—Influence and degradation ability of antinutritives and secondary plant metabolites. J. Appl. Microbiol. 2015, 119, 1075–1088. [Google Scholar] [CrossRef]
- García-Cano, I.; Rocha-Mendoza, D.; Ortega-Anaya, J.; Wang, K.; Kosmerl, E.; Jiménez-Flores, R. Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl. Microbiol. Biotechnol. 2019, 103, 5243–5257. [Google Scholar] [CrossRef]
- Ren, Y.; Li, L. Effects of extracellular proteases and its inhibitors on the gel characteristics of soy protein induced by lactic acid bacteria. Int. J. Food Sci. Tech. 2022, 57, 1587–1597. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Schweiggert-Weisz, U.; Eisner, P. Immunoreactivity, sensory and physicochemical properties of fermented soy protein isolate. Food Chem. 2016, 205, 229–238. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Hu, X. In vitro digestibility, protein composition and techno-functional properties of Saskatchewan grown yellow field peas (Pisum sativum L.) as affected by processing. Food Res. Int. 2017, 92, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Sozer, N.; Melama, L.; Silbir, S.; Rizzello, C.G.; Flander, L.; Poutanen, K. Lactic acid fermentation as a pre-treatment process for faba bean flour and its effect on textural, structural and nutritional properties of protein-enriched gluten-free faba bean breads. Foods 2019, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of Bioactive Peptides by Lactobacillus Species: From Gene to Application. Front. Microbiol. 2018, 9, 2354. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Zhang, Q.; Huang, J.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Does lactic fermentation influence soy yogurt protein digestibility: A comparative study between soymilk and soy yogurt at different pH. J. Sci. Food Agric. 2019, 99, 861–867. [Google Scholar] [CrossRef]
- Emkani, M.; Oliete, B.; Saurel, R. Pea Protein Extraction Assisted by Lactic Fermentation: Impact on Protein Profile and Thermal Properties. Foods 2021, 10, 549. [Google Scholar] [CrossRef]
- Lampart-Szczapa, E.; Konieczny, P.; Nogala-Kałucka, M.; Walczak, S.; Kossowska, I.; Malinowska, M. Some functional properties of lupin proteins modified by lactic fermentation and extrusion. Food Chem. 2006, 96, 290–296. [Google Scholar] [CrossRef]
- Schlegel, K.; Leidigkeit, A.; Eisner, P.; Schweiggert-Weisz, U. Technofunctional and Sensory Properties of Fermented Lupin Protein Isolates. Foods 2019, 8, 678. [Google Scholar] [CrossRef]
- Wu, H.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Mung bean (Vigna radiata) as probiotic food through fermentation with Lactobacillus plantarum B1-6. LWT 2015, 63, 445–451. [Google Scholar] [CrossRef]
- Xu, Y.; Coda, R.; Holopainen-Mantila, U.; Laitila, A.; Katina, K.; Tenkanen, M. Impact of in situ produced exopolysaccharides on rheology and texture of fava bean protein concentrate. Food Res. Int. 2019, 115, 191–199. [Google Scholar] [CrossRef]
- Chandra-Hioe, M.V.; Wong, C.H.; Arcot, J. The potential use of fermented chickpea and faba bean flour as food ingredients. Plant Foods Hum. Nutr. 2016, 71, 90–95. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Starkute, V.; Zadeike, D.; Juodeikiene, G. The Nutritional and Safety Challenges Associated with Lupin Lacto-Fermentation. Front Plant Sci. 2016, 7, 951. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Caspar, F.; Malcolmson, L.J.; Bellido, A.-S. Phenolics and antioxidant activity of lentil and pea hulls. Int. Food Res. J. 2011, 44, 436–441. [Google Scholar] [CrossRef]
