Health Benefits of Postbiotics Produced by E. coli Nissle 1917 in Functional Yogurt Enriched with Cape Gooseberry (Physalis peruviana L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Chemicals and Reagents
2.1.2. Microbial Strains and Cells Line
2.1.3. Plant Materials
2.2. Preparation of Cape Gooseberry Juice (CGJ)
2.3. A Dual Acidification Process for the Preparation of Functional Yogurt
2.4. Preparation of Yogurt Water Extracts (Postbiotic)
2.5. Determination of Phenolic Profiles of YCG by HPLC
2.6. Antimicrobial Activity of YCG Supernatant
2.6.1. Estimation of Minimum Inhibitory Concentrations (MICs) of YCG Supernatants
2.6.2. Determination of Minimum Bactericidal Concentrations (MBC) or Minimum Fungicidal Concentration (MFC) of YCG Supernatant
2.7. Cytotoxicity Assay of YCG Supernatant
2.8. Total Phenolic Content Assay
2.9. Antioxidant Capacity
2.9.1. DPPH Inhibition Assay
2.9.2. Determination of Nitric Oxide (NO•) Radical Scavenging
2.10. E.coli Nissle 1917 (EcN) Counts
2.11. Sensory Evaluation
2.12. Statistical Analysis
3. Results
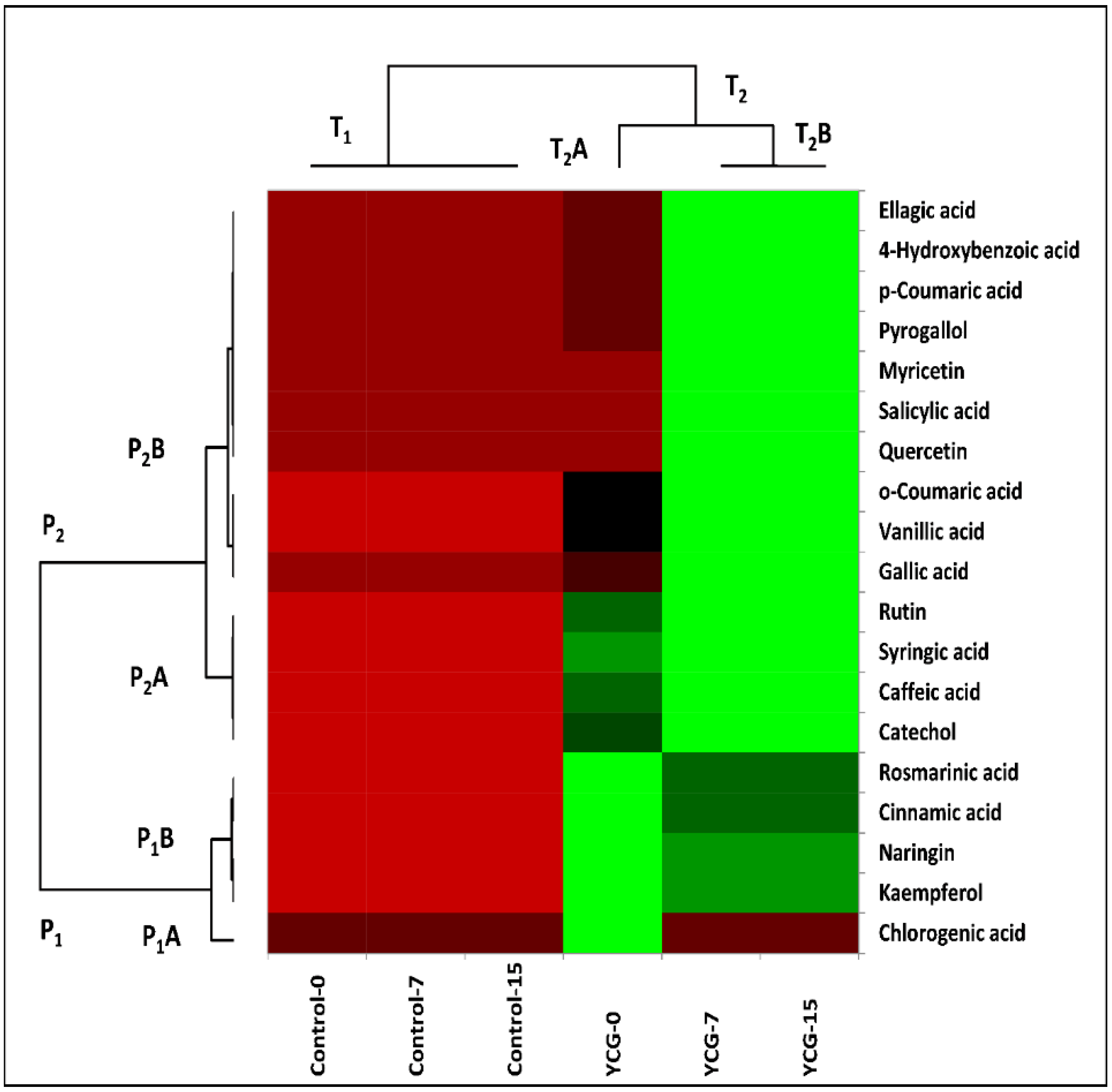
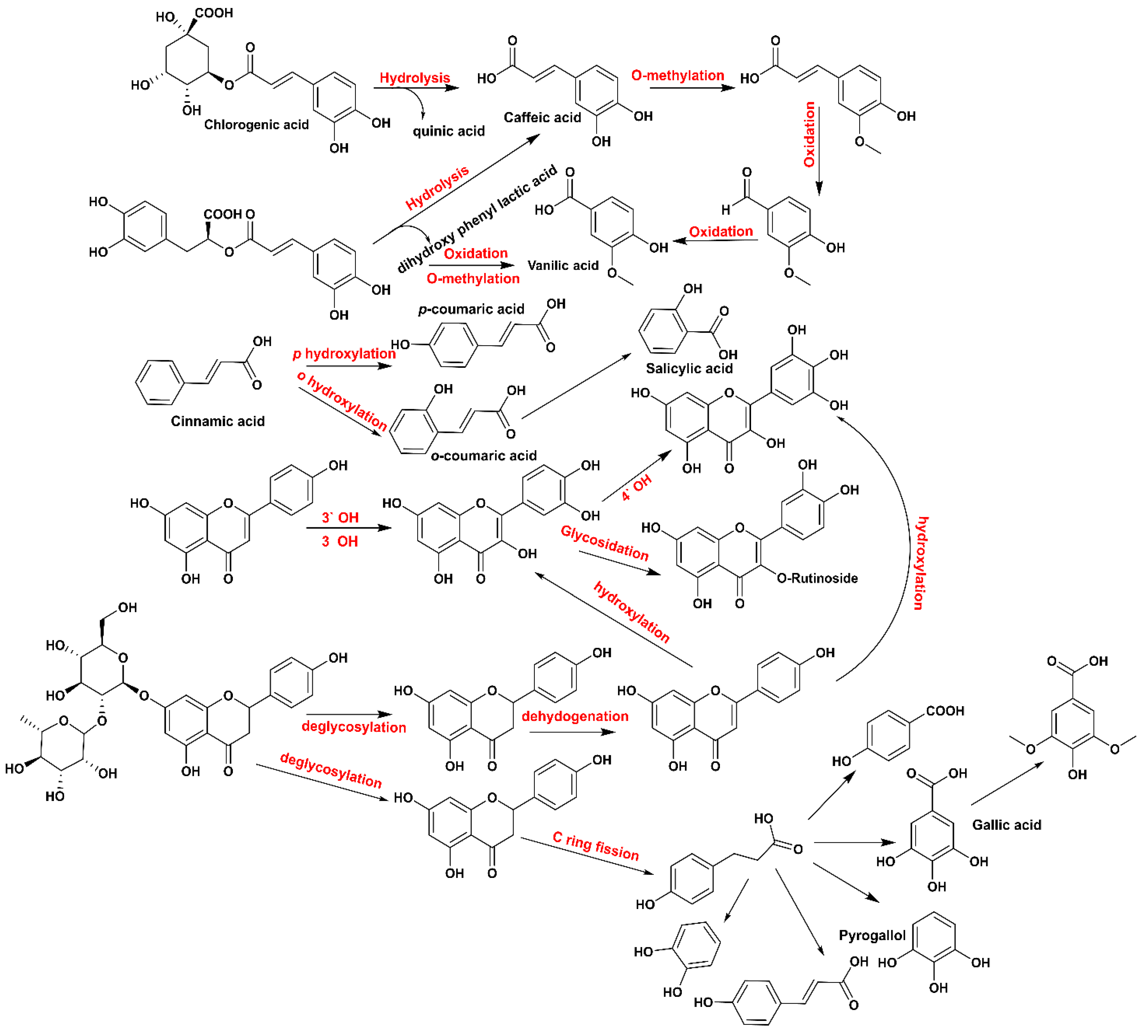
3.1. Phenolic Profiles of Yogurt
3.2. Antimicrobial Activity of YCG Supernatant
3.2.1. Minimum Inhibitory Concentrations (MIC) of YCG Supernatant
3.2.2. Minimum Bactericidal Concentrations (MBC) and Minimum Fungicidal Concentration (MFC) of YCG Supernatant
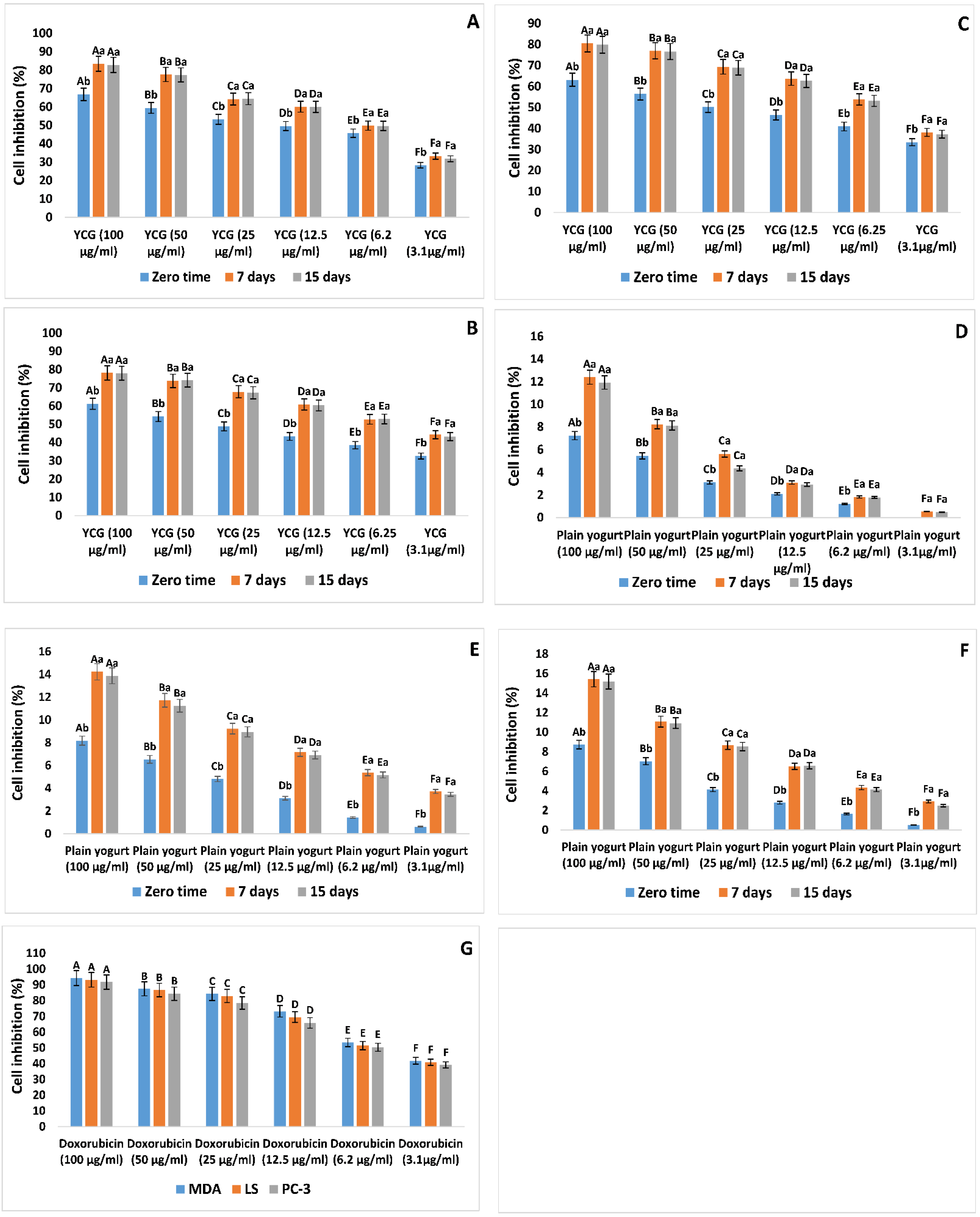
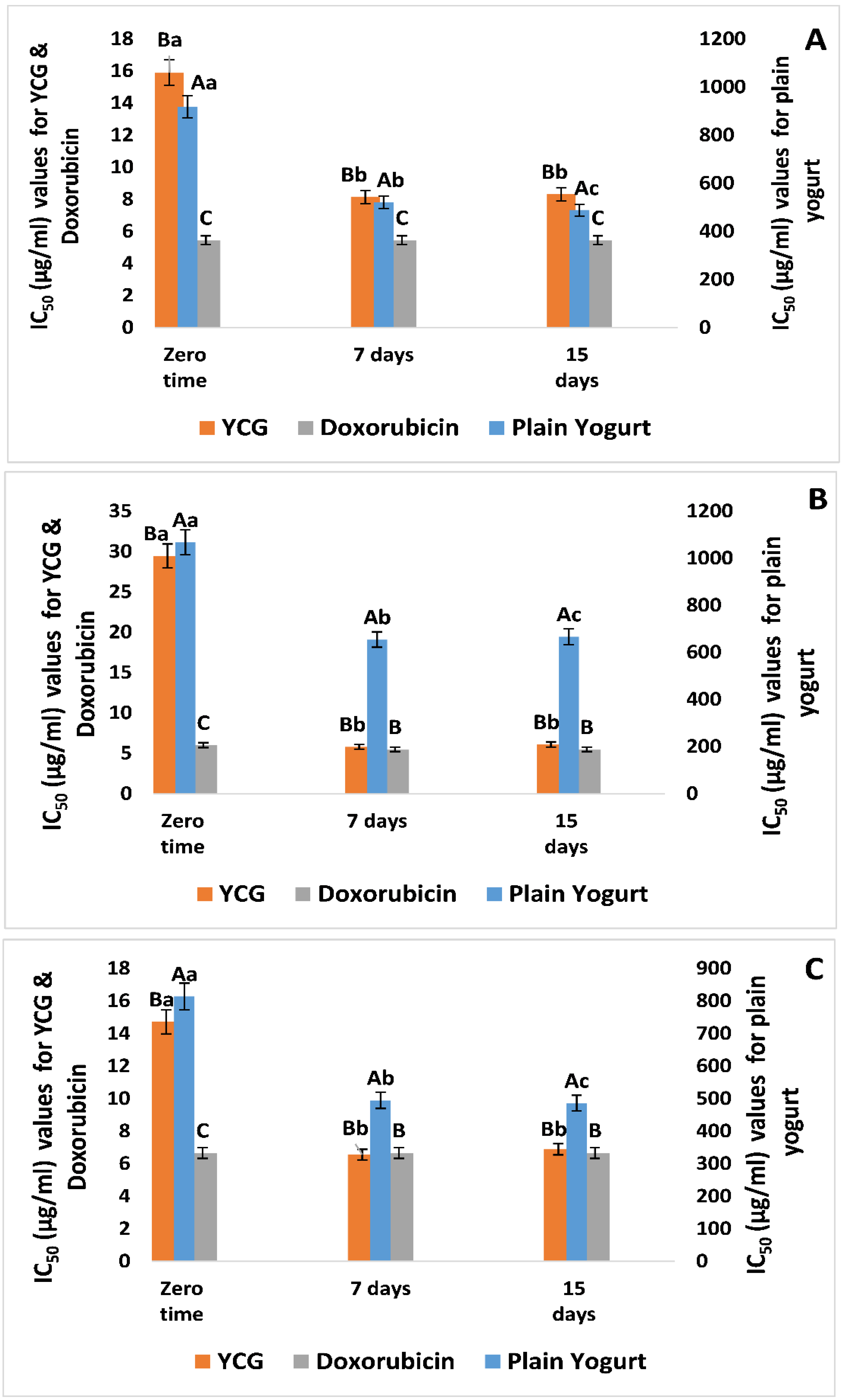
3.3. Cytotoxic Characteristics of YCG Supernatants
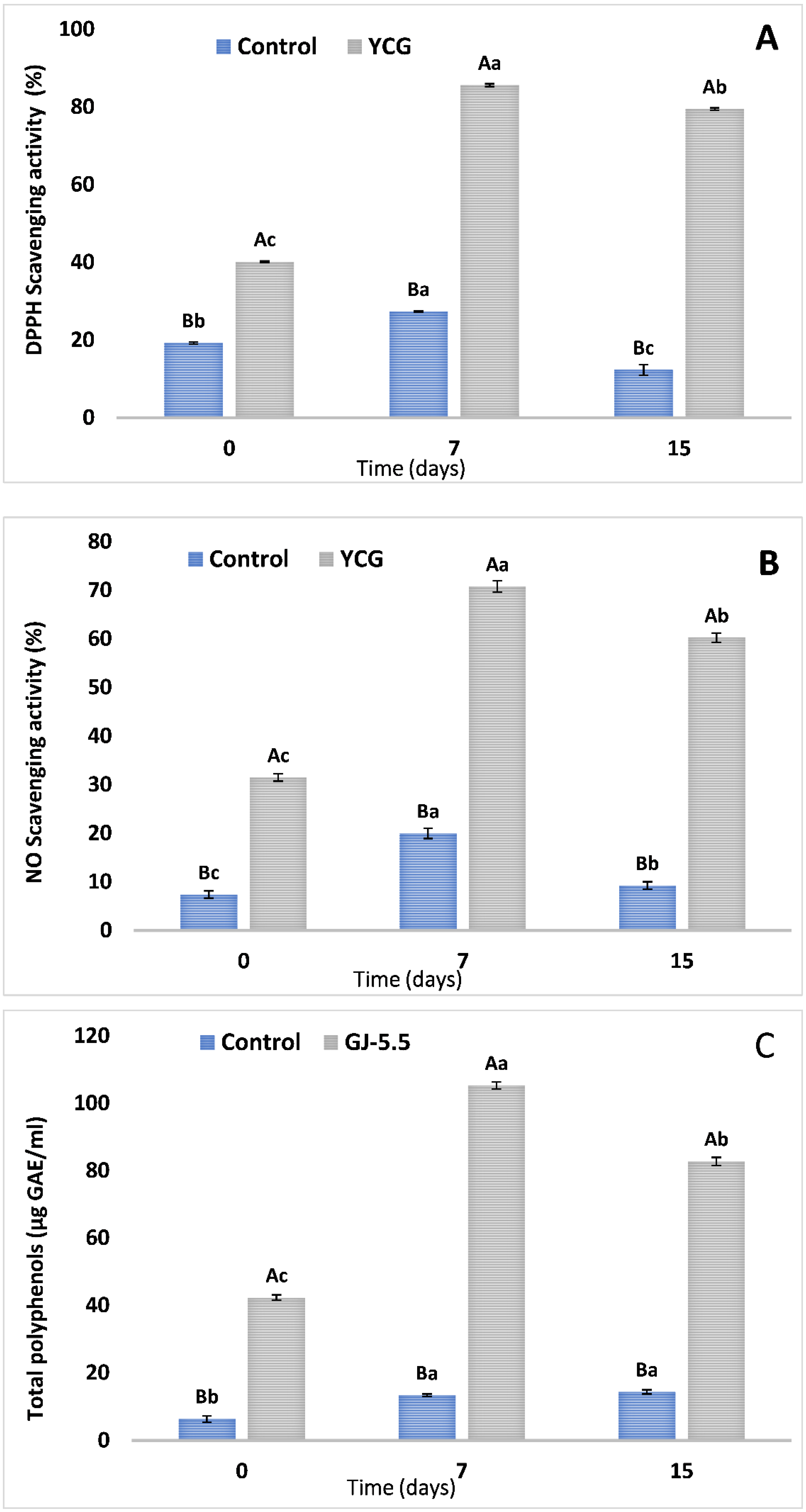
3.4. Assessment of Antioxidant Activities and Total Phenolic Components in Yogurt
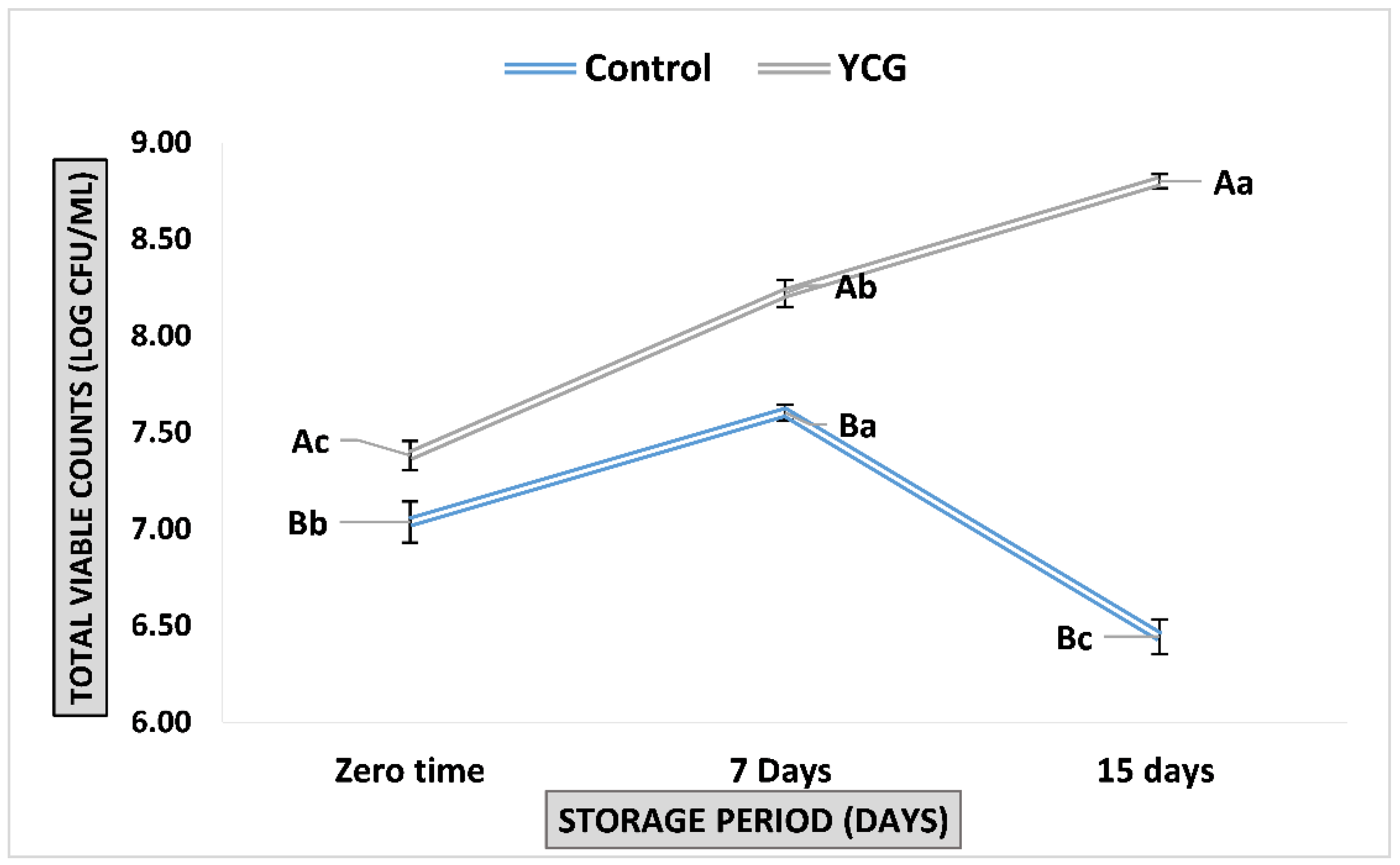
3.5. Total Viable Counts of E.coli Nissle 1917
3.6. Sensory Evaluation of Yogurt Samples
3.7. Multivariate Analysis of YCG Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazhand, A.; Souto, E.B.; Lucarini, M.; Souto, S.B.; Durazzo, A.; Santini, A. Ready to Use Therapeutical Beverages: Focus on Functional Beverages Containing Probiotics, Prebiotics and Synbiotics. Beverages 2020, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Bimbo, F.; Bonanno, A.; Nocella, G.; Viscecchia, R.; Nardone, G.; De Devitiis, B.; Carlucci, D. Consumers’ acceptance and preferences for nutrition-modified and functional dairy products: A systematic review. Appetite 2017, 113, 141–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltrán-Barrientos, L.; Hernández-Mendoza, A.; Torres-Llanez, M.; González-Córdova, A.; Vallejo-Córdoba, B. Invited review: Fermented milk as antihypertensive functional food. J. Dairy Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandylis, P.; Pissaridi, K.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A. Dairy and non-dairy probiotic beverages. Curr. Opin. Food Sci. 2016, 7, 58–63. