Formation, Losses, Preservation and Recovery of Aroma Compounds in the Winemaking Process
Abstract
1. Introduction
2. Formation of Volatile Substances during the Winemaking Process
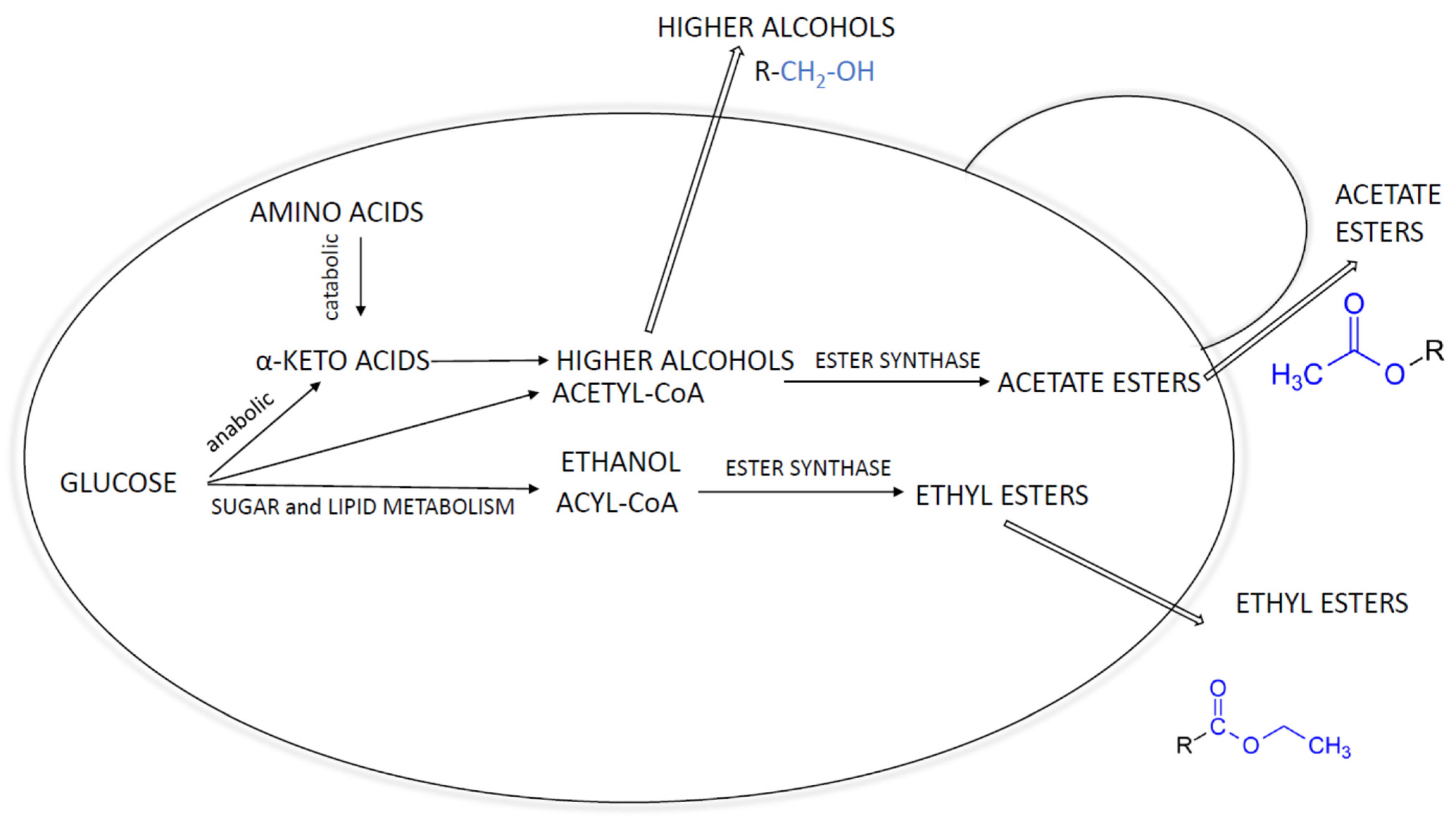
2.1. Higher Alcohols
2.2. Esters
2.3. Releasing Ester from Yeast
2.4. Sulphur Compounds
Thiols
2.5. Volatile Acidity
3. Factors Affecting the Composition of Fermentation Gases
3.1. Temperature
3.2. Nutrition
3.3. Yeast
3.4. Skin Contact
4. Fermentation Aroma Losses
Aroma Loss Caused by Stripping CO2
5. Capture and Aroma Recovery Techniques
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perestrelo, R.; Silva, C.; Gonçalves, C.; Castillo, M.; Câmara, J.S. An Approach of the MadeiraWine Chemistry. Beverages 2020, 6, 12. [Google Scholar] [CrossRef]
- Mina, M.; Tsaltas, D. Contribution of Yeast in Wine Aroma and Flavour; IntechOpen: London, UK, 2017. [Google Scholar]
- Lin, J.; Massonnet, M.; Cantu, D. The genetic basis of grape and wine aroma. Hortic. Res. 2019, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Ferreira, V.; Escudero, A.; Marques, J.C.; Cacho, J. Quantitative gas chromatography—Olfactometry and chemical quantitative study of the aroma of four Madeira wines. Anal. Chim. Acta 2006, 563, 180–187. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; Silva, L.M.A.; Ribeiro, P.R.V.; de Brito, E.S.; Zocolo, G.J.; Souza-Leão, P.C.; Marques, A.T.B.; Quintela, A.L.; Larsen, F.H.; Canuto, K.M. H-1 NMR and LC-MS-based metabolomic approach for evaluation of the seasonality and viticultural practices in wines from Sao Francisco River Valley, a Brazilian semi-arid region. Food Chem. 2019, 289, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Barros, A.S.; Rocha, S.M.; Câmara, J.S. Establishment of the varietal profile of Vitis vinifera L. grape varieties from different geographical regions based on HS-SPME/GC-qMS combined with chemometric tools. Microchem. J. 2014, 116, 107–117. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Song, H.; Tao, Y.; Russo, N. Phenolic matrix effect on aroma formation of terpenes during simulated wine fermentation—Part I: Phenolic acids. Food Chem. 2021, 341, 128–288. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arribas, M.V.; Polo, M.C. Winemaking biochemistry and microbiology: Current knowledge and future trends. Crit. Rev. Food Sci. Nutr. 2005, 45, 265–286. [Google Scholar] [CrossRef]
- Lukic, I.; Horvat, I. Differentiation of Commercial PDO Wines Produced in Istria (Croatia) According to Variety and Harvest Year Based on HS-SPME-GC/MS Volatile Aroma Compound Profiling. Food Technol. Biotechnol. 2017, 55, 95–108. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Jiang, Z.-T.; Tan, J.; Li, R. Geographical Origin Traceability of Red Wines Based on Chemometric Classification via Organic Acid Profiles. J. Food Qual. 2017, 2017, 2038073. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Camara, J.S. A useful approach for the differentiation of wines according to geographical origin based on global volatile patterns. J. Sep. Sci. 2014, 37, 1974–1981. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C-6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef]
- Mouret, J.R.; Morakul, S.; Nicolle, P.; Athes, V.; Sablayrolles, J. Gas-liquid transfer of aroma compounds during winemaking fermentations. LWT Food Sci. Technol. 2012, 49, 238–244. [Google Scholar] [CrossRef]
- Benito, A.; Calderon, F.; Benito, S. The Influence of Non-Saccharomyces Species on Wine Fermentation Quality Parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef]
- Kuchen, B.; Maturano, Y.P.; Mestre, M.V.; Combina, M.; Toro, M.E.; Vazquez, F. Selection of Native Non-Saccharomyces Yeasts with Biocontrol Activity against Spoilage Yeasts in Order to Produce Healthy Regional Wines. Fermentation 2019, 5, 60. [Google Scholar] [CrossRef]
- Benito, A.; Calderon, F.; Benito, S. Combined Use of S. pombe and L. thermotolerans in Winemaking. Beneficial Effects Determined through the Study of Wines’ Analytical Characteristics. Molecules 2016, 21, 1744. [Google Scholar] [CrossRef]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.-D.; Rauhut, D. Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef]
- Du Plessis, H.; Du Toit, M.; Nieuwoudt, H.; Van Der Rijst, M.; Hoff, J.; Jolly, N. Modulation of Wine Flavor using Hanseniaspora uvarum in Combination with Different Saccharomyces cerevisiae, Lactic Acid Bacteria Strains and Malolactic Fermentation Strategies. Fermentation 2019, 5, 64. [Google Scholar] [CrossRef]
- Benito, S. The Management of Compounds that Influence Human Health in Modern Winemaking from an HACCP Point of View. Fermentation 2019, 5, 33. [Google Scholar] [CrossRef]
- Vilela, A. Lachancea thermotolerans, the Non-Saccharomyces Yeast that Reduces the Volatile Acidity of Wines. Fermentation 2018, 4, 56. [Google Scholar] [CrossRef]
