Abstract
Large-scale worldwide production of plastics requires the use of large quantities of fossil fuels, leading to a negative impact on the environment. If the production of plastic continues to increase at the current rate, the industry will account for one fifth of global oil use by 2050. Bioplastics currently represent less than one percent of total plastic produced, but they are expected to increase in the coming years, due to rising demand. The usage of bioplastics would allow the dependence on fossil fuels to be reduced and could represent an opportunity to add some interesting functionalities to the materials. Moreover, the plastics derived from bio-based resources are more carbon-neutral and their manufacture generates a lower amount of greenhouse gasses. The substitution of conventional plastic with renewable plastic will therefore promote a more sustainable economy, society, and environment. Consequently, more and more studies have been focusing on the production of interesting bio-based building blocks for bioplastics. However, a coherent review of the contribution of fermentation technology to a more sustainable plastic production is yet to be carried out. Here, we present the recent advancement in bioplastic production and describe the possible integration of bio-based monomers as renewable precursors. Representative examples of both published and commercial fermentation processes are discussed.
1. Introduction
Plastics represent a heterogeneous group of polymers with a high molecular weight that has the characteristics of stability, durability, and resilience [1,2,3]. They have been vastly produced for many purposes and applications nowadays, including food and drink packaging, household items, healthcare and pharmaceuticals, and vehicles. In 2019, global plastic production reached a staggering 368 million tons. The major production occurred in Asia, with 51%, followed by North America, Europe, Middle East and Africa, Latin America, and the Commonwealth of Independent States with 19, 16, 7, 4, and 3%, respectively [4]. The highest plastic demand was found in the packaging sector (39.6%) followed by building and construction (20.4%), automotive (9.6%), electrical and electronic (6.2%), household/leisure/sport (4.1%), agricultural (3.4%), and others (16.7%) [4]. The reasons for the incredible success of plastics can be found in their cheap cost, diversity, easy processability, light weight and barrier properties, to mention just a few. However, their large diffusion comes with significant challenges, involving waste management and environmental issues. In fact, by 2050, the plastic industry will be responsible for 20% of global oil use [5] with a total emission of 2.8 gigatons of CO2 per year (corresponding to the emissions of 615,500-megawatt coal plants) [6]. Moreover, large amounts of post-consumer plastic waste are generated annually, as a result of the widespread use of plastic products and the lack of a more circular value chain. Especially during the COVID-19 pandemic, single-use plastic waste, such as face masks, gloves, and packaging for food and e-commerce parcel deliveries, has been skyrocketing. The amount of plastic waste generated globally was estimated to be 1.6 million tons/day during the outbreak, which reached a total of about 585 million tons at the end of 2020 [7]. The management of plastic waste has thus become one of the biggest problems in the world. Globally, less than 10% of plastic is recycled [3]; the rest is either incinerated, generating greenhouse gases and toxic pollutants, or ends up in landfills. Moreover, large fractions are often mismanaged and end up leaking into the environment, with the risk of contaminating both terrestrial and marine ecosystems [1,3,8], even affecting animal and human living by entering the food chains. Notably, more than 150 million tons of plastic waste leaked into the world’s oceans during the last decades [9] and almost 27 million tons accumulated in landfills in only a year (during 2018) [10]. Therefore, it is urgent and imperative to tackle this problem. In fact, despite the technological advancements in mechanical and (more recently) chemical recycling, effective recycling is still challenging, due to the complexity of sorting and processing highly diverse plastic waste materials. More specifically, current recycling often fails to effectively process plastic mixtures and blends, multilayers, as well as food-contaminated plastic waste, leading to an improper management of post-consumer plastic streams. Moreover, there is a risk of recycled plastics losing their physical properties (due to the degradation of the plastic fibers that occurs with each recycling) and having unsatisfactory appearances compared to virgin plastic [11]. As a result, despite increased attention from media, policymakers, industry, consumers and recyclers, in 2018, only 32.5% of European post-consumer plastic waste was collected to be recycled [4], indicating the ineffectiveness of existing infrastructure and technology.