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, M.S.A.; Aqil, F.; Singh, M. Microbial Applications in Agriculture and the Environment: A Broad Perspective. In Microbes and Microbial Technology: Agricultural and Environmental Applications; Ahmad, I., Ahmad, F., Pichtel, J., Eds.; Springer: New York, NY, USA, 2011; pp. 1–27. [Google Scholar]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size-and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [Green Version]
- Riaz, S.; Raza, Z.A.; Majeed, M.I.; Jan, T. Synthesis of zinc sulfide nanoparticles and their incorporation into poly (hydroxybutyrate) matrix in the formation of a novel nanocomposite. Mater. Res. Express 2018, 5, 055027. [Google Scholar] [CrossRef]
- Terzić-Vidojević, A.; Veljović, K.; Tolinački, M.; Živković, M.; Lukić, J.; Lozo, J.; Fira, Đ.; Jovčić, B.; Strahinić, I.; Begović, J.; et al. Diversity of non-starter lactic acid bacteria in autochthonous dairy products from Western Balkan Countries—Technological and probiotic properties. Food Res. Int. 2020, 136, 109494. [Google Scholar] [CrossRef]
- Schultz, M. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm. Bowel Dis. 2008, 14, 1012–1018. [Google Scholar] [CrossRef]
- Grozdanov, L.; Raasch, C.; Schulze, J.; Sonnenborn, U.; Gottschalk, G.; Hacker, J.; Dobrindt, U. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J. Bacteriol. 2004, 186, 5432–5441. [Google Scholar] [CrossRef] [Green Version]
- Caleja, C.; Barros, L.; Antonio, A.L.; Carocho, M.; Oliveira, M.B.; Ferreira, I.C. Fortification of yogurts with different antioxidant preservatives: A comparative study between natural and synthetic additives. Food Chem. 2016, 210, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Ramos, L.R.; Santos, J.S.; Daguer, H.; Valese, A.C.; Cruz, A.G.; Granato, D. Analytical optimization of a phenolic-rich herbal extract and supplementation in fermented milk containing sweet potato pulp. Food Chem. 2017, 221, 950–958. [Google Scholar] [CrossRef]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Basu, A. Phenolic compounds potential health benefits and toxicity. In Utilisation of Bioactive Compounds from Agricultural and Food Production Waste; Routledge: New York, NY, USA, 2017; pp. 27–59. [Google Scholar]
- de Llano, D.G.; Gil-Sánchez, I.; Esteban-Fernández, A.; Ramos, A.M.; Fernández-Díaz, M.; Cueva, C.; Moreno-Arribas, M.V.; Bartolomé, B. Reciprocal beneficial effects between wine polyphenols and probiotics: An exploratory study. Eur. Food Res. Technol. 2017, 243, 531–538. [Google Scholar] [CrossRef]
- Erkaya, T.; Dağdemir, E.; Şengül, M. Influence of Cape gooseberry (Physalis peruviana L.) addition on the chemical and sensory characteristics and mineral concentrations of ice cream. Food Res. Int. 2012, 45, 331–335. [Google Scholar] [CrossRef]