- Nikolaus, M.; Doris, R.; Andrii, T. Sulfane Sulfur Compounds as Source of Reappearance of Reductive Off-Odors in Wine. Fermentation 2022, 8, 53. [Google Scholar]
- Polaskova, P.; Herszage, J.; Ebeler, S.E. Wine flavor: Chemistry in a glass. Chem. Soc. Rev. 2008, 37, 2478–2489. [Google Scholar] [CrossRef] [PubMed]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Swiegers, J.; Bartowsky, E.; Henschke, P.; Pretorius, I. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Ebeler, S.E.; Thorngate, J.H. Wine Chemistry and Flavor: Looking into the Crystal Glass. J. Agric. Food Chem. 2009, 57, 8098–8108. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Palomo, E.S.; Díaz-Maroto, M.; Viñas, M.G.; Soriano-Pérez, A.; Pérez-Coello, M. Aroma profile of wines from Albillo and Muscat grape varieties at different stages of ripening. Food Control 2007, 18, 398–403. [Google Scholar] [CrossRef]
- Hjelmeland, A.K.; Ebeler, S.E. Glycosidically Bound Volatile Aroma Compounds in Grapes and Wine: A Review. Am. J. Enol. Vitic. 2015, 66, 1–11. [Google Scholar] [CrossRef]
- Capone, D.L.; Sefton, M.A.; Jeffery, D.W. Analytical investigations to relate important wine odorant 3-mercaptohexan-1-ol to its precursors. Abstr. Pap. Am. Chem. Soc. 2010, 240, 1390–1395. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology: The Chemistry of Wine Stabilization and Treatments; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; Volume 2. [Google Scholar]
- Nykanen, L. Formation and occurrence of flavor compounds in wine and distilled alcoholic beverages. Am. J. Enol. Vitic. 1986, 37, 84–96. [Google Scholar]
- Jacques, K.A.; Lyons, P.; Kelsall, D.R. The Alcohol Textbook: A Reference for the Beverage, Fuel and Industrial Alcohol Industries; Nottingham University Press: Nottingham, UK, 1999; Volume 3. [Google Scholar]
- Bamforth, C.W.; Cook, D.J. Food, Fermentation, and Micro-Organisms; Wiley-Blackwell: Hoboken, NJ, USA, 2019; Volume 2. [Google Scholar]
- Hui, Y.H. Handbook of Food Products Manufacturing; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kulp, K.; Lorenz, K. Handbook of Dough Fermentations; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Baca, E.; Chrostowski, J. Powstawanie i wpływ wyższych alkoholi i estrów na aromat piwa. Przem. Ferm. Owoc.-Warz. 1970, 3, 5–8. [Google Scholar]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Hazelwood, L.A.; Daran, J.-M.; van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Bachman, B. Technologia Spirytusu i Drożdży; PWN: Łódź, Poland, 1958; Volume 1. [Google Scholar]
- Bakker, J.; Clarke, R.J. Wine Flavour Chemistry; Wiley-Blackwell: Oxford, UK, 2012; Volume 2. [Google Scholar]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Ribereau-Gayon, J.; Peynaud, E.; Sudraud, P.; Riberau-Gayon, P. Traité D’oenologie: Sciences et Techniques du Vin, Tomee I: Analyse et Controle des Vins; Dunod: Paris, France, 1972. [Google Scholar]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C.G. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Nordström, K. Formation of Ethyl Acetate in Fermentation with Brewer’s Yeast: IV. Metabolism of Acetyl-Coenzyme A. J. Inst. Brew. 1963, 69, 142–153. [Google Scholar] [CrossRef]
- Neven, H.; Delvaux, F.; Derdelinckx, G. Flavor evolution of top fermented beers. MBAA Tech. Q. Master Brew. Assoc. Am. 1997, 34, 115–118. [Google Scholar]
- Yoshioka, K.; Hashimoto, N. Acetyl-CoA of Brewers’ Yeast and Formation of Acetate Esters. Agric. Biol. Chem. 1984, 48, 207–209. [Google Scholar]
- Calderbank, J.; Hammond, J.R.M. Influence of Higher Alcohol Availability on Ester Formation by Yeast. J. Am. Soc. Brew. Chem. 1994, 52, 84–90. [Google Scholar] [CrossRef]
- Stewart, G.G.; Younis, O.S. Effect of Malt Wort, Very-High-Gravity Malt Wort, and Very-High-Gravity Adjunct Wort on Volatile Production in Saccharomyces Cerevisiae. J. Am. Soc. Brew. Chem. 1999, 57, 39–45. [Google Scholar]
- Lee, S.; Villa, K.; Patino, H. Yeast Strain Development for Enhanced Production of Desirable Alcohols/Esters in Beer. J. Am. Soc. Brew. Chem. 1995, 53, 153–156. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Fukushige, T.; Yonezawa, T.; Sone, H. Genetic and physiological analysis of branched-chain alcohols and isoamyl acetate production in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2002, 59, 501–508. [Google Scholar] [PubMed]
- Marchesini, S.; Poirier, Y. Futile cycling of intermediates of fatty acid biosynthesis toward peroxisomal beta-oxidation in Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 32596–32601. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.T.; Kirsop, B.H. The origin of the medium chain length fatty acids present in beer. J. Inst. Brew. 1977, 83, 241–243. [Google Scholar] [CrossRef]
- Schneider, J.; Krottenthaler, M.; Back, W.; Weisser, H. Study on the membrane filtration of mash with particular respect to the quality of wort and beer. J. Inst. Brew. 2005, 111, 380–387. [Google Scholar] [CrossRef]
