Effect of Volatile Fatty Acids Accumulation on Biogas Production by Sludge-Feeding Thermophilic Anaerobic Digester and Predicting Process Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acclimation of Thermophilic Inoculum and Samples Preparation
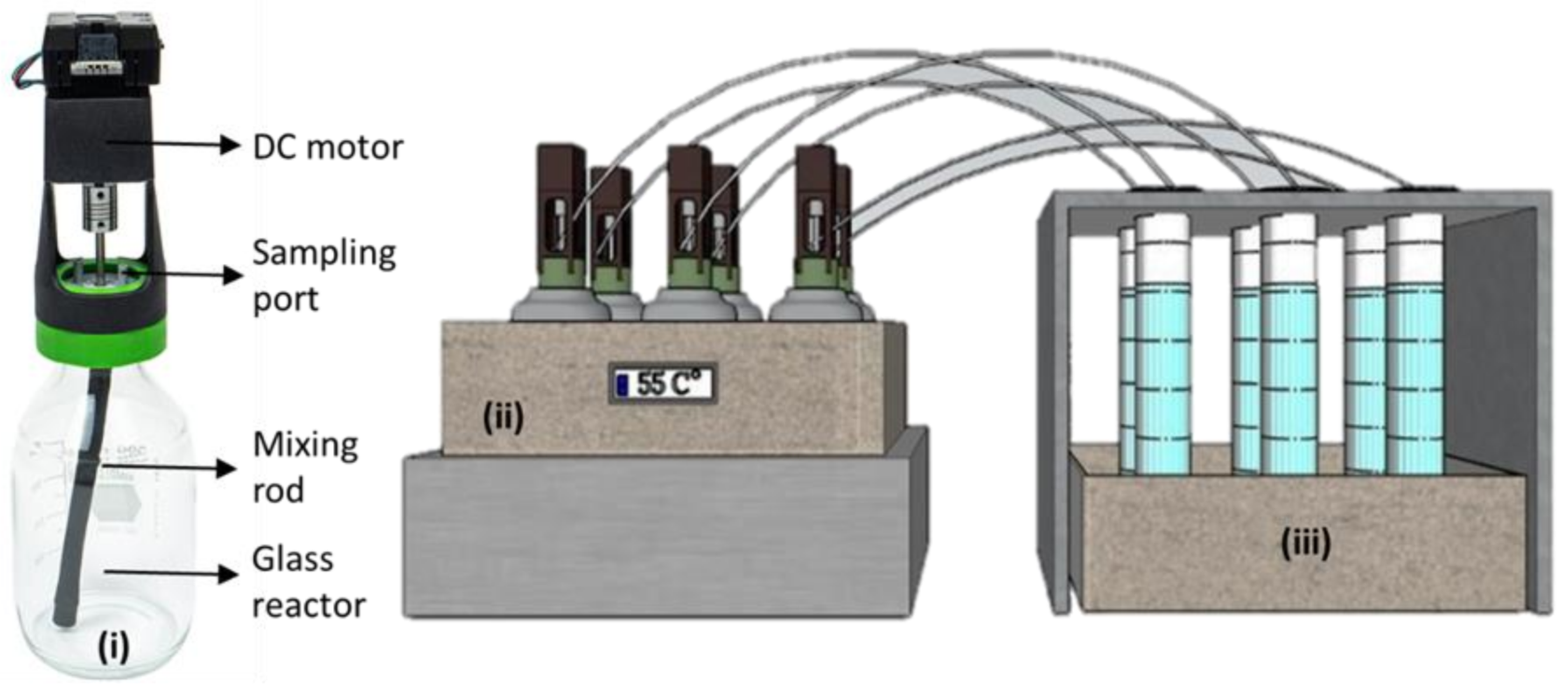
2.2. Preparing of Reactor Configurations
2.3. Analytical Methods
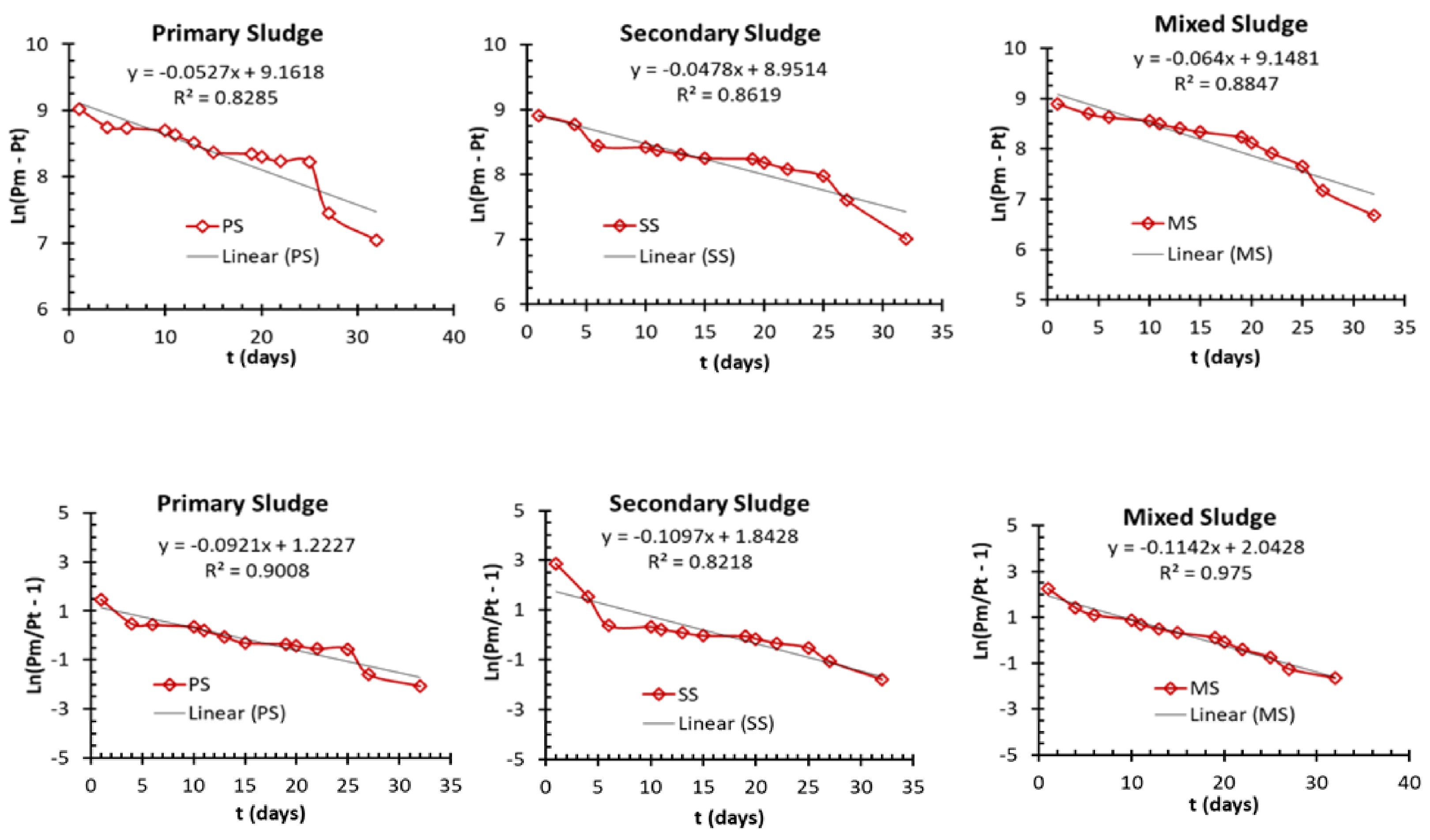
2.4. Kinetic Model Analysis
3. Results
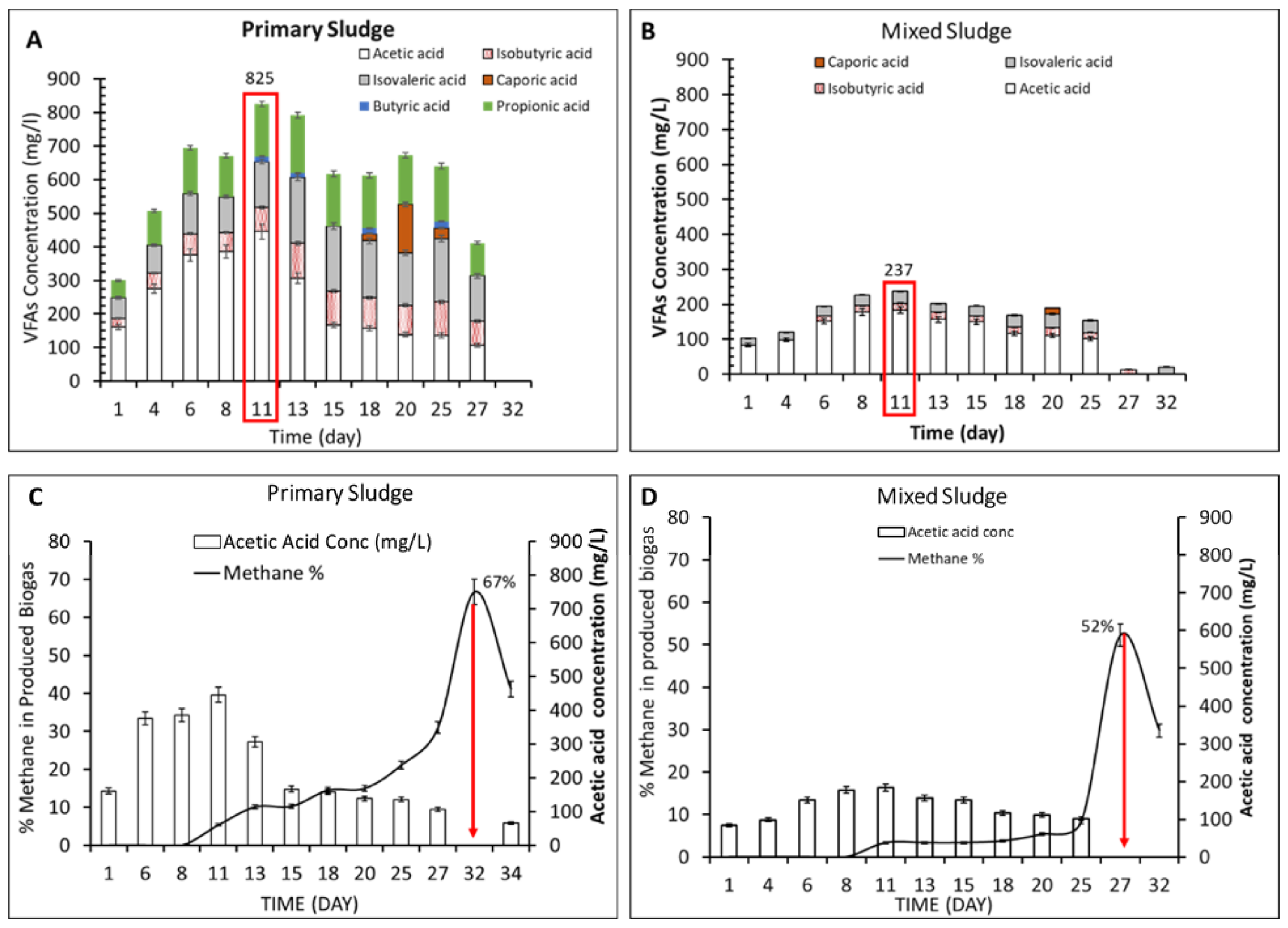
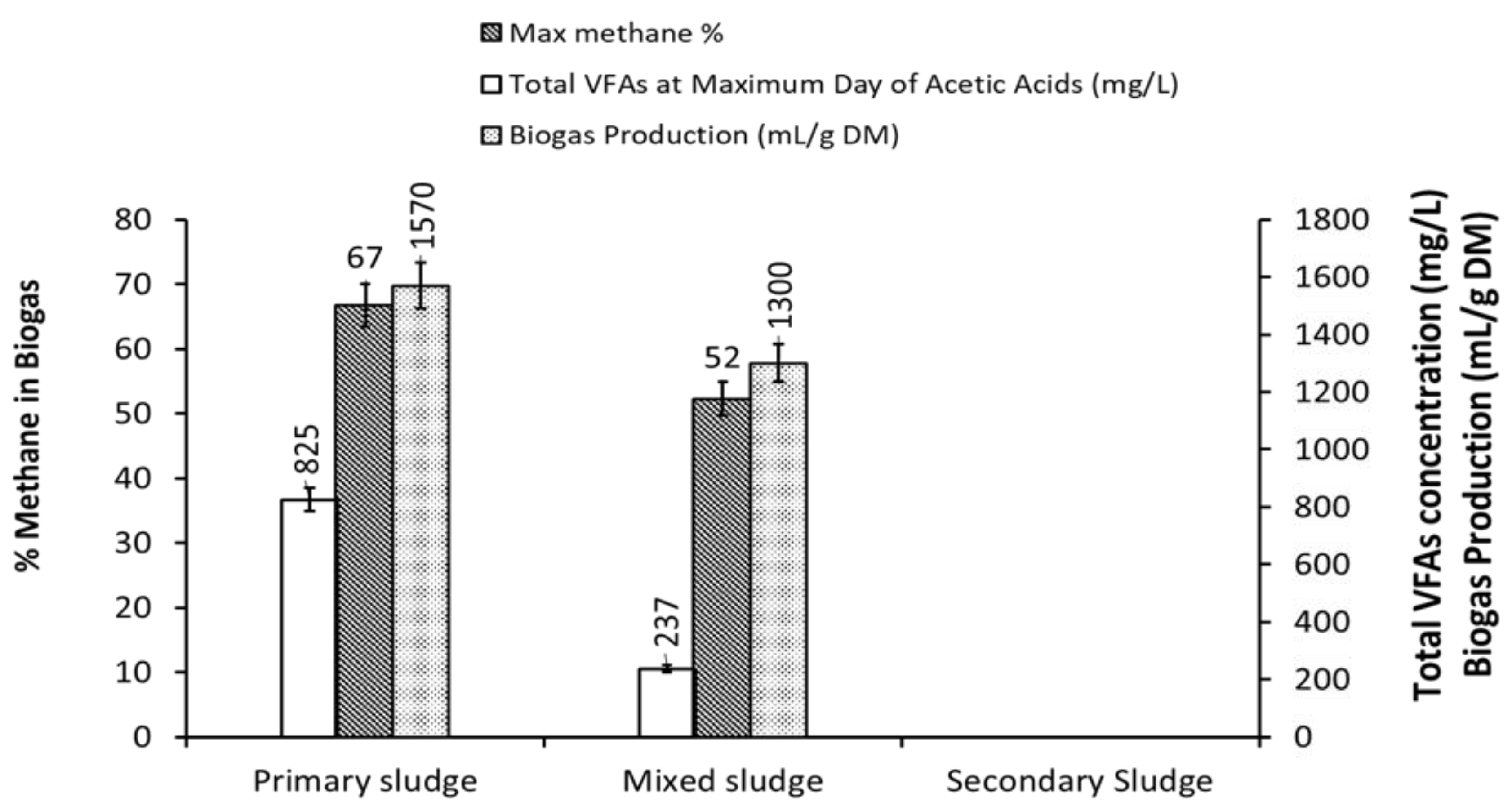
3.1. Effect of VFAs Accumulation on Methane Production
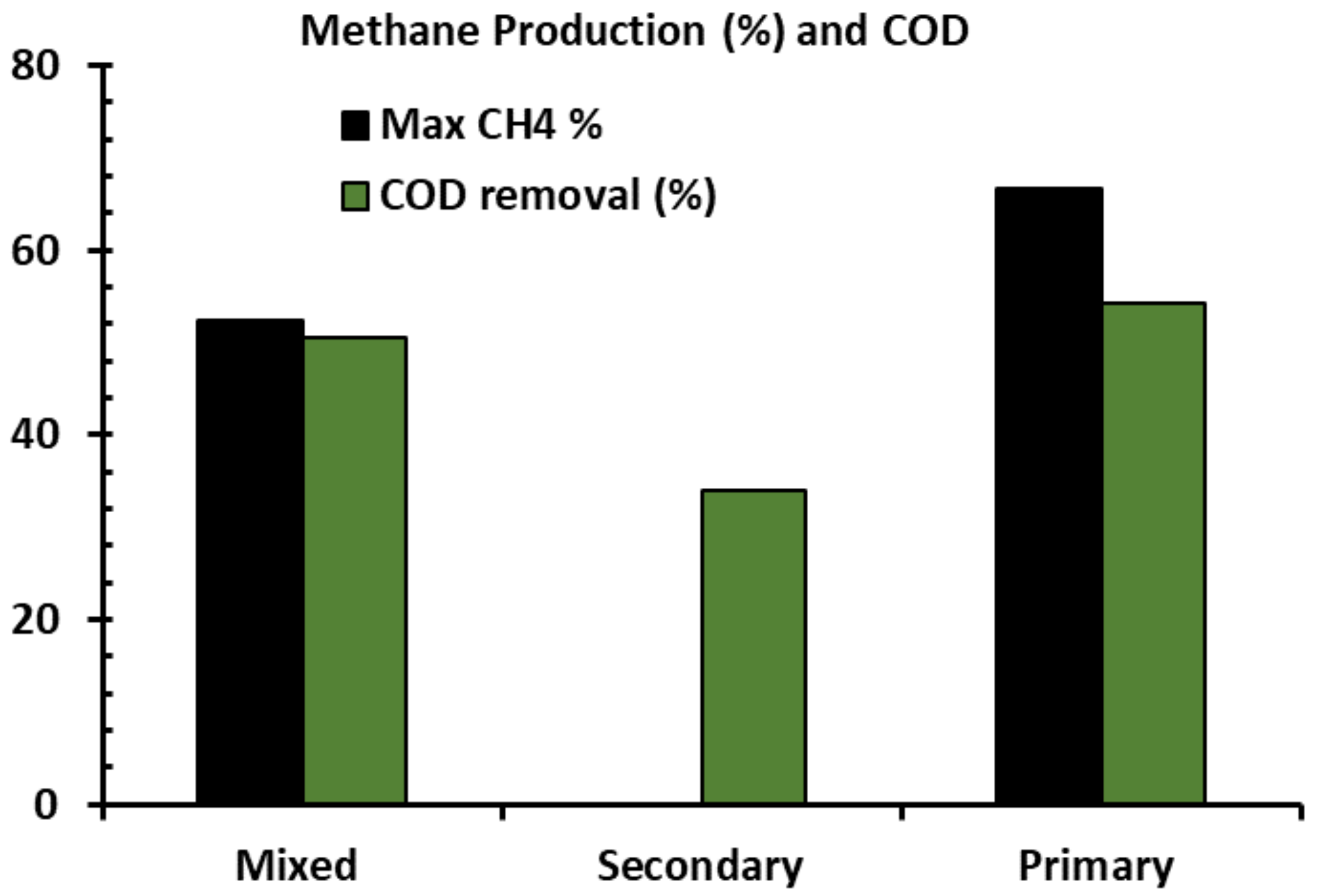
3.2. Solids and COD Removal over the Operation
3.3. Potential of Power Generation from Sludge as TAD Feedstock
3.4. Kinetic Models Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Das, P.; Khan, S.; AbdulQuadir, M.; Thaher, M.; Waqas, M.; Easa, A.; Attia, E.S.M.; Al-Jabri, H. Energy Recovery and Nutrients Recycling from Municipal Sewage Sludge. Sci. Total Environ. 2020, 715, 136775. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.M.S.; Hai, F.I.; Lu, W.; Al-Mamun, A.; Dhar, B.R. A Review of Mechanisms Underlying the Impacts of (Nano)Microplastics on Anaerobic Digestion. Bioresour. Technol. 2021, 329, 124894. [Google Scholar]
- Baawain, M.S.; Al-Mamun, A.; Omidvarborna, H.; Al-Sabti, A.; Choudri, B.S. Public Perceptions of Reusing Treated Wastewater for Urban and Industrial Applications: Challenges and Opportunities. Environ. Dev. Sustain. 2018, 22, 1859–1871. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Chen, Z.; Wei, D.; Song, Y.; Ma, Y.; Zhang, H. Achieving Biogas Production and Efficient Pollutants Removal from Nitrogenous Fertilizer Wastewater Using Combined Anaerobic Digestion and Autotrophic Nitrogen Removal Process. Bioresour. Technol. 2021, 339, 125659. [Google Scholar] [CrossRef] [PubMed]
- Baawain, M.; Al-Mamun, A.; Omidvarborna, H.; Al-Sulaimi, I.N. Measurement, Control, and Modeling of H2S Emissions from a Sewage Treatment Plant. Int. J. Environ. Sci. Technol. 2019, 16, 2721–2732. [Google Scholar] [CrossRef]
- Baawain, M.; Al-Mamun, A.; Omidvarborna, H.; Al-Jabri, A. Assessment of Hydrogen Sulfide Emission from a Sewage Treatment Plant Using AERMOD. Environ. Monit. Assess. 2017, 189, 263. [Google Scholar] [CrossRef]
