Evaluation of the Biocontrol Potential of a Commercial Yeast Starter against Fuel-Ethanol Fermentation Contaminants
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Inoculums Preparation
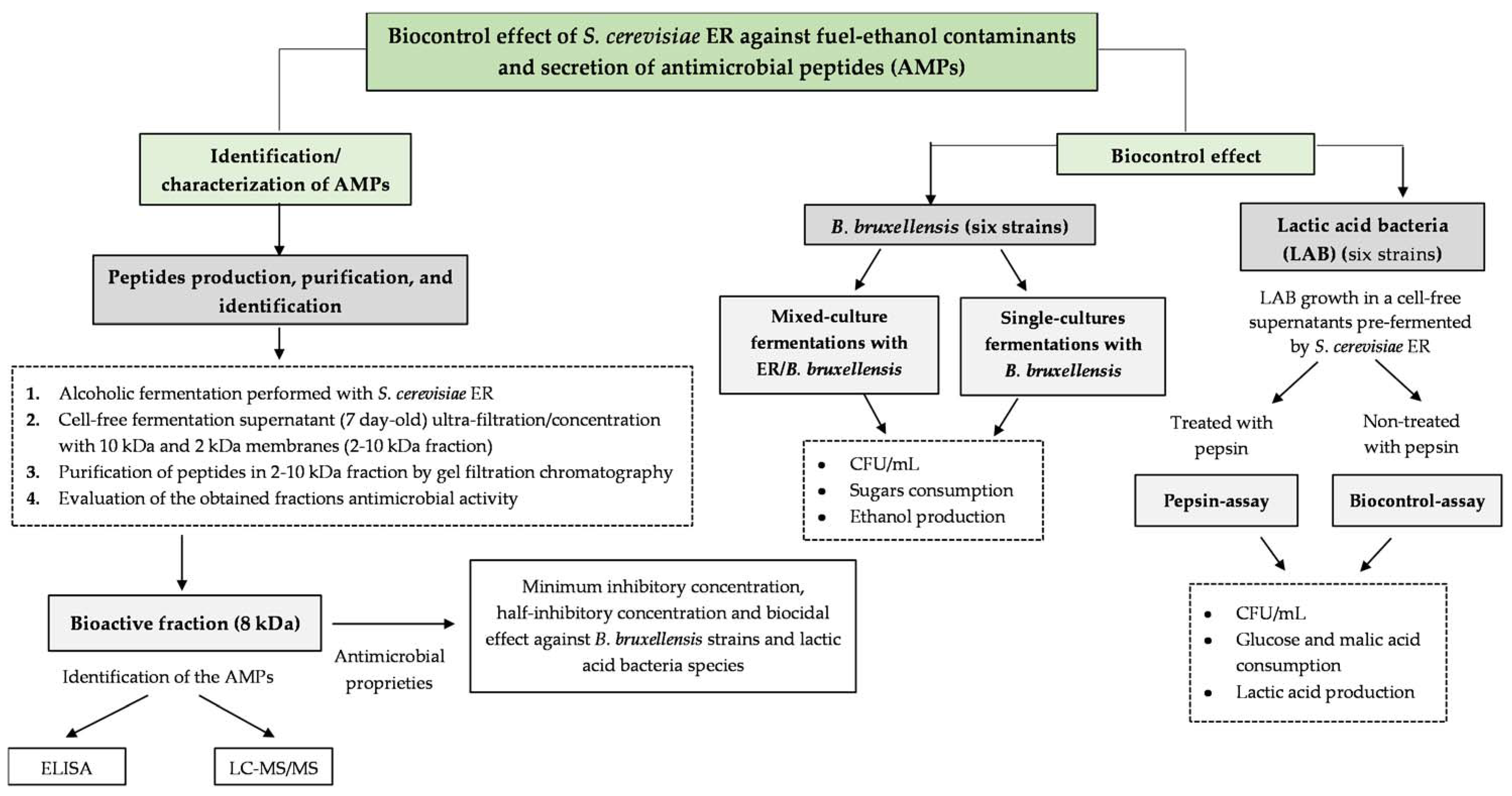
2.2. Alcoholic Fermentations Performed with Single- and Mixed-Cultures of S. cerevisiae ER and B. bruxellensis
2.3. Lactic Acid Bacteria (LAB) Growth in a Cell-Free Supernatant Pre-Fermented by S. cerevisiae ER
2.4. Determination of Sugars, Ethanol, Malic Acid, and Lactic Acid by High-Performance Liquid Chromatography (HPLC)
2.5. Statistical Analysis of Results
2.6. Identification of Peptides Secreted by S. cerevisiae ER during Alcoholic Fermentation
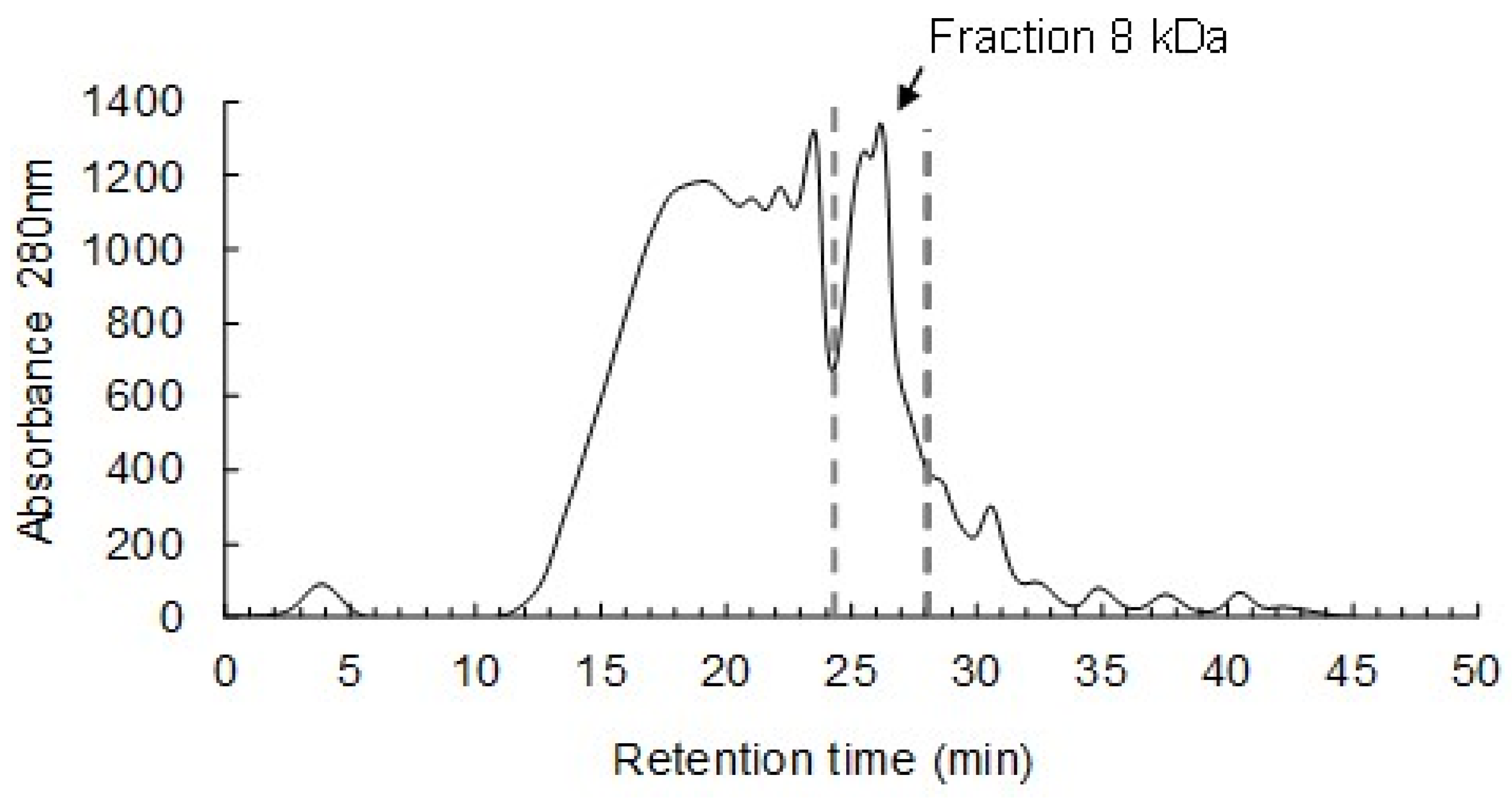
2.6.1. Purification of Peptides by Gel-Filtration Chromatography and Determination of Their Inhibitory Effect
2.6.2. AMPs Identification by Indirect Enzyme-Linked Immunosorbent Assay (ELISA)
2.6.3. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS) Analysis
2.7. Determination of Minimum Inhibitory Concentration (MIC), Half-Inhibitory Concentration (IC50), and Biocidal Effect of the AMPs Secreted by S. cerevisiae ER
3. Results and Discussion
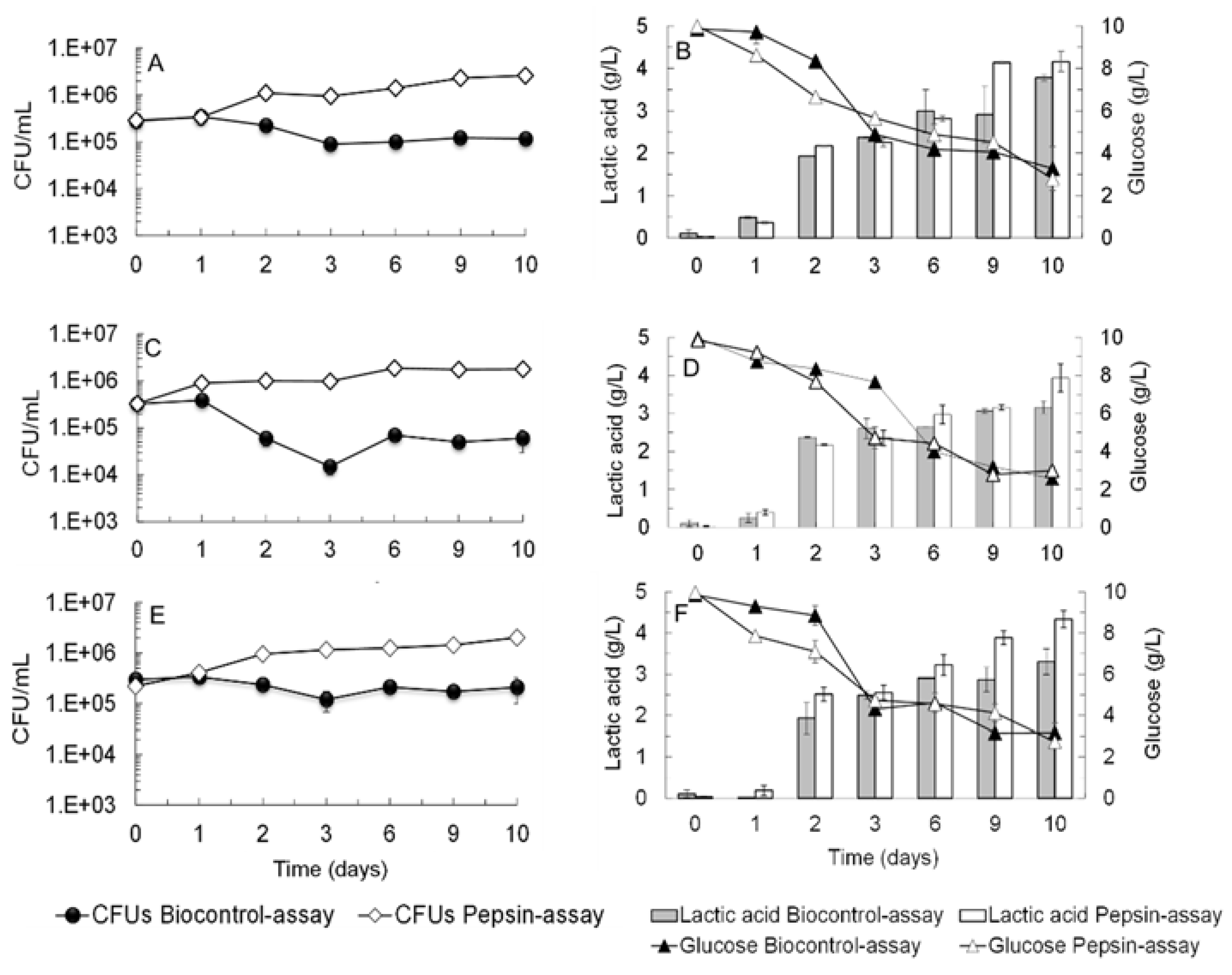
3.1. Biocontrol Potential of Saccharomyces Cerevisiae Ethanol Red (ER) against Brettanomyces bruxellensis Strains and LAB
3.2. Identification of the AMPs Secreted by S. cerevisiae ER by Indirect ELISA and Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS)
3.3. Minimum Inhibitory Concentration (MIC), Half-Inhibitory Concentration (IC50) and Biocidal Effect of the AMPs Secreted by S. cerevisiae ER against Fuel-Ethanol Microbial Contaminants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramos, M.D.N.; Milessi, T.S.; Candido, R.G.; Mendes, A.A.; Aguiar, A. Enzymatic catalysis as a tool in biofuels production in Brazil: Current status and perspectives. Energy Sustain. Develop. 2022, 68, 103–119. [Google Scholar] [CrossRef]
- Manochio, C.; Andrade, B.R.; Rodriguez, R.P.; Moraes, B.S. Ethanol from biomass: A comparative overview. Renew. Sustain. Energy Rev. 2017, 80, 743–755. [Google Scholar] [CrossRef]
