Bio-Based Processes for Material and Energy Production from Waste Streams under Acidic Conditions
Abstract
1. Introduction
2. Moderate Acidic pH Bioprocesses (pH 4–6)
2.1. Anaerobic Volatile Fatty Acids Production by Mixed Culture Fermentation
2.2. Aerobic Volatile Fatty Acid Production by Pure Culture Fermentation
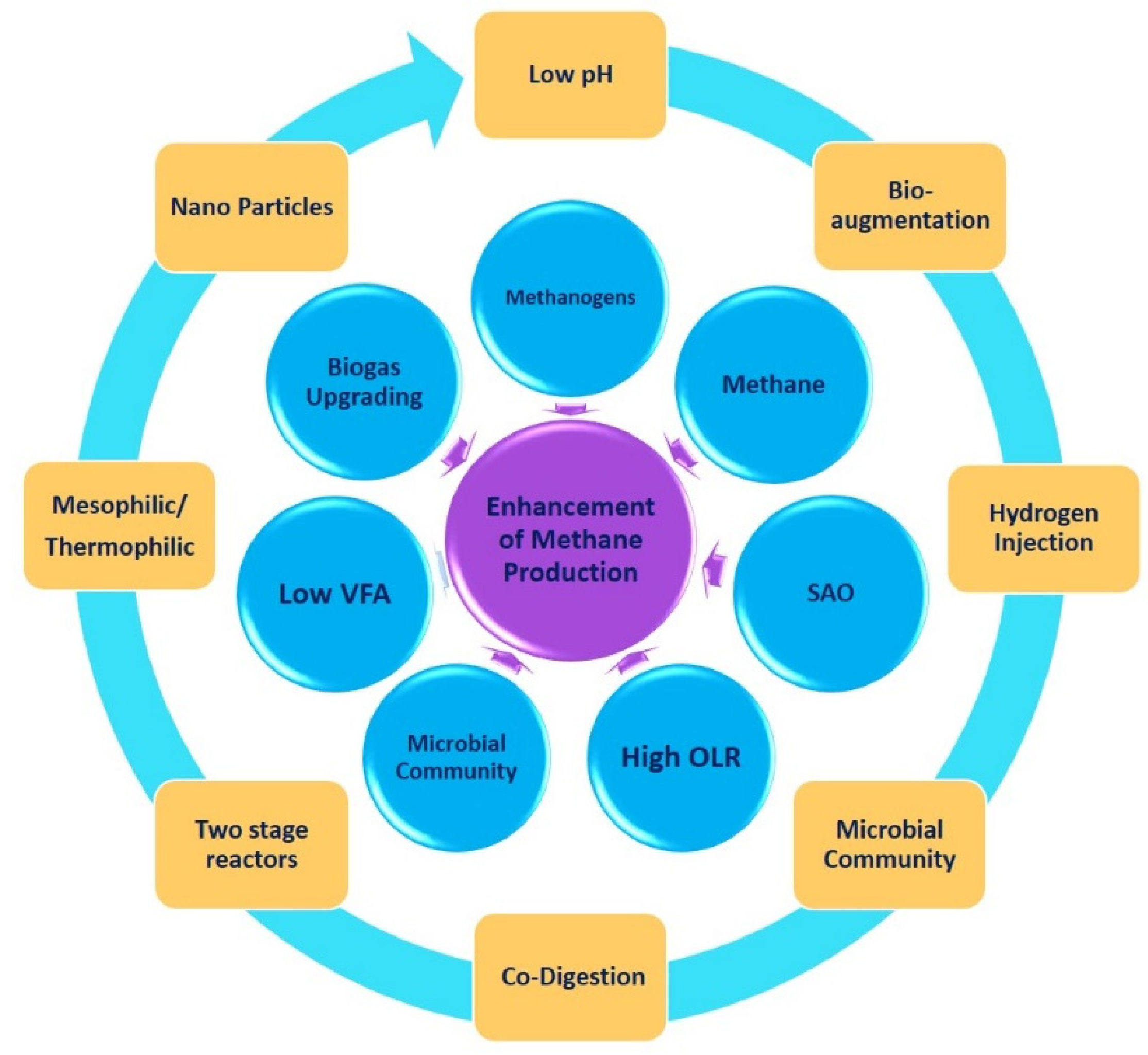
2.3. Biomethane
2.4. Biohydrogen
2.5. Metal Solution Produced by Organic Acids
3. Extreme Acidic pH Bioprocess (pH 1–3)
4. Future Perspectives and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mussatto, S.I. Challenges in Building a Sustainable Biobased Economy. Ind. Crops Prod. 2017, 106, 1–2. [Google Scholar] [CrossRef]
- Van Schoubroeck, S.; Van Dael, M.; Van Passel, S.; Malina, R. A review of sustainability indicators for biobased chemicals. Renew. Sustain. Energy Rev. 2018, 94, 115–126. [Google Scholar] [CrossRef]
- Chen, Y.; Nielsen, J. ScienceDirect Biobased organic acids production by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016, 37, 165–172. [Google Scholar] [CrossRef]
- Sedlakova-Kadukova, J.; Marcincakova, R.; Mrazikova, A.; Willner, J.; Fornalczyk, A. Closing the loop: Key role of iron in metal-bearing waste recycling. Arch. Metall. Mater. 2017, 62, 1459–1466. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Cheng, Y.-K. Effects of sulfur dosage and inoculum size on pilot-scale thermophilic bioleaching of heavy metals from sewage sludge. Chemosphere 2019, 234, 346–355. [Google Scholar] [CrossRef]
- Wei, X.; Liu, D.; Huang, W.; Huang, W.; Lei, Z. Simultaneously enhanced Cu bioleaching from E-wastes and recovered Cu ions by direct current electric field in a bioelectrical reactor. Bioresour. Technol. 2020, 298, 122566. [Google Scholar] [CrossRef]
- Yu, Z.; Han, H.; Feng, P.; Zhao, S.; Zhou, T.; Kakade, A.; Kulshrestha, S.; Majeed, S.; Li, X. Recent advances in the recovery of metals from waste through biological processes. Bioresour. Technol. 2020, 297, 122416. [Google Scholar] [CrossRef]
- European Commission. The European Green Deal; European Commission: Luxembourg, 2019. [Google Scholar]
- Vidra, A.; Németh, Á. Bio-produced acetic acid: A review. Period. Polytech. Chem. Eng. 2018, 62, 57–67. [Google Scholar] [CrossRef]
- Qu, Y.; Lian, B. Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresour. Technol. 2013, 136, 16–23. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Li, L.; Yuan, Z. Acclimation of acid-tolerant methanogenic propionate-utilizing culture and microbial community dissecting. Bioresour. Technol. 2018, 250, 117–123. [Google Scholar] [CrossRef]
- Sołowski, G.; Konkol, I.; Cenian, A. Methane and hydrogen production from cotton waste by dark fermentation under anaerobic and micro-aerobic conditions. Biomass Bioenergy 2020, 138, 105576. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Influence of initial uncontrolled pH on acidogenic fermentation of brewery spent grains to biohydrogen and volatile fatty acids production: Optimization and scale-up. Bioresour. Technol. 2021, 319, 124233. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Yang, Y.-H. Microbial production of volatile fatty acids: Current status and future perspectives. Rev. Environ. Sci. Bio./Technol. 2017, 16, 327–345. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Spekreijse, J.; Lammens, T.; Parisi, C.; Ronzon, T.; Vis, M. Insights into the European Market of bio-based chemicals. In Analysis Based on Ten Key Product Categories; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Varjani, S.; Liu, Y.; Deng, L.; Cheng, C. Selective production of volatile fatty acids at different pH in an anaerobic membrane bioreactor. Bioresour. Technol. 2019, 283, 120–128. [Google Scholar] [CrossRef]