- Gupta, Y. Anti-nutritional and toxic factors in food legumes: A review. Plant Foods Hum. Nutr. 1987, 37, 201–228. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Luc, G.; Leprelle, C.; Drover, J.C.; Harrison, J.E.; Olson, M. Phenolics, phytic acid, and phytase in Canadian-grown low-tannin faba bean (Vicia faba L.) genotypes. J. Agric. Food Chem. 2011, 59, 3763–3771. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.S.; McNeill, V.; Gänzle, M.G. Levansucrase and sucrose phoshorylase contribute to raffinose, stachyose, and verbascose metabolism by lactobacilli. Food Microbiol. 2012, 31, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef]
- Egounlety, M.; Aworh, O. Effect of soaking, dehulling, cooking and fermentation with Rhizopus oligosporus on the oligosaccharides, trypsin inhibitor, phytic acid and tannins of soybean (Glycine max Merr.), cowpea (Vigna unguiculata L. Walp) and groundbean (Macrotyloma geocarpa Harms). J. Food Eng. 2003, 56, 249–254. [Google Scholar] [CrossRef]
- Coda, R.; Varis, J.; Verni, M.; Rizzello, C.G.; Katina, K. Improvement of the protein quality of wheat bread through faba bean sourdough addition. LWT 2017, 82, 296–302. [Google Scholar] [CrossRef]
- Curiel, J.A.; Coda, R.; Centomani, I.; Summo, C.; Gobbetti, M.; Rizzello, C.G. Exploitation of the nutritional and functional characteristics of traditional Italian legumes: The potential of sourdough fermentation. Int. J. Food Microbiol. 2015, 196, 51–61. [Google Scholar] [CrossRef]
- Robinson, G.; Balk, J.; Domoney, C. Improving pulse crops as a source of protein, starch and micronutrients. Nutr. Bull. 2019, 44, 202–215. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Lai, L.-R.; Hsieh, S.-C.; Huang, H.-Y.; Chou, C.-C. Effect of lactic fermentation on the total phenolic, saponin and phytic acid contents as well as anti-colon cancer cell proliferation activity of soymilk. J. Biosci. Bioeng. 2013, 115, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Fernández, D.; Hernández, T.; Estrella, I.; Muñoz, R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J. Sci. Food Agric. 2005, 85, 297–304. [Google Scholar] [CrossRef]
- McCue, P.P.; Shetty, K. A model for the involvement of lignin degradation enzymes in phenolic antioxidant mobilization from whole soybean during solid-state bioprocessing by Lentinus edodes. Process Biochem. 2005, 40, 1143–1150. [Google Scholar] [CrossRef]
- Osawa, R.; Kuroiso, K.; Goto, S.; Shimizu, A. Isolation of tannin-degrading lactobacilli from humans and fermented foods. Appl. Environ. Microbiol. 2000, 66, 3093–3097. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Curiel, J.A.; Landete, J.M.; De las Rivas, B.; De Felipe, F.L.; Gómez-Cordovés, C.; Mancheño, J.M.; Muñoz, R. Food phenolics and lactic acid bacteria. Int. J. Food Microbiol. 2009, 132, 79–90. [Google Scholar] [CrossRef]
- Granito, M.; Frias, J.; Doblado, R.; Guerra, M.; Champ, M.; Vidal-Valverde, C. Nutritional improvement of beans (Phaseolus vulgaris) by natural fermentation. Eur. Food Res. Technol. 2002, 214, 226–231. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Singh, N. Pulse Chemistry and Technology; Royal Society of Chemistry: Cambridge, UK, 2012. [Google Scholar]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef]
- Sharma, N.; Angural, S.; Rana, M.; Puri, N.; Kondepudi, K.K.; Gupta, N. Phytase producing lactic acid bacteria: Cell factories for enhancing micronutrient bioavailability of phytate rich foods. Trends Food Sci. Technol. 2020, 96, 1–12. [Google Scholar] [CrossRef]
- Rekha, C.; Vijayalakshmi, G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. 2010, 109, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Sparvoli, F.; Bollini, R.; Lubrano, V.; Longo, V.; Pucci, L. The impact of sourdough fermentation on non-nutritive compounds and antioxidant activities of flours from different Phaseolus vulgaris L. genotypes. J. Food Sci. 2019, 84, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Van Vo, B.; Bui, D.P.; Nguyen, H.Q.; Fotedar, R. Optimized fermented lupin (Lupinus angustifolius) inclusion in juvenile barramundi (Lates calcarifer) diets. Aquaculture 2015, 444, 62–69. [Google Scholar] [CrossRef]