- Sumper, M. Control of Fatty-Acid Biosynthesis by Long-Chain Acyl CoAs and by Lipid Membranes. Eur. J. Biochem. 1974, 49, 469–475. [Google Scholar] [CrossRef]
- Dufour, J.-P.; Malcorps, P.; Silcock, P. Control of ester synthesis during brewery fermentation. Brew. Yeast Ferment. Perform. 2003, 2003, 213–233. [Google Scholar] [CrossRef]
- Aeyraepaeae, T.; Lindstroem, I. Aspects of the Influence of Exogenous Fatty Acids on the Fatty Acid Metabolism of Yeast; European Brewery Convention (EBC): Brussels, Belgium, 1979; pp. 507–517. [Google Scholar]
- Furukawa, K.; Yamada, T.; Mizoguchi, H.; Hara, S. Increased ethyl caproate production by inositol limitation in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2003, 95, 448–454. [Google Scholar] [CrossRef]
- Malcorps, P.; Dufour, J.P. Short-chain and medium-chain aliphatic-ester synthesis in Saccharomyces cerevisiae. Eur. J. Biochem. 1992, 210, 1015–1022. [Google Scholar] [CrossRef]
- Fujii, T.; Yoshimoto, H.; Tamai, Y. Acetate ester production by Saccharomyces cerevisiae lacking the ATF1 gene encoding the alcohol acetyltransferase. J. Ferm. Bioeng. 1996, 81, 538–542. [Google Scholar] [CrossRef]
- Fujii, T.; Nagasawa, N.; Iwamatsu, A.; Bogaki, T.; Tamai, Y.; Hamachi, M. Molecular cloning, sequence analysis, and expression of the yeast alcohol acetyltransferase gene. Appl. Environ. Microbiol. 1994, 60, 2786–2792. [Google Scholar] [CrossRef]
- Nagasawa, N.; Bogaki, T.; Iwamatsu, A.; Hamachi, M.; Kumagai, C. Cloning and nucleotide sequence of the alcohol acetyltransferase II gene (ATF2) from Saccharomyces cerevisiae Kyokai No. 7. Biosci. Biotechnol. Biochem. 1998, 62, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Yoshimoto, H.; Nagasawa, N.; Bogaki, T.; Tamai, Y.; Hamachi, M. Nucleotide sequences of alcohol acetyltransferase genes from lager brewing yeast, Saccharomyces carlsbergensis. Yeast 1996, 12, 593–598. [Google Scholar] [CrossRef]
- Athenstaedt, K.; Zweytick, D.; Jandrositz, A.; Kohlwein, S.D.; Daum, G. Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J. Bacteriol. 1999, 181, 6441–6448. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, P.; Malcorps, P.; Almeida, A.S.; Ferreira, A.; Meyer, A.M.; Dufour, J.P. Analysis of Free Fatty Acids, Fusel Alcohols, and Esters in Beer: An Alternative to CS2 Extraction. J. Am. Soc. Brew. Chem. 1994, 52, 127–134. [Google Scholar] [CrossRef]
- Mason, A.B.; Dufour, J.P. Alcohol acetyltransferases and the significance of ester synthesis in yeast. Yeast 2000, 16, 1287–1298. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Lilly, M.; Bauer, F.F.; Lambrechts, M.G.; Swiegers, J.H.; Cozzolino, D.; Pretorius, I.S. The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast 2006, 23, 641–659. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Verstrepen, K.; Van Laere, S.D.M.; Voet, A.; Van Dijck, P.; Delvaux, F.R.; Thevelein, J. The Saccharomyces cerevisiae EHT1 and EEB1 genes encode novel enzymes with medium-chain fatty acid ethyl ester synthesis and hydrolysis capacity. J. Biol. Chem. 2006, 281, 4446–4456. [Google Scholar] [CrossRef]
- Peddie, H.A.B. Ester Formation in Brewery Fermentations. J. Inst. Brew. 1990, 96, 327–331. [Google Scholar] [CrossRef]
- Olzhausen, J.; Schubbe, S.; Schuller, H.J. Genetic analysis of coenzyme A biosynthesis in the yeast Saccharomyces cerevisiae: Identification of a conditional mutation in the pantothenate kinase gene CAB1. Curr. Genet. 2009, 55, 163–173. [Google Scholar] [CrossRef]
- White, W.H.; Gunyuzlu, P.L.; Toyn, J.H. Saccharomyces cerevisiae is capable of de novo pantothenic acid biosynthesis involving a novel pathway of beta-alanine production from spermine. J. Biol. Chem. 2001, 276, 10794–10800. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Yamamoto, N.; Kiyokawa, Y.; Yanagiuchi, T.; Wakai, Y.; Kitamoto, K.; Inoue, Y.; Kimura, A. Balance of activities of alcohol acetyltransferase and esterase in Saccharomyces cerevisiae is important for production of isoamyl acetate. Appl. Environ. Microbiol. 1998, 64, 4076–4078. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, H. Yeast Esterases and Aroms Esters in Alcoholic Beverages. J. Inst. Brew. 1981, 87, 296–300. [Google Scholar] [CrossRef]
- Nykanen, L.; Nykanen, I.; Suomalainen, H. Distribution of the esters Produced during Sugar Fermentation between Yeast-Cell and Medium. J. Inst. Brew. 1977, 83, 32–34. [Google Scholar] [CrossRef]
- Dufour, J.P. Higher Alcohols, Acids and Ester Secretion during Yeast Growth; Academic Press: Cambridge, MA, USA, 1994; p. 140. [Google Scholar]
- Swiegers, J.H.; Francis, I.L.; Herderich, M.J.; Pretorius, I.S. Meeting consumer expectations through management in vineyard and winery: The choice of yeast for fermentation offers great potential to adjust the aroma of Sauvignon Blanc wine. Aust. N. Z. Wine Ind. J. 2006, 21, 34–42. [Google Scholar]
- VCF Online. Available online: http://www.vcf-online.nl/VcfHome.cfm (accessed on 5 May 2019).