- Yu, X.; Yin, J.; Shen, D.; Shentu, J.; Long, Y.; Chen, T. Improvement of acidogenic fermentation for volatile fatty acid production from protein-rich substrate in food waste. Waste Manag. 2018, 74, 177–184. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Schnürer, A.; Cetecioglu, Z. Volatile fatty acids production via mixed culture fermentation: Revealing the link between pH, inoculum type and bacterial composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef]
- de Lemos Chernicharo, C.A. Anaerobic Reactors; IWA Publishing: London, UK, 2015; Volume 6, ISBN 9781843391647. [Google Scholar]
- Meng, Y.; Mumme, J.; Xu, H.; Wang, K. A biologically inspired variable-pH strategy for enhancing short-chain fatty acids (SCFAs) accumulation in maize straw fermentation. Bioresour. Technol. 2016, 201, 329–336. [Google Scholar] [CrossRef]
- Joyce, A.; Ijaz, U.Z.; Nzeteu, C.; Vaughan, A.; Shirran, S.L.; Botting, C.H.; Quince, C.; O’Flaherty, V.; Abram, F. Linking microbial community structure and function during the acidified anaerobic digestion of grass. Front. Microbiol. 2018, 9, 540. [Google Scholar] [CrossRef] [PubMed]
- Weissbrodt, D.G.; Laureni, M.; van Loosdrecht, M.C.M.; Comeau, Y.; Chen, G.; Ekama, G.A.; van Loosdrecht, M.C.M.; Brdjanovic, D. (Eds.) Basic microbiology and metabolism. In Biological Wastewater Treatment: Principles, Modeling and Design; IWA Publishing: London, UK, 2020; ISBN 9781789060362. [Google Scholar]
- Zhang, B.; Zhang, L.-L.; Zhang, S.-C.; Shi, H.-Z.; Cai, W.-M. The Influence of pH on Hydrolysis and Acidogenesis of Kitchen Wastes in Two-phase Anaerobic Digestion. Environ. Technol. 2005, 26, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.; Chwialkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Volatile fatty acids production during mixed culture fermentation—The impact of substrate complexity and pH. Chem. Eng. J. 2017, 326, 901–910. [Google Scholar] [CrossRef]
- Feng, K.; Wang, Q.; Li, H.; Zhang, Y.; Deng, Z.; Liu, J.; Du, X. Effect of fermentation type regulation using alkaline addition on two-phase anaerobic digestion of food waste at different organic load rates. Renew. Energy 2020, 154, 385–393. [Google Scholar] [CrossRef]
- van Aarle, I.M.; Perimenis, A.; Lima-Ramos, J.; de Hults, E.; George, I.F.; Gerin, P.A. Mixed inoculum origin and lignocellulosic substrate type both influence the production of volatile fatty acids during acidogenic fermentation. Biochem. Eng. J. 2015, 103, 242–249. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, X.; Fu, B.; Chen, J.; Yu, H.-Q. Acidogenic fermentation of proteinaceous sewage sludge: Effect of pH. Water Res. 2012, 46, 799–807. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, X.; Zhang, P.; Wan, J.; Guo, H.; Ghasimi, D.S.M.; Morera, X.C.; Zhang, T. Overview of key operation factors and strategies for improving fermentative volatile fatty acid production and product regulation from sewage sludge. J. Environ. Sci. (China) 2020, 87, 93–111. [Google Scholar] [CrossRef]
- Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Production of volatile fatty acids through co-digestion of sewage sludge and external organic waste: Effect of substrate proportions and long-term operation. Waste Manag. 2020, 112, 30–39. [Google Scholar] [CrossRef]
