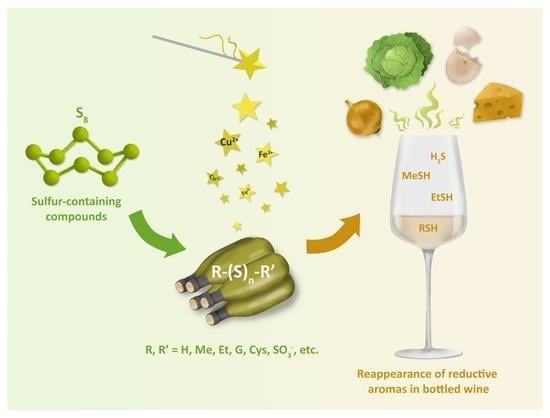
Sulfane Sulfur Compounds as Source of Reappearance of Reductive Off-Odors in Wine
Abstract
:1. Introduction
- A description of sulfane sulfur precursors and a discussion of their importance;
- Possible reactions of chemicals and enzymatic formation of sulfane sulfur precursors, including the role of the sulfur metabolism of Saccharomyces cerevisiae yeasts;
- Chemical reactions and their mechanisms which lead to the formation of reductive off-odors in wine from sulfane sulfur precursors after bottling.
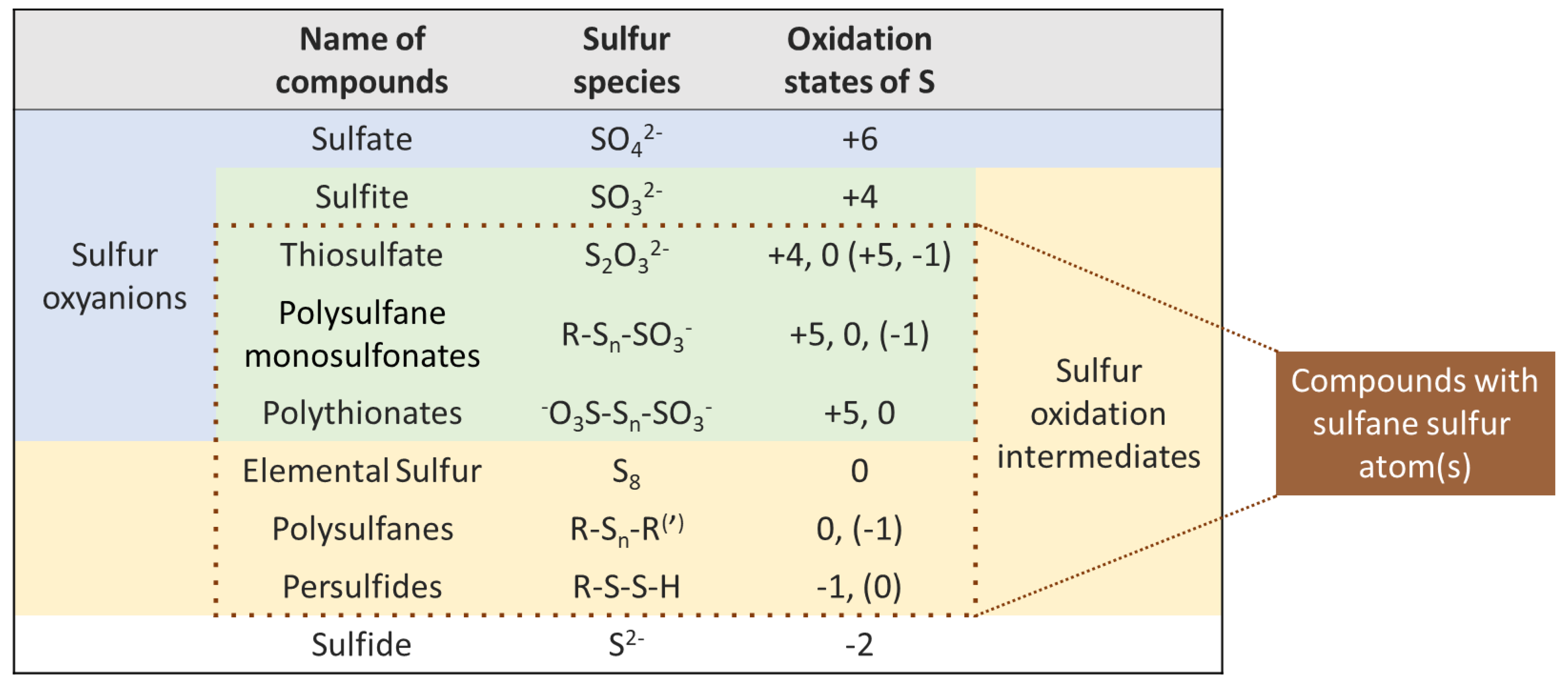
2. Definition of Sulfane Sulfur Compounds
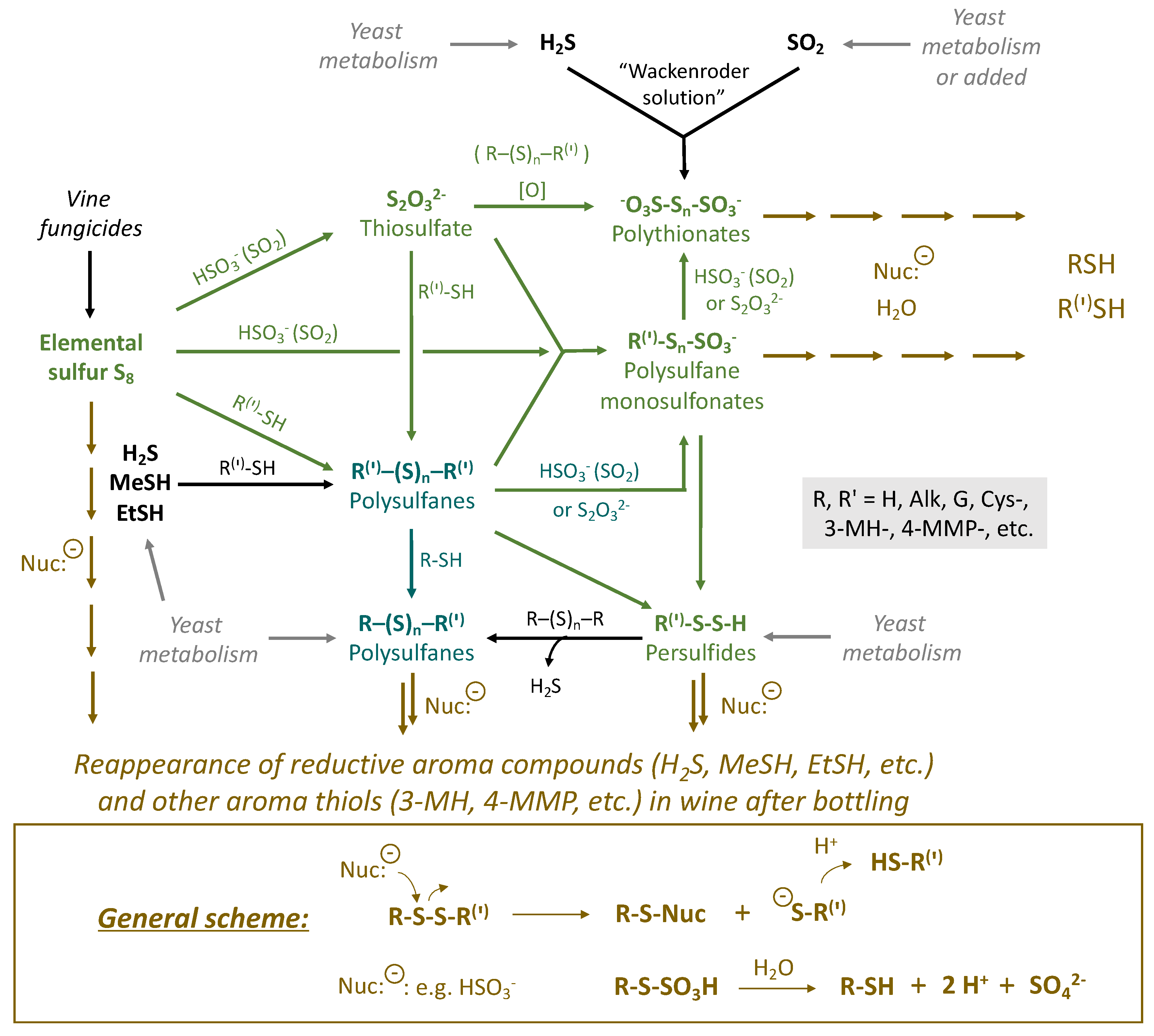
3. Sulfane Sulfur Compounds in Wine and Their Transformations
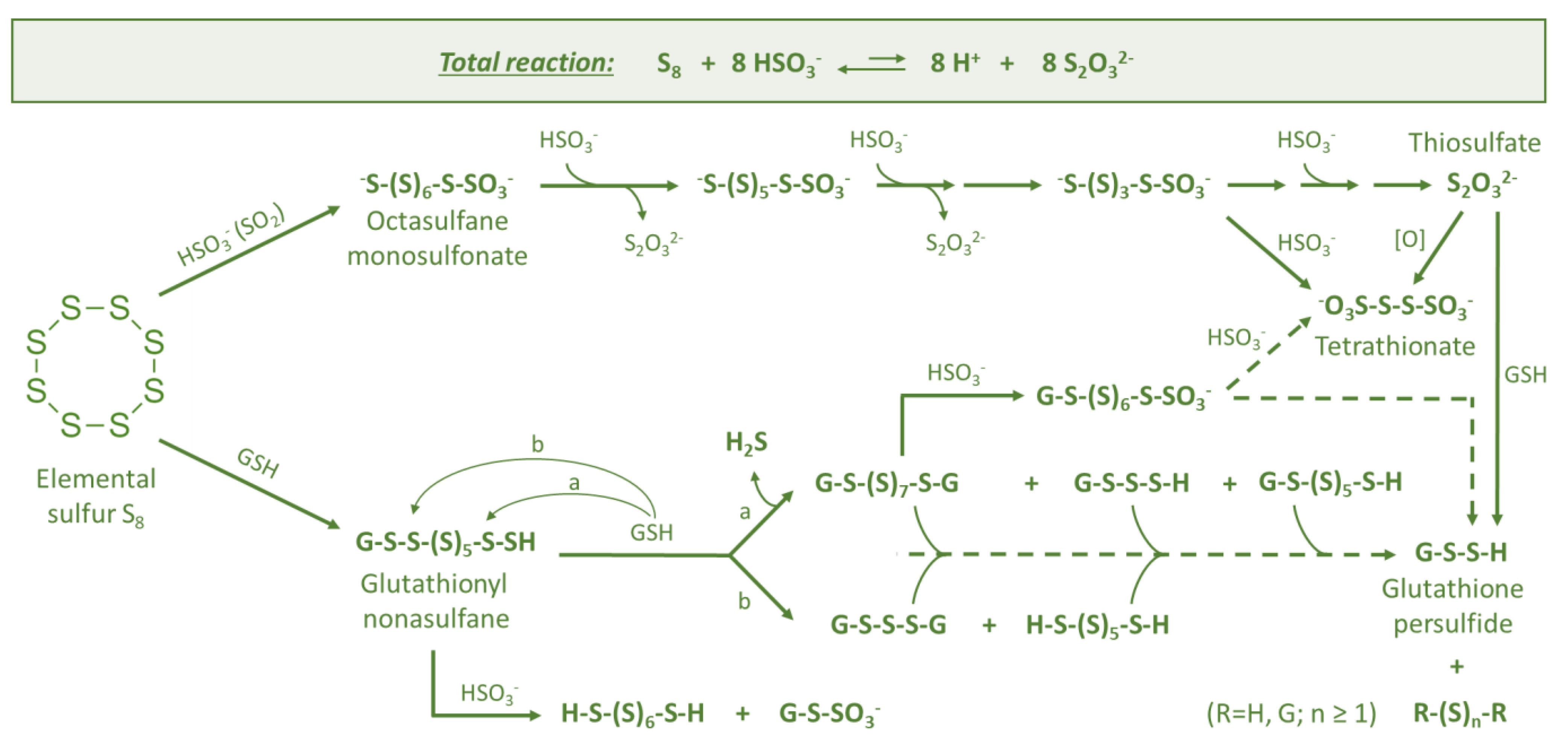
3.1. Elemental Sulfur (S8)
3.2. Thiosulfate (S2O32−)
3.3. Persulfides (R-S-S-H)
3.4. Polysulfanes (R-Sn-R(′))
- (a)
- Sulfitolysis: formation of polysulfane monosulfonates and polythionates, which will be discussed in the next subsections;
- (b)
- Thiosulfatolysis: results in polysulfane monosulfonates with sulfur chain elongation;
- (c)
- Thiolysis by H2S: trapping of malodorous H2S, which can reappear later due to the subsequent reactions, e.g., with HS-containing nucleophiles;
- (d)
- Thiolysis by MeSH: trapping of malodorous MeSH, which can be released later due to the consequent reactions, e.g., sulfitolysis;
- (e)
- Thiolysis by glutathione persulfide: formation of polysulfanes with longer sulfur chains;
- (f)
- Thiolysis with 4-MMP: trapping of varieatal thiol 4-MMP, which can reappear later due to the subsequent reactions, e.g., with HS-containing nucleophiles.