To change the current paradigm of a linear plastic life cycle (produce, use, throw out), the emerging practice is to keep the material in the loop as long as possible, using plastic waste as a renewable resource to enhance its circularity. In other words, as plastic waste has defined composition and abundance, it should re-enter the economy as valuable commodity, instead of being discarded as waste after each use. This, together with a better reutilization of the plastic items, will help to reduce waste and minimize the carbon footprint from new plastic production, as well as conserve natural resources [8]. Through improved recycling technologies, such as biochemical recycling, the non-biodegradable petroleum-based polymers are expected to be recirculated back infinitely to their value chain. Post-consumer plastic will be subjected to chemical or biological depolymerization and then reutilized as feedstock for new plastic materials with the same (or even improved) function and properties. Enzymatic depolymerization has been extensively investigated to open up the infinite recycling of plastic. Engineered leaf-branch compost cutinase [12] and IsPETase [13], for instance, were reported increase in activity and stability, leading to more efficient production of recyclable polyethylene terephthalate (PET) monomers. Another strategy using microbial mixed consortia is proposed for use in plastic degradation. The complex interaction and cross-feeding mechanism could promote the degradation of a recalcitrant substrate such as plastic [3]. These technologies show tremendous potential and will help to develop a more circular plastic value chain that positively impacts economy, society, and environment.
It is worth noting that, while several current chemical recycling proposes to down-cycle plastic waste into products with different/lower functions (i.e., converting plastic waste into fuels, or plastic bottles into carpeting materials, shop counters or park benches, etc.), this strategy does not really allow the material to be kept in the loop, within the same value chain. Despite the importance of these solutions, new technologies are now starting to look into recycling strategies that lead to improved value or property of the plastic materials (upcycling). A good example could be the substitution of (fossil-based) terephthalate in PET with bio-based furan dicarboxylic acid (FDCA) in poly(ethylene furanoate) (PEF), which is not only more biodegradable, but also provides higher barrier properties when used in plastic bottles [14,15].
Due to the complexity and diversity of the plastic value chain, scientific studies have tried to collect different information about topics, such as commercial applications, emerging renewable plastics, rational designs, material properties and characterizations, including definitions of bio-based and biodegradable plastics [2,8,16,17]. Some articles also summarize approaches and technology to produce green building blocks via chemical and biological routes [11,18,19,20]. However, it is imperative to have a comprehensive outlook on how biotechnological processes can contribute to the renewable plastic sector, while keeping a focus on the progress towards actual commercialization. In this review, we analyze the current status of renewable plastic from both, the research and commercial sectors. We highlight the contribution of fermentation technology to the production of bio-building blocks for more easily recyclable and renewable plastics, keeping the material in the loop. This review aims to provide an overview on how fermentation technology can contribute to the improvement of the plastic sector, making it more circular and sustainable.
2. Renewable Plastics
The importance of renewable plastics and their potential to substitute conventional (fossil-based) plastics has been highlighted for decades. However, only in the last decade has the dedication of both the academic and commercial sectors resulted in a more sensitive growth of its market share. The recent report from European Bioplastics and nova-Institute illustrates the rising global bioplastic production, reaching 2.87 million tons in 2025, with a 36% growth from 2020 [21]. The new report [22] predicts that the global bioplastic production will more than triple over next 5 years, reaching 7.59 million tons in 2026. Currently, over 64% of bioplastic is the biodegradable kind (1.5 million tons), while the rest are non-biodegradable bio-based (Figure 1). In fact, bioplastic does not automatically mean biodegradable. According to European Bioplastics, a plastic material is defined as a bioplastic “if it is either bio-based, biodegradable, or features both properties” (Figure 2). Bio-based refers to a material/product that is (partly) derived from biomass, such as corn, sugarcane, or cellulose. It is also important to underline that “bio-based” is not synonym of biodegradable either. In other words, biodegradability does not depend on the origin of a material but rather on its chemical structure and bonds. The clear advantage of bio-based plastic is that it reduces the use of fossil fuel resources by substituting them with renewable feedstock (such as biomass), leading to a smaller carbon footprint or even potential carbon neutrality.