- Breda, C.C.; Soares, B.; Francisco, R.; Tavanti, R.; Gomes Viana, D.; Da, O.; Freddi, S.; Piedade, A.R.; Mahl, D.; Traballi, R.C.; et al. Successive Sewage Sludge Fertilization: Recycling for Sustainable Agriculture. Waste Manag. 2020, 109, 38–50. [Google Scholar] [CrossRef]
- Barua, S.; Zakaria, B.S.; Al-Mamun, A.; Dhar, B.R. Anodic Performance of Microbial Electrolysis Cells in Response to Ammonia Nitrogen. J. Environ. Eng. Sci. 2018, 14, 37–43. [Google Scholar] [CrossRef]
- Tarpani, R.R.Z.; Alfonsín, C.; Hospido, A.; Azapagic, A. Life Cycle Environmental Impacts of Sewage Sludge Treatment Methods for Resource Recovery Considering Ecotoxicity of Heavy Metals and Pharmaceutical and Personal Care Products. J. Environ. Manag. 2020, 260, 109643. [Google Scholar] [CrossRef]
- Al-Wahaibi, A.; Osman, A.I.; Al-Muhtaseb, A.H.; Alqaisi, O.; Baawain, M.; Fawzy, S.; Rooney, D.W. Techno-Economic Evaluation of Biogas Production from Food Waste via Anaerobic Digestion. Sci. Rep. 2020, 10, 15719. [Google Scholar] [CrossRef]
- Alwahaibi, B.M.; Al-Mamun, A.; Said Baawain, M.; Sana, A. Biological Efficiency and Control of a Membrane Bioreactor and Conventional Activated Sludge Process for Treating Municipal Wastewater. SQU J. Agric. Mar. Sci. 2020, 25, 41–49. [Google Scholar] [CrossRef]
- Barua, S.; Zakaria, B.S.; Chung, T.; Hai, F.I.; Haile, T.; Al-Mamun, A.; Dhar, B.R. Microbial Electrolysis Followed by Chemical Precipitation for Effective Nutrients Recovery from Digested Sludge Centrate in WWTPs. Chem. Eng. J. 2019, 361, 256–265. [Google Scholar] [CrossRef]
- Fahimi, A.; Federici, S.; Depero, L.E.; Valentim, B.; Vassura, I.; Ceruti, F.; Cutaia, L.; Bontempi, E. Evaluation of the Sustainability of Technologies to Recover Phosphorus from Sewage Sludge Ash Based on Embodied Energy and CO2 Footprint. J. Clean. Prod. 2021, 289, 125762. [Google Scholar] [CrossRef]
- Al-Mamun, A.; Jafary, T.; Baawain, M.S.; Rahman, S.; Choudhury, M.R.; Tabatabaei, M.; Lam, S.S. Energy Recovery and Carbon/Nitrogen Removal from Sewage and Contaminated Groundwater in a Coupled Hydrolytic-Acidogenic Sequencing Batch Reactor and Denitrifying Biocathode Microbial Fuel Cell. Environ. Res. 2020, 183, 109273. [Google Scholar] [CrossRef]