- Puente, L.A.; Pinto-Muñoz, C.A.; Castro, E.S.; Cortés, M. Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: A review. Food Res. Int. 2011, 44, 1733–1740. [Google Scholar] [CrossRef]
- Narváez-Cuenca, C.E.; Mateus-Gómez, Á.; Restrepo-Sánchez, L.P. Antioxidant capacity and total phenolic content of air-dried cape gooseberry (Physalis peruviana L.) at different ripeness stages. Agron. Colomb. 2014, 32, 232–237. [Google Scholar] [CrossRef]
- Tammu, J.; Ramana, K.V. Pharmacological review on Physalis species: A potential herbal cure-all. World J. Pharm. Res. 2015, 4, 247–256. [Google Scholar]
- Ramadan, M.F. Bioactive phytochemicals, nutritional value, and functional properties of cape gooseberry (Physalis peruviana): An overview. Food Res. Int. 2011, 44, 1830–1836. [Google Scholar] [CrossRef]
- De Souza, E.L.; de Albuquerque, T.M.R.; dos Santos, A.S.; Massa, N.M.L.; de Brito Alves, J.L. Potential interactions among phenolic compounds and probiotics for mutual boosting of their health-promoting properties and food functionalities—A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1645–1659. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Goñi, I. Dietary polyphenols and human gut microbiota: A review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Possemiers, S.; Bolca, S.; Verstraete, W.; Heyerick, A. The intestinal microbiome: A separate organ inside the body with the metabolic potential to influence the bioactivity of botanicals. Fitoterapia 2011, 82, 53–66. [Google Scholar] [CrossRef]
- Wegh, C.A.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and their potential applications in early life nutrition and beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [Green Version]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and flavanones are more bioavailable than previously perceived: A review of recent evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef]
- Stevens, Y.; Rymenant, E.V.; Grootaert, C.; Camp, J.V.; Possemiers, S.; Masclee, A.; Jonkers, D. The intestinal fate of citrus flavanones and their effects on gastrointestinal health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.A.; Serag, H.M.; Qadir, M.S.; Ramadan, M.F. Cape gooseberry (Physalis peruviana) juice as a modulator agent for hepatocellular carcinoma-linked apoptosis and cell cycle arrest. Biomed. Pharmacother. 2017, 94, 1129–1137. [Google Scholar] [CrossRef]
- Margean, A.; Lupu, M.I.; Alexa, E.; Padureanu, V.; Canja, C.M.; Cocan, I.; Negrea, M.; Calefariu, G.; Poiana, M.-A. An Overview of Effects Induced by Pasteurization and High-Power Ultrasound Treatment on the Quality of Red Grape Juice. Molecules 2020, 25, 1669. [Google Scholar] [CrossRef] [Green Version]