- McGorrin, R.J. The Significance of Volatile Sulfur Compounds in Food Flavors An Overview. In Volatile Sulfur Compounds in Food; American Chemical Society: Washington, DC, USA, 2011; Volume 1068, pp. 3–31. [Google Scholar] [CrossRef]
- Mussinan, C.J.; Keelan, M.E. Sulfur-compounds in Foods—An overview. In Sulfur Compounds in Foods; American Chemical Society: Washington, DC, USA, 1994; pp. 1–6. [Google Scholar]
- Vermeulen, C.; Gijs, L.; Collin, S. Sensorial contribution and formation pathways of thiols in foods: A review. Food Rev. Int. 2005, 21, 69–137. [Google Scholar] [CrossRef]
- Van, L.-N.; Bouchet, S.; Bjorn, E. Determination of Sub-Nanomolar Levels of Low Molecular Mass Thiols in Natural Waters by Liquid Chromatography Tandem Mass Spectrometry after Derivatization with p-(Hydroxymercuri)Benzoate and Online Preconcentration. Anal. Chem. 2015, 87, 1089–1096. [Google Scholar]
- Tominaga, T.; Furrer, A.; Henry, R.; Dubourdieu, D. Identification of new volatile thiols in the aroma of Vitis vinifera L. var. Sauvignon blanc wines. Flavour Fragr. J. 1998, 13, 159–162. [Google Scholar] [CrossRef]
- Benkwitz, F.; Tominaga, T.; Kilmartin, P.A.; Lund, C.; Wohlers, M.; Nicolau, L. Identifying the Chemical Composition Related to the Distinct Aroma Characteristics of New Zealand Sauvignon blanc Wines. Am. J. Enol. Vitic. 2012, 63, 62–72. [Google Scholar] [CrossRef]
- Tominaga, T.; Darriet, P.; Dubourdieu, D. Identification of 3-mercaptohexyl acetate in Sauvignon wine, a powerful aromatic compound exhibiting box-tree odor. Vitis 1996, 35, 207–210. [Google Scholar]
- Jiranek, V.; Langridge, P.; Henschke, P.A. Validation of Bismuth-Containing Indicator Media for Predicting H2S-Producing Potential of Saccharomyces cerevisiae Wine Yeasts Under Enological Conditions. Am. J. Enol. Vitic. 1995, 46, 269–273. [Google Scholar]
- Spiropoulos, A.; Tanaka, J.; Flerianos, I.; Bisson, L.F. Characterization of hydrogen sulfide formation in commercial and natural wine isolates of Saccharomyces. Am. J. Enol. Vitic. 2000, 51, 233–248. [Google Scholar]
- Mendes-Ferreira, A.; Mendes-Faia, A.; Leão, C. Survey of hydrogen sulphide production by wine yeasts. J. Food Prot. 2002, 65, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Rauhut, D. Yeasts−production of sulfur compounds. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood Academic: Chur, Switzerland, 1993; pp. 183–223. [Google Scholar]
- Vos, P.J.A.; Gray, R.S. The origin and control of hydrogen sulphide during fermentation of grape must. Am. J. Enol. Vitic. 1979, 30, 187–197. [Google Scholar]
- Henschke, P.A.; Jiranek, V. Hydrogen sulfide formation during fermentation: Effect of nitrogen composition in model grape must. In International Symposium on Nitrogen in Grapes and Wine; ASEV: Seattle, WA, USA, 1991. [Google Scholar]
- Hallinan, C.P.; Saul, D.J.; Jiranek, V. Differential utilization of sulfur compounds for H2S liberation by nitrogen-starved wine yeast. Aust. J. Grape Wine Res. 1999, 5, 82–90. [Google Scholar] [CrossRef]
- Fleet, G.M. (Ed.) Yeast−growth during fermentation. In Wine Microbiology and Biotechnology; Harwood Academic: Chur, Switzerland, 1993; pp. 27–54. [Google Scholar]
- Park, S.K.; Boulton, R.B.; Noble, A.C. Formation of hydrogen sulfide and glutathione during fermentation of white grape musts. Am. J. Enol. Vitic. 2000, 51, 91–97. [Google Scholar]
- Moreira, N.; Mendes, F.; Pereira, O.; de Pinho, P.G.; Hogg, T.; Vasconcelos, I. Volatile sulphur compounds in wines related to yeast metabolism and nitrogen composition of grape musts. Anal. Chim. Acta 2002, 458, 157–167. [Google Scholar] [CrossRef]
- Giudici, P.; Kunkee, R.E. The Effect of Nitrogen Deficiency and Sulfur-Containing Amino Acids on the Reduction of Sulfate to Hydrogen Sulfide by Wine Yeasts. Am. J. Enol. Vitic. 1994, 45, 107–112. [Google Scholar]
- Jiranek, V.; Langridge, P.; Henschke, P.A. Determination of sulphite reductase activity and its response to assimilable nitrogen status in a commercial Saccharomyces cerevisiae wine yeast. J. Appl. Bacteriol. 1996, 81, 329–336. [Google Scholar] [CrossRef]
- Tominaga, T.; Blanchard, L.; Darriet, A.P.; Dubourdieu, D. A powerful aromatic volatile thiol, 2-furanmethanethiol, exhibiting roast coffee aroma in wines made from several Vitis vinifera grape varieties. J. Agric. Food Chem. 2000, 48, 1799–1802. [Google Scholar] [CrossRef]
- Liu, H.-F.; Wu, B.-H.; Fan, P.-G.; Xu, H.-Y.; Li, S.-H. Inheritance of sugars and acids in berries of grape (Vitis vinifera L.). Euphytica 2007, 153, 99–107. [Google Scholar] [CrossRef]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Silva, R.D.; Chaves, S.R.; Sousa, M.J.; Corte-Real, M. The impact of acetate metabolism on yeast fermentative performance and wine quality: Reduction of volatile acidity of grape musts and wines. Appl. Microbiol. Biotechnol. 2011, 89, 271–280. [Google Scholar] [CrossRef]
- Jackson, D.I.; Lombard, P.B. Environmental and Management Practices Affecting Grape Composition and Wine Quality—A Review. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar]
- Ramey, D.; Bertrand, A.; Ough, C.S.; Singleton, V.L.; Sanders, E. Effects of skin contact temperature on Chardonnay must and wine composition. Am. J. Enol. Vitic. 1986, 37, 99–106. [Google Scholar]
- Arnold, R.A.; Noble, A.C. Effect of pomace contact on the flavor of Chardonnay wine. Am. J. Enol. Vitic. 1979, 30, 179–181. [Google Scholar]
- Test, S.L.; Noble, A.C.; Schmidt, J.O. Effect of pomace contact on Chardonnay musts and wines. Am. J. Enol. Vitic. 1986, 37, 133–136. [Google Scholar]