- Renewable Fuels Association (RFA). Ethanol Industry Statistics, Washington, DC, USA. 2015. Available online: www.ethanolrfa.org (accessed on 9 May 2022).
- Basílio, A.C.M.; Araújo, P.R.L.; Morais, J.O.F.; da Silva Filho, E.A.; de Morais, M.A., Jr.; Simões, D.A. Detection and identification of wild yeast contaminants of the industrial fuel ethanol fermentation process. Curr. Microbiol. 2008, 5, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Brexó, R.P.; Sant’Ana, A.S. Microbial interactions during sugar cane must fermentation for bioethanol production: Does quorum sensing play a role? Crit. Rev. Biotechnol. 2018, 38, 231–244. [Google Scholar] [CrossRef]
- Beckner, M.; Ivey, M.; Phister, T. Microbial contamination of fuel ethanol fermentations. Lett. Appl. Microbiol. 2011, 53, 387–394. [Google Scholar] [CrossRef]
- Liberal, A.T.S.; Basílio, A.C.M.; Brasileiro, B.T.R.V.; da Silva Filho, E.A.; Simões, D.A.; de Morais, M.A., Jr. Identification of the yeast Dekkera bruxellensis as major contaminant in continuous fuel ethanol fermentation. J. Appl. Microbiol. 2007, 102, 538–547. [Google Scholar]
- Skinner, K.A.; Leathers, T.D. Bacterial contaminants of fuel ethanol production. J. Ind. Microbiol. Biotechnol. 2004, 31, 401–408. [Google Scholar] [CrossRef]
- Abbott, D.A.; Hynes, S.H.; Ingledew, W.M. Growth rates of Dekkera/Brettanomyces yeasts hinder their ability to compete with Saccharomyces cerevisiae in batch corn mash fermentations. Appl. Microbiol. Biotechnol. 2005, 66, 641–647. [Google Scholar] [CrossRef]
- Passoth, V.; Blomqvist, J.; Schnurer, J. Dekkera bruxellensis and Lactobacillus vini form a stable ethanol producing consortium in a commercial alcohol process. Appl. Environ. Microbiol. 2007, 73, 4354–4356. [Google Scholar] [CrossRef]
- Lucena, B.T.; dos Santos, B.M.; Moreira, J.L.; Moreira, A.P.; Nunes, A.C.; Azevedo, V.; Miyoshi, A.; Thompson, F.L.; de Morais, M.A., Jr. Diversity of lactic acid bacteria of the bioethanol process. BMC Microbiol. 2010, 10, 298. [Google Scholar] [CrossRef]
- Ngang, J.E.; Letourneau, F.; Wolniewicz, E.; Villa, P. Inhibition of beet molasses alcoholic fermentation by lactobacilli. Appl. Microbiol. Biotechnol. 2004, 33, 490–493. [Google Scholar]