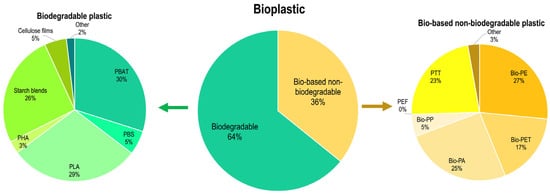
Figure 1.
Global production capacities of bioplastics (adapted from European Bioplastics and nova-Institute [22]).
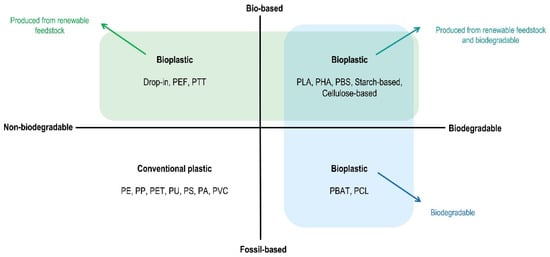
Figure 2.
Plastic material categorized system (adapted from European Bioplastics [23]).
Polybutylene adipate-co-terephthalate (PBAT), poly(lactic acid) (PLA), and starch blends, which are biodegradable plastics, shared the highest global production in 2021, with 19.2%, 18.9, and 16.4%, respectively. These three accounted for 85% of total biodegradable plastic, as seen in Figure 1. While polyhydroxyalkanoates (PHA) (biodegradable bio-based) represented only 1.8% of the worldwide production, it is expected to have a significant growth rate in the coming years [22,24]. Another important degradable plastic, namely polybutylene succinate (PBS), reached 3.5% production capacities.
According to the latest forecast from the nova-Institute [22], PBAT is likely to quadruple during the next 5 years, while PBS will also show a significant growth. The bio-based non-biodegradable plastics, including the drop-ins (such as bio-polyethylene (bio-PE), bio-polypropylene (bio-PP), bio-polyethylene terephthalate (bio-PET), bio-polyamides (bio-PA), and bio-polyurethanes (bio-PU)), PEF, and poly(trimethyleneterephthalate) (PTT) constitute a significant percentage of the bio-plastic market (around 36% in 2021). It is worth noting that only three years before, the nova-Institute report (2018) presented the opposite situation, with the majority of the bioplastics coming from non-degradable polymers (56.8%), thus showing a new interesting trend towards more biodegradable solutions (Figure 3).
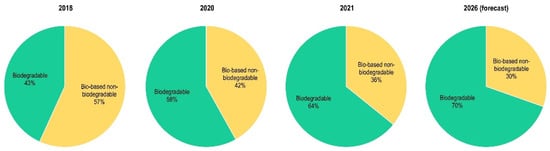
Figure 3.
Bioplastics market trend from 2018–2026 (adapted from European Bioplastics and nova-Institute [21,22,25]).
A new promising polymer is PEF, which has advanced properties for food and drink packaging, can be 100% bio-based and is expected to enter the market in 2023 [21]. It is sometimes presented as the “rising star” among bioplastics, but due to its energy-intensive production process it is not yet established on the market.
In conclusion, it is clear that, because of their renewability, technical properties, and advanced functionality, which allow great application windows, bioplastics show a growing demand and are expected to integrate and (on the long run) replace fossil-based plastics in the coming years.
