1. Introduction
An important requirement for skincare cosmetics is the maintenance of the skin barrier. The skin barrier protects the human body from attacks by the external environment (e.g., pathogens, physical traumas, and ultraviolet radiation) [
1,
2], prevents water transpiration from the surface of the skin, and retains moisture [
2]. To moisten the skin, it is important to use different types of moisturizers in appropriate combinations, such as emollients, humectants, and occlusives [
2,
3]. In addition to this physical approach, it is important to maintain the barrier ability of the skin itself.
The skin is the largest organ of the human body, and its outermost layer is the epidermis, consisting of a highly stratified epithelium [
4]. The basal layer of the skin epidermis (stratum basale), which is attached to the basement membrane, consists of mitotically active stem cells that produce undifferentiated keratinocytes. Keratinocytes are mitotically active and produce cells that sequentially differentiate into three histologically different outer layers—the innermost spinous layer (stratum spinosum), the granulosum layer (stratum granulosum), and finally the horny layer [stratum corneum (SC)]—by a finely tuned process [
4]. The physiological skin barrier is mainly attributable to the SC and stratum granulosum [
5]. SC consists of enucleated corneocytes in a lipid matrix. Keratinocytes in the granulosum form tight junctions (TJs) between cells, which form a strong barrier for the paracellular transfer of molecules [
5]. Disruption of such structures is harmful for the skin; therefore, substances that can support the integrity of the skin are very useful as ingredients in cosmetics. Since TJ consists of several structural proteins, including claudins [
6,
7], occludin [
8], and ZO-1 [
9], which forms protein complexes tethering adjacent cells, agents upregulating these proteins might be candidates for such ingredients.
In the granulosum layer, TJs between keratinocytes have been reported to be tightened by the stimulation of their toll-like receptor 2 (TLR2) [
10,
11]. TLR2 is a cell surface receptor that mainly recognizes microbes, including Gram-positive bacteria, and plays an important role in innate immunity [
12]. Although the main cell types expressing TLR2 are immune cells such as macrophages, monocytes, dendritic cells, and neutrophils [
9], TLR2 is also expressed in epidermal keratinocytes [
13], intestinal epidermal cells [
14], and pulmonary alveolar epithelial cells [
15]. When TLR2 is stimulated in immune cells, the immune response is triggered, including phagocytosis of pathogens, cytokine secretion, and presentation of antigens to professional immune cells, which results in inflammation [
12]. By contrast, stimulation of TLR2 in epithelia is not directly related to inflammation but tightens the cellular barrier, which is another aspect of the defense mechanism of the skin [
10,
11].
Yuki et al. [
10] and Kuo et al. [
11] reported that TLR2 agonists are responsible for the rapid and chronic tightening of TJs in skin keratinocytes, suggesting that TLR2 agonists without pathogenic properties might be useful for skincare purposes by stimulating the skin’s self-defense mechanism. Moreover, Kuo et al. [
11] demonstrated that the stimulation of TLR2 plays a role in the wound repair response in atopic dermatitis (AD) patients. Therefore, appropriate TLR2 agonists are thought to be beneficial not only as cosmetics but also as AD therapeutics. However, in their studies, either peptidoglycan prepared from pathogenic
Staphylococcus aureus or the synthetic ligand Pam3CSK4 was used as a TLR2 agonist. Moreover, to the best of our knowledge, no compounds with TLR2-stimulating activity suitable for manufacturing cosmetics have been reported to date. In the present study, we tried to produce soluble TLR2 agonists using natural raw materials that are suitable for use in cosmetics. As a result, we found that the fermentation of plant extracts supplemented with milk components by appropriate lactic acid bacteria could produce significant amounts of soluble TLR2 agonists. Since such ferments contain soluble TLR2 agonists that can strengthen the barrier function of the cellular sheet of keratinocytes, they might be novel ingredients of skincare cosmetics.