- Neelakantam, V.; Narendranath, N.V.; Power, R. Relationship between pH and medium dissolved solids in terms of growth and metabolism of Lactobacilli and Saccharomyces cerevisiae during ethanol production. Appl. Microbiol. Biotechnol. 2005, 71, 2239–2243. [Google Scholar]
- Bayrock, D.P.; Ingledew, W.M. Inhibition of yeast by lactic acid bacteria in continuous culture: Nutrient depletion and/or acid toxicity? J. Ind. Microbiol. Biotechnol. 2004, 31, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.L.; Paulillo, S.C.; Godoy, A.; Cherubin, R.A.; Lorenzi, M.S.; Giometti, F.H.C.; Bernardino, C.D.; Amorim Neto, H.B.D.; Amorim, H.V.D. Ethanol production in Brazil: A bridge between science and industry. Braz. J. Microbiol. 2016, 47, 64–76. [Google Scholar] [CrossRef]
- Simpson, W.J.; Hammond, J.R.M. The response of brewing yeasts to acid washing. J. Inst. Brew. 1989, 95, 347–354. [Google Scholar] [CrossRef]
- Seo, S.O.; Park, S.K.; Jung, S.C.; Ryu, C.M.; Kim, J.S. Anti-Contamination Strategies for Yeast Fermentations. Microorganisms 2020, 8, 274. [Google Scholar] [CrossRef]
- Cunningham, S.; Stewart, G.G. Effects of high-gravity brewing and acid washing on brewers’ yeast. J. Am. Soc. Brew. Chem. 1998, 56, 12–18. [Google Scholar] [CrossRef]
- Tang, Y.; An, M.; Zhong, Y.; Shigeru, M.; Wu, X.; Kida, K. Continuous ethanol fermentation from non-sulfuric acid-washed molasses using traditional stirred tank reactors and the flocculating yeast strain KF-7. J. Biosci. Bioeng. 2010, 109, 41–46. [Google Scholar] [CrossRef]
- Broda, M.; Narendranath, W. Ammonia disinfection of corn grains intended for ethanol fermentation. Acta Sci. Pol. Technol. Aliment. 2009, 8, 33–38. [Google Scholar]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Urea hydrogen peroxide reduces the numbers of lactobacilli, nourishes yeast, and leaves no residues in the ethanol fermentation. Appl. Environ. Microbiol. 2000, 66, 4187–4192. [Google Scholar] [CrossRef][Green Version]