- Olukomaiya, O.O.; Adiamo, O.Q.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chem. 2020, 315, 126238. [Google Scholar] [CrossRef]
- CHU, G.M.; Ohmori, H.; Kawashima, T.; Funaba, M.; Matsui, T. Brewer’s yeast efficiently degrades phytate phosphorus in a corn-soybean meal diet during soaking treatment. Anim. Sci. J. 2009, 80, 433–437. [Google Scholar] [CrossRef]
- Kasprowicz-Potocka, M.; Borowczyk, P.; Zaworska, A.; Nowak, W.; Frankiewicz, A.; Gulewicz, P. The Effect of Dry Yeast Fermentation on Chemical Composition and Protein Characteristics of Blue Lupin Seeds. Food Technol. Biotechnol. 2016, 54, 360–366. [Google Scholar] [CrossRef]
- De Angelis, M.; Gallo, G.; Corbo, M.R.; McSweeney, P.L.; Faccia, M.; Giovine, M.; Gobbetti, M. Phytase activity in sourdough lactic acid bacteria: Purification and characterization of a phytase from Lactobacillus sanfranciscensis CB1. Int. J. Food Microbiol. 2003, 87, 259–270. [Google Scholar] [CrossRef]
- Zhao, H.-M.; Guo, X.-N.; Zhu, K.-X. Impact of solid state fermentation on nutritional, physical and flavor properties of wheat bran. Food Chem. 2017, 217, 28–36. [Google Scholar] [CrossRef]
- Vats, P.; Banerjee, U.C. Production studies and catalytic properties of phytases (myo-inositolhexakisphosphate phosphohydrolases): An overview. Enzyme Microb. Technol. 2004, 35, 3–14. [Google Scholar] [CrossRef]
- Khokhar, S.; Apenten, R.K.O. Antinutritional factors in food legumes and effects of processing. Role Food Agric. For. Fish. Hum. Nutr. 2003, 4, 82–116. [Google Scholar]
- Lin, C.-Y.; Tsai, C.-Y.; Lin, S.-H. Effects of soy components on blood and liver lipids in rats fed high-cholesterol diets. World J. Gastroenterol. WJG 2005, 11, 5549. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.A.; Berhow, M.A.; Singletary, K.W. Inhibition of Akt signaling and enhanced ERK1/2 activity are involved in induction of macroautophagy by triterpenoid B-group soyasaponins in colon cancer cells. Carcinogenesis 2006, 27, 298–306. [Google Scholar] [CrossRef]
- Ishii, Y.; Tanizawa, H. Effects of soyasaponins on lipid peroxidation through the secretion of thyroid hormones. Biol. Pharm. Bull 2006, 29, 1759–1763. [Google Scholar] [CrossRef] [PubMed]
- Hubert, J.; Berger, M.; Nepveu, F.; Paul, F.; Daydé, J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008, 109, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Gacek, M. Soy and legume seeds as sources of isoflavones: Selected individual determinants of their consumption in a group of perimenopausal women. Menopausal Rev. 2014, 13, 27. [Google Scholar] [CrossRef]
- Dowling, S.; Regan, F.; Hughes, H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010, 104, 1091–1098. [Google Scholar] [CrossRef]
- Csaba, G. Effect of endocrine disruptor phytoestrogens on the immune system: Present and future. Acta Microbiol. Immunol. Hung. 2018, 65, 1–14. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Favero, B.T.; Morzelle, M.C.; Franchin, M.; Alvarez-Parrilla, E.; de la Rosa, L.A.; Geraldi, M.V.; Maróstica Júnior, M.R.; Shahidi, F.; Schwember, A.R. Is chickpea a potential substitute for soybean? Phenolic bioactives and potential health benefits. Int. J. Mol. Sci. 2019, 20, 2644. [Google Scholar] [CrossRef]
- Wang, L.-J.; Li, D.; Zou, L.; Dong Chen, X.; Cheng, Y.-Q.; Yamaki, K.; Li, L.-T. Antioxidative activity of douchi (a Chinese traditional salt-fermented soybean food) extracts during its processing. Int. J. Food Prop. 2007, 10, 385–396. [Google Scholar] [CrossRef][Green Version]