- Al-Mamun, A.; Baawain, M.S. Accumulation of Intermediate Denitrifying Compounds Inhibiting Biological Denitrification on Cathode in Microbial Fuel Cell. J. Environ. Heal. Sci. Eng. 2015, 13, 81. [Google Scholar] [CrossRef] [Green Version]
- Bezirgiannidis, A.; Chatzopoulos, P.; Tsakali, A.; Ntougias, S.; Melidis, P. Renewable Energy Recovery from Sewage Sludge Derived from Chemically Enhanced Precipitation. Renew. Energy 2020, 162, 1811–1818. [Google Scholar] [CrossRef]
- Ryue, J.; Lin, L.; Kakar, F.L.; Elbeshbishy, E.; Al-Mamun, A.; Dhar, B.R. A Critical Review of Conventional and Emerging Methods for Improving Process Stability in Thermophilic Anaerobic Digestion. Energy Sustain. Dev. 2020, 54, 72–84. [Google Scholar] [CrossRef]
- Park, M.; Kim, N.; Jung, S.; Jeong, T.Y.; Park, D. Optimization and Comparison of Methane Production and Residual Characteristics in Mesophilic Anaerobic Digestion of Sewage Sludge by Hydrothermal Treatment. Chemosphere 2021, 264, 128516. [Google Scholar] [CrossRef]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors Influencing Volatile Fatty Acids Production from Food Wastes via Anaerobic Digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef] [Green Version]
- El-Fadel, M.; Saikaly, P.; Ghanimeh, S. Startup and Stability of Thermophilic Anaerobic Digestion of OFMSW. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2685–2721. [Google Scholar] [CrossRef]
- Etta, A.E.B.; James, E.; Ben, A.; Tiku, D.R. Biogas Generation from Co-Digestion of Four Substrates; Water Hyacinth, Cassava Peels, Poultry Droppings and Cow Dung. Annu. Res. Rev. Biol. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Blasius, J.P.; Contrera, R.C.; Maintinguer, S.I.; Alves de Castro, M.C.A. Effects of Temperature, Proportion and Organic Loading Rate on the Performance of Anaerobic Digestion of Food Waste. Biotechnol. Rep. 2020, 27, e00503. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, M.; Miao, H.; Huang, Z.; Gao, S.; Ruan, W. In Situ Volatile Fatty Acids Influence Biogas Generation from Kitchen Wastes by Anaerobic Digestion. Bioresour. Technol. 2014, 163, 186–192. [Google Scholar] [CrossRef]