3.5. Polythionates (−O3S-Sn-SO3−)
3.6. Polysulfane Monosulfonates (R-Sn-SO3−)
4. Biosynthesis of Sulfane Sulfur Compounds by Microorganisms during Fermentation and the Enzymes Involved
5. Proteins with Various Sulfane Sulfur Groups
6. Final Remarks
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Mestres, M.; Busto, O.; Guasch, J. Analysis of organic sulfur compounds in wine aroma. J. Chromatogr. A 2000, 881, 569–581. [Google Scholar] [CrossRef]
- Siebert, T.; Bramley, B.; Solomon, M. Technical notes. Hydrogen sulfide: Aroma detection threshold study in white and red wine. Tech. Rev. 2009, 183, 14–16. [Google Scholar]
- Siebert, T.E.; Solomon, M.R.; Pollnitz, A.P.; Jeffery, D.W. Selective Determination of Volatile Sulfur Compounds in Wine by Gas Chromatography with Sulfur Chemiluminescence Detection. J. Agric. Food Chem. 2010, 58, 9454–9462. [Google Scholar] [CrossRef] [PubMed]
- Goniak, O.J.; Noble, A.C. Sensory Study of Selected Volatile Sulfur Compounds in White Wine. Am. J. Enol. Vitic. 1987, 38, 223–227. [Google Scholar]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Fracassetti, D.; Vigentini, I. Occurrence and Analysis of Sulfur Compounds in Wine. In Grapes and Wines; Jordão, A.M., Cosme, F., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Tarasov, A.; Garzelli, F.; Schuessler, C.; Fritsch, S.; Loisel, C.; Pons, A.; Patz, C.-D.; Rauhut, D.; Jung, R. Wine Storage at Cellar vs. Room Conditions: Changes in the Aroma Composition of Riesling Wine. Molecules 2021, 26, 6256. [Google Scholar] [CrossRef]
- Rauhut, D.; Kürbel, H.; MacNamara, K.; Grossmann, M. Headspace GC-SCD monitoring of low volatile sulfur compounds during fermentation and in wine. Analusis 1998, 26, 142–144. [Google Scholar] [CrossRef] [Green Version]
- Rauhut, D. Impact of volatile sulfur compounds on wine quality. In Sulfur Transport and Assimilation in Plants; Davidian, J.-C., Grill, D., De Kok, L.J., Stulen, I., Hawkesford, M.J., Schnug, E., Rennenberg, H., Eds.; Backhuys Publishers: Leiden, The Netherlands, 2003; pp. 121–131. [Google Scholar]
- Rauhut, D.; Kürbel, H.; Fischer, S.; Beisert, B. Simultaneous analysis of N- and S-compounds as screening for the identification of off-flavours in wine. In Recent Highlights in Flavor Chemistry and Biology; Hofmann, T., Meyerhof, W., Schieberle, P., Eds.; Deutsche Forschungsanstalt für Lebensmittelchemie: Garching, Germany, 2007; pp. 314–317. [Google Scholar]
- Rauhut, D.; Beisert, B.; Berres, M.; Gawron-Scibek, M.; Kürbel, H. Pulse flame photometric detection: An innovative technique to analyse volatile sulfur compounds in wine and other beverages. In State of the Art in flavour Chemistry and Biology; Hofmann, T., Rothe, M., Schieberle, P., Eds.; Deutsche Forschungsanstalt für Lebensmittelchemie: Garching, Germany, 2005; pp. 363–368. [Google Scholar]
- Goode, J.; Harrop, S. Wine faults and their prevalence: Data from the world’s largest blind tasting. In Proceedings of the 20th Entretiens Scientifiques Lallemand, Horsens, Denmark, 15 May 2008; pp. 7–9. [Google Scholar]
- Müller, N.; Rauhut, D. Recent Developments on the Origin and Nature of Reductive Sulfurous Off-Odours in Wine. Fermentation 2018, 4, 62. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, T.M.; Grieshaber, M.K. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J. 2008, 275, 3352–3361. [Google Scholar] [CrossRef]
- Jiang, G.; Li, M.; Wen, Y.; Zeng, W.; Zhao, Q.; Chen, C.; Yuan, H.; Liu, C.; Liu, C. Visualization of Sulfane Sulfur in Plants with a Near-Infrared Fluorescent Probe. ACS Sens. 2019, 4, 434–440. [Google Scholar] [CrossRef]
- Koike, S.; Ogasawara, Y. Sulfur Atom in its Bound State Is a Unique Element Involved in Physiological Functions in Mammals. Molecules 2016, 21, 1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauhut, D. Usage and Formation of Sulphur Compounds. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 255–291. ISBN 978-3-319-60021-5. [Google Scholar]
- Thomas, D.; Surdin-Kerjan, Y. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1997, 61, 503–532. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.B.; Sacks, G.L. Brine-Releasable Hydrogen Sulfide in Wine: Mechanism of Release from Copper Complexes and Effects of Glutathione. J. Agric. Food Chem. 2021, 69, 13164–13172. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Walker, M.E.; Fedrizzi, B.; Gardner, R.C.; Jiranek, V. Hydrogen sulfide and its roles in Saccharomyces cerevisiae in a winemaking context. FEMS Yeast Res. 2017, 17, fox058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vela, E.; Hernández-Orte, P.; Franco-Luesma, E.; Ferreira, V. The effects of copper fining on the wine content in sulfur off-odors and on their evolution during accelerated anoxic storage. Food Chem. 2017, 231, 212–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreitman, G.Y.; Elias, R.J.; Jeffery, D.W.; Sacks, G.L. Loss and formation of malodorous volatile sulfhydryl compounds during wine storage. Crit. Rev. Food Sci. Nutr. 2019, 59, 1728–1752. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.L. Sulfane sulfur. In Methods in Enzymology; Sulfur and Sulfur Amino Acids; Academic Press: Cambridge, MA, USA, 1987; Volume 143, pp. 25–29. [Google Scholar]
- Iciek, M.; Bilska-Wilkosz, A.; Górny, M. Sulfane sulfur—New findings on an old topic. Acta Biochim. Pol. 2019, 66, 533–544. [Google Scholar] [CrossRef]
- Westley, J.; Adler, H.; Westley, L.; Nishida, C. The sulfurtransferases. Fundam. Appl. Toxicol. 1983, 3, 377–382. [Google Scholar] [CrossRef]
- Toohey, J.I. Sulfur signaling: Is the agent sulfide or sulfane? Anal. Biochem. 2011, 413, 1–7. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Ignarro, L.J.; Nagy, P.; Wink, D.A.; Kevil, C.G.; Feelisch, M.; Cortese-Krott, M.M.; Bianco, C.L.; Kumagai, Y.; Hobbs, A.J.; et al. Biological hydropersulfides and related polysulfides—A new concept and perspective in redox biology. FEBS Lett. 2018, 592, 2140–2152. [Google Scholar] [CrossRef] [Green Version]
- Bekker, M.Z.; Kreitman, G.Y.; Jeffery, D.W.; Danilewicz, J.C. Liberation of Hydrogen Sulfide from Dicysteinyl Polysulfanes in Model Wine. J. Agric. Food Chem. 2018, 66, 13483–13491. [Google Scholar] [CrossRef] [PubMed]
- Ida, T.; Sawa, T.; Ihara, H.; Tsuchiya, Y.; Watanabe, Y.; Kumagai, Y.; Suematsu, M.; Motohashi, H.; Fujii, S.; Matsunaga, T.; et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7606–7611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wróbel, M.; Lewandowska, I.; Bronowicka-Adamska, P.; Paszewski, A. The level of sulfane sulfur in the fungus Aspergillus nidulans wild type and mutant strains. Amino Acids 2008, 37, 565. [Google Scholar] [CrossRef] [PubMed]