- Sung, J.H.; Choi, S.J.; Lee, S.W.; Park, K.H.; Moon, T.W. Isoflavones found in Korean soybean paste as 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Biosci., Biotechnol., Biochem. 2004, 68, 1051–1058. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Kim, J.-D.; Zheng, J.; Row, K.H. Comparisons of isoflavones from Korean and Chinese soybean and processed products. Biochem. Eng. J. 2007, 36, 49–53. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, W.; Shan, Y.; Zhi-Qiang, E.; Wang, L.-Q. Study on the inhibition of fermented soybean to cancer cells. J. Northeast Agric. Univ. (Engl. Ed.) 2009, 16, 25–28. [Google Scholar]
- De Olmos, A.R.; Garro, M.S. Metabolic profile of Lactobacillus paracasei subsp. paracasei CRL 207 in solid state fermentation using commercial soybean meal. Food Biosci. 2020, 35, 100584. [Google Scholar] [CrossRef]
- Delgado, S.; Guadamuro, L.; Flórez, A.B.; Vázquez, L.; Mayo, B. Fermentation of commercial soy beverages with lactobacilli and bifidobacteria strains featuring high β-glucosidase activity. Innov. Food Sci. Emerg. Technol. 2019, 51, 148–155. [Google Scholar] [CrossRef]
- Budryn, G.; Klewicka, E.; Grzelczyk, J.; Gałązka-Czarnecka, I.; Mostowski, R. Lactic acid fermentation of legume seed sprouts as a method of increasing the content of isoflavones and reducing microbial contamination. Food Chem. 2019, 285, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Spano, G.; Russo, P.; Lonvaud-Funel, A.; Lucas, P.; Alexandre, H.; Grandvalet, C.; Coton, E.; Coton, M.; Barnavon, L.; Bach, B. Biogenic amines in fermented foods. Eur. J. Clin. Nutr. 2010, 64, S95–S100. [Google Scholar] [CrossRef]
- Suzzi, G.; Torriani, S. Biogenic amines in foods. Front. Microbiol. 2015, 6, 472. [Google Scholar] [CrossRef]
- Stojanović, Z.; Kos, J. Detection of metabolites of microbial origin in beverages with harmful effect on human health—Biogenic amines and mycotoxins. In Safety Issues in Beverage Production; Grumezescu, A., Holban, A.M., Eds.; Elsevier: Cambridge, MA, USA, 2020; pp. 39–77. [Google Scholar]
- Anal, A.K.; Perpetuini, G.; Petchkongkaew, A.; Tan, R.; Avallone, S.; Tofalo, R.; Van Nguyen, H.; Chu-Ky, S.; Ho, P.H.; Phan, T.T. Food safety risks in traditional fermented food from South-East Asia. Food Control 2020, 109, 106922. [Google Scholar] [CrossRef]
- Leuschner, R.G.; Heidel, M.; Hammes, W.P. Histamine and tyramine degradation by food fermenting microorganisms. Int. J. Food Microbiol. 1998, 39, 1–10. [Google Scholar] [CrossRef]
- Rahate, K.A.; Madhumita, M.; Prabhakar, P.K. Nutritional composition, anti-nutritional factors, pretreatments-cum-processing impact and food formulation potential of faba bean (Vicia faba L.): A comprehensive review. LWT 2021, 138, 110796. [Google Scholar] [CrossRef]
- Khazaei, H.; Purves, R.W.; Hughes, J.; Link, W.; O’Sullivan, D.M.; Schulman, A.H.; Björnsdotter, E.; Geu-Flores, F.; Nadzieja, M.; Andersen, S.U. Eliminating vicine and convicine, the main anti-nutritional factors restricting faba bean usage. Trends Food Sci. Technol. 2019, 91, 549–556. [Google Scholar] [CrossRef]
- Doblado, R.; Zielinski, H.; Piskula, M.; Kozlowska, H.; Muñoz, R.; Frías, J.; Vidal-Valverde, C. Effect of processing on the antioxidant vitamins and antioxidant capacity of Vigna sinensis Var. Carilla. J. Agric. Food Chem. 2005, 53, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Yu, R.-C.; Chou, C.-C. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006, 23, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Li, W.; Wang, T. Effect of solid-state fermentation with Bacillus subtilis lwo on the proteolysis and the antioxidative properties of chickpeas. Int. J. Food Microbiol. 2021, 338, 108988. [Google Scholar] [CrossRef]
- Frias, J.; Miranda, M.L.; Doblado, R.; Vidal-Valverde, C. Effect of germination and fermentation on the antioxidant vitamin content and antioxidant capacity of Lupinus albus L. var. Multolupa. Food Chem. 2005, 92, 211–220. [Google Scholar] [CrossRef]