- McDonnell, G.; Russell, D. Antiseptics and desinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Messik, C.R.; Pendland, S.L.; Moshirfar, M.; Fiscella, R.G.; Losnedahl, K.J.; Schriever, C.A.; Schreckenberger, P.C. In-vitro activity of polyheamethylene biguanide (PHMB) against fungal isolates associated with infective keratitis. J. Antimicrob. Chemother. 1999, 44, 297–298. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; White, G.F.; Morby, A.P. The response of Escherichia coli to exposure to the biocide polyhexamethylene biguanide. Microbiology 2006, 152, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Elsztein, C.; Scavuzzi de Menezes, J.A.; Morais, M.A., Jr. Polyhexamethyl biguanide can eliminate contaminant yeasts from fuel-ethanol fermentation process. J. Ind. Microbiol. 2008, 35, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Aquarone, E. Penicillin and tetracycline as contamination control agents in alcoholic fermentation of sugarcane molasses. Appl. Environ. Microbiol. 1960, 8, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Day, W.H.; Serjak, W.C.; Stratton, J.R.; Stone, L. Contamination inhibition, antibiotics as contamination-control agents in grain alcohol fermentations. J. Agric. Food. Chem. 1954, 2, 252–258. [Google Scholar] [CrossRef]
- Hynes, S.H.; Kjarsgaard, D.M.; Thomas, K.C.; Ingledew, W.M. Use of virginiamycin to control the growth of lactic acid bacteria during alcohol fermentation. J. Ind. Microbiol. Biotechnol. 1997, 18, 284–291. [Google Scholar] [CrossRef]
- Kumar, A.; Pal, D. Antibiotic resistance and wastewater: Correlation, impact and critical human health challenges. J. Environ. Chem. Eng. 2018, 6, 52–58. [Google Scholar] [CrossRef]
- Gil, G.; del Mónaco, S.; Cerrutti, P.; Galvagno, M. Selective antimicrobial activity of chitosan on beer spoilage bacteria and brewing yeasts. Biotechnol. Lett. 2004, 26, 569–574. [Google Scholar] [CrossRef]
- Radler, F. Possible use of nisin in winemaking. I. Action of nisin against lactic acid bacteria and wine yeasts in solid and liquid media. Am. J. Enol. Vitic. 1990, 41, 1–6. [Google Scholar]