2. Materials and Methods
2.1. Lactic Acid Bacteria
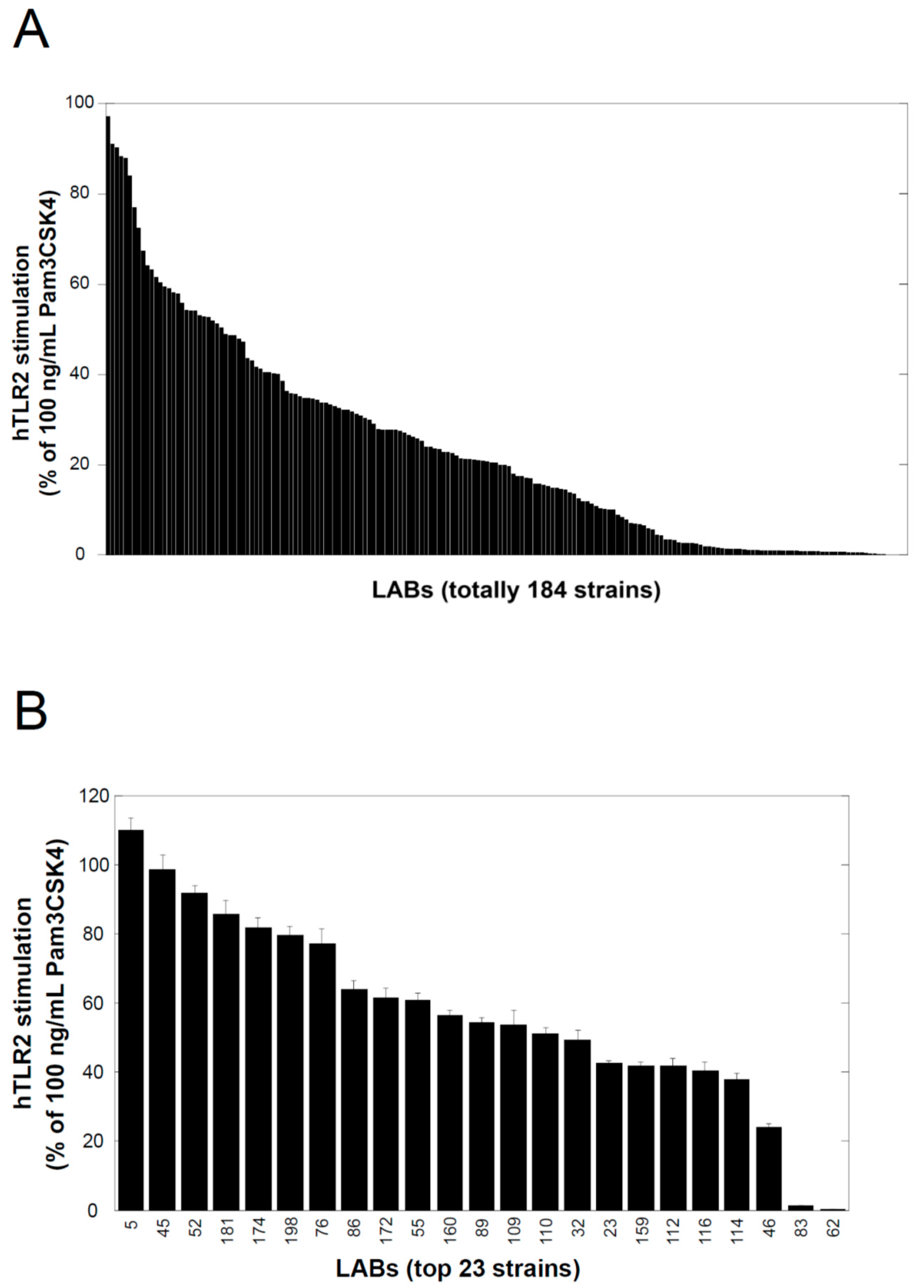
The lactic acid bacteria used in this study were kindly provided by Prof. Taku Miyamoto at Kurashiki Sakuyo University (Okayama, Japan); they were isolated from a variety of traditional fermented foods worldwide, and their culture supernatants were examined using a TLR2-stimulating assay described below. Lactobacillus delbrueckii subsp. lactis TL24 (TL24), whose culture supernatant has strong TLR2-stimulating activity, was isolated from Georgian traditional Sulguni cheese. The species of this strain was identified by comparing the 16S ribosomal RNA and gyrase B gene sequences of the strain with those of other sequenced bacteria available in the DNA sequence database [DDBJ/ENA(EMBL)/GenBank].