- Shori, A.; Baba, A. Antioxidant activity and inhibition of key enzymes linked to type-2 diabetes and hypertension by Azadirachta indica-yogurt. J. Saudi Chem. Soc. 2013, 17, 295–301. [Google Scholar] [CrossRef]
- Taher, M.; El-Daly, N.M.; El-Khateeb, A.Y.; Hassan, S.M.; Elsherbiny, E.A. Chemical Composition, Antioxidant, Antitumor and Antifungal Activities of Methanolic Extracts of Coleus blumei, Plectranthus amboinicus and Salvia splendens (Lamiaceae). J. Agric. Chem. Biotechnol. 2021, 12, 177–187. [Google Scholar] [CrossRef]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Drissi Kaitouni, L.B.; Merzouki, M.; Benlemlih, M. Phenolic profile (HPLC-UV) of olive leaves according to extraction procedure and assessment of antibacterial activity. Biotechnol. Rep. 2019, 23, e00347. [Google Scholar] [CrossRef]
- Jovanovic, M.; Petrovic, M.; Miocinovic, J.; Zlatanovic, S.; Lalicic Petronijevic, J.; Mitic-Culafic, D.; Gorjanovic, S. Bioactivity and Sensory Properties of Probiotic Yogurt Fortified with Apple Pomace Flour. Foods 2020, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Helvaci, S.; Kokdil, G.; Kawai, M.; Duran, N.; Duran, G.; Guvenc, A. Antimicrobial activity of the extracts and physalin D from Physalis alkekengi and evaluation of antioxidant potential of physalin D. Pharm. Biol. 2010, 48, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.A.; Tadros, L.K.; Dawood, D.H. Phytochemical constituents, antioxidant activity and safety evaluation of Kei-apple fruit (Dovyalis caffra). Food Chem. 2018, 265, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.A.; MennatAllah, E.; Tadros, L.K.; Sanad, M.I. The effects of new formulations based on Gum Arabic on antioxidant capacity of tomato (Solanum lycopersicum L.) fruit during storage. J. Food Meas. Charact. 2020, 14, 2489–2502. [Google Scholar] [CrossRef]
- Lee, S.-B.; Cosmas, B.; Park, H.-D. The Antimutagenic and Antioxidant Activity of Fermented Milk Supplemented with Cudrania tricuspidata Powder. Foods 2020, 9, 1762. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Hadi, J.; Gutierrez-Maddox, N.; Li, Y.; Leung, I.K.; Gao, Y.; Shu, Q.; Quek, S.-Y. Sensory, Microbiological and Physicochemical Characterisation of Functional Manuka Honey Yogurts Containing Probiotic Lactobacillus reuteri DPC16. Foods 2020, 9, 106. [Google Scholar] [CrossRef] [Green Version]
- Mingshu, L.; Kai, Y.; Qiang, H.; Dongying, J. Biodegradation of gallotannins and ellagitannins. J. Basic Microbiol. 2006, 46, 68–84. [Google Scholar] [CrossRef]