- Liu, N.; Jiang, J.; Yan, F.; Gao, Y.; Meng, Y.; Aihemaiti, A.; Ju, T. Enhancement of Volatile Fatty Acid Production and Biogas Yield from Food Waste Following Sonication Pretreatment. J. Environ. Manag. 2018, 217, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Bhui, I.; Mathew, A.K.; Chaudhury, S.; Balachandran, S. Influence of Volatile Fatty Acids in Different Inoculum to Substrate Ratio and Enhancement of Biogas Production Using Water Hyacinth and Salvinia. Bioresour. Technol. 2018, 270, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Usman, M.; Alsareii, S.A.; Harraz, F.A.; Al-Assiri, M.S.; Jalalah, M.; Li, X.; Salama, E.S. Synergistic Ammonia and Fatty Acids Inhibition of Microbial Communities during Slaughterhouse Waste Digestion for Biogas Production. Bioresour. Technol. 2021, 337, 125383. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Briceño, C.I.; Ross, A.B.; Camargo-Valero, M.A. Mass and Energy Integration Study of Hydrothermal Carbonization with Anaerobic Digestion of Sewage Sludge. Renew. Energy 2021, 167, 473–483. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Sung, S.; Raskin, L.; Khanal, S.K. Anaerobic Co-Digestion of Various Organic Wastes: Kinetic Modeling and Synergistic Impact Evaluation. Bioresour. Technol. 2022, 343, 126063. [Google Scholar] [CrossRef]
- Sedighi, A.; Karrabi, M.; Shahnavaz, B.; Mostafavinezhad, M. Bioenergy Production from the Organic Fraction of Municipal Solid Waste and Sewage Sludge Using Mesophilic Anaerobic Co-Digestion: An Experimental and Kinetic Modeling Study. Renew. Sustain. Energy Rev. 2022, 153, 111797. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Jeon, B.H.; Jeung, J.H.; Rene, E.R.; Banu, J.R.; Ravindran, B.; Vu, C.M.; Ngo, H.H.; Guo, W.; Chang, S.W. Thermophilic Anaerobic Digestion of Model Organic Wastes: Evaluation of Biomethane Production and Multiple Kinetic Models Analysis. Bioresour. Technol. 2019, 280, 269–276. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environmental Federation. Standard Methods for Examination of Water and Wastewater. An. Hidrol. Médica 2012, 5, 185–186. [Google Scholar]