2.2. Normal Human Epidermal Keratinocytes
The normal human epidermal keratinocytes used in this study, NHEK (NB), were purchased from Kurabo (Osaka, Japan) and were cultured in DermaLife K keratinocyte medium (Lifeline Cell Technology, Frederick, MD, USA) at 37 °C and 5% CO2 in a humidified chamber. All experiments were performed with cells passaged six times or less.
2.3. Plant Extracts and Milk Materials
A variety of plant materials, namely, from asparagus (stem of edible part), broccoli (stem of edible part), cabbage (edible part), carrot (edible part, root), daikon radish (edible part, root), Chinese cabbage (edible part), apple (fruit), eggplant (fruit), cucumber (fruit), lemon balm (leaf), orange (fruit), cherry (leaf), tomato (fruit), and grape (fruit), were subjected to hot water extraction. Unless otherwise mentioned, the plant bodies were mixed with purified water at a weight ratio of 1:2 and autoclaved (121 °C for 15 min). After the temperature was lowered to 40 °C or lower, the plant extracts were squeezed through a polypropylene mesh. The extract was stored at −20 °C or lower until further use. Among these plants, we chose asparagus as the main target for extensive examination, because (1) asparagus extracts have been used in cosmetics as skin conditioning agents [
16]; (2) since the bottom part of the spear of asparagus is normally trimmed and discarded before shipment, the usage of these parts meets the policy of sustainable development goal (SDG) 12, namely, “responsible consumption and production” [
17].
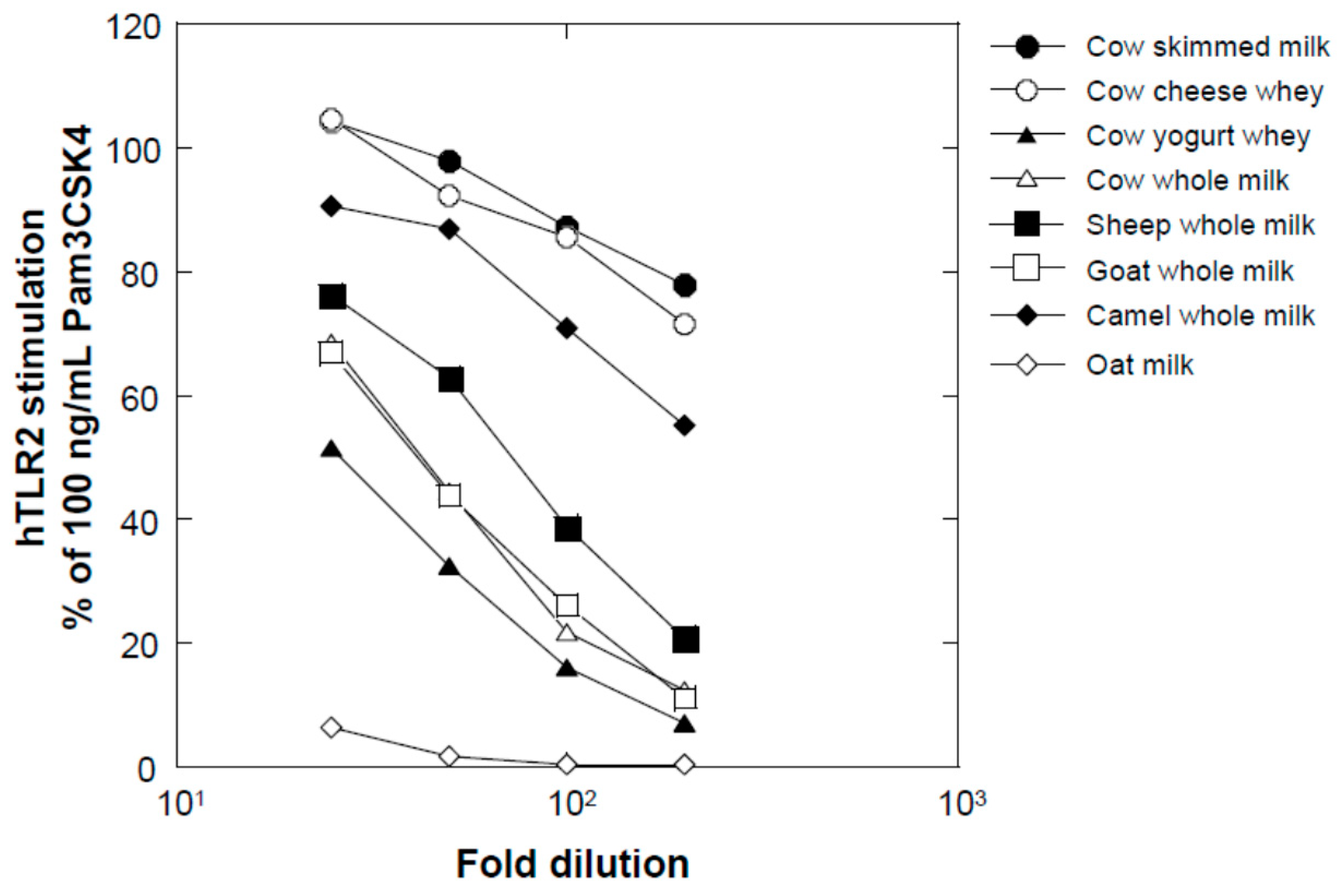
Several milk powders, namely, cow skimmed milk powder (Takanashi Milk Products, Kanagawa, Japan), cow whole milk powder (Yotsuba Milk Products, Hokkaido, Japan), goat whole milk powder (Meyenberg, Turlock, CA, USA), camel whole milk powder (Aadvik Foods & Products Pvt. Ltd., Rajasthan, India), and cow cheese whey powder (Yotsuba Milk Products), were used in this study. Sheep whole milk powder was produced by lyophilizing sheep whole milk purchased from Matsuyama Farm (Hokkaido, Japan). Yogurt whey powder was produced by lyophilizing whey liquid obtained from plain yogurt (Takanashi Milk Products). Oat milk powder (GODO Co., Ltd., Tokyo, Japan) was used as a milk-like plant-derived material.
2.4. Bacterial Culture and Fermentation