- Araujo, L.D.; Vannevel, S.; Buica, A.; Callerot, S.; Fedrizzi, B.; Kilmartin, P.A.; du Toit, W.J. Indications of the prominent role of elemental sulfur in the formation of the varietal thiol 3-mercaptohexanol in Sauvignon blanc wine. Food Res. Int. 2017, 98, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Jastrzembski, J.A.; Allison, R.B.; Friedberg, E.; Sacks, G.L. Role of Elemental Sulfur in Forming Latent Precursors of H2S in Wine. J. Agric. Food Chem. 2017, 65, 10542–10549. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Heinrich, H. Reaktion von schwefliger Säure mit elementarem Schwefel. Über Säuren des Schwefels, XI. Angew. Chem. 1958, 70, 572. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Li, H.; Liu, H.; Xia, Y.; Xun, L. The Complete Pathway for Thiosulfate Utilization in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2018, 84, e01241-18. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Xia, Y.; Liu, H.; Liu, H.; Xun, L. The Mechanisms of Thiosulfate Toxicity against Saccharomyces cerevisiae. Antioxidants 2021, 10, 646. [Google Scholar] [CrossRef]
- Melideo, S.L.; Jackson, M.R.; Jorns, M.S. Biosynthesis of a Central Intermediate in Hydrogen Sulfide Metabolism by a Novel Human Sulfurtransferase and Its Yeast Ortholog. Biochemistry 2014, 53, 4739–4753. [Google Scholar] [CrossRef]
- Huang, C.-W. Identification of Yeast Genes Affecting Production of Hydrogen Sulfide and Volatile Thiols from Cysteine Treatment during Fermentation. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 2017. [Google Scholar]
- Starkenmann, C.; Chappuis, C.J.-F.; Niclass, Y.; Deneulin, P. Identification of Hydrogen Disulfanes and Hydrogen Trisulfanes in H2S Bottle, in Flint, and in Dry Mineral White Wine. J. Agric. Food Chem. 2016, 64, 9033–9040. [Google Scholar] [CrossRef]
- Jastrzembski, J.A. Mass Spectrometry for the High-Throughput Quantification and Mechanistic Investigation of Odor-Active Volatiles in Grapes and Wine. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 2017. [Google Scholar] [CrossRef]
- Kumar, M.R.; Farmer, P.J. Characterization of Polysulfides, Polysulfanes, and Other Unique Species in the Reaction between GSNO and H2S. Molecules 2019, 24, 3090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohwerder, T.; Sand, W. The sulfane sulfur of persulfides is the actual substrate of the sulfur-oxidizing enzymes from Acidithiobacillus and Acidiphilium spp. Microbiol. Read. Engl. 2003, 149, 1699–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobet, R.A.; Noble, A.C.; Boulton, R.B. Kinetics of the ethanethiol and diethyl disulfide interconversion in wine-like solutions. J. Agric. Food Chem. 1990, 38, 449–452. [Google Scholar] [CrossRef]
- Kreitman, G.Y.; Danilewicz, J.C.; Jeffery, D.W.; Elias, R.J. Reaction Mechanisms of Metals with Hydrogen Sulfide and Thiols in Model Wine. Part 2: Iron- and Copper-Catalyzed Oxidation. J. Agric. Food Chem. 2016, 64, 4105–4113. [Google Scholar] [CrossRef]
- Kreitman, G.Y.; Danilewicz, J.C.; Jeffery, D.W.; Elias, R.J. Copper(II)-Mediated Hydrogen Sulfide and Thiol Oxidation to Disulfides and Organic Polysulfanes and Their Reductive Cleavage in Wine: Mechanistic Elucidation and Potential Applications. J. Agric. Food Chem. 2017, 65, 2564–2571. [Google Scholar] [CrossRef]
- Nardin, T.; Larcher, R.; van Leeuwen, K.A.; Pilkington, L.I.; Roman, T.; Malacarne, M.; Fedrizzi, B. Effect of antioxidant supplementation on the polysulfides of white wines. LWT 2020, 134, 110132. [Google Scholar] [CrossRef]
- Nedjma, M.; Hoffmann, N. Hydrogen Sulfide Reactivity with Thiols in the Presence of Copper(II) in Hydroalcoholic Solutions or Cognac Brandies: Formation of Symmetrical and Unsymmetrical Dialkyl Trisulfides. J. Agric. Food Chem. 1996, 44, 3935–3938. [Google Scholar] [CrossRef]
- Nishibori, N.; Kuroda, A.; Yamada, O.; Goto-Yamamoto, N. Factors Affecting Dimethyl Trisulfide Formation in Wine. Food Sci. Technol. Res. 2017, 23, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Pilkington, L.I.; Deed, R.C.; Parish-Virtue, K.; Huang, C.-W.; Walker, M.E.; Jiranek, V.; Barker, D.; Fedrizzi, B. Iterative synthetic strategies and gene deletant experiments enable the first identification of polysulfides in Saccharomyces cerevisiae. Chem. Commun. 2019, 55, 8868–8871. [Google Scholar] [CrossRef]
- van Leeuwen, K.A.; Nardin, T.; Barker, D.; Fedrizzi, B.; Nicolini, G.; Larcher, R. A novel LC-HRMS method reveals cysteinyl and glutathionyl polysulfides in wine. Talanta 2020, 218, 121105. [Google Scholar] [CrossRef]
- Arapitsas, P.; Ugliano, M.; Perenzoni, D.; Angeli, A.; Pangrazzi, P.; Mattivi, F. Wine metabolomics reveals new sulfonated products in bottled white wines, promoted by small amounts of oxygen. J. Chromatogr. A 2016, 1429, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Dekker, S.; Fedrizzi, B.; van Leeuwen, K.A.; Nardin, T.; Dell’Anna, C.; Larcher, R. Time course accumulation of polysulfides in Chardonnay and model juice fermentations. Food Chem. 2022, 371, 131341. [Google Scholar] [CrossRef] [PubMed]
- Hallinan, C.P.; Saul, D.J.; Jiranek, V. Differential utilisation of sulfur compounds for H2S liberation by nitrogen-starved wine yeasts. Aust. J. Grape Wine Res. 1999, 5, 82–90. [Google Scholar] [CrossRef]
- Benchoam, D.; Semelak, J.A.; Cuevasanta, E.; Mastrogiovanni, M.; Grassano, J.S.; Ferrer-Sueta, G.; Zeida, A.; Trujillo, M.; Möller, M.N.; Estrin, D.A.; et al. Acidity and nucleophilic reactivity of glutathione persulfide. J. Biol. Chem. 2020, 295, 15466–15481. [Google Scholar] [CrossRef]
- Dupraz, S.; Ménez, B.; Guyot, F. Fast Determination of the Main Reduced Sulfur Species in Aquatic Systems by a Direct and Second-Derivative Spectrophotometric Method. J. Chem. 2019, 2019, e1039487. [Google Scholar] [CrossRef] [Green Version]
- Nikolantonaki, M.; Waterhouse, A.L. A Method To Quantify Quinone Reaction Rates with Wine Relevant Nucleophiles: A Key to the Understanding of Oxidative Loss of Varietal Thiols. J. Agric. Food Chem. 2012, 60, 8484–8491. [Google Scholar] [CrossRef]
- Steudel, R.; Eckert, B. Solid Sulfur AllotropesSulfur Allotropes. In Elemental Sulfur and Sulfur-Rich Compounds I; Steudel, R., Ed.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–80. ISBN 978-3-540-44855-6. [Google Scholar]
- Kafantaris, C. On the Reactivity of Nanoparticulate Elemental Sulfur: Experimentation and Field Observations. Ph.D. Thesis, Indiana Univerity, Bloomington, IN, USA, 2017. [Google Scholar]
- Rauhut, D.; Kürbel, H. The production from H2S from elemental sulfur residues during fermentation and its influence on the formation of sulfur metabolites causing off-flavours in wine. Vitic. Enol. Sci 1994, 49, 27–36. [Google Scholar]