- Björnsson, L.; Murto, M.; Mattiasson, B. Evaluation of parameters for monitoring an anaerobic co-digestion process. Appl. Microbiol. Biotechnol. 2000, 54, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Phuleria, H.C.; Chandel, M.K. Estimation of Energy Recovery Potential of Sewage Sludge in India: Waste to Watt Approach. J. Clean. Prod. 2020, 276, 122538. [Google Scholar] [CrossRef]
- Deshusses, M.; Soupir, M.; Han, N.; Manser, D. Kinetics of Inactivation of Indicator Pathogens during Thermophilic Anaerobic Digestion Effect s of Solids Ret Ent Ion T Ime and Feeding Frequency on Performance and Pat Hogen Fat e in Semi. Water Res. 2010, 44, 5965–5972. [Google Scholar] [CrossRef]
- Li, L.; Kong, X.; Yang, F.; Li, D.; Yuan, Z.; Sun, Y. Biogas Production Potential and Kinetics of Microwave and Conventional Thermal Pretreatment of Grass. Appl. Biochem. Biotechnol. 2012, 166, 1183–1191. [Google Scholar] [CrossRef]
- Johnravindar, D.; Wong, J.W.C.; Chakraborty, D.; Bodedla, G.; Kaur, G. Food Waste and Sewage Sludge Co-Digestion Amended with Different Biochars: VFA Kinetics, Methane Yield and Digestate Quality Assessment. J. Environ. Manag. 2021, 290, 112457. [Google Scholar] [CrossRef]
- Jiménez, A.M.; Borja, R.; Martín, A.; Raposo, F. Kinetic Analysis of the Anaerobic Digestion of Untreated Vinasses and Vinasses Previously Treated with Penicillium Decumbens. J. Environ. Manag. 2006, 80, 303–310. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Pérez-Elvira, S.I.; Fdz-Polanco, F. Application of Simplified Models for Anaerobic Biodegradability Tests. Evaluation of Pre-Treatment Processes. Chem. Eng. J. 2010, 160, 607–614. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; Visvanathan, C.; Abeynayaka, A. Effect of Organic Loading Rate on VFA Production, Organic Matter Removal and Microbial Activity of a Two-Stage Thermophilic Anaerobic Membrane Bioreactor. Bioresour. Technol. 2011, 102, 5353–5360. [Google Scholar] [CrossRef]