2.1. Poly(ethylene furanoate) (PEF)
PEF, co-polymerized from 2,5-furandicarboxylic acid (FDCA) and ethylene glycol (EG), is a furan-derived analog to PET and was reported to have greater mechanical properties, lower oxygen/carbon dioxide/water permeability, higher glass transition temperature, and slower chain mobility [15]. Fully bio-based PEF can be produced by the oxidation of 5-hydroxymethylfurfural (5-HMF), which is usually derived from the dehydration product of hexoses, to FDCA [26]. FDCA can also be produced from other sources such as levulinic acid or bioethanol [27], and new studies also suggest the possibility of using C5 sugars such as xylose, converted to furfural and then to FDCA [28]. This would mean that, in principle, FDCA can be obtained from 2G feedstock such as lignocellulosic biomass, valorizing both, the cellulose and hemicellulose fraction. EG, the other significant component of PEF, can also be produced from renewable sugars or produced directly through engineered microorganisms [29]. Bio-EG or propylene glycol can be obtained from sorbitol, for instance. Sorbitol is currently produced by a number of companies and is mainly used in the food industry, but it can also represent an important feedstock for commodity chemicals, such as propylene glycol, EG, glycerol, lactic acid, 1,4-sorbitan and 2,5-anhydrosugars [30]. The Dutch process technology firm Avantium has started up a 10 Mt/y demonstration facility to produce bio-based EG and has received EU funding (Horizon 2020 EGePLANT project) to assess the technoeconomic and environmental sustainability of the process [31,32]. Moreover, recent studies reported enzymatic degradation of PET to EG and terephthalic acid (TA) [12]. So another approach would be the utilization of more sustainable recycled EG [33]. With its improved properties and the utilization of bio-based building blocks, PEF has the potential to become the ideal substitute for conventional packaging plastic, once the minimum selling price becomes more competitive. This would, however, require large-scale production and process optimization, as the estimated selling price for PEF is currently expected to be 4–5 times higher than that for PET [33]. According to a recent study by Roux and Varrone [33], however, these costs can significantly decrease if higher recycling rates would be applied.
In addition to the comparison of PEF and PET in terms of properties and costs, the comparison of their biodegradation is also important for the circular economy scenario. Weinberger et al. reported that PEF is 1.7 times faster than PET in enzymatical hydrolysis by Humicola insolens cutinase [34]. However, PEF still has lower biodegradability compared to the entirely aliphatic renewable polyesters such as PBS and poly(butylene adipate) (PBA) [30], and is in fact listed among the non-degradable bioplastics (see Figure 2). Attempts toward improving its biodegradability have been reported by co-polymerization with other bio-based monomers, e.g., lactic acid and succinic acid. Poly(ethylene 2,5-furandicarboxylate)-co-poly-(lactic acid) (PEF-co-PLA) [35], poly(ethylene 2,5-furandicarboxylate-co-ethylene succinate) (PEF-co-PES) [36], and poly(ethylene furanoate-co-ethylene adipate) (PEFAd) [37] are examples of co-polyesters that were developed. Matos et al. reported that the incorporation of only 8 mol% of lactyl units into the PEF-co-PLA backbone improved the degradability substantially compared to PEF homo-polyester, without affecting its thermal properties [35]. Co-polymer PEFAd with 90 and 95 mol% of ethylene adipate (EAd) was degraded completely after 22 and 25 days, respectively, using Rhizopus oryzae and Pseudomonas cepacia lipases. Moreover, the coloration during the synthesis was reduced with the reducing ethylene furanoate (EF) content, as it was white-yellowish at more than 90% EAd, while it was red or light brown at the lower EAd percentage. The decomposition temperature of the PEFAd was about 400 °C, thus presenting good thermal stability [37]. These approaches could, therefore, be beneficial for packaging applications, even though they pose the risk of complicating the recycling process.
2.2. Poly(trimethylene terephthalate) (PTT)
PTT reached 8.1% of global bioplastic production in 2021, which puts it in the top three most produced bio-based non-biodegradable plastics [22]. It is a semi-crystalline thermoplastic that can be easily molded and spun to fiber; thus, it has notable applications in carpet and textile fibers. The characteristics of PTT are similar to PET in terms of mechanical and thermo-physical properties and to polybutylene terephthalate (PBT) in terms of molding properties. Thus, it has good flexibility and chemical resistance [38,39]. Interestingly, PTT could be theoretically produced in already existing PET production sites [40].