- Schütz, M.; Kunkee, R.E. Formation of Hydrogen Sulfide from Elemental Sulfur during Fermentation by Wine Yeast. Am. J. Enol. Vitic. 1977, 28, 137–144. [Google Scholar]
- Thomas, C.S.; Gubler, W.D.; Silacci, M.W.; Miller, R. Changes in Elemental Sulfur Residues on Pinot noir and Cabernet Sauvignon Grape Berries during the Growing Season. Am. J. Enol. Vitic. 1993, 44, 205. [Google Scholar]
- Kwasniewski, M.T.; Sacks, G.L.; Wilcox, W.F. Persistence of Elemental Sulfur Spray Residue on Grapes during Ripening and Vinification. Am. J. Enol. Vitic. 2014, 65, 453. [Google Scholar] [CrossRef] [Green Version]
- Kritzinger, E.C.; Bauer, F.F.; du Toit, W.J. Role of Glutathione in Winemaking: A Review. J. Agric. Food Chem. 2013, 61, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Dahl, C.; Friedrich, C.; Kletzin, A. Sulfur Oxidation in Prokaryotes. In eLS; American Cancer Society: Atlanta, GA, USA, 2008; ISBN 978-0-470-01590-2. [Google Scholar]
- Dahl, C.; Hell, R.; Leustek, T.; Knaff, D. Introduction to Sulfur Metabolism in Phototrophic Organisms. In Sulfur Metabolism in Phototrophic Organisms; Hell, R., Dahl, C., Knaff, D., Leustek, T., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2008; pp. 1–14. ISBN 978-1-4020-6863-8. [Google Scholar]
- Arapitsas, P.; Guella, G.; Mattivi, F. The impact of SO2 on wine flavanols and indoles in relation to wine style and age. Sci. Rep. 2018, 8, 858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alessandri, M.P. Nutritional and Genetic Aspects of Sulfite Excretion during Fermentation by Wine Strains of Saccharomyces cerevisiae. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2018. [Google Scholar]
- Schmidt, M. Über Säuren des Schwefels. I. zur Kenntnis der wasserfreien Thioschwefelsäure. Z. Für Anorg. Allg. Chem. 1957, 289, 141–157. [Google Scholar] [CrossRef]
- Miethe, G. Untersuchungen zum Zerfall und zur Analytik der Zersetzungsprodukte von Natriumthiosulfat-Injektionslösungen. Ph.D. Dissertation, Humbolt-Universität Berlin, Berlin, Germany, 2003. [Google Scholar]
- Breuer, P.L.; Jeffrey, M.I. The reduction of copper(II) and the oxidation of thiosulfate and oxysulfur anions in gold leaching solutions. Hydrometallurgy 2003, 70, 163–173. [Google Scholar] [CrossRef]
- Funahashi, E.; Saiki, K.; Honda, K.; Sugiura, Y.; Kawano, Y.; Ohtsu, I.; Watanabe, D.; Wakabayashi, Y.; Abe, T.; Nakanishi, T.; et al. Finding of thiosulfate pathway for synthesis of organic sulfur compounds in Saccharomyces cerevisiae and improvement of ethanol production. J. Biosci. Bioeng. 2015, 120, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Benchoam, D.; Cuevasanta, E.; Möller, M.N.; Alvarez, B. Hydrogen Sulfide and Persulfides Oxidation by Biologically Relevant Oxidizing Species. Antioxidants 2019, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Cuevasanta, E.; Lange, M.; Bonanata, J.; Coitiño, E.L.; Ferrer-Sueta, G.; Filipovic, M.R.; Alvarez, B. Reaction of Hydrogen Sulfide with Disulfide and Sulfenic Acid to Form the Strongly Nucleophilic Persulfide. J. Biol. Chem. 2015, 290, 26866–26880. [Google Scholar] [CrossRef] [Green Version]
- Benchoam, D.; Cuevasanta, E.; Möller, M.N.; Alvarez, B. Persulfides, at the crossroads between hydrogen sulfide and thiols. Essays Biochem. 2020, 64, 155–168. [Google Scholar] [CrossRef]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Aroca, A.; Gotor, C.; Romero, L.C. Hydrogen Sulfide Signaling in Plants: Emerging Roles of Protein Persulfidation. Front. Plant Sci. 2018, 9, 1369. [Google Scholar] [CrossRef] [Green Version]
- Vojtovič, D.; Luhová, L.; Petřivalský, M. Something smells bad to plant pathogens: Production of hydrogen sulfide in plants and its role in plant defence responses. J. Adv. Res. 2021, 27, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Corvino, A.; Frecentese, F.; Magli, E.; Perissutti, E.; Santagada, V.; Scognamiglio, A.; Caliendo, G.; Fiorino, F.; Severino, B. Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants 2021, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Ran, M.; Wang, T.; Shao, M.; Chen, Z.; Liu, H.; Xia, Y.; Xun, L. Sensitive Method for Reliable Quantification of Sulfane Sulfur in Biological Samples. Anal. Chem. 2019, 91, 11981–11986. [Google Scholar] [CrossRef] [PubMed]
- Pluth, M.D.; Bailey, T.S.; Hammers, M.D.; Hartle, M.D.; Henthorn, H.A.; Steiger, A.K. Natural Products Containing Hydrogen Sulfide Releasing Moieties. Synlett 2015, 26, 2633–2643. [Google Scholar] [CrossRef]
- Dahl, C. Sulfur Metabolism in Phototrophic Bacteria. In Modern Topics in the Phototrophic Prokaryotes: Metabolism, Bioenergetics, and Omics; Hallenbeck, P.C., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 27–66. ISBN 978-3-319-51365-2. [Google Scholar]
- Dekker, S.; Nardin, T.; Mattana, M.; Fochi, I.; Larcher, R. Identification and characterisation of thiolated polysulfides in must and wine using online SPE UHPLC-HRMS. Anal. Bioanal. Chem. 2020, 412, 5229–5245. [Google Scholar] [CrossRef]
- Müller, N. Iminiumsalz-Strukturen bei der durch Pyridoxalphosphat (Vitamin B6) katalysierten Bildung von Aromastoffen und Fehlaromen im Wein. Z. Nat. B 2018, 73, 521–533. [Google Scholar] [CrossRef]
- Kelly, D.P. Thermodynamic aspects of energy conservation by chemolithotrophic sulfur bacteria in relation to the sulfur oxidation pathways. Arch. Microbiol. 1999, 171, 219–229. [Google Scholar] [CrossRef]
- Steudel, R.; Holdt, G.; Göbel, T.; Hazeu, W. Chromatographic Separation of Higher Polythionates SnO (n = 3…22) and Their Detection in Cultures of Thiobacillus ferroxidans; Molecular Composition of Bacterial Sulfur Secretions. Angew. Chem. Int. Ed. Engl. 1987, 26, 151–153. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A.; Nordstrom, D.K.; Cunningham, K.M.; Ball, J.W. Sulfur geochemistry of hydrothermal waters in Yellowstone National Park, Wyoming, USA. II. Formation and decomposition of thiosulfate and polythionate in Cinder Pool. J. Volcanol. Geotherm. Res. 2000, 97, 407–423. [Google Scholar] [CrossRef]
- Lens, P.; Pol, L.H. Environmental Technologies to Treat Sulfur Pollution—Principles and Applications; IWA Publishing: London, UK, 2005. [Google Scholar] [CrossRef]
- Rickard, D. Sulfidic Sediments and Sedimentary Rocks, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 65, ISBN 978-0-444-52989-3. [Google Scholar]
- BRENDA BRaunschweig ENzyme DAtabase. Available online: https://www.brenda-enzymes.org (accessed on 10 November 2021).
- KEGG Kyoto Encyclopedia of Genes and Genomes. Available online: https://www.genome.jp/kegg/ (accessed on 10 November 2021).