- Wang, C.C.; Chang, C.W.; Chu, C.P.; Lee, D.J.; Chang, B.-V.; Liao, C.S.; Tay, J.H. Using Filtrate of Waste Biosolids to Effectively Produce Bio-Hydrogen by Anaerobic Fermentation. Water Res. 2003, 37, 2789–2793. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Ye, H.; Wang, Y.; Luo, W.; Chang, J.S.; Li, Q.; He, N. Anaerobic Co-Digestion of Sewage Sludge and Food Waste for Hydrogen and VFA Production with Microbial Community Analysis. Waste Manag. 2018, 78, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vassalle, L.; Passos, F.; Rosa-Machado, A.T.; Moreira, C.; Reis, M.; Pascoal de Freitas, M.; Ferrer, I.; Mota, C.R. The Use of Solar Pre-Treatment as a Strategy to Improve the Anaerobic Biodegradability of Microalgal Biomass in Co-Digestion with Sewage. Chemosphere 2022, 286, 131929. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, K.L.; Torres-Lozada, P.; Chaparro, T.R. Beverage Wastewater Treatment by Anaerobic Digestion in Two-Stages for Organic Matter Removal and Energy Production. Biomass Bioenergy 2021, 154, 106260. [Google Scholar] [CrossRef]
- Helenas Perin, J.K.; Biesdorf Borth, P.L.; Torrecilhas, A.R.; Santana da Cunha, L.; Kuroda, E.K.; Fernandes, F. Optimization of Methane Production Parameters during Anaerobic Co-Digestion of Food Waste and Garden Waste. J. Clean. Prod. 2020, 272, 123130. [Google Scholar] [CrossRef]
- Zwain, H.M.; Alzubaidi, S.A.; Kheudhier, Z.A. Effect of Temperature on Compartmental Profile of Solid Content in a Modified Anaerobic Inclining-Baffled Reactor Treating Recycled Paper Mill Effluent. AIP Conf. Proc. 2019, 2124, 30003. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, D.; Dai, X.; Lou, Z.; Yuan, H.; Zhu, N. The Synthetic Effect on Volatile Fatty Acid Disinhibition and Methane Production Enhancement by Dosing FeCl3 in a Sludge Thermophilic Anaerobic Digestion System. RSC Adv. 2016, 6, 21090–21098. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Li, Y.Y.; Liu, Y.; Zhao, Y. Influence of Zero Valent Scrap Iron (ZVSI) Supply on Methane Production from Waste Activated Sludge. Chem. Eng. J. 2015, 263, 461–470. [Google Scholar] [CrossRef]
- Nayak, J.K.; Amit; Ghosh, U.K. An Innovative Mixotrophic Approach of Distillery Spent Wash with Sewage Wastewater for Biodegradation and Bioelectricity Generation Using Microbial Fuel Cell. J. Water Process Eng. 2018, 23, 3. [Google Scholar] [CrossRef]