- Conte, A.; Sinigaglia, M.; Del Nobile, M.A. Use of lemon extract to inhibit the growth of malolactic bacteria. J. Food Prot. 2007, 70, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Aniche, G.N.; Uwakwe, G.U. Potential use of Garcinia kola as hop substitute in lager beer brewing. World J. Microbiol. Biotechnol. 1990, 6, 323–327. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, H.; Francisco, D.; Gori, K.; Arneborg, N.; Gírio, F. Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl. Microbiol. Biotechnol. 2010, 86, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Albergaria, H.; Branco, P.; Francisco, D.; Coutinho, R.; Monteiro, M.; Malfeito-Ferreira, M.; Arneborg, N.; Almeida, M.G.; Caldeira, J. Dominance of Saccharomyces cerevisiae in wine fermentations: Secretion of antimicrobial peptides and microbial interactions. In Proceedings of the 2nd International Conference on Microbial Diversity: Microbial Interactions in Complex Ecosystems, Turin, Italy, 23–25 October 2013. [Google Scholar]
- Branco, P.; Francisco, D.; Chambon, C.; Hébraud, M.; Arneborg, N.; Almeida, M.G.; Caldeira, J.; Albergaria, H. Identification of novel GAPDH-derived antimicrobial peptides secreted by Saccharomyces cerevisiae and involved in wine microbial interactions. Appl. Microbiol. Biotechnol. 2014, 98, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.; Francisco, D.; Monteiro, M.; Almeida, M.G.; Caldeira, J.; Arneborg, N.; Prista, C.; Albergaria, H. Antimicrobial properties and death-inducing mechanisms of saccharomycin, a biocide secreted by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, B.P.J.; Victor, P.A. Yeast for Fermentation. Patent WO/2011/035392, 31 March 2011. Available online: https://www.freepatentsonline.com/WO2011035392.html (accessed on 12 February 2021).
- Pérez-Nevado, F.; Albergaria, H.; Hogg, T.; Gírio, F. Cellular death of two non-Saccharomyces wine-related yeasts during mixed fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 2006, 108, 336–345. [Google Scholar]
- Fry, J.C. One-Way Analysis of Variance in Biological Data Analysis: A Practical Approach, 1st ed.; Oxford University Press: Oxford, UK, 1993; pp. 1–39. [Google Scholar]
- Branco, P.; Coutinho, R.; Malfeito-Ferreira, M.; Prista, C.; Albergaria, H. Wine Spoilage Control: Impact of Saccharomycin on Brettanomyces bruxellensis and Its Conjugated Effect with Sulfur Dioxide. Microorganisms 2021, 9, 2528. [Google Scholar] [CrossRef]