- Namiesnik, J.; Vearasilp, K.; Kupska, M.; Ham, K.-S.; Kang, S.-G.; Park, Y.-K.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Antioxidant activities and bioactive components in some berries. Eur. Food Res. Technol. 2013, 237, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.; Lin, C.; Wu, S.; Lin, D.; Wang, S.; Miaw, C.; Ng, L. Antioxidative and hepatoprotective effects of Physalis peruviana extract against acetaminophen-induced liver injury in rats. Pharm. Biol. 2008, 46, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Deng, K.J.; Zang, L.L.; Lan, X.H.; Zhong, Z.H.; Xiong, B.Q.; Zhang, Y.; Zheng, X.L. Antioxidant Components from Cape Gooseberry. J. Food Processing Preserv. 2016, 40, 893–898. [Google Scholar] [CrossRef]
- Muñoz, P.; Parra, F.; Simirgiotis, M.J.; Sepúlveda Chavera, G.F.; Parra, C. Chemical Characterization, Nutritional and Bioactive Properties of Physalis peruviana Fruit from High Areas of the Atacama Desert. Foods 2021, 10, 2699. [Google Scholar] [CrossRef]
- Bel-Rhlid, R.; Thapa, D.; Kraehenbuehl, K.; Hansen, C.E.; Fischer, L. Biotransformation of caffeoyl quinic acids from green coffee extracts by Lactobacillus johnsonii NCC 533. Amb Express 2013, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Wu, H.; He, Y.; Pan, W.; Yan, Z.; Liao, Y.; Peng, W.; Gan, L.; Zhang, Y.; Su, W. Simultaneously quantitative analysis of naringin and its major human gut microbial metabolites naringenin and 3-(4′-hydroxyphenyl) propanoic acid via stable isotope deuterium-labeling coupled with RRLC-MS/MS method. Molecules 2019, 24, 4287. [Google Scholar] [CrossRef] [Green Version]
- Raimondi, S.; Anighoro, A.; Quartieri, A.; Amaretti, A.; Tomás-Barberán, F.A.; Rastelli, G.; Rossi, M. Role of bifidobacteria in the hydrolysis of chlorogenic acid. Microbiologyopen 2015, 4, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Guo, Z.; Cai, Z. Combination of β-elimination and liquid chromatography/quadrupole time-of-flight mass spectrometry for the determination of O-glycosylation sites. Talanta 2009, 78, 358–363. [Google Scholar] [CrossRef]
- Cavia-Saiz, M.; Busto, M.D.; Pilar-Izquierdo, M.C.; Ortega, N.; Perez-Mateos, M.; Muñiz, P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: A comparative study. J. Sci. Food Agric. 2010, 90, 1238–1244. [Google Scholar] [CrossRef]
- Lin, S.; Zhu, Q.; Wen, L.; Yang, B.; Jiang, G.; Gao, H.; Chen, F.; Jiang, Y. Production of quercetin, kaempferol and their glycosidic derivatives from the aqueous-organic extracted residue of litchi pericarp with Aspergillus awamori. Food Chem. 2014, 145, 220–227. [Google Scholar] [CrossRef]
- Burapan, S.; Kim, M.; Han, J. Curcuminoid demethylation as an alternative metabolism by human intestinal microbiota. J. Agric. Food Chem. 2017, 65, 3305–3310. [Google Scholar] [CrossRef]
- Aura, A.-M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Thilakarathna, W.W.; Langille, M.G.; Rupasinghe, H.V. Polyphenol-based prebiotics and synbiotics: Potential for cancer chemoprevention. Curr. Opin. Food Sci. 2018, 20, 51–57. [Google Scholar] [CrossRef]