- Clauser, N.M.; González, G.; Mendieta, C.M.; Kruyeniski, J.; Area, M.C.; Vallejos, M.E. Biomass waste as sustainable raw material for energy and fuels. Sustainability 2021, 13, 794. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Loh, K.C. Review and perspectives of enhanced volatile fatty acids production from acidogenic fermentation of lignocellulosic biomass wastes. Bioresour. Bioprocess. 2021, 8, 68. [Google Scholar] [CrossRef]
- She, Y.; Hong, J.; Zhang, Q.; Chen, B.Y.; Wei, W.; Xin, X. Revealing microbial mechanism associated with volatile fatty acids production in anaerobic acidogenesis of waste activated sludge enhanced by freezing/thawing pretreatment. Bioresour. Technol. 2020, 302, 122869. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Cetecioglu, Z. Butyric acid dominant volatile fatty acids production: Bio-Augmentation of mixed culture fermentation by Clostridium butyricum. J. Environ. Chem. Eng. 2020, 8, 104496. [Google Scholar] [CrossRef]
- Zigová, J.; Šturdík, E. Advances in biotechnological production of butyric acid. J. Ind. Microbiol. Biotechnol. 2000, 24, 153–160. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Nghiem, L.D.; Hai, F.I.; Deng, L.J.; Wang, J.; Wu, Y. Optimization of process parameters for production of volatile fatty acid, biohydrogen and methane from anaerobic digestion. Bioresour. Technol. 2016, 219, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Allied Market Research. World Acetic Acid Market—Opportunities and Forecasts, 2019–2026; Allied Market Research: Portland, OR, USA, 2020. [Google Scholar]
- Trček, J.; Barja, F. Updates on quick identification of acetic acid bacteria with a focus on the 16S-23S rRNA gene internal transcribed spacer and the analysis of cell proteins by MALDI-TOF mass spectrometry. Int. J. Food Microbiol. 2015, 196, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, D.; Niu, D.; Zhao, Y. Acetic acid production from food wastes using yeast and acetic acid bacteria micro-aerobic fermentation. Bioprocess Biosyst. Eng. 2015, 38, 863–869. [Google Scholar] [CrossRef]
- Trček, J.; Mira, N.P.; Jarboe, L.R. Adaptation and tolerance of bacteria against acetic acid. Appl. Microbiol. Biotechnol. 2015, 99, 6215–6229. [Google Scholar] [CrossRef]
- Trcek, J.; Toyama, H.; Czuba, J.; Misiewicz, A.; Matsushita, K. Correlation between acetic acid resistance and characteristics of PQQ-dependent ADH in acetic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 70, 366–373. [Google Scholar] [CrossRef]
- Trček, J.; Jernejc, K.; Matsushita, K. The highly tolerant acetic acid bacterium Gluconacetobacter europaeus adapts to the presence of acetic acid by changes in lipid composition, morphological properties and PQQ-dependent ADH expression. Extremophiles 2007, 11, 627–635. [Google Scholar] [CrossRef]
- Matsushita, K.; Takaki, Y.; Shinagawa, E.; Ameyama, M.; Osao, A. Ethanol Oxidase Respiratory Chain of Acetic Acid Bacteria. Reactivity with Ubiquinone of Pyrroloquinoline Qui none-dependent Alcohol Dehydrogenases Purified from Aceto-bacter aceti and Gluconobacter suboxydans. Biosci. Biotechnol. Biochem. 1992, 56, 304–310. [Google Scholar] [CrossRef]
- Vashisht, A.; Thakur, K.; Kauldhar, B.S.; Kumar, V.; Yadav, S.K. Waste valorization: Identification of an ethanol tolerant bacterium Acetobacter pasteurianus SKYAA25 for acetic acid production from apple pomace. Sci. Total Environ. 2019, 690, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Perumpuli, P.A.B.N.; Watanabe, T.; Toyama, H. Identification and characterization of thermotolerant acetic acid bacteria strains isolated from coconut water vinegar in Sri Lanka. Biosci. Biotechnol. Biochem. 2014, 78, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Tanamool, V.; Chantarangsee, M.; Soemphol, W. Simultaneous vinegar fermentation from a pineapple by-product using the co-inoculation of yeast and thermotolerant acetic acid bacteria and their physiochemical properties. 3 Biotech 2020, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Nayak, J. Acetic Acid Production and Purification: Critical Review Towards Process Intensification. Sep. Purif. Rev. 2017, 46, 44–61. [Google Scholar] [CrossRef]