- Martínez-Gil, A.M.; Garde-Cerdán, T.; Lorenzo, C.; Lara, J.F.; Pardo, F.; Salinas, M.R. Volatile Compounds Formation in Alcoholic Fermentation from Grapes Collected at 2 Maturation Stages: Influence of Nitrogen Compounds and Grape Variety. J. Food Sci. 2012, 77, C71–C79. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Xu, Y.; Qin, L.; Wang, Y. Effects of different βD-glycosidases on bound aroma compounds in Muscat grape determined by HS-SPME and GC-MS. J. Inst. Brew. 2010, 116, 70–77. [Google Scholar] [CrossRef]
- Dennis, E.G.; Keyzers, R.; Kalua, C.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape Contribution to Wine Aroma: Production of Hexyl Acetate, Octyl Acetate, and Benzyl Acetate during Yeast Fermentation Is Dependent upon Precursors in the Must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef]
- Ferreira, V.; Fernández, P.; Cacho, J.F. A study of factors affecting wine volatile composition and its application in discriminant analysis. LWT Food Sci. Technol. 1996, 29, 251–259. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Camarasa, C.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.-M.; Dequin, S.; Mouret, J.-R. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.; Agosin, E. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Beltran, G.; Novo, M.; Leberre, V.; Sokol, S.; Labourdette, D.; Guillamon, J.-M.; Mas, A.; François, J.; Rozes, N. Integration of transcriptomic and metabolic analyses for understanding the global responses of low-temperature winemaking fermentations. Fems Yeast Res. 2006, 6, 1167–1183. [Google Scholar] [CrossRef]
- Torija, M.-J.; Beltran, G.; Novo, M.; Poblet, M.; Guillamon, J.; Mas, A.; Rozès, N. Effects of fermentation temperature and Saccharomyces species on the cell fatty acid composition and presence of volatile compounds in wine. Int. J. Food Microbiol. 2003, 85, 127–136. [Google Scholar] [CrossRef]
- Besada-Lombana, P.B.; Fernandez-Moya, R.; Fenster, J.; Da Silva, N.A. Engineering Saccharomyces cerevisiae fatty acid composition for increased tolerance to octanoic acid. Biotechnol. Bioeng. 2017, 114, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Legras, J.L.; Erny, C.; Le Jeune, C.; Lollier, M.; Adolphe, Y.; Demuyter, C.; Delobel, P.; Blondin, B.; Karst, F. Activation of Two Different Resistance Mechanisms in Saccharomyces cerevisiae upon Exposure to Octanoic and Decanoic Acids. Appl. Environ. Microbiol. 2010, 76, 7526–7535. [Google Scholar] [CrossRef] [PubMed]
- Mouret, J.; Camarasa, C.; Angenieux, M.; Aguera, E.; Perez, M.; Farines, V.; Sablayrolles, J. Kinetic analysis and gas-liquid balances of the production of fermentative aromas during winemaking fermentations: Effect of assimilable nitrogen and temperature. Food Res. Int. 2014, 62, 1–10. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Modulation of volatile sulfur compounds by wine yeast. Appl. Microbiol. Biotechnol. 2007, 74, 954–960. [Google Scholar] [CrossRef]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Carrau, F.M.; Medina, K.; Farina, L.; Boido, E.; Henschke, P.A.; Dellacassa, E. Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: Effects of yeast assimilable nitrogen on two model strains. Fems Yeast Res. 2008, 8, 1196–1207. [Google Scholar] [CrossRef]
- Jiménez-Martí, E.; Aranda, A.; Mendes-Ferreira, A.; Mendes-Faia, A.; Del Olmo, M.L. The nature of the nitrogen source added to nitrogen depleted vinifications conducted by a Saccharomyces cerevisiae strain in synthetic must affects gene expression and the levels of several volatile compounds. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2007, 92, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Ugliano, M.; Varela, C.; Siebert, T.; Pretorius, I.S.; Henschke, P.A. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Appl. Microbiol. Biotechnol. 2007, 77, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Siebert, T.E.; Varela, C.; Pretorius, I.S.; Henschke, P.A. Effect of ammonium nitrogen supplementation of grape juice on wine volatiles and non-volatiles composition of the aromatic grape variety Albarino. Food Chem. 2012, 133, 124–131. [Google Scholar] [CrossRef]
- Torrea, D.; Varela, C.; Ugliano, M.; Ancin-Azpilicueta, C.; Francis, L.; Henschke, P.A. Comparison of inorganic and organic nitrogen supplementation of grape juice—Effect on volatile composition and aroma profile of a Chardonnay wine fermented with Saccharomyces cerevisiae yeast. Food Chem. 2011, 127, 1072–1083. [Google Scholar] [CrossRef]
- Ugliano, M.; Travis, B.; Francis, I.L.; Henschke, P.A. Volatile Composition and Sensory Properties of Shiraz Wines as Affected by Nitrogen Supplementation and Yeast Species: Rationalizing Nitrogen Modulation of Wine Aroma. J. Agric. Food Chem. 2010, 58, 12417–12425. [Google Scholar] [CrossRef]
- Garde-Cerdan, T.; Ancin-Azpilicueta, C. Effect of the addition of different quantities of amino acids to nitrogen-deficient must on the formation of esters, alcohols, and acids during wine alcoholic fermentation. LWT-Food Sci. Technol. 2008, 41, 501–510. [Google Scholar] [CrossRef]
- Hernandez-Orte, P.; Bely, M.; Cacho, J.; Ferreira, V. Impact of ammonium additions on volatile acidity, ethanol, and aromatic compound production by different Saccharomyces cerevisiae strains during fermentation in controlled synthetic media. Aust. J. Grape Wine Res. 2006, 12, 150–160. [Google Scholar] [CrossRef]
- Beltran, G.; Esteve-Zarzoso, B.; Rozès, N.; Mas, A.; Guillamón, J.M. Influence of the timing of nitrogen additions during synthetic grape must fermentations on fermentation kinetics and nitrogen consumption. J. Agric. Food Chem. 2005, 53, 996–1002. [Google Scholar] [CrossRef]