Lactic acid bacteria were cultured in De Man, Rogosa, and Sharpe (MRS) medium (Difco) at their preferred temperature (30 °C or 37 °C, depending on the strains) under static conditions for 24 h and used as the seed culture (MRS seed). For fermentation under static conditions, an MRS seed equivalent to 2% of the fermentation volume was added to the plant extracts with or without milk components after sterilization (110 °C × 20 min), and fermentation was performed at the bacteria preferred temperature (30 °C or 37 °C) for 24 h. For fermentation by TL24 using a 5 L jar fermenter (Bioneer-Neo, Marubishi Bioengineering, Tokyo, Japan), an MRS seed equivalent to 2% of the fermentation volume was added to a sterilized asparagus extract containing 3% skimmed milk, and the mixture was incubated at 37 °C for 24 h in static conditions to obtain an asparagus seed culture. After the vessel of the fermenter was filled with 2.5 L of asparagus extract and 3% skimmed milk, sterilized at 110 °C for 20 min, and cooled to 37 °C, 50 mL of the asparagus seed culture was inoculated. Fermentation was performed under mild stirring conditions (80 rpm) at 37 °C for 24 h.
2.5. TLR2- and TLR4-Stimulating Activities
TLR2-stimulating activity was determined using a reporter cell system, HEK BlueTM hTLR2 cells (InvivoGen, Toulouse, France), according to the manufacturer’s instructions. In brief, the cells were cultivated in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with a mixture of selective antibiotics for keeping the persistent expression of transgenes (HEK Blue selection, InvivoGen), in addition to other antibiotics for preventing the growth of microorganisms (penicillin, Fujifilm-Wako, Osaka, Japan; streptomycin, Fujifilm-Wako; and normocin, InvivoGen), and 10% fetal bovine serum (Equitech Bio, Kerrville, TX, USA) at 37 °C in a 5% CO2 atmosphere in a humidified chamber. For the assay, the cells were seeded in each well of a 96-well cell culture plate at a density of 2 × 105 cells/mL in 100 µL of the culture medium. After 2 d, 100 µL of the test samples, sterilized by membrane filtration (pore size = 0.2 µm) and diluted in the culture medium, was added to each well. As positive and negative controls, 200 ng/mL of Pam3CSK4 (final concentration 100 ng/mL) and the medium were used, respectively. After an additional 1 d of cultivation, 20 µL of the conditioned medium from each well was transferred to a fresh 96-well assay plate, 180 µL of Quanti Blue Solution (InvivoGen) was added, and the plate was incubated for 30 min at 37 °C. The absorbance of each well was determined at 655 nm using a microplate reader (Multiskan GO, Thermo Fisher Scientific, Waltham, MA, USA), and the average value of the negative control wells was subtracted from the values of all wells. TLR2-stimulating activity is expressed as the relative value to the average values of the positive control. When examining TLR4-stimulating activity, HEK Blue hTLR4 cells (InvivoGen) and 1000 ng/mL LPS (Lipopolysaccharide from Escherichia coli 0111:B4; Sigma-Aldrich) were used instead of HEK Blue hTLR2 and 100 ng/mL Pam3CSK4, respectively.