- Bendich, A.; Machlin, L.; Scandurra, O.; Burton, G.; Wayner, D. The antioxidant role of vitamin C. Adv. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Villacrés, E.; Quelal, M.B.; Jácome, X.; Cueva, G.; Rosell, C.M. Effect of debittering and solid-state fermentation processes on the nutritional content of lupine (Lupinus mutabilis Sweet). Int. J. Food Sci. Tech. 2020, 55, 2589–2598. [Google Scholar] [CrossRef]
- Torres, A.; Frías, J.; Granito, M.; Vidal-Valverde, C. Fermented pigeon pea (Cajanus cajan) ingredients in pasta products. J. Agric. Food Chem. 2006, 54, 6685–6691. [Google Scholar] [CrossRef]
- Niki, E. Evidence for beneficial effects of vitamin E. KJM Korean J. Intern. Med. 2015, 30, 571. [Google Scholar] [CrossRef]
- Schowen, R.L. Principles of biochemistry 2nd ed. (Lehninger, Albert L.; Nelson, David L.; Cox, Michael M.). J. Chem. Educ. 1993, 70, A223. [Google Scholar] [CrossRef]
- James, S.; Nwabueze, T.U.; Ndife, J.; Onwuka, G.I.; Usman, M.A.A. Influence of fermentation and germination on some bioactive components of selected lesser legumes indigenous to Nigeria. J. Agric. Food Res. 2020, 2, 100086. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez, J. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Coda, R.; Chamlagain, B.; Edelmann, M.; Varmanen, P.; Piironen, V.; Katina, K. Fermentation of cereal, pseudo-cereal and legume materials with Propionibacterium freudenreichii and Levilactobacillus brevis for vitamin B12 fortification. LWT 2021, 137, 110431. [Google Scholar] [CrossRef]
- Watanabe, F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef]
- Anzani, C.; Boukid, F.; Drummond, L.; Mullen, A.M.; Álvarez, C. Optimising the use of proteins from rich meat co-products and non-meat alternatives: Nutritional, technological and allergenicity challenges. Food Res. Int. 2020, 137, 109575. [Google Scholar] [CrossRef]
- Moore, L.E.; Stewart, P.H.; deShazo, R.D. Food allergy: What we know now. Am. J. Med. Sci. 2017, 353, 353–366. [Google Scholar] [CrossRef]
- Licandro, H.; Ho, P.H.; Nguyen, T.K.C.; Petchkongkaew, A.; Van Nguyen, H.; Chu-Ky, S.; Nguyen, T.V.A.; Lorn, D.; Waché, Y. How fermentation by lactic acid bacteria can address safety issues in legumes food products? Food Control 2020, 110, 106957. [Google Scholar] [CrossRef]
- Untersmayr, E.; Jensen-Jarolim, E. The role of protein digestibility and antacids on food allergy outcomes. J. Allergy Clin. Immunol. 2008, 121, 1301–1308. [Google Scholar] [CrossRef]
- Yang, A.; Zuo, L.; Cheng, Y.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Degradation of major allergens and allergenicity reduction of soybean meal through solid-state fermentation with microorganisms. Food Funct. 2018, 9, 1899–1909. [Google Scholar] [CrossRef]
- Song, Y.-S.; Frías, J.; Martinez-Villaluenga, C.; Vidal-Valdeverde, C.; de Mejia, E.G. Immunoreactivity reduction of soybean meal by fermentation, effect on amino acid composition and antigenicity of commercial soy products. Food Chem. 2008, 108, 571–581. [Google Scholar] [CrossRef] [PubMed]
- AT, D.; Action, E.C. Scientific concepts of functional foods in Europe: Consensus document. Br. J. Nutr. 1999, 81, S1–S27. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Shin, D.-H.; Jung, S.-J.; Chae, S.-W. Functional properties of microorganisms in fermented foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Tamang, J. Health benefits of Natto. In Health Benefits of Fermented Foods; Tamang, J., Ed.; CRC Press: New York, NY, USA, 2015; pp. 433–453. [Google Scholar]
- Tamang, J.P. Naturally fermented ethnic soybean foods of India. J. Ethn. Foods 2015, 2, 8–17. [Google Scholar] [CrossRef]