- Bisson, L.F.; Karpel, J.E. Genetics of Yeast Impacting Wine Quality. Annu. Rev. Food Sci. Technol. 2010, 1, 139–162. [Google Scholar] [CrossRef]
- Ugliano, M.; Bartowsky, E.J.; McCarthy, J.; Moio, L.; Henschke, P.A. Hydrolysis and transformation of grape glycosidically bound volatile compounds during fermentation with three Saccharomyces yeast strains. J. Agric. Food Chem. 2006, 54, 6322–6331. [Google Scholar] [CrossRef]
- Vilanova, M.; Sieiro, C. Contribution by Saccharomyces cerevisiae yeast to fermentative flavour compounds in wines from cv. Albarino. J. Ind. Microbiol. Biotechnol. 2006, 33, 929–933. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wondra, M.; Berovic, M. Analyses of aroma components of chardonnay wine fermented by different yeast strains. Food Technol. Biotechnol. 2001, 39, 141–148. [Google Scholar]
- Molina, A.M.; Guadalupe, V.; Varela, C.; Swiegers, J.H.; Pretorius, I.S.; Agosin, E. Differential synthesis of fermentative aroma compounds of two related commercial wine yeast strains. Food Chem. 2009, 117, 189–195. [Google Scholar] [CrossRef]
- Vilela, A. Use of Nonconventional Yeasts for Modulating Wine Acidity. Fermentation 2019, 5, 27. [Google Scholar] [CrossRef]
- Vilela-Moura, A.; Schuller, D.; Mendes-Faia, A.; Côrte-Real, M. Reduction of volatile acidity of wines by selected yeast strains. Appl. Microbiol. Biotechnol. 2008, 80, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Lafon-Lafourcade, S.; MP, R.G. Utilisation de levains mixtes dans l élaboration’des vins de pourriture noble en vue de réduire lácidité volatile. Comptes Rendues Acad. Agric. 1981, 67, 616–622. [Google Scholar]
- Lencioni, L.; Taccari, M.; Ciani, M.; Domizio, P. Zygotorulaspora florentina and Starmerella bacillaris in multistarter fermentation with Saccharomyces cerevisiae to reduce volatile acidity of high sugar musts. Aust. J. Grape Wine Res. 2018, 24, 368–372. [Google Scholar] [CrossRef]
- Orte, P.H.; Guitart, A.; Ferreira, V.; Gracia, J.; Cacho, J. Effect of maceration time and the addition of enzymes on the amino acid composition of musts and wines and its influence on wine aroma. Food Sci. Technol. Int. 1998, 4, 407–418. [Google Scholar] [CrossRef]
- Suriano, S.; Basile, T.; Tarricone, L.; Di Gennaro, D.; Tamborra, P. Effects of skin maceration time on the phenolic and sensory characteristics of Bombino Nero rose wines. Ital. J. Agron. 2015, 10, 21–29. [Google Scholar] [CrossRef]
- Şener, H. Effect of Temperature and Duration of Maceration on Colour and Sensory Properties of Red Wine—A Review. S. Afr. J. Enol. Vitic. 2018, 39, 227–234. [Google Scholar] [CrossRef]
- Colagrande, O. Gené se des odeurs et de goût anormaux des vins. Rev. Oenol. 1989, 53, 25–27. [Google Scholar]
- Baumes, R.; Bayonove, C.; Barillere, J.M.; Samson, A.; Cordonnier, R.E. La maceration pelliculaire dans la vinification en blanc−incidence sur la composition volatile des vins. Vitis 1989, 28, 31–48. [Google Scholar]
- Panighel, A.; Flamini, R. Applications of solid-phase microextraction and gas chromatography/mass spectrometry (SPME-GC/MS) in the study of grape and wine volatile compounds. Molecules 2014, 19, 21291–21309. [Google Scholar] [CrossRef] [PubMed]
- Charters, S.; Pettigrew, S. The dimensions of wine quality. Food Qual. Prefer. 2007, 18, 997–1007. [Google Scholar] [CrossRef]
- Rodriguez-Bencomo, J.J.; Muñoz-González, C.; Andújar-Ortiz, I.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M.Á. Assessment of the effect of the non-volatile wine matrix on the volatility of typical wine aroma compounds by headspace solid phase microextraction/gas chromatography analysis. J. Sci. Food Agric. 2011, 91, 2484–2494. [Google Scholar] [CrossRef]
- Morakul, S. Etude et Modélisation de la Composition du gaz Fermentaire en Conditions Oenologiques: Intérêt pour le Contrôle de la Fermentation. Ph.D. Thesis, Centre International d’Etudes Superieures en Sciences Agronomiques, Montpellier Supagro, Montpellier, France, 2011. [Google Scholar]
- Lezaeta, A.; Bordeu, E.; Agosin, E.; Perez-Correa, J.; Varela, P. White wines aroma recovery and enrichment: Sensory-led aroma selection and consumer perception. Food Res. Int. 2018, 108, 595–603. [Google Scholar] [CrossRef]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Description of Alcoholic Fermentation Kinetics: Its Variability and Significance. Am. J. Enol. Vitic. 1990, 41, 319–324. [Google Scholar]
- Miller, G.C.; Amon, J.M.; Simpson, R.F. Loss of aroma compounds in carbon dioxide effluent during white wine fermentation. Food Technol. Aust. 1987, 39, 246. [Google Scholar]
- Hodson, E. Effects of Capture and Return on Chardonnay (Vitis vinifera L.) fermentation volatiles. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2004. [Google Scholar]
- Muller, C.J.; Wahlstrom, V.L.; Fugelsang, K.C. Capture and Use of Volatile Flavor Constituents Emitted during Wine Fermentation. In Beer and Wine Production: Analysis, Characterization, and Technological Advances; Gump, B.H., Ed.; American Chemical Society: San Francisco, CA, USA, 1993; pp. 219–232. [Google Scholar]
- Sablayrolles, J.M. Control of alcoholic fermentation in winemaking: Current situation and prospect. Food Res. Int. 2009, 42, 418–424. [Google Scholar] [CrossRef]
- Killian, E.; Ough, C.S. Fermentation Esters—Formation and Retention as Affected by Fermentation Temperature. Am. J. Enol. Vitic. 1979, 30, 301–305. [Google Scholar]