- Matsushita, K.; Inoue, T.; Adachi, O.; Toyama, H. Acetobacter aceti possesses a proton motive force-dependent efflux system for acetic acid. J. Bacteriol. 2005, 187, 4346–4352. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chang, Y.; Zhang, R.; Song, J.; Xu, Y.; Liu, J.; Wang, M. Two-stage oxygen supply strategy based on energy metabolism analysis for improving acetic acid production by Acetobacter pasteurianus. J. Ind. Microbiol. Biotechnol. 2018, 45, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Yang, G. pH Control in Anaerobic Treatment of Industrial Wastewater. J. Environ. Eng. 1992, 118, 551–567. [Google Scholar] [CrossRef]
- Bräuer, S.L.; Cadillo-Quiroz, H.; Yashiro, E.; Yavitt, J.B.; Zinder, S.H. Isolation of a novel acidiphilic methanogen from an acidic peat bog. Nature 2006, 442, 192–194. [Google Scholar] [CrossRef]
- Taconi, K.A.; Zappi, M.E.; Todd French, W.; Brown, L.R. Methanogenesis under acidic pH conditions in a semi-continuous reactor system. Bioresour. Technol. 2008, 99, 8075–8081. [Google Scholar] [CrossRef]
- Ugurlu, A.; Forster, C.F. The impact of shock loadings on the performance of thermophilic anaerobic filters with porous and non-porous packings. Bioresour. Technol. 1992, 39, 23–30. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Liu, Y.; Zhao, Z.; Zhao, Z.; Liu, S.; Zhao, H.; Quan, X. Enhancement of anaerobic methanogenesis at a short hydraulic retention time via bioelectrochemical enrichment of hydrogenotrophic methanogens. Bioresour. Technol. 2016, 218, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, D.; Lu, X.-Y.; Shen, Z.; Chen, J.; Lee, P.K.H. Pyrosequencing of mcrA and archaeal 16S rRNA genes reveals diversity and substrate preferences of methanogen communities in anaerobic digesters. Appl. Environ. Microbiol. 2015, 81, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Kurade, M.B.; Xiong, J.-Q.; Govindwar, S.P.; Roh, H.-S.; Saratale, G.D.; Jeon, B.-H.; Lim, H. Uptake and biodegradation of emerging contaminant sulfamethoxazole from aqueous phase using Ipomoea aquatica. Chemosphere 2019, 225, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Ahring, B.K. Lignin degradation under anaerobic digestion: Influence of lignin modification—A review. Biomass Bioenergy 2019, 128, 105325. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Zhao, Y.; Xu, C.; Zhang, K.; Li, J.; Yan, L.; Gu, J.-D.; Wei, D.; Wang, W. Establishing practical strategies to run high loading corn stover anaerobic digestion: Methane production performance and microbial responses. Bioresour. Technol. 2020, 310, 123364. [Google Scholar] [CrossRef]
- Shimada, T.; Morgenroth, E.; Tandukar, M.; Pavlostathis, S.G.; Smith, A.; Raskin, L.; Kilian, R.E. Syntrophic acetate oxidation in two-phase (acid–methane) anaerobic digesters. Water Sci. Technol. 2011, 64, 1812–1820. [Google Scholar] [CrossRef]
- Xiao, K.K.; Guo, C.H.; Zhou, Y.; Maspolim, Y.; Wang, J.Y.; Ng, W.J. Acetic acid inhibition on methanogens in a two-phase anaerobic process. Biochem. Eng. J. 2013, 75, 1–7. [Google Scholar] [CrossRef]
- Dalkılıc, K.; Ugurlu, A. Biogas production from chicken manure at different organic loading rates in a mesophilic-thermopilic two stage anaerobic system. J. Biosci. Bioeng. 2015, 120, 315–322. [Google Scholar] [CrossRef]