2.6. Transcellular Molecular Transfer in Keratinocytes
NHEK (NB) cells were seeded in a BioCoat Control Culture insert (0.4 µm PET membrane, 24 wells; Corning, NY, USA) and cultured for 4 d until they reached confluence at 37 °C and 5% CO2 in a humidified chamber. To examine the effect on transcellular molecular transfer, both the inner and the outer solutions were changed to solutions containing 1.8 mM CaCl2, which was used to stimulate the differentiation of keratinocytes, with or without diluted test samples. After 3 d of incubation, 1/50 volume of 1% fluorescein sodium was added to the inner solution (final concentration = 0.02%). After 30 min at 37 °C, the outer solution was collected and diluted 10-fold with PBS, and its fluorescence was determined using a real-time PCR apparatus (Thermal Cycler Dice Real-time System II; Takara Bio, Shiga, Japan). To evaluate whether TLR2-stimulating activity was the underlying mechanism, 1 µg/mL of neutralizing antibody against human TLR2 (Clone 383936; R&D Systems, Minneapolis, MN, USA) was added to the samples.
2.7. Statistics
Statistical differences between the control group and each test group were analyzed using the Dunnett’s test. If statistical comparisons between all groups were necessary, Scheffe’s test was employed. All statistical analyses were performed using the KaleidaGraph software (Synergy Software, Ver4.5, Reading, PA, USA). Differences between groups were considered statistically significant when the significance probability was less than 0.05.
4. Discussion
In this study, we sought cosmetically applicable novel agonists for TLR2. TLR2 is a cell surface receptor involved in innate immunity and plays an important role in recognizing microbes, including Gram-positive bacteria [
12]. In keratinocytes, the main role of TLR2 is to tighten cell–cell junctions, rather than to stimulate inflammation [
10,
11]. Therefore, TLR2 agonists are thought to be novel cosmetic ingredients. In this study, we tried to identify appropriate lactic acid bacterial strains and their culture conditions to obtain soluble TLR2 agonists possibly suitable for cosmetics. Although the MRS medium is rich and appropriate for the culture of a variety of lactic acid bacteria, some of its components are thought to be unsuitable for the production of cosmetics. For example, the MRS medium contains “peptone,” which is a digest of proteins, but in many cases, details of peptone in commercial MRS medium are not disclosed. It also contains beef extract and manganese, which are not normal ingredients in cosmetics. Furthermore, the odor of the MRS medium is not appropriate for cosmetic application, and its price is high for industrial application. Therefore, we tried to formulate a medium for lactic acid bacteria fermentation containing only compounds from natural sources, suitable for use in cosmetics.