- Athès, V.; Lillo, M.P.Y.L.; Bernard, C.; Pérez-Correa, R.; Souchon, I. Comparison of experimental methods for measuring infinite dilution volatilities of aroma compounds in water/ethanol mixtures. J. Agric. Food Chem. 2004, 52, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.M.; Birkmyre, L.; Paterson, A.; Piggott, J.R. Headspace concentrations of ethyl esters at different alcoholic strengths. J. Sci. Food Agric. 1998, 77, 121–126. [Google Scholar] [CrossRef]
- Tsachaki, M.; Gady, A.-L.; Kalopesas, M.; Linforth, R.; Athès, V.; Marin, M.; Taylor, A.J. Effect of ethanol, temperature, and gas flow rate on volatile release from aqueous solutions under dynamic headspace dilution conditions. J. Agric. Food Chem. 2008, 56, 5308–5315. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias-Cervantes, M.; Champion, D.; Bongard, S.; Voilley, A. Effects of the nature and concentration of substrates in aqueous solutions on the solubility of aroma compounds. Flavour Fragr. J. 2005, 20, 265–273. [Google Scholar] [CrossRef]
- Nahon, D.F.; Koren, P.A.N.Y.; Roozen, J.P.; Posthumus, M.A. Flavor release from mixtures of sodium cyclamate, sucrose, and an orange aroma. J. Agric. Food Chem. 1998, 46, 4963–4968. [Google Scholar] [CrossRef]
- Ferreira, V.; Pena, C.; Escudero, A.; Cacho, J. Losses of volatile compounds during fermentation. Z. Lebensm. -Unters. Und-Forsch. 1996, 202, 318–323. [Google Scholar] [CrossRef]
- Guerrini, L.; Masella, P.; Spugnoli, P.; Spinelli, S.; Calamai, L.; Parenti, A. A Condenser to Recover Organic Volatile Compounds during Vinification. Am. J. Enol. Vitic. 2016, 67, 163–168. [Google Scholar] [CrossRef]
- D’Alberti, V.; Cammalleri, I.; La Bella, S.; Ragusa, M.; Pavan, M.; Ragusa, R. Production of Algae with CO2 from Wine Fermentation: An Important Way to Reduce Emissions. Experimental Tests on 4 Algal Strains. In Proceedings of the 23rd European Biomass Conference and Exhibition (EU BC and E) 2015, Vienna, Austria, 1–4 June 2015. [Google Scholar]
- Morakul, S.; Mouret, J.-R.; Nicolle, P.; Trelea, I.C.; Sablayrolles, J.-M.; Athes, V. Modelling of the gas-liquid partitioning of aroma compounds during wine alcoholic fermentation and prediction of aroma losses. Process Biochem. 2011, 46, 1125–1131. [Google Scholar] [CrossRef]
- Todd, D.F.; Castronovo, C.; Fugelsang, K.C.; Gump, B.H.; Muller, C.J. Ethanol emissions control from wine fermentation tanks using charcoal adsorption. A Pilot Study. Calif. Agric. Technol. Inst. 1990. Available online: http://hdl.handle.net/10211.3/180244 (accessed on 20 January 2022).
- Zoecklein, B.W.; Herns, R.; Whiton, R.S.; Mansfield, A. Capture and return of chardonnay volatiles during fermentation. Am. J. Enol. Vitic. 2000, 51, 432. [Google Scholar]
- Fontanille, P.; Larroche, C. Production of Food Additives. In Comprehensive Food Fermentation and Biotechnology; Asiatech Publishers Inc.: New Delhi, India, 2010; pp. 1071–1096. [Google Scholar]
- Berger, R.G. Aroma Biotechnology; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Schreiber, W.; Scharpf, L.J.; Katz, I. Flavors and Fragrances: The Chemistry Challenges; Chemtech: Washington, DC, USA, 1997; Volume 3, pp. 58–62. [Google Scholar]
- Fleming, H. Consider membrane pervaporation. Chem. Eng. Proc. 1992, 88, 46–52. [Google Scholar]
- Lethanh, M.; Voilley, A.; Tanluu, R.P. The influence of the composition of model liquid culture medium on vapor liquid partition coefficient of aroma substances. Sci. Aliment. 1993, 13, 699–710. [Google Scholar]
- Wright, A.J.; Pyle, D.L. An investigation into the use of the spinning cone column for in situ ethanol removal from a yeast broth. Process Biochem. 1996, 31, 651–658. [Google Scholar] [CrossRef]
- Jolly, D.R.P. Wine flavour extraction with liquid carbon dioxide. Process Biochem. 1981, 16, 36–40. [Google Scholar]
- Bengtson, G.; Boddeker, K.W.; Hanssen, H.-P.; Urbasch, I. Recovery of 6-pentyl-alpha-pyrone from Trichoderma viride culture medium by perevaporation. Biotechnol. Tech. 1992, 6, 23–26. [Google Scholar] [CrossRef]
- Schafer, T.; Bengtson, G.; Pingel, H.; Boddeker, K.W.; Crespo, J.P.S.G. Recovery of aroma compounds from a wine-must fermentation by organophilic pervaporation. Biotechnol. Bioeng. 1999, 62, 412–421. [Google Scholar] [CrossRef]
- Groot, W.J.; Kraayenbrink, M.R.; Waldram, R.H.; van der Lans, R.G.J.M.; Luyben, K.C.A.M. Ethanol production in an integrated process of fermentation and ethanol recovery by pervaporation. Bioprocess Eng. 1992, 8, 99–111. [Google Scholar] [CrossRef]
- Bengtsson, E.; Tragardh, G.; Hallstrom, B. Concentration of apple juice aroma from evaporator condensate using pervaporation. Food Sci. Technol. -Lebensm. -Wiss. Technol. 1992, 25, 29–34. [Google Scholar]
- Ishii, N.; Matsumura, M.; Kataoka, H.; Tanaka, H.; Araki, K. Diacetyl fermentation coupled with pervaporation using oleyl alcohol supported liquid membrane. Bioprocess Eng. 1995, 13, 119–123. [Google Scholar] [CrossRef]
- Karlsson, H.O.; Loureiro, S.; Trägårdh, G. Aroma compound recovery with pervaporation—Temperature effects during pervaporation of a muscat wine. J. Food Eng. 1995, 26, 177–191. [Google Scholar] [CrossRef]
- Moutounet, M.; Escudier, J.L.; Jouret, C. Production of spirits by pervaporation. Comparison with still distillation. Food Sci. Technol. -Lebensm. -Wiss. Technol. 1992, 25, 71–73. [Google Scholar]
- Lamer, T.; Spinnler, H.; Souchon, I.; Voilley, A. Extraction of benzaldehyde from fermentation broth by pervaporation. Process Biochem. 1996, 31, 533–542. [Google Scholar] [CrossRef]
- Rajagopalan, N.; Cheryan, M. Pervaporation of grape juice aroma. J. Membr. Sci. 1995, 104, 243–250. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food; Springer: New York, NY, USA, 2010; Volume 2, p. 596. [Google Scholar]