Monomers of PTT comprise 1,3-propanediol (1,3-PDO) and TA, which can be polymerized to PTT via a (trans-)esterification process. Partially bio-based PTT has been produced since the year 2000, using bio-based 1,3-PDO from engineered bacterial strains, and has been commercialized as CorterraTM by Shell or Sorona® by DuPont [18,41]. Bio-based 1,3-PDO can also be obtained from 2G feedstock, through mixed culture fermentation from crude glycerol [42]. TA can currently be derived from bio-based feedstock by starting at fermentation to bioethanol. Then, the bioethanol is converted to bio-ethylene by dehydration using solid acid catalysts and consequently passed through several steps, including oxidation and hydration to EG, which finally is converted to TA through polycondensation [43]. He et al. [44] demonstrated the process to produce TA from lignocellulosic biomass, with a conversion yield of 72.8%. They employed selective catalytic pyrolysis to form p-xylene intermediate, which was subsequently oxidized to TA using the metal oxide catalysts. The biological transformation of p-xylene to TA using engineered Escherichia coli was also reported, with a high conversion yield of 96.7 mol% [45]. One-pot TA synthesis from renewable methane, derived from biogas, was proposed by Zhang et al. [46]. 4-Methyl-1,4-cyclohexadiene-1-carboxylic acid was formed by cycloaddition of propiolic acid with isoprene; both can be synthesized from methane. Then, the catalytic oxidations of cycloadduct leads to TA formation. Moreover, as mentioned, TA derived from PET depolymerization can be the integrated building block of PTT (a process recently described as “plastic biorefinery”), promoting the sustainable upcycling of plastic waste [33].
2.3. Drop-In Plastic
Drop-in plastic is produced using the same pathway as petrochemical plastics, but the monomers are derived from biomass instead of fossil-based feedstock. Therefore, it can be processed with the same technology and equipment as the conventional ones. The drop-in plastics that play an important role in the bioplastic market are bio-PA, bio-PE, bio-PET, and bio-PP, representing 9.1, 9.5, 6.2, and 1.9% of global bioplastic production, respectively [22].
2.3.1. Bio-Polyamides (Bio-PA)
Polyamides are polymers of repeating units of aliphatic, semi-aromatic or aromatic molecules linked via amide bonds. Typically, caprolactam (a cyclic amide of caproic acid) is used as monomer in nylon 6, while polycondensation of hexamethylene diamine and adipic acid (or hexanedioic acid) is used in the case of nylon 6,6. Bio-PA or bio-nylon is based on sebacic acid and undecylenic acid produced from castor oil. Sebacic acid-based bio-PA includes PA6-10, PA10-10, and PA10-12, which are prepared by step-growth polymerizing the diacid with diamine, such as hexamethylenediamine (C6) and decamethylenediamine (C10) [47]. Undecylenic acid-based bio-PA, PA11 is produced by firstly hydrolyzing castor oil to ricinoleic acid. Then, catalysis using methanol and high-temperature treatment are performed in order to produce 11-undecenoic acid, which is converted to 11-bromoundecanoic acid by bromine peroxide and reacted with ammonia to finally obtain 11-aminoundecanoic acid as a monomer of PA11 [43]. The commercialized bio-PA is produced by several manufacturers such as Evonik (PA6/10, PA10/10, PA10/12; trade name VESTAMID® Terra), RadiciGroup (PA6/10, PA10/10, PA10/12; trade name Radilon™), or Arkema (PA11; trade name Rilsan™) [48].
2.3.2. Bio-Polyethylene (Bio-PE)
Bio-PE is produced from the polymerization of bio-ethylene in the presence of hydrogen to control the chain length. The monomer, bio-ethylene, is obtained from bioethanol through a dehydration process, using a solid catalyst at high temperature [43]. Bio-PE has been commercialized on a large scale for a decade and the companies that are key players in the bio-PE market are Braskem, the joint venture Dow and Crystalsev, Solvay, Nova Chemicals, and Petrobras [49]. Ethanol fermentation from bacteria and yeast greatly contributes to the bio-PE production process.