- Hnasko, R.; Lin, A.; McGarvey, J.A.; Stanker, L.H. A rapid method to improve protein detection by indirect ELISA. Biochem. Biophys. Res. 2011, 410, 726–731. [Google Scholar] [CrossRef]
- Branco, P.; Sabir, F.; Diniz, M.; Carvalho, L.; Albergaria, H.; Prista, C. Biocontrol of Brettanomyces/Dekkera bruxellensis in alcoholic fermentations using saccharomycin-overproducing Saccharomyces cerevisiae strains. Appl. Microbiol. Biotechnol. 2019, 103, 3073–3083. [Google Scholar] [CrossRef]
- Stanley, G.A.; Hobley, T.J.; Pamment, N.B. Effect of acetaldehyde on Saccharomyces cerevisiae and Zymomonas mobilis subjected to environmental shocks. Biotechnol. Bioeng. 1997, 53, 71–78. [Google Scholar] [CrossRef]
- Birch, R.M.; Walker, G.M. Influence of magnesium ions on heat shock and ethanol stress responses of Saccharomyces cerevisiae. Enzym. Microb. Technol. 2000, 26, 678–687. [Google Scholar] [CrossRef]
- Rozpędowska, E.; Hellborg, L.; Ishchuk, O.P.; Orhan, F.; Galafassi, S.; Merico, A.; Woolfit, M.; Compagno, C.; Piskur, J. Parallel evolution of the make-accumulate-consume strategy in Saccharomyces and Dekkera yeasts. Nat. Commun. 2011, 2, 302. [Google Scholar] [CrossRef] [PubMed]
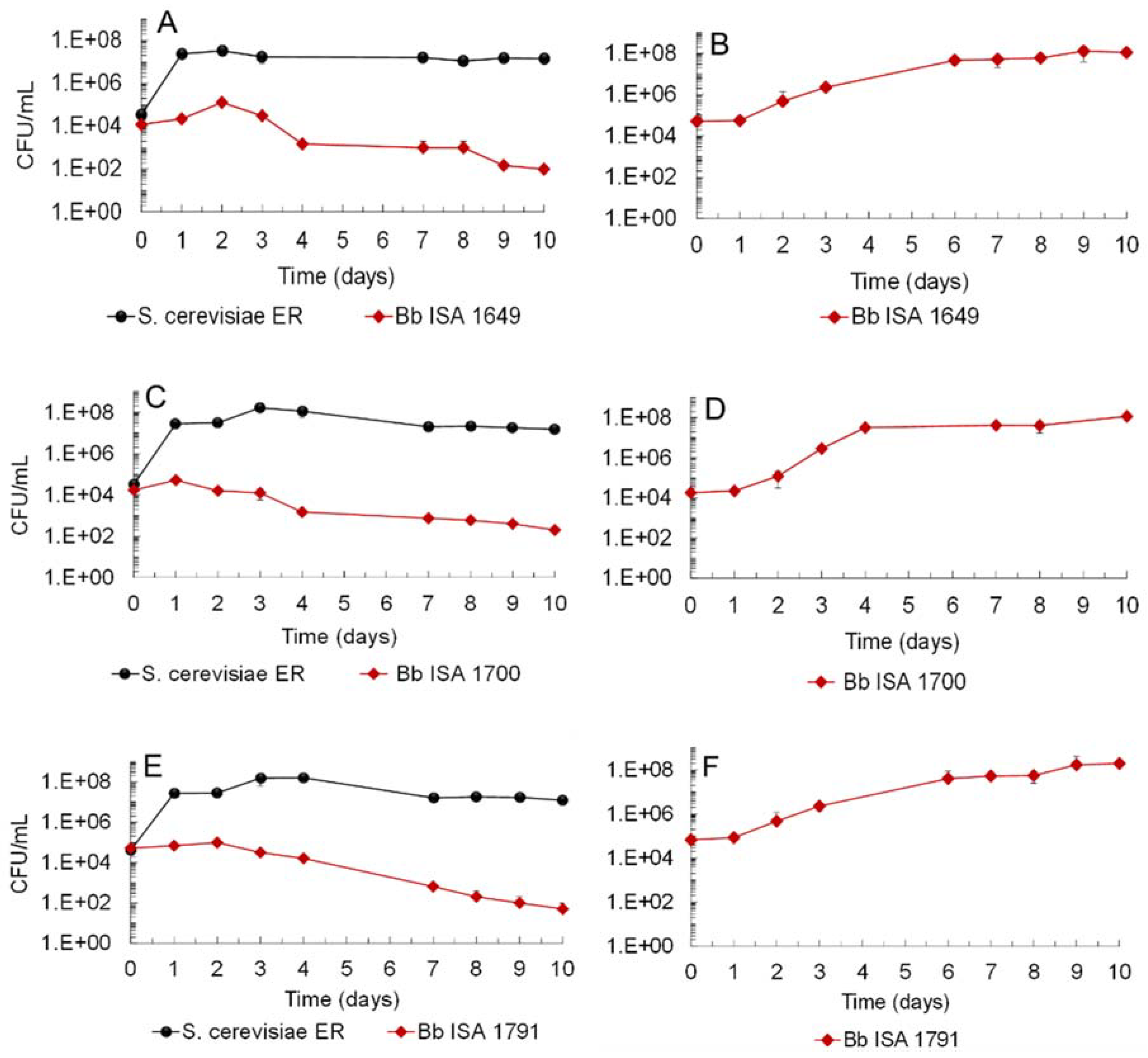
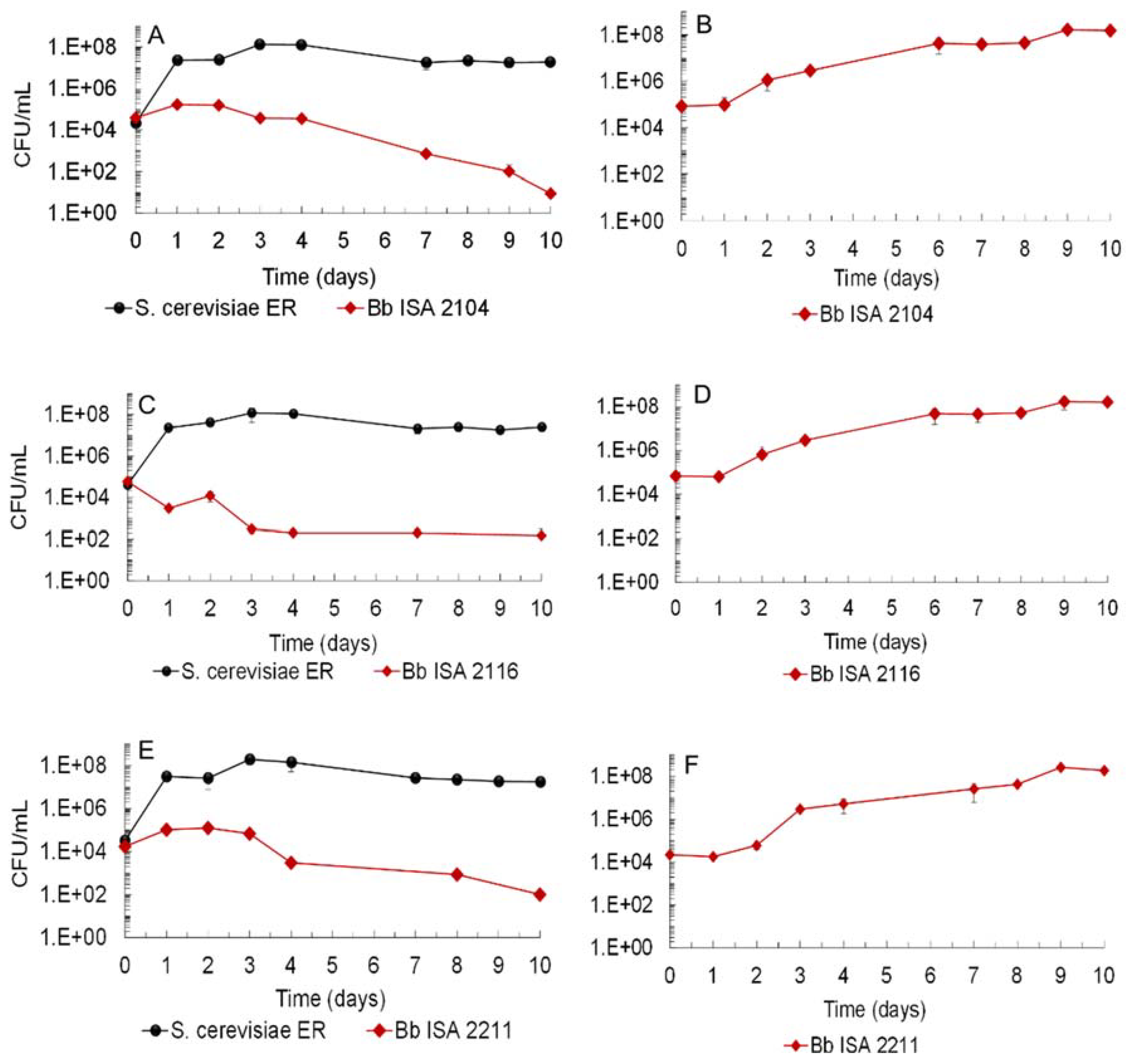


| Time (Days) | Sugars Concentration (g/L) in Single- and in Mixed-Culture Fermentations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single ISA 1649 | Mixed Sc/ISA 1649 | Single ISA 1700 | Mixed ER/ISA 1700 | Single ISA 1791 | Mixed ER/ISA 1791 | Single ISA 2104 | Mixed ER/ISA 2104 | Single ISA 2116 | Mixed ER/ISA 2116 | Single ISA 2211 | Mixed ER/ISA 2211 | |
| 0 | 219.40 ± 1.5 | 222.5 ± 0.3 | 228.9 ± 5.2 | 222.5 ± 0.3 | 218.2 ± 3.9 | 222.5 ± 0.3 | 235.3 ± 6.2 | 222.5 ± 0.3 | 225.9 ± 1.6 | 222.5 ± 0.3 | 243.3 ± 0.8 | 222.5 ± 0.3 |
| 1 | 216.5 ± 5.7 | 162.9 ± 6.6 | 222.2 ± 4.5 | 180.8 ± 8.8 | 212.1 ± 1.6 | 197.8 ± 5.9 | 221.3 ± 6.3 | 198.9 ± 0.2 | 215.0 ± 2.4 | 195.8 ± 4.5 | 221.8 ± 0.5 | 189.5 ± 2.8 |
| 2 | 209.5 ± 3.9 | 59.8 ± 1.6 | 218.6 ± 0.7 | 58.8 ± 2.4 | 209.5 ± 1.0 | 61.5 ± 4.4 | 220.2 ± 0.7 | 51.3 ± 0.0 | 210.8 ± 4.1 | 68.7 ± 6.6 | 221.5 ± 0.5 | 58.9 ± 0.7 |