- Pradhan, N.; d’Ippolito, G.; Dipasquale, L.; Esposito, G.; Panico, A.; Lens, P.N.L.; Fontana, A. Simultaneous synthesis of lactic acid and hydrogen from sugars via capnophilic lactic fermentation by Thermotoga neapolitana cf capnolactica. Biomass Bioenergy 2019, 125, 17–22. [Google Scholar] [CrossRef]
- Poggi-Varaldo, H.M.; Munoz-Paez, K.M.; Escamilla-Alvarado, C.; Robledo-Narváez, P.N.; Ponce-Noyola, M.T.; Calva-Calva, G.; Ríos-Leal, E.; Galíndez-Mayer, J.; Estrada-Vázquez, C.; Ortega-Clemente, A.; et al. Biohydrogen, biomethane and bioelectricity as crucial components of biorefinery of organic wastes: A review. Waste Manag. Res. 2014, 32, 353–365. [Google Scholar] [CrossRef]
- Muñoz-Páez, K.M.; Alvarado-Michi, E.L.; Buitrón, G.; Valdez-Vazquez, I. Distinct effects of furfural, hydroxymethylfurfural and its mixtures on dark fermentation hydrogen production and microbial structure of a mixed culture. Int. J. Hydrogen Energy 2019, 44, 2289–2297. [Google Scholar] [CrossRef]
- Kim, C. Potential of anaerobic digestion for material recovery and energy production in waste biomass from a poultry slaughterhouse. Waste Manag. 2016, 34, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Nasirian, N.; Almassi, M.; Minaei, S.; Widmann, R. Development of a method for biohydrogen production from wheat straw by dark fermentation. Int. J. Hydrogen Energy 2011, 36, 411–420. [Google Scholar] [CrossRef]
- Li, Y.; Yang, G.; Li, L.; Sun, Y. Bioaugmentation for overloaded anaerobic digestion recovery with acid-tolerant methanogenic enrichment. Waste Manag. 2018, 79, 744–751. [Google Scholar] [CrossRef]
- Giovannini, G.; Donoso-Bravo, A.; Jeison, D.; Chamy, R.; Ruíz-Filippi, G.; Vande Wouver, A. A review of the role of hydrogen in past and current modelling approaches to anaerobic digestion processes. Int. J. Hydrogen Energy 2016, 41, 17713–17722. [Google Scholar] [CrossRef]
- Lakaniemi, A.-M.; Koskinen, P.E.P.; Nevatalo, L.M.; Kaksonen, A.H.; Puhakka, J.A. Biogenic hydrogen and methane production from reed canary grass. Biomass Bioenergy 2011, 35, 773–780. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Deng, L.; Liu, Z.; Jiang, H.; Wang, F. Biohydrogen production from fermentation of cotton stalk hydrolysate by Klebsiella sp. WL1316 newly isolated from wild carp (Cyprinus carpio L.) of the Tarim River basin. Appl. Microbiol. Biotechnol. 2018, 102, 4231–4242. [Google Scholar] [CrossRef]
- Chi, C.H.; Chen, K.W.; Huang, J.J.; Chuang, Y.C.; Wu, M.H. Gas composition in Clostridium septicum gas gangrene. J. Formos. Med. Assoc. 1995, 94, 757–759. [Google Scholar]
- Nasirian, N.; Almassi, M. Optimization of biological hydrogen production process using stepwise regression method. Int. J. Biosci. 2014, 6655, 289–299. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass—An overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, X.; Dai, R.; Wang, Y.; Ma, P. Effect of low temperature of thermal pretreatment on anaerobic digestion of textile dyeing sludge. Bioresour. Technol. 2017, 243, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef] [PubMed]
- Nissilä, M.E.; Li, Y.-C.; Wu, S.-Y.; Puhakka, J.A. Dark Fermentative Hydrogen Production from Neutralized Acid Hydrolysates of Conifer Pulp. Appl. Biochem. Biotechnol. 2012, 168, 2160–2169. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Borowski, S.; Domański, J. Dark fermentative hydrogen production from hydrolyzed sugar beet pulp improved by iron addition. Bioresour. Technol. 2020, 314, 123713. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Wiktorowska-Sowa, E.; Piotrowski, J.; Salamon, A.; Kaźmierczak, W.; Błaszczyk, M.K.; Barberan, A.; Chen, Y.; et al. Dynamics and Complexity of Dark Fermentation Microbial Communities Producing Hydrogen From Sugar Beet Molasses in Continuously Operating Packed Bed Reactors. Front. Microbiol. 2021, 11, 3303. [Google Scholar] [CrossRef]