2.3.3. Bio-Polyethylene Terephthalate (Bio-PET)
For bio-PET, both monomers, EG and TA, can be produced from sustainable feedstock. EG accounts for 30% of PET; therefore, the commercial bio-PET usually means 100% bio-based ethylene glycol (bio-EG) [50]. For example, the “PlantBottleTM” from the Coca-Cola Company consists of 100% bio-EG (30% of the total material) and conventional petroleum-derived TA [51]. Recently, Coca-Cola revealed that they have now created a 100% bio-based beverage bottle including bio-EG from sugarcane and bio-based terephthalic acid (bio-TA) from plant (corn)-based paraxylene. Their goal is to use 3 million tons less of virgin plastic from oil-based sources by 2025 [52]. The biosynthesis of EG has been performed by several pathways. Bio-EG can be obtained by high-pressure and high-temperature hydrolysis of ethylene oxide [53], obtained via oxidation of bio-ethylene [49]. Cabulong et al. and Wang et al. explained the biosynthesis of EG by recombinant E. coli via a pentose pathway using xylose as a substrate, which currently reaches 98% of the theoretical yield [54,55]. Metabolically engineered E. coli modified to have enzymes decarboxylase, ethanolamine oxidase, and glycolaldehyde reductase converting glucose to EG (through serine-biosynthesis pathway) was reported by Pereira et al. [56]. The EG production route from gases, including CO2, CO, and H2, via the Wood-Ljungdahl pathway of carbon fixing acetogenic bacteria was also studied [57]. In addition, EG can be produced by yeast Saccharomyces cerevisiae, using D-xylose as a substrate and the two key enzymes, phosphofructokinase and fructose-bisphosphate aldolase in the glycolytic pathway [58].
Notably, also TA can be biologically produced. It can be biosynthesized, for instance, by initially converting bio-based isobutylene, obtained from iso-butanol, to isooctane, according the technology introduced by Gevo [59]. Then, the cyclization of two isooctane molecules via dehydrogenation is performed to produce p-xylene, which is finally converted to TA [51]. Carraher et al. [60] studied the production of renewable TA using a combination of biological and chemical processes. First, they produced cis,cis-mucononic acid by fermentation of sugar or lignin monomers; this acid was then isomerized to trans,trans-muconic acid, followed by the reaction with bio-based ethylene, obtained through Diels–Alder cycloaddition and dehydrogenation, to finally produce bio-TA. Another interesting technology for bio-TA is bioconversion of furan derivatives, e.g., 2,5-di-methylfuran, furfural, and hydroxymethylfurfural, which can be derived from lignocellulosic biomass [61,62,63].
2.3.4. Bio-Polypropylene (Bio-PP)
Bio-PP is the polymer of bio-propylene, which has been reported to be produced via several methods. Chen and Patel [64] explained the process scheme of bio-PP production starting from fermentation of glucose to iso-butanol, then dehydration to bio-butylene, isomerization to 2-butylene, and metathesis with ethylene to finally obtain bio-propylene as a monomer of bio-PP. Another method could be to convert bio-based n-butanol to 1-butene, which is then mixed with ethylene and heated under catalytic conditions to produce monomer bio-propylene [43,65]. The acetone–butanol–ethanol (ABE) fermentation technology plays an important role in bio-PP production as it provides butanol for starting the process.
2.3.5. Bio-Polyurethane (Bio-PU)
PU is the polymer derived from the condensation of (poly)isocyanates (-NCO) and polyols (exothermic reactions) [66]. It is a heterogenous class of polymer formed using different types of monomers, such as adipic acid, 1,4-butanediol (1,4-BDO), EG, and the isocyanates (methylene diphenyl diisocyanate and toluene diisocyanate) [67]. The polyols can be derived from bio-based feedstock via fermentation or chemical reaction, but the isocyanates are usually non-renewable [68]. Plant oils (e.g., jatropha oil, sunflower oil, castor oil, rape seed oil, etc.) have been used to prepare bio-PU [69]. Sahoo et al. reported preparing bio-PU by reacting castor-oil-based polyol with partially bio-based polyisocyanate for coating application [70].
2.4. Starch-Based Plastic
The excessive use of petrochemical plastic, which becomes a considerable concern due to its sustainability and effect on the environment, is the major driving force toward biodegradable starch-based materials. Starch is an inexpensive and biodegradable natural product. Therefore, it has been gaining interest as a material for obtaining bioplastics for a long time and currently represents the third largest market share (16.4%) [22]. Due to its abundance, starch is a high potential polymer for substituting petrochemical plastic.