- Pedre, B.; Dick, T.P. 3-Mercaptopyruvate sulfurtransferase: An enzyme at the crossroads of sulfane sulfur trafficking. Biol. Chem. 2021, 402, 223–237. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Kreinbring, C.A.; Hill, M.; Liu, C.; Petsko, G.A.; McCune, C.D.; Berkowitz, D.B.; Liu, D.; Ringe, D. Crystal Structures of Cystathionine β-Synthase from Saccharomyces cerevisiae: One Enzymatic Step at a Time. Biochemistry 2018, 57, 3134–3145. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, N.; Wróbel, M. H2S, Polysulfides, and Enzymes: Physiological and Pathological Aspects. Biomolecules 2020, 10, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selles, B.; Moseler, A.; Rouhier, N.; Couturier, J. Rhodanese domain-containing sulfurtransferases: Multifaceted proteins involved in sulfur trafficking in plants. J. Exp. Bot. 2019, 70, 4139–4154. [Google Scholar] [CrossRef] [PubMed]
- Andreeßen, C.; Gerlt, V.; Steinbüchel, A. Conversion of cysteine to 3-mercaptopyruvic acid by bacterial aminotransferases. Enzyme Microb. Technol. 2017, 99, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xin, Y.; Xuan, G.; Zhao, R.; Liu, H.; Xia, Y.; Xun, L. Escherichia coli Uses Separate Enzymes to Produce H2S and Reactive Sulfane Sulfur From L-cysteine. Front. Microbiol. 2019, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Knoll, C.; du Toit, M.; Schnell, S.; Rauhut, D.; Irmler, S. Cloning and characterisation of a cystathionine β/γ-lyase from two Oenococcus oeni oenological strains. Appl. Microbiol. Biotechnol. 2011, 89, 1051–1060. [Google Scholar] [CrossRef]
- Matoba, Y.; Noda, M.; Yoshida, T.; Oda, K.; Ezumi, Y.; Yasutake, C.; Izuhara-Kihara, H.; Danshiitsoodol, N.; Kumagai, T.; Sugiyama, M. Catalytic specificity of the Lactobacillus plantarum cystathionine γ-lyase presumed by the crystallographic analysis. Sci. Rep. 2020, 10, 14886. [Google Scholar] [CrossRef]
- Pripis-Nicolau, L.; de Revel, G.; Bertrand, A.; Lonvaud-Funel, A. Methionine catabolism and production of volatile sulphur compounds by Oenococcus oeni. J. Appl. Microbiol. 2004, 96, 1176–1184. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Brand, J.; Ortiz-Julien, A.; Camarasa, C.; Divol, B. Nitrogen metabolism in three non-conventional wine yeast species: A tool to modulate wine aroma profiles. Food Microbiol. 2021, 94, 103650. [Google Scholar] [CrossRef]
- Benito, S.; Ruiz, J.; Belda, I.; Kiene, F.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Santos, A.; Rauhut, D. Application of Non-Saccharomyces Yeasts in Wine Production. In Non-Conventional Yeasts: From Basic Research to Application; Sibirny, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 75–89. ISBN 978-3-030-21110-3. [Google Scholar]
- Ferreira, R.B.; Piçarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R. The wine proteins. Trends Food Sci. Technol. 2001, 12, 230–239. [Google Scholar] [CrossRef]
- Claus, H.; Mojsov, K. Enzymes for Wine Fermentation: Current and Perspective Applications. Fermentation 2018, 4, 52. [Google Scholar] [CrossRef] [Green Version]