- Cieslik, M.; Dach, J.; Lewicki, A.; Smurzyńska, A.; Janczak, D.; Pawlicka-Kaczorowska, J.; Boniecki, P.; Cyplik, P.; Czekała, W.; Jóźwiakowski, K. Methane fermentation of the maize straw silage under meso- and thermophilic conditions. Energy 2016, 115, 1495–1502. [Google Scholar] [CrossRef]
- Kozłowski, K.; Lewicki, A.; Malińska, K.; Wei, Q. Current State, Challenges and Perspectives of Biological Production of Hydrogen in Dark Fermentation Process in Poland. J. Ecol. Eng. 2019, 20, 146–160. [Google Scholar] [CrossRef]
- Chong, M.-L.; Sabaratnam, V.; Shirai, Y.; Hassan, M.A. Biohydrogen production from biomass and industrial wastes by dark fermentation. Int. J. Hydrogen Energy 2009, 34, 3277–3287. [Google Scholar] [CrossRef]
- Fomina, M.A.; Alexander, I.J.; Colpaert, J.V.; Gadd, G.M. Solubilization of toxic metal minerals and metal tolerance of mycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 851–866. [Google Scholar] [CrossRef]
- Reed, D.W.; Fujita, Y.; Daubaras, D.L.; Jiao, Y.; Thompson, V.S. Bioleaching of rare earth elements from waste phosphors and cracking catalysts. Hydrometallurgy 2016, 166, 34–40. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Faraji, F.; Golmohammadzadeh, R.; Rashchi, F.; Alimardani, N. Fungal bioleaching of WPCBs using Aspergillus niger: Observation, optimization and kinetics. J. Environ. Manag. 2018, 217, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Sedlakova-Kadukova, J.; Marcincakova, R.; Luptakova, A.; Vojtko, M.; Fujda, M.; Pristas, P. Comparison of three different bioleaching systems for Li recovery from lepidolite. Sci. Rep. 2020, 10, 14594. [Google Scholar] [CrossRef]
- Mouna, H.M.; Baral, S.S. A bio-hydrometallurgical approach towards leaching of lanthanum from the spent fluid catalytic cracking catalyst using Aspergillus niger. Hydrometallurgy 2019, 184, 175–182. [Google Scholar] [CrossRef]
- Urík, M.; Bujdoš, M.; Milová-Žiaková, B.; Mikušová, P.; Slovák, M.; Matúš, P. Aluminium leaching from red mud by filamentous fungi. J. Inorg. Biochem. 2015, 152, 154–159. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, N.; Li, Y.; Luo, D.; Wu, P.; Dang, Z. Rapid and green process for valuable materials recovery from waste liquid crystal displays. Resour. Conserv. Recycl. 2020, 153, 104544. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M.; Baniasadi, M. Use of adapted metal tolerant Aspergillus niger to enhance bioleaching efficiency of valuable metals from spent lithium-ion mobile phone batteries. J. Clean. Prod. 2018, 197, 1546–1557. [Google Scholar] [CrossRef]
- Arshadi, M.; Nili, S.; Yaghmaei, S. Ni and Cu recovery by bioleaching from the printed circuit boards of mobile phones in non-conventional medium. J. Environ. Manag. 2019, 250, 109502. [Google Scholar] [CrossRef]
- Díaz-Martínez, M.E.; Argumedo-Delira, R.; Sánchez-Viveros, G.; Alarcón, A.; Mendoza-López, M.R. Microbial Bioleaching of Ag, Au and Cu from Printed Circuit Boards of Mobile Phones. Curr. Microbiol. 2019, 76, 536–544. [Google Scholar] [CrossRef]
- Kaliyaraj, D.; Rajendran, M.; Angamuthu, V.; Antony, A.R.; Kaari, M.; Thangavel, S.; Venugopal, G.; Joseph, J.; Manikkam, R. Bioleaching of heavy metals from printed circuit board (PCB) by Streptomyces albidoflavus TN10 isolated from insect nest. Bioresour. Bioprocess. 2019, 6, 47. [Google Scholar] [CrossRef]