- Utami, I.; Redjeki, S.; Astuti, D.H. Sani Biogas Production and Removal COD - BOD and TSS from Wastewater Industrial Alcohol (Vinasse) by Modified UASB Bioreactor. In Proceedings of the MATEC Web of Conferences, Kuala Lumpur, Malaysia, 7–8 March 2016; Volume 58. [Google Scholar]
- Villamil, J.A.; Mohedano, A.F.; Rodriguez, J.J.; de la Rubia, M.A. Valorisation of the Liquid Fraction from Hydrothermal Carbonisation of Sewage Sludge by Anaerobic Digestion. J. Chem. Technol. Biotechnol. 2018, 93, 450–456. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.S.; Wong, J.W.C.; Bui, X.T. A Critical Review on Various Feedstocks as Sustainable Substrates for Biosurfactants Production: A Way towards Cleaner Production. Microb. Cell Fact. 2021, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Nayak, J.K.; Ghosh, U.K. Post Treatment of Microalgae Treated Pharmaceutical Wastewater in Photosynthetic Microbial Fuel Cell (PMFC) and Biodiesel Production. Biomass Bioenergy 2019, 131, 105415. [Google Scholar] [CrossRef]
- Samal, K.; Roshan, R.; Bhunia, P. Science of the Total Environment Design and Development of a Hybrid Macrophyte Assisted Vermi Fi Lter for the Treatment of Dairy Wastewater: A Statistical and Kinetic Modelling Approach. Sci. Total Environ. 2018, 645, 156–169. [Google Scholar] [CrossRef] [PubMed]

| pH | COD (mg/L) | BOD5 (mg/L) | SS (mg/L) | VSS (mg/L) | Partial Alkalinity mg/L as CaCO3 | Total Alkalinity mg/L as CaCO3 | NH3-N Aqueous (mg/L) | |
|---|---|---|---|---|---|---|---|---|
| PS | 7.72 ± 0.02 | 10400 ± 50.5 | 324 ± 45.5 | 11880 ± 100 | 10692 ± 110 | 342.86 ± 22.2 | 657.14 ± 23.5 | 50.85 ± 12.5 |
| SS | 7.76 ± 0.02 | 3368 ± 42.2 | 52.5 ± 65.3 | 2800 ± 150 | 2380 ± 120 | 28.57 ± 20.1 | 57.14 ± 12.5 | 70.24 ± 18 |
| MS | 7.54 ± 0.01 | 7648 ± 100 | 108 ± 50.2 | 6800 ± 105 | 6120 ± 125 | 64.29 ± 30.3 | 92.86 ± 20.1 | 11.98 ± 17.6 |
| Configuration | Type of Feedstock | COD Removal (%) | Methane Production (% CH4) | Power Generation (KWh) | Ref |
|---|---|---|---|---|---|
| Mesophilic AD | Mixed food waste | NA | 30 | 0.000927 | [10] |
| Rice waste | 64 | 0.002809 | [10] | ||
| Date fruit waste | <20 | 0.001224 | [10] | ||
| Legume beans waste | 40 | 0.001417 | [10] | ||
| UASB reactor with co-digestion | Sewage sludge and microalgal biomass pretreated by solar thermal system | 70 | 67.5 | - | [43] |