- Jeong, H.-R.; Jo, Y.N.; Jeong, J.H.; Jin, D.E.; Song, B.G.; Choi, S.J.; Shin, D.-H.; Heo, H.J. Antiamnesic effects of ethyl acetate fraction from chestnut (Castanea crenata var. dulcis) inner skin on Aβ25–35-induced cognitive deficits in mice. J. Med. Food 2012, 15, 1051–1056. [Google Scholar] [CrossRef]
- Omizu, Y.; Tsukaoto, C.; Chettri, R.; Tamang, J.P. Determination of saponin contents in raw soybean and fermented soybean foods of India. J. Sci. Ind. Res. 2011, 70, 533–538. [Google Scholar]
- Sanjukta, S.; Rai, A.K. Production of bioactive peptides during soybean fermentation and their potential health benefits. Trends Food Sci. Technol. 2016, 50, 1–10. [Google Scholar] [CrossRef]
- Park, K.-B.; Oh, S.-H. Enhancement of γ-aminobutyric acid production in Chungkukjang by applying a Bacillus subtilis strain expressing glutamate decarboxylase from Lactobacillus brevis. Biotechnol. Lett. 2006, 28, 1459–1463. [Google Scholar] [CrossRef]
- Gänzle, M.G. Food fermentations for improved digestibility of plant foods—An essential ex situ digestion step in agricultural societies? Curr. Opin. Food Sci. 2020, 32, 124–132. [Google Scholar] [CrossRef]
- Arteaga, V.G.; Leffler, S.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Sensory profile, functional properties and molecular weight distribution of fermented pea protein isolate. CRFS Curr. Res. Food Sci. 2021, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Jakobsone, I.; Juodeikiene, G.; Vidmantiene, D.; Pugajeva, I.; Bartkevics, V. Effect of lactic acid fermentation of lupine wholemeal on acrylamide content and quality characteristics of wheat-lupine bread. Int. J. Food Sci. Nutr. 2013, 64, 890–896. [Google Scholar] [CrossRef]
- Parca, F.; Koca, Y.O.; Aydın, U. Nutritional and Antinutritional factors of some pulses seed and their effects on human health. Int. J. Second. 2018, 5, 331–342. [Google Scholar] [CrossRef]
- Cabuk, B.; Stone, A.K.; Korber, D.R.; Tanaka, T.; Nickerson, M.T. Effect of Lactobacillus plantarum Fermentation on the Surface and Functional Properties of Pea Protein-Enriched Flour. Food Technol. Biotechnol. 2018, 56, 411–420. [Google Scholar] [CrossRef]
- Bureau, S.; Ruiz, D.; Reich, M.; Gouble, B.; Bertrand, D.; Audergon, J.-M.; Renard, C.M. Rapid and non-destructive analysis of apricot fruit quality using FT-near-infrared spectroscopy. Food Chem. 2009, 113, 1323–1328. [Google Scholar] [CrossRef]
- Konieczny, P.; Uchman, W. Comparative characterization of surface hydrophobicity and other physico-chemical properties of selected protein prepartions. Electron. J. Pol. Agric. Univ. Series Food Sci. Technol. 2002, 5. [Google Scholar]
- Sharif, H.R.; Williams, P.A.; Sharif, M.K.; Abbas, S.; Majeed, H.; Masamba, K.G.; Safdar, W.; Zhong, F. Current progress in the utilization of native and modified legume proteins as emulsifiers and encapsulants—A review. Food Hydrocoll. 2018, 76, 2–16. [Google Scholar] [CrossRef]
- Shi, Y.; Singh, A.; Kitts, D.; Pratap-Singh, A. Lactic acid fermentation: A novel approach to eliminate unpleasant aroma in pea protein isolates. LWT 2021, 150, 111927. [Google Scholar] [CrossRef]
- Bartkiene, E.; Juodeikiene, G.; Vidmantiene, D.; Viskelis, P.; Urbonaviciene, D. Nutritional and quality aspects of wheat sourdough bread using L. luteus and L. angustifolius flours fermented by Pedioccocus acidilactici. Int. J. Food Sci. Tech. 2011, 46, 1724–1733. [Google Scholar] [CrossRef]
- Perri, G.; Coda, R.; Rizzello, C.G.; Celano, G.; Ampollini, M.; Gobbetti, M.; De Angelis, M.; Calasso, M. Sourdough fermentation of whole and sprouted lentil flours: In situ formation of dextran and effects on the nutritional, texture and sensory characteristics of white bread. Food Chem. 2021, 355, 129638. [Google Scholar] [CrossRef]