- Liasi, S.A.; Azmi, T.I.; Hassan, M.D.; Shuhaimi, M.; Rosfarizan, M.; Ariff, A.B. Antimicrobial activity and antibiotic sensitivity of three isolates of lactic acid bacteria from fermented fish product, Budu. Malays. J. Microbiol. 2009, 5, 33–37. [Google Scholar]
- Kareem, K.Y.; Hooi Ling, F.; Teck Chwen, L.; May Foong, O.; Anjas Asmara, S. Inhibitory activity of postbiotic produced by strains of Lactobacillus plantarum using reconstituted media supplemented with inulin. Gut Pathog. 2014, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
- Kantouch, A.; El-Sayed, A.A.; Salama, M.; El-Kheir, A.A.; Mowafi, S. Salicylic acid and some of its derivatives as antibacterial agents for viscose fabric. Int. J. Biol. Macromol. 2013, 62, 603–607. [Google Scholar] [CrossRef]
- Mason, T.L. Inactivation of red beet β-glucan synthase by native and oxidized phenolic compounds. Phytochemistry 1987, 26, 2197–2202. [Google Scholar] [CrossRef]
- Simões, C.C.; Araújo, D.B.d.; Araújo, R.P.C.d. Study, in vitro and ex vivo, of the action of different concentrations of propolis extracts against microorganisms present in human saliva. Rev. Bras. Farmacogn. 2008, 18, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram-Positive Bacterial Infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.; El-Ghorab, A.; Ghanem, K. Volatile compounds, antioxidants, and anticancer activities of Cape gooseberry fruit (Physalis peruviana L.): An in vitro study. J. Arab Soc. Med. Res. 2015, 10, 56–64. [Google Scholar] [CrossRef]
- Xu, Y.-M.; Wijeratne, E.K.; Babyak, A.L.; Marks, H.R.; Brooks, A.D.; Tewary, P.; Xuan, L.-J.; Wang, W.-Q.; Sayers, T.J.; Gunatilaka, A.L. Withanolides from aeroponically grown Physalis peruviana and their selective cytotoxicity to prostate cancer and renal carcinoma cells. J. Nat. Prod. 2017, 80, 1981–1991. [Google Scholar] [CrossRef]
- Ahn, H.; Im, E.; Lee, D.Y.; Lee, H.-J.; Jung, J.H.; Kim, S.-H. Antitumor effect of Pyrogallol via miR-134 mediated S phase arrest and inhibition of PI3K/AKT/Skp2/cMyc signaling in hepatocellular carcinoma. Int. J. Mol. Sci. 2019, 20, 3985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Al-Olayan, E.M.; El-Khadragy, M.F.; Omer, S.A.; Shata, M.T.M.; Kassab, R.B.; Abdel Moneim, A.E. The beneficial effect of Cape gooseberry juice on carbon tetrachloride-induced neuronal damage. CNS Neurol. Disord.-Drug Targets 2016, 15, 344–350. [Google Scholar] [CrossRef]
- Blum, U. Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on allelopathic interactions. J. Chem. Ecol. 1998, 24, 685–708. [Google Scholar] [CrossRef]
- Lourens-Hattingh, A.; Viljoen, B.C. Yogurt as probiotic carrier food. Int. Dairy J. 2001, 11, 1–17. [Google Scholar] [CrossRef]
- Correia, I.; Nunes, A.; Duarte, I.F.; Barros, A.; Delgadillo, I. Sorghum fermentation followed by spectroscopic techniques. Food Chem. 2005, 90, 853–859. [Google Scholar] [CrossRef]