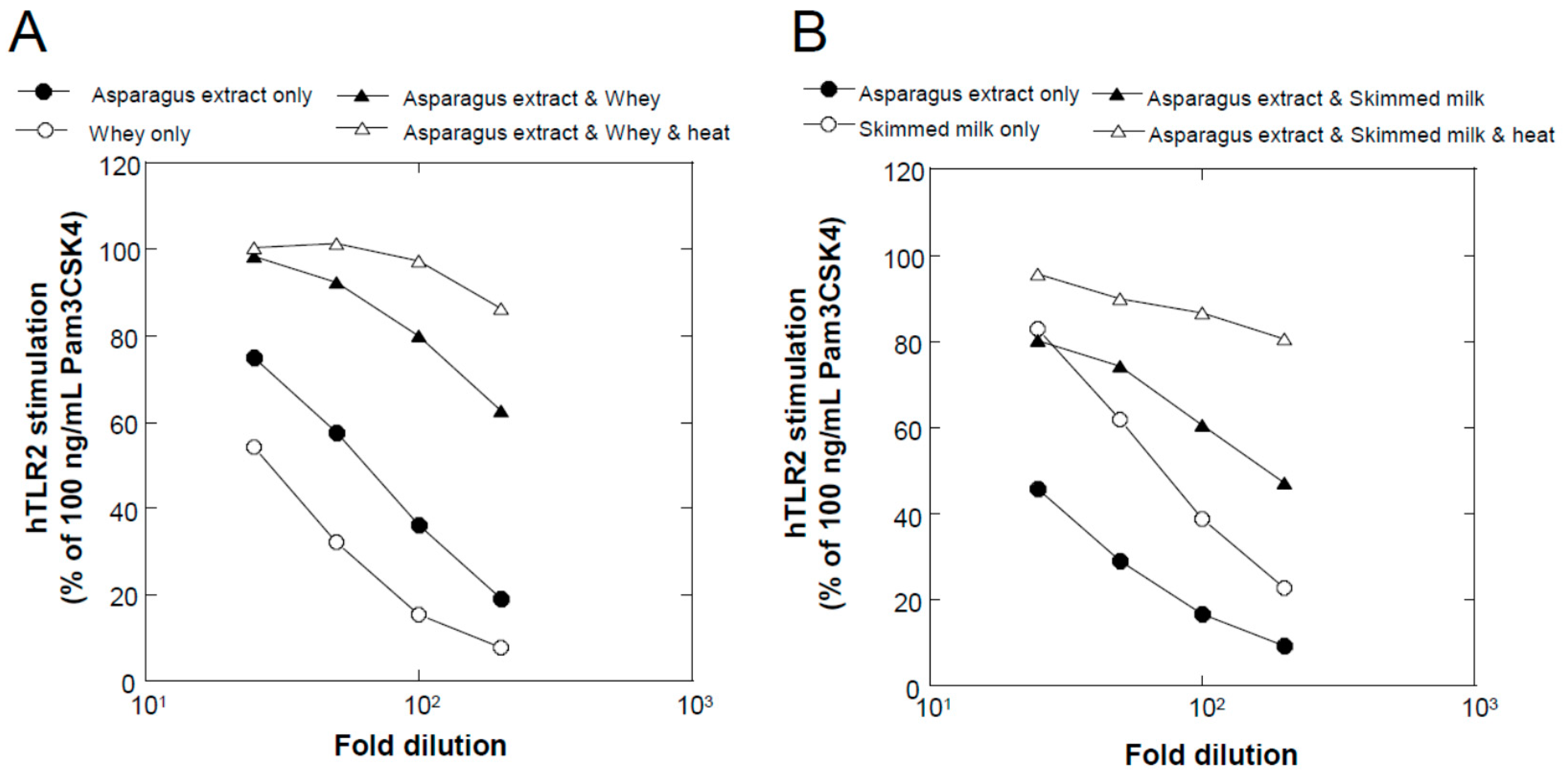
We found that a combination of plant extracts and milk components can be used. One of the best media was the combination of a hot water extract of asparagus edible stem and milk components. Plant and milk components should not necessarily be restricted to asparagus and cow milk, respectively, because a variety of plant and milk materials could support the production of soluble TLR2 agonists. Because the activity of the ferments when asparagus extract or milk components were used individually was inferior to that obtained in their mixture, the combined usage of these materials appeared to be appropriate. Based on the results of TLR2-stimulating and paracellular molecular transfer studies, the appropriate content of the ferments in cosmetics might be 2% or higher.