| 3 | 208.9 ± 3.3 | 15.1 ± 2.2 | 216.6 ± 3.2 | 10.8 ± 1.1 | 210.0 ± 1.0 | 17.7 ± 1.2 | 218.8 ± 0.3 | 19.5 ± 4.2 | 199.4 ± 7.4 | 9.4 ± 0.3 | 221.0 ± 0.4 | 14.2 ± 0.6 |
| 4 | - | 5.4 ± 0.2 | 168.9 ± 5.8 | 8.2 ± 0.5 | - | 6.8 ± 0.5 | - | 8.0 ± 1.4 | - | 8.0 ± 0.0 | 195.0 ± 9.0 | 7.9 ± 1.4 |
| 6 | 163.0 ± 15.4 | - | - | - | 201.3 ± 6.9 | - | 210.6 ± 1.3 | - | 187.8 ± 7.1 | - | - | - |
| 7 | 144.3 ± 7.6 | 4.8 ± 0.4 | 146.9 ± 4.8 | 7.9 ± 0.0 | 157.0 ± 1.3 | 4.8 ± 0.3 | 203.5 ± 9.2 | 4.2 ± 0.2 | 148.9 ± 8.1 | 0.55 ± 0.1 | 157.0 ± 9.2 | 0.3 ± 0.0 |
| 8 | 123.3 ± 9.6 | - | 139.1 ± 2.9 | 139.3 ± 1.6 | - | 183.1 ± 0.4 | - | 139.3 ± 5.7 | - | 143.0 ± 10.9 | - | |
| 9 | - | - | - | 110.3 ± 4.0 | - | 177.2 ± 2.5 | - | 112.3 ± 9.6 | - | - | - | |
| 10 | 107.9 ± 6.7 | 4.5 ± 1.6 | 125.9 ± 3.9 | 2.0 ± 0.0 | 102.5 ± 4.8 | 0.6 ± 0.2 | 173.1 ± 1.9 | 0.6 ± 0.0 | 91.4 ± 1.7 | 0.27 ± 0.0 | 75.48 ± 4.4 | 0.2 ± 0.0 |
| Time (Days) | Ethanol Concentration (g/L) in Single- and in Mixed-Culture Fermentations | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single ISA 1649 | Mixed ER/ISA 1649 | Single ISA 1700 | Mixed ER/ISA 1700 | Single ISA 1791 | Mixed ER/ISA 1791 | Single ISA 2104 | Mixed ER/ISA 2104 | Single ISA 2116 | Mixed ER/ISA 2116 | Single ISA 2211 | Mixed ER/ISA 2211 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 0 | 16.7 ± 1.0 | 0.9 ± 0.1 | 20.9 ± 1.2 | 0 | 22.2 ± 3.2 | 0 | 21.8 ± 0.4 | 0 | 21.2 ± 1.8 | 0 | 17.8 ± 0.5 |
| 2 | 0 | 67.0 ± 11.5 | 2.9 ± 0.1 | 82.7 ± 16.4 | 0 | 69.4 ± 15.6 | 0 | 67.2 ± 0.0 | 0 | 75.6 ± 2.4 | 0 | 74.9 ± 0.6 |
| 3 | 0 | 80.9 ± 17.2 | 3.0 ± 0.2 | 87.5 ± 13.2 | 0 | 84.7 ± 14 | 0 | 73.9 ± 2.6 | 0 | 93.1 ± 11.4 | 1.2 ± 0.1 | 96.4 ± 9.9 |
| 4 | - | 93.0 ± 9.0 | 4.4 ± 0.1 | 99.8 ± 3.9 | - | 92.0 ± 7.4 | - | 85.0 ± 1.6 | - | 110.4 ± 0.0 | 2.9 ± 0.1 | 97.3 ± 8.1 |
| 6 | 13.7 ± 3.5 | - | - | - | 12.8 ± 2.3 | - | 6.2 ± 0.4 | - | 8.7 ± 2.1 | - | - | - |
| 7 | 22.6 ± 3.2 | 96.4 ± 8.8 | 12.1 ± 0.6 | 104.6 ± 5.8 | 22.6 ± 0.2 | 99.8 ± 1.2 | 9.0 ± 0.9 | 96.7 ± 2.2 | 14.0 ± 4.1 | 103.4 ± 2.0 | 12.6 ± 0.7 | 97.8 ± 2.0 |
| 8 | 29.6 ± 1.8 | 96.9 ± 8.4 | 17.0 ± 0.2 | 109.1 ± 10 | 31.2 ± 1.5 | 101.6 ± 3.0 | 8.8 ± 0.1 | 101.5 ± 1.6 | 33.5 ± 2.0 | 102.2 ± 2.4 | 32.2 ± 3.3 | 98.14 ± 3.2 |