- Willner, J.; Fornalczyk, A. Extraction of metals from electronic waste by bacterial leaching. Environ. Prot. Eng. 2013, 39, 197–208. [Google Scholar] [CrossRef]
- Akbari, S.; Ahmadi, A. Recovery of copper from a mixture of printed circuit boards (PCBs) and sulphidic tailings using bioleaching and solvent extraction processes. Chem. Eng. Process. Process Intensif. 2019, 142, 107584. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Moscardini, E.; Altimari, P.; Abo Atia, T.; Toro, L. Leaching of electrodic powders from lithium ion batteries: Optimization of operating conditions and effect of physical pretreatment for waste fraction retrieval. Waste Manag. 2017, 60, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.L.; Newman, D.K. Geomicrobiology; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Mražíková, A.; Marcinčáková, R.; Kaduková, J.; Velgosová, O. Influence of bacterial culture to copper bioleaching from printed circuit boards. Inz. Miner. 2013, 14, 59–62. [Google Scholar]
- Dorado, A.D.; Solé, M.; Lao, C.; Alfonso, P.; Gamisans, X. Effect of pH and Fe(III) ions on chalcopyrite bioleaching by an adapted consortium from biogas sweetening. Miner. Eng. 2012, 39, 36–38. [Google Scholar] [CrossRef][Green Version]
- Auerbach, R.; Bokelmann, K.; Stauber, R.; Gutfleisch, O.; Schnell, S.; Ratering, S. Critical raw materials—Advanced recycling technologies and processes: Recycling of rare earth metals out of end of life magnets by bioleaching with various bacteria as an example of an intelligent recycling strategy. Miner. Eng. 2019, 134, 104–117. [Google Scholar] [CrossRef]
- Ma, X.; Gao, M.; Yin, Z.; Zhu, W.; Liu, S.; Wang, Q. Lactic acid and animal feeds production from Sophora flavescens residues by Rhizopus oryzae fermentation. Process Biochem. 2020, 92, 401–408. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, L.; Peng, J.; Ge, Y.; Tian, Z.; Sun, J.; Cheng, H.; Zhou, H. Cleaner utilization of electroplating sludge by bioleaching with a moderately thermophilic consortium: A pilot study. Chemosphere 2019, 232, 345–355. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, W.; Liu, Y.; Jia, H.; Zhou, J.; Wei, P.; Zhou, H. Bioleaching of dewatered electroplating sludge for the extraction of base metals using an adapted microbial consortium: Process optimization and kinetics. Hydrometallurgy 2020, 191, 105227. [Google Scholar] [CrossRef]
- Priya, A.; Hait, S. Extraction of metals from high grade waste printed circuit board by conventional and hybrid bioleaching using Acidithiobacillus ferrooxidans. Hydrometallurgy 2018, 177, 132–139. [Google Scholar] [CrossRef]
- Arshadi, M.; Mousavi, S.M. Multi-objective optimization of heavy metals bioleaching from discarded mobile phone PCBs: Simultaneous Cu and Ni recovery using Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2015, 147, 210–219. [Google Scholar] [CrossRef]
- Sodha, A.B.; Tipre, D.R.; Dave, S.R. Optimisation of biohydrometallurgical batch reactor process for copper extraction and recovery from non-pulverized waste printed circuit boards. Hydrometallurgy 2020, 191, 105170. [Google Scholar] [CrossRef]
- Velgosová, O.; Kaduková, J.; Marcinčáková, R.; Mrážiková, A.; Fröhlich, L. The Role of Main Leaching Agents Responsible for Ni Bioleaching from spent Ni-Cd Batteries. Sep. Sci. Technol. 2014, 49, 438–444. [Google Scholar] [CrossRef]