- Toohey, J.I. Sulphane sulphur in biological systems: A possible regulatory role. Biochem. J. 1989, 264, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S Signals Through Protein S-Sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dóka, É.; Pader, I.; Bíró, A.; Johansson, K.; Cheng, Q.; Ballagó, K.; Prigge, J.R.; Pastor-Flores, D.; Dick, T.P.; Schmidt, E.E.; et al. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2016, 2, e1500968. [Google Scholar] [CrossRef] [Green Version]
- Bekker, M.Z.; Nandorfy, D.E.; Kulcsar, A.C.; Faucon, A.; Bindon, K.; Smith, P.A. Comparison of remediation strategies for decreasing ‘reductive’ characters in Shiraz wines. Aust. J. Grape Wine Res. 2021, 27, 52–65. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Appearance of Reduced Aromas during Bottle Storage. In Understanding Wine Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; ISBN 978-1-118-73072-0. [Google Scholar]
- Lajin, B.; Goessler, W. Exploring the sulfur species in wine by HPLC-ICPMS/MS. Anal. Chim. Acta 2019, 1092, 1–8. [Google Scholar] [CrossRef]
- Roullier-Gall, C.; Hemmler, D.; Gonsior, M.; Li, Y.; Nikolantonaki, M.; Aron, A.; Coelho, C.; Gougeon, R.D.; Schmitt-Kopplin, P. Sulfites and the wine metabolome. Food Chem. 2017, 237, 106–113. [Google Scholar] [CrossRef]
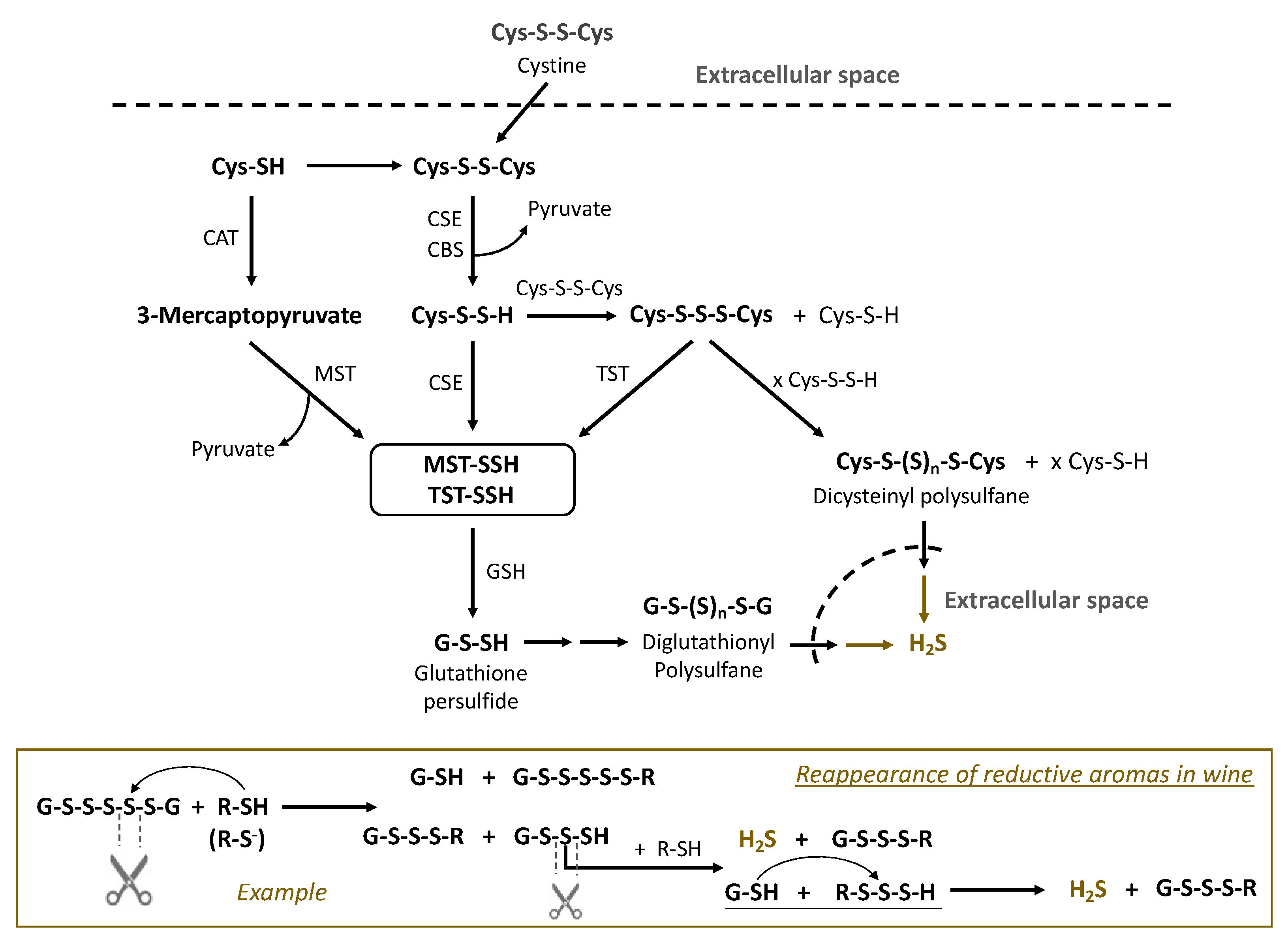
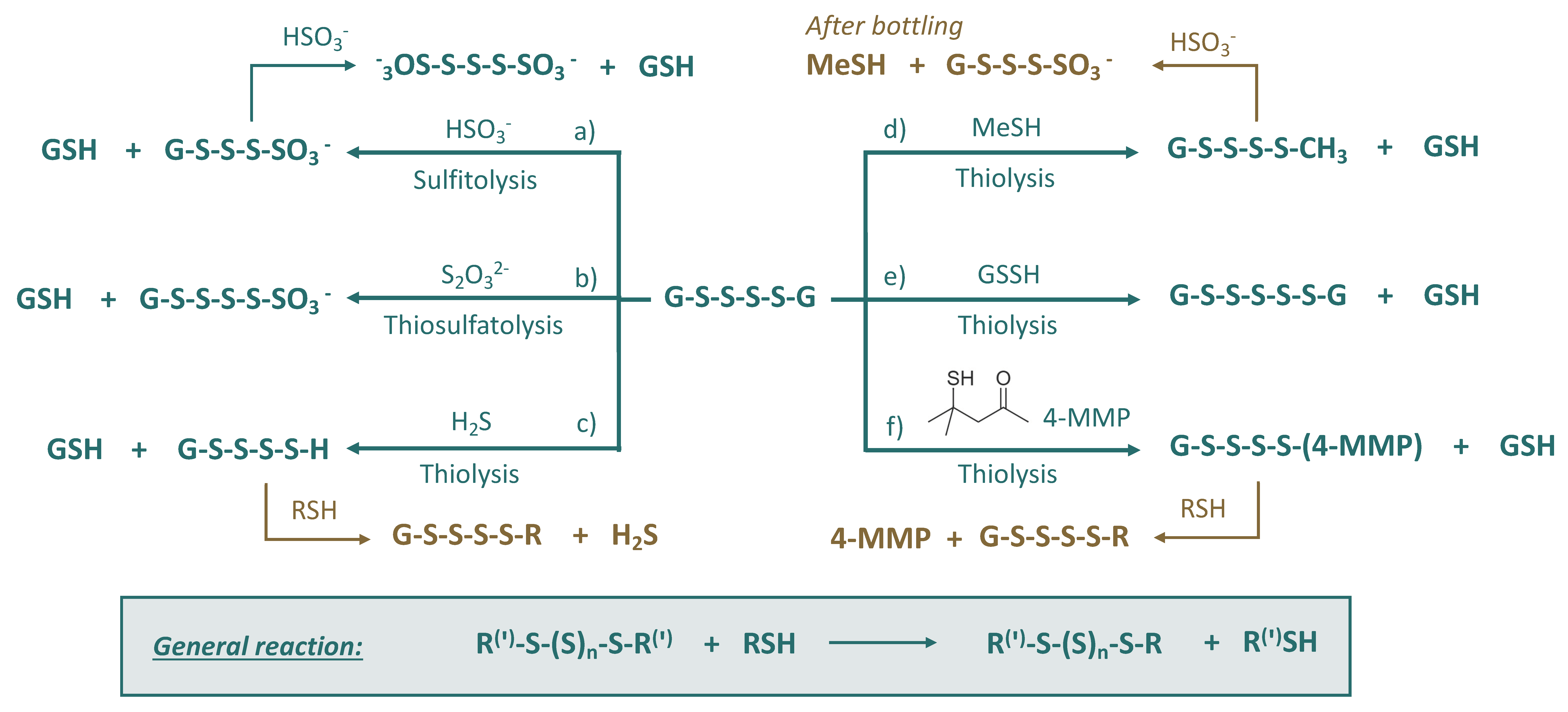
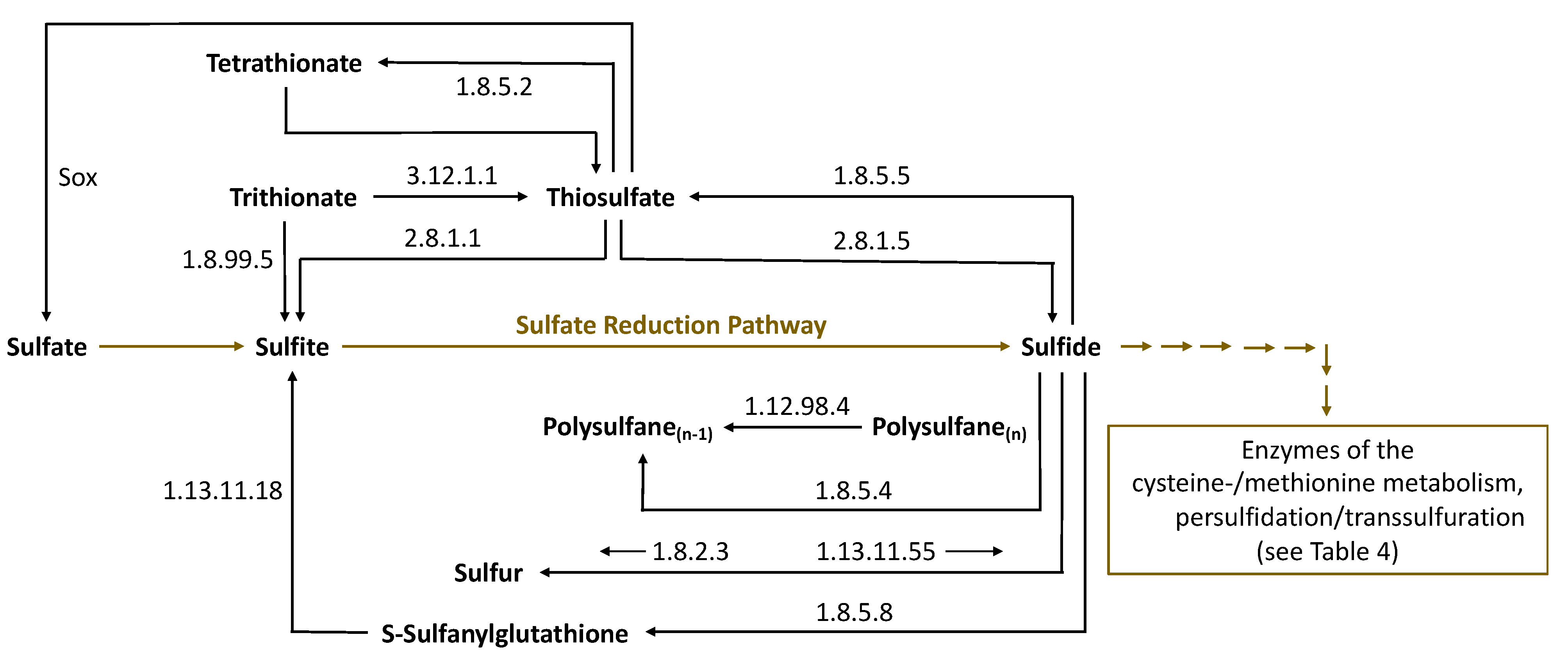
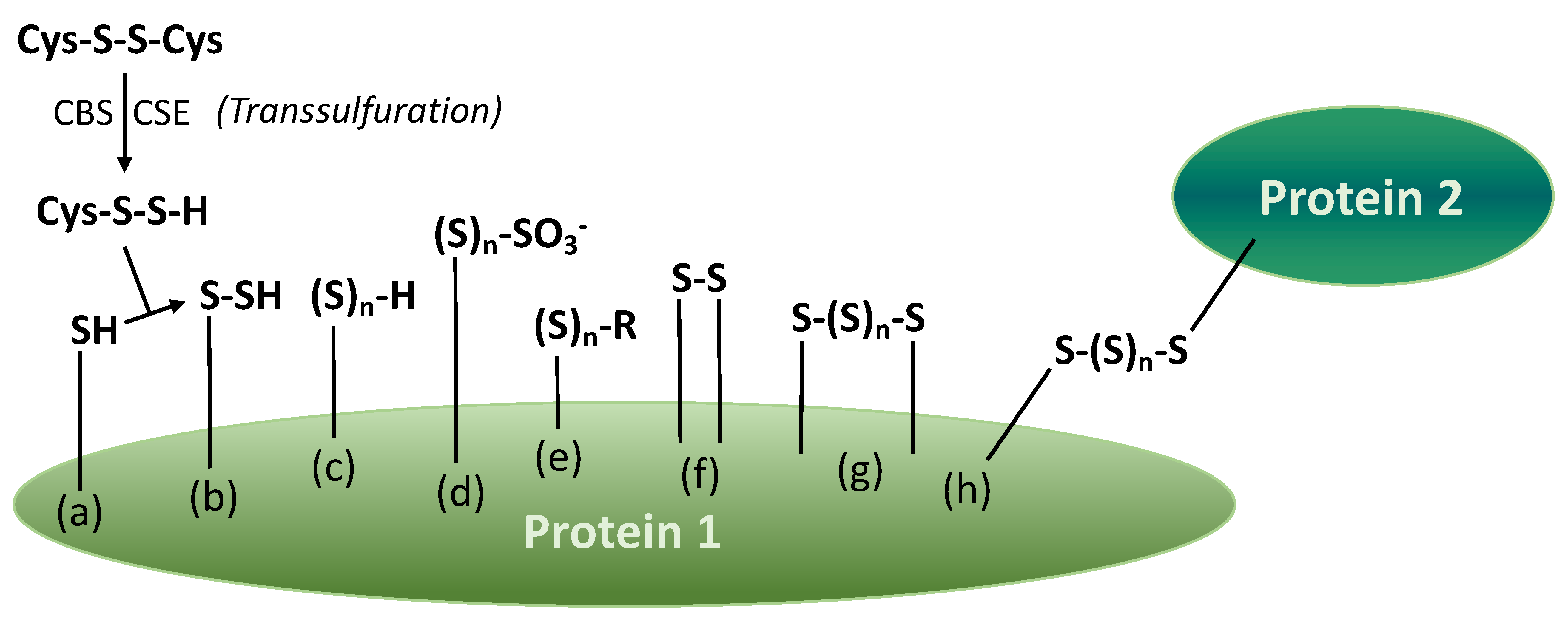
| Sulfane Sulfur Compounds | General Formula | Identification in Wine/Origin | Examples | General Remarks | References |
|---|---|---|---|---|---|
| Elemental sulfur | Sn (homocycles, n = 8) | Yes (fungicide from vineyard) | Octasulfur (S8) | Reactions of sulfur: thiolysis, sulfitolysis, thiosulfatolysis | [31,32,33] |
| Thiosulfate | S2O32− | No | - | Hypothetical formation due to sulfitolysis of sulfur: S8 + SO2 (HSO3−) | [33,34,35] |
| Persulfides | R-S-S-H | Yes (yeast metabolism) | G-S-S-H Cys-S-S-H | [20,35,36] | |
| Inorganic polysulfanes | H-Sn-H (n ≥ 2) | Yes (from precursors in must) | H2S2 H2S3 | Di- and trisulfane “flintstone aroma” | [20,37,38] |
| Organic monosubstituted polysulfanes | R-Sn-H (n ≥ 2) | No (from grape precursors/yeast metabolism) | G-S-(S)x-SH | Hypothetically they can be formed as intermediates, but not stable due to high reactivity | [32,39,40,41] |
| Organic disubstituted polysulfanes | R-Sn-R(′) (n ≥ 2) | Yes (grape precursors/yeast metabolism) | Cys-S-Sn-S-Cys, Me-S-Sn-S-Me G-S-Sn-S-G 3MH-S-Sn-S-G | Symmetric and asymmetric polysulfanes | [20,22,28,42,43,44,45,46,47,48,49] |
| Polythionates | −O3S-Sn-SO3− (n ≥ 1, can be > 20) | Yes (from elemental sulfurreactions) | Tri-, tetra-, penta-, hexathionates | Hypothetical formation also due to “Wackenroder’s reaction” H2S + SO2 | [13,32,39] |
| ––––(Poly)sulfane monosulfonates | R-Sn-SO3− (n ≥ 1) | Yes (from polysulfanes reactions) | G-S-(S)7-SO3− G-Sn-SO3− Cys-Sn-SO3− (n = 1–3) | Formation due to sulfitolysis | [28,32,39,50,51] |
| S-Nucleophile | Formula | pKa (s) |
|---|---|---|
| Hydrogen sulfide | H2S/HS−/S2− | 7.0/12.9 |