| UASB and AD combined with subsequent autotrophic nitrogen removal over nitrite process | Nitrogenous fertilizer Wastewater | 98.4 | - | - | [4] |
| Acidogenic AD reactor | Beverage wastewater | 25 | 35 | - | [44] |
| Co-digestion fluidized-bed AD | Food waste and garden waste | - | 65 | - | [45] |
| Thermophilic AD | Sewage primary sludge | 54.21 | 67 | 0.00012 | This Study |
| Parameters | PS | SS | MS |
|---|---|---|---|
| Measured final biogas yield (mL biogas/g VS) for 100 days, Pm | 10,200 | 7800 | 8080 |
| First-order kinetic model | |||
| Pm (mL biogas/g VS) | 9526 | 7718.6 | 9396.6 |
| k (d−1) | 0.0527 | 0.0478 | 0.064 |
| R2 | 0.829 | 0.862 | 0.884 |
| Error % for measured vs. predicted Pm | 6 | 1 | 16 |
| Logistic model | |||
| Pm (mL biogas/g VS) | 9526 | 7718.6 | 9396.6 |
| Rm (mL biogas/g VS/d) | 102 | 78 | 80.8 |
| λ (d) | 8.4 | 1.29 | ≈0 |
| R2 | 0.901 | 0.821 | 0.975 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sulaimi, I.N.; Nayak, J.K.; Alhimali, H.; Sana, A.; Al-Mamun, A. Effect of Volatile Fatty Acids Accumulation on Biogas Production by Sludge-Feeding Thermophilic Anaerobic Digester and Predicting Process Parameters. Fermentation 2022, 8, 184. https://doi.org/10.3390/fermentation8040184
Al-Sulaimi IN, Nayak JK, Alhimali H, Sana A, Al-Mamun A. Effect of Volatile Fatty Acids Accumulation on Biogas Production by Sludge-Feeding Thermophilic Anaerobic Digester and Predicting Process Parameters. Fermentation. 2022; 8(4):184. https://doi.org/10.3390/fermentation8040184
Chicago/Turabian StyleAl-Sulaimi, Intisar Nasser, Jagdeep Kumar Nayak, Halima Alhimali, Ahmed Sana, and Abdullah Al-Mamun. 2022. "Effect of Volatile Fatty Acids Accumulation on Biogas Production by Sludge-Feeding Thermophilic Anaerobic Digester and Predicting Process Parameters" Fermentation 8, no. 4: 184. https://doi.org/10.3390/fermentation8040184
APA StyleAl-Sulaimi, I. N., Nayak, J. K., Alhimali, H., Sana, A., & Al-Mamun, A. (2022). Effect of Volatile Fatty Acids Accumulation on Biogas Production by Sludge-Feeding Thermophilic Anaerobic Digester and Predicting Process Parameters. Fermentation, 8(4), 184. https://doi.org/10.3390/fermentation8040184