- Schultz, W.G.; Randall, J.M. Liquid carbon dioxide for selective aroma extraction. Food Technol. 1970, 24, 94–98. [Google Scholar]
- Medina, I.; Martinez, J.L. Dealcoholisation of cider by supercritical extraction with carbon dioxide. J. Chem. Technol. Biotechnol. 1997, 68, 14–18. [Google Scholar] [CrossRef]
- Perrut, M.; da Ponte, M.N. Fraccionement, Application A L’extraction Supercritique des Arômes de Boissons Fermentees et Distillees. In Proceedings of the 3eme Colloque sur les Fluides Supercritiques, Grasse, France, 29–30 January 1996; Volume 3, p. 4560. [Google Scholar]
- Perrut, M.; da Ponte, M.N. Aromas from fermented and distilled beverages by liquid–fluid fractionation. In Proceedings of the Fourth International Symposium on Supercritical Fluids, Sendeai, Japan, 11–14 May 1997. [Google Scholar]
- Señoráns, F.J.; Ruiz-Rodríguez, A.; Ibañez, E.; Tabera, J.; Reglero, G. Optimization of countercurrent supercritical fluid extraction conditions for spirits fractionation. J. Supercrit. Fluids 2001, 21, 41–49. [Google Scholar] [CrossRef]
- Señoráns, F.J.; Ruiz-Rodríguez, A.; Ibáñez, E.; Tabera, J.; Reglero, G. Isolation of brandy aroma by countercurrent supercritical fluid extraction. J. Supercrit. Fluids 2003, 26, 129–135. [Google Scholar] [CrossRef]
- Gamse, T.; Rogler, I.; Marr, R. Supercritical CO2 extraction for utilisation of excess wine of poor quality. J. Supercrit. Fluids 1999, 14, 123–128. [Google Scholar] [CrossRef]
- Del Re, G.; Di Giacomo, G. Continuous fractionation of wine with dense carbon dioxide. In Proceedings of the 5th Meeting on Supercritical Fluids, Nice, France, 23–25 March 1998. [Google Scholar]
- Wiesenberger, A.; Marr, R.; Kolb, E.; Schildmann, J.A.; Weisrock, R. Process for Producing Alcoholreduced or Alcoholfree Beverages Made by Natural Fermentation. U.S. Patent Application No. 4867997, 19 September 1989. [Google Scholar]
- Berger, F.; Sagi, F.; Cerles, B. Procédé d’Extraction de l’ Arôme de Boissons Alcoolisées Obtenues à Partir de Fruits ou de Produits Assimilés. European Patent Application No. 0129459, 30 December 1986. [Google Scholar]
- Macedo, S.; Fernandes, S.; Lopes, J.A.; De Sousa, H.C.; Pereira, P.; Carmelo, P.J.; Menduiña, C.; Simões, P.C.; Da Ponte, M.N. Recovery of Wine-Must Aroma Compounds by Supercritical CO2. Food Bioprocess Technol. 2008, 1, 74–81. [Google Scholar] [CrossRef]

| Higher Alcohol | Amino Acid Precursor | Aromatic Notes | Content in Wines | Odour Threshold |
|---|---|---|---|---|
| 2-methyl-butan-2-ol | Isoleucine | Nail polish, solvent malt | 30–100 mg/L | 30 mg/L |
| 3-methyl-butan-1-ol | Leucine | Alcohol notes, nail varnish, solvent amilic notes, malt | 80–300 mg/L | 30 mg/L |
| 2-methyl-propan-1-ol | Valine | Solvent, chemical alcoholic, malt notes, wineosity notes | 50–150 mg/L | 40 mg/L |
| Phenylethanol | Phenylalanine | Floral, rose, honey notes, peach notes | 10–100 mg/L | 10–14 mg/L |
| Methionol | Methionine | Crushed potatoes | 0–5 mg/L | 1 mg/L |
| Propan-1-ol | Alcohol, ripe fruits | 10–50 mg/L | 306 mg/L | |
| Butan-1-ol | Medicinal | 1–10 mg/L | 150 mg/L |
| Esters | Aromatic Note | Detection Threshold |
|---|---|---|
| Ethyl butanoate | Pineapple, strawberries | 20 µg/L |
| Ethyl hexanoate | Green apples, strawberries, blackberries | 14 µg/L |
| Ethyl octanoate | Floral, fruity, soap | 2 µg/L |
| Ethyl decanoate | Floral, fruity, soap | 200–500 µg/L |
| Ethyl acetate | Unpleasant, solvent, fruity | 12–14 mg/L |
| Butyl acetate | Banana, floral, fruity | 1 mg/L |
| Ethyl propanoate | Cherries | 10 µg/L |
| 2-methylbutyl acetate | Fruity | 5 µg/L |
| 3-methylbutyl acetate | Bananas, ripe apples, candy | 2000–3000 µg/L |
| 2-phenylethyl acetate | Rose, fruity | 2000 µg/L |
| Hexyl acetate | Pears, plums, bananas, currants | 15 mg/L |
| Method | Advantages | Disadvantages |
|---|---|---|
| Pervaporation | Can be operated continuously at low temperature | Membrane fouling of nonporous membrane |
| Does not require any extraction step | ||
| Does not exert high stress on the active biomass | ||
| Extraction by supercritical carbon dioxide | Alternative to conventional extraction with solvents | Technical issue with recovery of aroma compounds from the high-pressure stream |
| Technology for clean chemistry The result is recyclable CO2 and the desired product | ||
| Condensation | Clean and simple technique | Condensates include some off–aromas—the need of fractionation |
| No widespread commercial applications of this technology | ||
| Charcoal adsorption traps | Energy saving | Initial investment and maintenance costs are high |
| Impossible to completely capture all aromatic substances |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prusova, B.; Humaj, J.; Sochor, J.; Baron, M. Formation, Losses, Preservation and Recovery of Aroma Compounds in the Winemaking Process. Fermentation 2022, 8, 93. https://doi.org/10.3390/fermentation8030093
Prusova B, Humaj J, Sochor J, Baron M. Formation, Losses, Preservation and Recovery of Aroma Compounds in the Winemaking Process. Fermentation. 2022; 8(3):93. https://doi.org/10.3390/fermentation8030093
Chicago/Turabian StylePrusova, Bozena, Jakub Humaj, Jiri Sochor, and Mojmir Baron. 2022. "Formation, Losses, Preservation and Recovery of Aroma Compounds in the Winemaking Process" Fermentation 8, no. 3: 93. https://doi.org/10.3390/fermentation8030093
APA StylePrusova, B., Humaj, J., Sochor, J., & Baron, M. (2022). Formation, Losses, Preservation and Recovery of Aroma Compounds in the Winemaking Process. Fermentation, 8(3), 93. https://doi.org/10.3390/fermentation8030093