- Boeck, T.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Nutritional properties and health aspects of pulses and their use in plant-based yogurt alternatives. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3858–3880. [Google Scholar] [CrossRef] [PubMed]
- Yousseef, M.; Lafarge, C.; Valentin, D.; Lubbers, S.; Husson, F. Fermentation of cow milk and/or pea milk mixtures by different starter cultures: Physico-chemical and sensorial properties. LWT 2016, 69, 430–437. [Google Scholar] [CrossRef]
- Zare, F.; Boye, J.; Champagne, C.; Orsat, V.; Simpson, B. Probiotic milk supplementation with pea flour: Microbial and physical properties. Food Bioprocess Technol. 2013, 6, 1321–1331. [Google Scholar] [CrossRef]
- Klost, M.; Giménez-Ribes, G.; Drusch, S. Enzymatic hydrolysis of pea protein: Interactions and protein fractions involved in fermentation induced gels and their influence on rheological properties. Food Hydrocoll. 2020, 105, 105793. [Google Scholar] [CrossRef]
- Vasilean, I.; Aprodu, I.; Garnai, M.; Munteanu, V.; Patrașcu, L. Preliminary Investigations into the Use of Amylases and Lactic Acid Bacteria to Obtain Fermented Vegetable Products. Foods 2021, 10, 1530. [Google Scholar] [CrossRef]
- Cheng, Y.; Thompson, L.; Brittin, H. Sogurt, a yogurt-like soybean product: Development and properties. J. Food Sci. 1990, 55, 1178–1179. [Google Scholar] [CrossRef]
- Ogodo, A.; Ugbogu, O.; Onyeagba, R. Variations in the Functional Properties of Soybean Flour Fermented with Lactic Acid Bacteria (LAB)-Consortium. Appli. Microbiol. Open Access 2018, 4, 2. [Google Scholar] [CrossRef]
- Roland, W.S.; Pouvreau, L.; Curran, J.; van de Velde, F.; de Kok, P.M. Flavor aspects of pulse ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef]
- Trindler, C.; Kopf-Bolanz, K.A.; Denkel, C. Aroma of peas, its constituents and reduction strategies–effects from breeding to processing. Food Chem. 2021, 376, 131892. [Google Scholar] [CrossRef]
- Fischer, E.; Cayot, N.; Cachon, R. Potential of Microorganisms to Decrease the “Beany” Off-Flavor: A Review. J. Agric. Food Chem. 2022, 70, 4493–4508. [Google Scholar] [CrossRef]
- El Youssef, C.; Bonnarme, P.; Fraud, S.; Péron, A.-C.; Helinck, S.; Landaud, S. Sensory Improvement of a Pea Protein-Based Product Using Microbial Co-Cultures of Lactic Acid Bacteria and Yeasts. Foods 2020, 9, 349. [Google Scholar] [CrossRef] [PubMed]
- Ben-Harb, S.; Saint-Eve, A.; Panouillé, M.; Souchon, I.; Bonnarme, P.; Dugat-Bony, E.; Irlinger, F. Design of microbial consortia for the fermentation of pea-protein-enriched emulsions. Int. J. Food Microbiol. 2019, 293, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Grujović, M.Ž.; Mladenović, K.G.; Semedo-Lemsaddek, T.; Laranjo, M.; Stefanović, O.D.; Kocić-Tanackov, S.D. Advantages and disadvantages of non-starter lactic acid bacteria from traditional fermented foods: Potential use as starters or probiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1537–1567. [Google Scholar] [CrossRef] [PubMed]
- Cogan, T.M.; Hill, C. Cheese starter cultures. In Cheese: Chemistry, Physics and Microbiology; Fox, P.F., Ed.; Springer: Boston, MA, USA, 1993; pp. 193–255. [Google Scholar]
- Ben-Harb, S.; Irlinger, F.; Saint-Eve, A.; Panouillé, M.; Souchon, I.; Bonnarme, P. Versatility of microbial consortia and sensory properties induced by the composition of different milk and pea protein-based gels. LWT 2020, 118, 108720. [Google Scholar] [CrossRef]
- Murat, C.; Bard, M.-H.; Dhalleine, C.; Cayot, N. Characterisation of odour active compounds along extraction process from pea flour to pea protein extract. Int. Food Res. J. 2013, 53, 31–41. [Google Scholar] [CrossRef]