| Microorganism | Inhibition Zone (mm) Supernatants. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plain Yogurt | YCG (µg/mL) | Amikacin (µg/mL) | Fluconazole (µg/mL) | |||||||
| 100 µg/mL | MIC | MBC | 30 µg/mL | MIC | MBC | 100 µg/mL | MIC | MBC | ||
| Zero Time | ||||||||||
| Gram (−) Bacteria | ||||||||||
| K.pneumoniae | - | 18.2 Bb* | 12.5 | 25 | 24.9 A | 6.15 | 12.5 | - | - | - |
| E.coli | - | 19.2 Bb | 25 | 50 | 29.1 A | 3.1 | 6.15 | - | - | - |
| E.cloacae | - | 17.1 Bb | 25 | 50 | 23.2 A | 12.5 | 25 | - | - | - |
| P. aeruginosa | - | 23.8 Bb | 12.5 | 25 | 25.3 A | 6.15 | 12.5 | - | - | - |
| P. vulgaris | - | 17.4 Bb | 12.5 | 25 | 21.5 A | 12.5 | 25 | - | - | - |
| Gram (+) bacteria | ||||||||||
| B. cereus | - | 23.2 Bb | 6.15 | 6.15 | 26.8 A | 6.15 | 12.5 | - | - | - |
| B.subtilis | - | 26.6 Bb | 6.15 | 12.5 | 29.4 A | 6.15 | 12.5 | - | - | - |
| S. aureus | - | 27.5 Ab | 6.15 | 12.5 | 24.5 B | 3.1 | 6.15 | - | - | - |
| E. faecalis | - | 23.7 Ab | 6.15 | 12.5 | 22.1 B | 6.15 | 12.5 | - | - | - |
| S. epidermidis | - | 22.3 Bb | 6.15 | 12.5 | 32.7 A | 3.1 | 6.15 | - | - | - |
| Fungi | ||||||||||
| C. parapsilosis | - | 11.6 Bb | 50 | 100 | - | - | - | 23.2 A | 12.5 | 25 |
| C. krusei | - | 12.1 Bb | 25 | 50 | - | - | - | 27.4 A | 6.15 | 12.5 |
| C. albicans | - | 12.5 Bb | 50 | 100 | - | - | - | 22.9 A | 12.5 | 25 |
| C. tropicalis | - | 10.1 Bb | 50 | 100 | - | - | - | 22.3 A | 25 | 50 |
| C. glabrata | - | 13.7 Bb | 25 | 50 | - | - | - | 30.2 A | 6.15 | 12.5 |
| 7 days | ||||||||||
| Gram (−) bacteria | ||||||||||
| K.pneumoniae | - | 22.7 Ba | 1 2.5 | 25 | 24.9 A | 6.15 | 12.5 | - | - | - |
| E.coli | - | 24.4 Ba | 6.15 | 12.5 | 29.1 A | 3.1 | 6.15 | - | - | - |
| E.cloacae | - | 21.3 Ba | 12.5 | 12.5 | 23.2 A | 12.5 | 25 | - | - | - |
| P. aeruginosa | - | 26.7 Aa | 3.1 | 6.15 | 25.3 B | 6.15 | 12.5 | - | - | - |
| P. vulgaris | - | 22.5 Aa | 12.5 | 25 | 21.5 B | 12.5 | 25 | - | - | - |
| Gram (+) bacteria | ||||||||||
| B. cereus | - | 27.5 Aa | 6.15 | 6.15 | 26.8 A | 6.15 | 12.5 | - | - | - |
| B.subtilis | - | 28.8 Aa | 6.15 | 12.5 | 29.4 A | 6.15 | 12.5 | - | - | - |
| S. aureus | - | 29.5 Aa | 3.1 | 6.15 | 24.5 B | 3.1 | 6.15 | - | - | - |
| E. faecalis | - | 26.7 Aa | 3.1 | 6.15 | 22.1 B | 6.15 | 12.5 | - | - | - |
| S. epidermidis | - | 29.2 Ba | 6.15 | 12.5 | 32.7 A | 3.1 | 6.15 | - | - | - |
| Fungi | ||||||||||
| C. parapsilosis | - | 18.2 Ba | 12.5 | 25 | - | - | - | 23.2 A | 12.5 | 25 |
| C. krusei | - | 19.1 Ba | 12.5 | 25 | - | - | - | 27.4 A | 6.15 | 12.5 |
| C. albicans | - | 18.5 Ba | 12.5 | 25 | - | - | - | 22.9 A | 12.5 | 25 |
| C. tropicalis | - | 18.1 Ba | 25 | 50 | - | - | - | 22.3 A | 25 | 50 |