| Disulfane | H2S2/HS2−/S22− | 5.0/9.7 |
| Trisulfane | H2S3/HS3−/S32− | 4.2/7.5 |
| Tetrasulfane | H2S4/HS4−/S42− | 3.8/6.3 |
| Pentasulfane | H2S5/HS5−/S52− | 3.5/5.7 |
| Thiosulfate | H2S2O3/HS2O3−/S2O32− | 0.6/1.7 |
| Sulfite | H2SO3/HSO3−/SO32−- | 1.8/7.2 |
| Sulfate | H2SO4/HSO4−/SO42− | −3.0/2.0 |
| Polythionates | H2SxO6/HSxO6−/SxO62− (x = 3–5) | -/−2.3 |
| Methanthiol | MeSH | 10.4 |
| Ethanthiol | EtSH | 10.6 |
| Glutathione | GSH/GS− | 8.94 |
| Glutathione persulfide | GS-SH/GS-S− | 5.45 |
| Enzyme | Abbreviation | E.C. Number | References |
|---|---|---|---|
| Cystathionine β-synthase | CBS | E.C. 4.2.1.22 | [73,74,90,91,92] |
| Cystathionine γ-lyase | CSE (CST, CTH) | E.C. 4.4.1.1 | [73,91,93] |
| Thosulfate sulfurtransferase (Rhodanese) | TST (Rhd) | E.C. 2.8.1.1 | [73,74,93,94] |
| 3-Mercaptopyruvate sulfurtransferase | MST (MpST) | E.C. 2.8.1.2 | [73,91,93] |
| Cysteine aminotransferase | CAT | E.C. 2.6.1.3 | [95,96] |
| Sulfane Sulfur Compound | Enzyme | Substrate | Product | Enzyme (BRENDA) |
|---|---|---|---|---|
| Thiosulfate | Dehydrogenase (quinone) | Thiosulfate | Tetrathionate | 1.8.5.2 |
| Thiosulfate | Reductase (quinone) | Sulfite + H2S | Thiosulfate | 1.8.5.5 |
| Thiosulfate | Dithiol- sulfurtransferase | Thiosulfate + Dithiothreitol + H2S | H2S | 2.8.1.5 |
| Tetrathionate | Thiosulfate dehydrogenase (quinone) | Thiosulfate | Tetrathionate | 1.8.5.2 |
| Trithionate | Trithionate hydrolase | Trithionate + H2O | Thiosulfate + Sulfate | 3.12.1.1 |
| Trithionate | Dissimilatory sulfite reductase | Trithionate + Protein-S-S-H | Sulfite/Thiosulfate (Trithionate pathway) | 1.8.99.5 |
| Sulfanylglutathione G-S-S-H | Eukaryotic sulfide quinone oxido-reductase (SQR) | GSH + Sulfide | G-S-S-H | 1.8.5.8 |
| Sulfanylglutathione G-S-S-H | Persulfide dioxygenase | G-S-S-H + H2O | G-S-H + Sulfite | 1.13.11.18 |
| Elemental Sulfur | Sulfide-cytochrome-c reductase | H2S | Elemental sulfur | 1.8.2.3 |
| Elemental Sulfur | Sulfur oxygenase/reductase | Elemental Sulfur + H2O + O2 | Sulfite + H2S | 1.13.11.55 |
| Polysulfane | Sulfhydrogenase | H2 + Polysulfane(n) (H2 + H-S-S-S-S-S-H) | H2S + Polysulfane(n−1) (H2S + H-S-S-S-S-H) | 1.12.98.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, N.; Rauhut, D.; Tarasov, A. Sulfane Sulfur Compounds as Source of Reappearance of Reductive Off-Odors in Wine. Fermentation 2022, 8, 53. https://doi.org/10.3390/fermentation8020053
Müller N, Rauhut D, Tarasov A. Sulfane Sulfur Compounds as Source of Reappearance of Reductive Off-Odors in Wine. Fermentation. 2022; 8(2):53. https://doi.org/10.3390/fermentation8020053
Chicago/Turabian StyleMüller, Nikolaus, Doris Rauhut, and Andrii Tarasov. 2022. "Sulfane Sulfur Compounds as Source of Reappearance of Reductive Off-Odors in Wine" Fermentation 8, no. 2: 53. https://doi.org/10.3390/fermentation8020053
APA StyleMüller, N., Rauhut, D., & Tarasov, A. (2022). Sulfane Sulfur Compounds as Source of Reappearance of Reductive Off-Odors in Wine. Fermentation, 8(2), 53. https://doi.org/10.3390/fermentation8020053