Starch is a plant-produced polysaccharide for energy storage purposes. It forms semi-crystalline granules and has both linear and branched structures. The linear chain is called amylose, which has glucose units linked together by -1,4 glycosidic linkage, whereas the branched chain is called amylopectin, consisting of short -1,4 chains linked by -1,6 glycosidic bonds at the branching, leading to the crystalline region of the starch granule. Starch-based plastic, or so-called thermoplastic starch, is processed via extrusion, during which starch granules undergo phase changes, including swelling, loss of birefringence, melting, and solubilization, because of high-shear and high-pressure conditions with plasticizers [71]. The extrusion techniques and conditions strongly affected the final properties of starch-based materials.
Since starch-based plastic has lower mechanical properties, thermal stability, and moisture tolerance than petroleum-based, blending and reinforcement have been performed to improve durability and decrease the cost of the material. The blending can be achieved with other plastic polymers (e.g., PLA, PBS, PCL, and PHA [2,72,73]), or natural polymers (e.g., cellulose and gelatin [71]). Marichelvam et al. [74] developed rice and corn starch-based bioplastic films using glycerol as a plasticizer, in the presence of gelatin and citric acid, to improve mechanical properties and shelf-life. They reported that the favorable properties of this starch-based plastic could substitute PE plastic bags as a packaging material.
Starch-based plastic is already commercialized, with many companies as key players; Novamont, for example, has been known for its Mater-Bi starch-based bioplastic since the early 1990s. In terms of future prospects, starch-based plastics can be produced from more sustainable and cheaper resources, such as microalgae after wastewater treatment application [75].
2.5. Cellulose-Based Plastic
Cellulose is the main component of lignocellulosic biomass, along with hemicellulose and lignin. It is a homopolymer composed of D-anhydroglucopyranose units linked by β-1,4 glycosidic bonds [76]. Its abundancy and eco-friendly properties make it the strong candidate to replace fossil-based polymers [77]. The processible cellulose-based plastic can be produced via a reaction with esters or ethers and modification with plasticizer [78]. Cellulose esters, e.g., cellulose acetate, cellulose propionate, cellulose butyrate, and nitrocellulose, have been used extensively to manufacture thermoplastic materials, while cellulose ethers have various applications as surface coatings [78].
An advantage of cellulose-based plastic is that it can be derived from agricultural waste, reducing the commercial cost of the material. For example, the cellulose-based material using carboxymethyl cellulose (CMC) from sugar cane bagasse, blended with gelatin, agar, and glycerol, showed promising properties for packaging applications in terms of permeability, mechanical strength, and biodegradability [79]. However, the hydrophilicity nature of cellulose limited its competitiveness with other plastics due to lower durability, gas-barrier capability, and waterproof properties. Several designs of cellulose composite have been reported to enhance cellulose-based polymer properties. Fabrication of a pure cellulose material combined with paper sheet and regenerated cellulose from NaOH/urea/cellulose solutions was reported to improve barrier properties for H2O and O2 [80]. Lignin–cellulose composite exhibited higher tensile strength, water stability, and thermal stability than conventional cellulose paper [81]. Integration of polyimine-based covalent adaptable networks with cellulose paper was also shown to develop cellulose-based materials’ properties by enhancing strength, gas-barrier, waterproof, malleability, processability, and recyclability properties [82].
Cellulose-based plastic is a rather mature technology and has shown rapid growth in manufacturing facilities and industries. Many companies engage in the cellulose-based plastic market, such as Celanese Corporation, Solvay, Daicel Corporation, AkzoNobel, Mitsubishi Rayon, Dow Company, SK chemicals Co., Ltd., and Eastman Chemical Company. The compound annual growth rate (CAGR) of cellulose-based plastic was reported to be 17.5% between 2020 and 2027 and it could reach a market size of 176.5 million USD in 2028 [83].
2.6. Poly(lactic acid) (PLA)
PLA is an aliphatic polyester that is both bio-based and biodegradable. It has a high growth rate in the plastic market, reaching 18.9% of the global production of bioplastics in 2021 [22]. PLA is mainly used in packaging applications and also in consumer goods, textiles, agriculture and horticulture, coatings and adhesives, biomedical settings, as well as electrics and electronics. Lactic acid, a well-known product from mature fermentation technology of various organic feedstock, is a building block of PLA. Ahmad et al. [84] published an extensive review about lactic fermentation from cheap feedstocks, such as food waste, sugar and starch materials, lignocellulose materials, microalgae, and glycerol. The study highlighted the contribution of fermentation technology and bio-based building blocks to the bioplastic production.