- Ubaldini, S.; Kadukova, J.; Mravzíková, A.; Fornari, P.; Luptáková, A.; Marcincakova, R.; Pizzichemi, P. Application of Innovative Processes for the Valorisation of Spent Alkaline Batteries. Chem. Eng. Trans. 2014, 39, 1609–1614. [Google Scholar]
- Marcinčáková, R.; Kaduková, J.; Mražíková, A.; Velgosová, O.; Luptáková, A.; Ubaldini, S. Metal bioleaching from spent lithium-ion batteries using acidophilic bacterial strains. Inz. Miner. 2016, 17, 117–120. [Google Scholar]
- Auerbach, R.; Ratering, S.; Bokelmann, K.; Gellermann, C.; Brämer, T.; Baumann, R.; Schnell, S. Bioleaching of valuable and hazardous metals from dry discharged incineration slag. An approach for metal recycling and pollutant elimination. J. Environ. Manag. 2019, 232, 428–437. [Google Scholar] [CrossRef]
- Pourhossein, F.; Mousavi, S.M. Enhancement of copper, nickel, and gallium recovery from LED waste by adaptation of Acidithiobacillus ferrooxidans. Waste Manag. 2018, 79, 98–108. [Google Scholar] [CrossRef]
- Pourhossein, F.; Mousavi, S.M. A novel step-wise indirect bioleaching using biogenic ferric agent for enhancement recovery of valuable metals from waste light emitting diode (WLED). J. Hazard. Mater. 2019, 378, 120648. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Boxall, N.J.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Cheng, K.Y.; Usher, K.M.; Lakaniemi, A.-M. Recent progress in biohydrometallurgy and microbial characterisation. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y.-P. Microbial leaching of heavy metals using Escherichia coli and evaluation of bioleaching mechanism. Bioresour. Technol. Rep. 2020, 9, 100368. [Google Scholar] [CrossRef]
- Natarajan, G.; Ting, Y.-P. Gold biorecovery from e-waste: An improved strategy through spent medium leaching with pH modification. Chemosphere 2015, 136, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Hui, L.; Wenjie, T.; Xiaoqing, W.; Xuemeng, W.; Xiaohui, J.; Ben, S.; Song, G.; Tang, Y. Leaching of valuable metals from red mud via batch and continuous processes by using fungi. Miner. Eng. 2015, 81, 1–4. [Google Scholar]
- Huang, T.; Wei, X.; Zhang, S. Bioleaching of copper sulfide minerals assisted by microbial fuel cells. Bioresour. Technol. 2019, 288, 121561. [Google Scholar] [CrossRef]
- Wei, X.; Li, J.; Huang, W.; Zheng, X.; Li, S.; Chen, X.; Liu, D. Comparative Study of Iron-Oxidizing and Sulfur-Oxidizing Bioleaching Processes for Heavy Metal Removal and Nutrient Leaching from Pig Manure. Water Air Soil Pollut. 2020, 231, 34. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cetecioglu, Z.; Atasoy, M.; Cenian, A.; Sołowski, G.; Trček, J.; Ugurlu, A.; Sedlakova-Kadukova, J. Bio-Based Processes for Material and Energy Production from Waste Streams under Acidic Conditions. Fermentation 2022, 8, 115. https://doi.org/10.3390/fermentation8030115
Cetecioglu Z, Atasoy M, Cenian A, Sołowski G, Trček J, Ugurlu A, Sedlakova-Kadukova J. Bio-Based Processes for Material and Energy Production from Waste Streams under Acidic Conditions. Fermentation. 2022; 8(3):115. https://doi.org/10.3390/fermentation8030115
Chicago/Turabian StyleCetecioglu, Zeynep, Merve Atasoy, Adam Cenian, Gaweł Sołowski, Janja Trček, Aysenur Ugurlu, and Jana Sedlakova-Kadukova. 2022. "Bio-Based Processes for Material and Energy Production from Waste Streams under Acidic Conditions" Fermentation 8, no. 3: 115. https://doi.org/10.3390/fermentation8030115
APA StyleCetecioglu, Z., Atasoy, M., Cenian, A., Sołowski, G., Trček, J., Ugurlu, A., & Sedlakova-Kadukova, J. (2022). Bio-Based Processes for Material and Energy Production from Waste Streams under Acidic Conditions. Fermentation, 8(3), 115. https://doi.org/10.3390/fermentation8030115