| C. glabrata | - | 19.7 Ba | 12.5 | 25 | - | - | - | 30.2 A | 6.15 | 12.5 |
| 15 days | ||||||||||
| Gram (−) bacteria | ||||||||||
| K.pneumoniae | - | 22.3 Ba | 12.5 | 25 | 24.9 A | 6.15 | 12.5 | - | - | - |
| E.coli | - | 24.0 Ba | 6.15 | 12.5 | 29.1 A | 3.1 | 6.15 | - | - | - |
| E.cloacae | - | 20.8 Ba | 12.5 | 12.5 | 23.2 B | 12.5 | 25 | - | - | - |
| P. aeruginosa | - | 26.5 Aa | 3.1 | 6.15 | 25.3 B | 6.15 | 12.5 | - | - | - |
| P. vulgaris | - | 22.1 Aa | 12.5 | 25 | 21.5 A | 12.5 | 25 | - | - | - |
| Gram (+) bacteria | ||||||||||
| B. cereus | - | 27.1 Aa | 6.15 | 6.15 | 26.8 A | 6.15 | 12.5 | - | - | - |
| B.subtilis | - | 28.2 Aa | 6.15 | 12.5 | 29.4 A | 6.15 | 12.5 | - | - | - |
| S. aureus | - | 29.0 Aa | 3.1 | 6.15 | 24.5 B | 3.1 | 6.15 | - | - | - |
| E. faecalis | - | 26.1 Aa | 3.1 | 6.15 | 22.1 B | 6.15 | 12.5 | - | - | - |
| S. epidermidis | - | 29.6 Ba | 6.15 | 12.5 | 32.7 A | 3.1 | 6.15 | - | - | - |
| Fungi | ||||||||||
| C. parapsilosis | - | 17.8 Ba | 12.5 | 25 | - | - | - | 23.2 A | 12.5 | 25 |
| C. krusei | - | 18.8 Ba | 12.5 | 25 | - | - | - | 27.4 A | 6.15 | 12.5 |
| C. albicans | - | 18.1 Ba | 12.5 | 25 | - | - | - | 22.9 A | 12.5 | 25 |
| C. tropicalis | - | 17.7 Ba | 25 | 50 | - | - | - | 22.3 A | 25 | 50 |
| C. glabrata | - | 19.4 Ba | 12.5 | 25 | - | - | - | 30.2 A | 6.15 | 12.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, M.S.; Qiu, L.; Taher, M.A.; Zaki, A.A.; Abou-Zeid, N.A.; Dawood, D.H.; Shalabi, O.M.A.K.; Khojah, E.; Elawady, A.A. Health Benefits of Postbiotics Produced by E. coli Nissle 1917 in Functional Yogurt Enriched with Cape Gooseberry (Physalis peruviana L.). Fermentation 2022, 8, 128. https://doi.org/10.3390/fermentation8030128
Darwish MS, Qiu L, Taher MA, Zaki AA, Abou-Zeid NA, Dawood DH, Shalabi OMAK, Khojah E, Elawady AA. Health Benefits of Postbiotics Produced by E. coli Nissle 1917 in Functional Yogurt Enriched with Cape Gooseberry (Physalis peruviana L.). Fermentation. 2022; 8(3):128. https://doi.org/10.3390/fermentation8030128
Chicago/Turabian StyleDarwish, Mohamed Samir, Longxin Qiu, Mohamed A. Taher, Ahmed A. Zaki, Noha A. Abou-Zeid, Dawood H. Dawood, Ola M. A. K. Shalabi, Ebtihal Khojah, and Asmaa A. Elawady. 2022. "Health Benefits of Postbiotics Produced by E. coli Nissle 1917 in Functional Yogurt Enriched with Cape Gooseberry (Physalis peruviana L.)" Fermentation 8, no. 3: 128. https://doi.org/10.3390/fermentation8030128
APA StyleDarwish, M. S., Qiu, L., Taher, M. A., Zaki, A. A., Abou-Zeid, N. A., Dawood, D. H., Shalabi, O. M. A. K., Khojah, E., & Elawady, A. A. (2022). Health Benefits of Postbiotics Produced by E. coli Nissle 1917 in Functional Yogurt Enriched with Cape Gooseberry (Physalis peruviana L.). Fermentation, 8(3), 128. https://doi.org/10.3390/fermentation8030128