The present results suggest that the TL24 ferments containing a soluble TLR2 agonist can be used as ingredients of cosmetics. By contrast, Kim et al. [
18] suggested that TLR2 might play a role in inducing inflammatory reactions in acne via stimulation by
Cutibacterium acnes, the pathogenic bacterium that causes acne. However, they only demonstrated that
C. acne could stimulate TLR2-transfected HEK293 reporter cells (comparable to our assay), and monocytes/macrophages were activated by
C. acne via TLR2, resulting in the production of inflammatory cytokines. Based on the observation that TLR2-positive cells appeared, and their number increased in acne lesions, they speculated that TLR2 was responsible for triggering an inflammatory response in acne. However, they could not detect TLR2-positive cells in early acne lesions for up to 6 h, though it is obvious that keratinocytes express TLR2 on their surfaces [
13]. Therefore, it is reasonable to assume that
C. acne does not trigger inflammation by TLR2 stimulation but augments an already existing inflammation by stimulating inflammatory cells. In fact, another commensal Gram-positive bacterium,
Staphylococcus epidermidis, has been reported to be beneficial in maintaining skin health, and
S. epidermidis does not trigger inflammation [
19], although it is thought to stimulate TLR2. Furthermore, it has been reported that TLR2 might play a role in wound repair in AD, rather than enhancing wounds [
11]. Moreover, lactic acid bacteria, their extracts, or ferments have been widely used to promote skin health, suggesting that their intrinsic property of TLR2 stimulation is not problematic for their usage in cosmetics [
20]. Collectively, these results suggest that TLR2 agonists are beneficial for non-inflamed skin, but we should be cautious about their use in the presence of inflammation. However, this is a speculation, and the safety aspects of soluble TLR2 agonists as cosmetic ingredients should be clinically evaluated in future studies.
Since TLR2 intrinsically recognizes cell surface components of Gram-positive bacteria, the ligand for TLR2 is normally thought to exist on the surface of bacteria, for example, peptide glycan, lipoteichoic acid, lipoprotein [
12]. Although some fractions of cell surface ligands for TLR2 might be shed into the extracellular space, research focusing on soluble ligands for TLR2 produced by lactic acid bacteria has not yet been conducted. Regarding soluble factors produced by lactic acid bacteria to tighten cell–cell junctions, soluble proteins produced by
Lactobacillus rhamnosus GG have been reported in assays involving cell sheets of the intestinal Caco2 cell line [
21]. However, the molecular nature of these proteins and their underlying mechanisms for tightening cell–cell junctions have not yet been described. Based on the present study, the soluble factors produced by
Lactobacillus rhamnosus GG may act as ligands for TLR2. Although, in this study, we focused on the application of soluble ligands for TLR2 to cosmetics, they might also be used as food ingredients, e.g.,
L. rhamnosus GG. Concerning the activation of TLRs in the gut, Gu et al. reported that the decrease in the barrier function of the gut induced by deoxynivalnol, a metabolite of fungi, was inhibited by TLR2 agonists [
22]. On the contrary, TLR4 stimulation in the gut was reported to possibly induce the so-called leaky gut in patients with alcoholic steatohepatitis [
23]. Therefore, the substances stimulating TLR2 but not TLR4 might be appropriate as food ingredients targeting the gut. The asparagus/milk ferments described in this study might have such a beneficial property.
Regarding substances responsible for the TLR2-stimulating activity of the ferments, we have not yet obtained decisive information. When we examined solvent extraction with ethyl acetate, the activity remained in the aqueous phase (data not shown), suggesting that the active components are highly hydrophilic. Synthetic lipopeptides, e.g., Pam3CSK4, have been widely used as specific agonists for TLR2, and their specificity to TLR2 was demonstrated based on X-ray crystallographic studies [
24,
25]. These peptides were designed based on the N-terminal structure of bacterial surface lipoprotein, and it was said that both the positively charged peptide chain and the structure of the lipid moiety were necessary [
26]. Another known ligand of TLR2, lipoteichoic acid, also contains hydrophobic lipid chains and hydrophilic repeated glycerophosphate units [
24]. Therefore, soluble agonists for TLR2 in the ferments might have both hydrophilic and hydrophobic structures in their molecules, although the exact structures must be elucidated in future studies.
This study is somewhat at its early stage and with some limitations. Therefore, future studies will include (1) the elucidation of the molecular structures of soluble TLR2 agonists produced by lactic acid bacteria, including TL24, (2) obtaining morphological evidence underlying the described effect on the paracellular transfer of molecules (e.g., immunostaining of TLR2 and TJ proteins), (3) the evaluation of TLR2 agonists’ effects in clinical situations, and (4) the assessment of their safety in depth, especially in a clinical setup.