| 9 | 46.6 ± 5.2 | 96.3 ± 7.0 | - | 109.6 ± 10 | 36.3 ± 1.2 | 100.7 ± 2.1 | 11.0 ± 0.5 | 100.6 ± 0.6 | 43.2 ± 4.7 | 102.1 ± 1.4 | - | 98.5 ± 2.3 |
| 10 | 49.9 ± 2.3 | 96.2 ± 5.0 | 22.8 ± 3.5 | 109.1 ± 10 | 44.7 ± 2.5 | 100.7 ± 2.0 | 14.9 ± 0.8 | 101.9 ± 1.6 | 47.7 ± 2.0 | 98.9 ± 0.6 | 40.5 ± 7.7 | 99.9 ± 4.6 |
| B. bruxellensis Strains | MIC (mg/mL) | IC50 (mg/mL) | Log of [CFU/mL] Reduction | |
|---|---|---|---|---|
| [Fraction 8 kDa] | ||||
| 1.50 mg/mL | 2.0 mg/mL | |||
| ISA 1649 | >2.0 | 0.45 | 0.00 | 0.56 |
| ISA 1700 | >2.0 | 0.70 | 0.00 | 0.03 |
| ISA 1791 | 2.0 | 0.28 | 0.00 | 0.06 |
| ISA 2104 | 2.0 | 0.30 | 0.00 | 0.18 |
| ISA 2116 | 1.50 | 0.30 | 1.00 | 1.50 |
| ISA 2211 | 1.50 | 0.40 | 1.20 | 1.81 |
| Lactic Acid Bacteria | MIC (mg/mL) | IC50 (mg/mL) | LOG of [CFU/mL] Reduction |
|---|---|---|---|
| [Fraction 8 kDa] | |||
| 2.0 mg/mL | |||
| L. brevis ISA 4385 | >2.0 | 1.71 | - |
| L. hilgardii ISA 4387 | 2.0 | 1.70 | 1.16 |
| L plantarum ISA 4395 | 2.0 | 1.50 | 1.06 |
| L. mesenteroides subsp. cremoris ISA 4383 | 2.0 | 1.17 | 0.67 |
| P. parvulus ISA 4401 | 2.0 | 1.20 | 1.90 |
| P. pentosaceus ISA 4379 | >2.0 | 1.58 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branco, P.; Diniz, M.; Albergaria, H. Evaluation of the Biocontrol Potential of a Commercial Yeast Starter against Fuel-Ethanol Fermentation Contaminants. Fermentation 2022, 8, 233. https://doi.org/10.3390/fermentation8050233
Branco P, Diniz M, Albergaria H. Evaluation of the Biocontrol Potential of a Commercial Yeast Starter against Fuel-Ethanol Fermentation Contaminants. Fermentation. 2022; 8(5):233. https://doi.org/10.3390/fermentation8050233
Chicago/Turabian StyleBranco, Patrícia, Mário Diniz, and Helena Albergaria. 2022. "Evaluation of the Biocontrol Potential of a Commercial Yeast Starter against Fuel-Ethanol Fermentation Contaminants" Fermentation 8, no. 5: 233. https://doi.org/10.3390/fermentation8050233
APA StyleBranco, P., Diniz, M., & Albergaria, H. (2022). Evaluation of the Biocontrol Potential of a Commercial Yeast Starter against Fuel-Ethanol Fermentation Contaminants. Fermentation, 8(5), 233. https://doi.org/10.3390/fermentation8050233