- Blagden, T.D.; Gilliland, S.E. Reduction of levels of volatile components associated with the “beany” flavor in soymilk by lactobacilli and streptococci. J. Food Sci. 2005, 70, M186–M189. [Google Scholar] [CrossRef]
- Pinthong, R.; Macrae, R.; Rothwell, J. The development of a soya-based yoghurt: I. Acid production by lactic acid bacteria. Int. J. Food Sci. Tech. 1980, 15, 647–652. [Google Scholar] [CrossRef]
- Zheng, Y.; Fei, Y.; Yang, Y.; Jin, Z.; Yu, B.; Li, L. A potential flavor culture: Lactobacillus harbinensis M1 improves the organoleptic quality of fermented soymilk by high production of 2,3-butanedione and acetoin. Food Microbiol. 2020, 91, 103540. [Google Scholar] [CrossRef]
- Schindler, S.; Zelena, K.; Krings, U.; Bez, J.; Eisner, P.; Berger, R.G. Improvement of the aroma of pea (Pisum sativum) protein extracts by lactic acid fermentation. Food Biotechnol. 2012, 26, 58–74. [Google Scholar] [CrossRef]
- Schindler, S.; Wittig, M.; Zelena, K.; Krings, U.; Bez, J.; Eisner, P.; Berger, R.G. Lactic fermentation to improve the aroma of protein extracts of sweet lupin (Lupinus angustifolius). Food Chem. 2011, 128, 330–337. [Google Scholar] [CrossRef]
- Ayad, E.H.; Verheul, A.; Engels, W.J.; Wouters, J.; Smit, G. Enhanced flavour formation by combination of selected lactococci from industrial and artisanal origin with focus on completion of a metabolic pathway. J. Appl. Microbiol. 2001, 90, 59–67. [Google Scholar] [CrossRef]
- Yvon, M.; Berthelot, S.; Gripon, J. Adding α-ketoglutarate to semi-hard cheese curd highly enhances the conversion of amino acids to aroma compounds. Int. Dairy J. 1998, 8, 889–898. [Google Scholar] [CrossRef]
- Smit, G.; Smit, B.A.; Engels, W.J. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Crafack, M.; Mikkelsen, M.B.; Saerens, S.; Knudsen, M.; Blennow, A.; Lowor, S.; Takrama, J.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H. Influencing cocoa flavour using Pichia kluyveri and Kluyveromyces marxianus in a defined mixed starter culture for cocoa fermentation. Int. J. Food Microbiol. 2013, 167, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Rui, X.; Zhang, Y.; Cai, F.; Chen, X.; Jiang, M. Production of tofu by lactic acid bacteria isolated from naturally fermented soy whey and evaluation of its quality. LWT 2017, 82, 227–234. [Google Scholar] [CrossRef]
- Laaksonen, O.; Kahala, M.; Marsol-Vall, A.; Blasco, L.; Järvenpää, E.; Rosenvald, S.; Virtanen, M.; Tarvainen, M.; Yang, B. Impact of lactic acid fermentation on sensory and chemical quality of dairy analogues prepared from lupine (Lupinus angustifolius L.) seeds. Food Chem. 2021, 346, 128852. [Google Scholar] [CrossRef]
- Schlegel, K.; Lidzba, N.; Ueberham, E.; Eisner, P.; Schweiggert-Weisz, U. Fermentation of Lupin Protein Hydrolysates—Effects on Their Functional Properties, Sensory Profile and the Allergenic Potential of the Major Lupin Allergen Lup an 1. Foods 2021, 10, 281. [Google Scholar] [CrossRef]
- Yi, C.; Li, Y.; Zhu, H.; Liu, Y.; Quan, K. Effect of Lactobacillus plantarum fermentation on the volatile flavors of mung beans. LWT 2021, 146, 111434. [Google Scholar] [CrossRef]
- Su, L.-W.; Cheng, Y.-H.; Hsiao, F.S.-H.; Han, J.-C.; Yu, Y.-H. Optimization of mixed solid-state fermentation of soybean meal by Lactobacillus species and Clostridium butyricum. Polish J. Microbiol. 2018, 67, 297. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Siedler, S.; Balti, R.; Neves, A.R. Bioprotective mechanisms of lactic acid bacteria against fungal spoilage of food. Curr. Opin. Biotechnol. 2019, 56, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Shimelis, E.A.; Rakshit, S.K. Influence of natural and controlled fermentations on α-galactosides, antinutrients and protein digestibility of beans (Phaseolus vulgaris L.). Int. J. Food Sci. Tech. 2008, 43, 658–665. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).