Lactic acid has two chiral stereoisomers—L- and D-lactic—which can be polymerized to the poly(L-lactic acid) (PLLA), poly(D-lactic acid) (PDLA), or poly(DL-lactic acid) (PDLLA) [24]. PLLA and PDLA are semi-crystalline, with a melting temperature of 170–180 °C, but the equivalent mixtures of PLLA and PDLA form a racemic crystallite, elevating the melting temperature and enhancing mechanical strength [2]. PLA production is usually accomplished by combining biological and chemical methods, starting from lactic acid fermentation, followed by lactide formation, and finally ring opening polymerization (ROP) of the lactide. The mentioned method can lead to high molecular weight PLA materials (which is more favorable than low molecular weight PLA, obtained from direct polymerization of lactic acid) [2,24].
PLA has the lowest price among other bioplastics, around 1.9 euro/kg [85]. Consequently, PLA production can be considered (relatively) competitive with traditional plastics, because of its availability, biodegradable properties, and economic feasibility, especially as it has the potential to substitute PP, as reflected in the increasing investments in PLA production in the US and EU [21]. Examples of companies that commercially manufacture PLA are NatureWorks (Bangkok, Thailand), Natur-Tec (Circle Pines, MN, USA), and TotalEnergies and Corbion joint venture (Amsterdam, The Netherlands) [86].
2.7. Polyhydroxyalkanoates (PHA)
PHA is a polyester family produced naturally inside the microbial cells as intracellular carbon and energy storage granules, typically when there is excess carbon and limited nitrogen or phosphorus sources [87]. Basically, PHAs are polymers of hydroxyalkanoate (HA) units, which can be homo-, co-, or terpolymers, depending on the monomer type. They are generally water insoluble, non-toxic, and have thermoplastic characteristics, making them suitable for various applications, ranging from medical, packaging, and agricultural [24]. PHA can be classified into short-chain length PHA (sclPHA) (4–5 carbons) and medium-chain length PHA (mclPHA) (6 carbons) [88]. Examples of well-known PHAs are poly(3-hydroxybutyrate) (PHB) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV). PHB is highly crystalline with good gas-barrier properties, but it is stiff, brittle and shows little resistance to thermal degradation [24,89]. On the other hand, the co-polymer PHBV has improved properties of elasticity, toughness, elongation, and reduced stiffness [90].
PHAs have a low environmental impact, being 100% bio-based, biodegradable, and biocompatible. They can be synthesized by fermentation using several bacteria and archaea, employing PHA polymerase encoded by PhaC [87]. Bacillus [91], Burkholderia [92], Comamonas [93], Cupriavidus [94], Haloferax [90], Ralstonia [95], Pannonibacter [96], Halomonas [97], Serratia [98], Pseudomonas [99], and mixed cultures [100,101] are a few examples reported as natural strains accumulating PHAs. Recombinants strains also show great potential [102,103]. In addition to bacterial strains, some microalgae and cyanobacteria also accumulate PHA granules in their cells. Despite the low PHA content, the advantage of using microalgae is that they can convert atmospheric CO2 to PHA by autotrophic metabolism [104]. For example, Microcystis aeruginosa, Chlorella pyrenoidosa, Synechococcus subsalsus, and Spirulina sp. LEB-18 are reported to produce PHB [105,106,107]. The properties of PHAs depend on different factors, such as the bacterial strains used, the feedstock, and the transient conditions applied during fermentation [108], which could be controlled to obtain the required polymer properties and favor the substitution of conventional plastic such as PP, PE, PS, PET, etc. [40]. PHAs are commercially available by, for example, Meredian (USA), Telles (USA), Kaneka (JP), Danimer Scientific (USA), Yield10 Bioscience (USA), etc.
Through fermentation technology and synthetic biology, the PHA production has been developed using various organic waste streams. For example, lipid-rich organic waste, whey, molasses, lignin and its derivatives, spent coffee, and food waste [109]. In addition, production of PHA from conventional plastic waste such as PE, PP, PS, and PET has been studied. The depolymerization of PET was performed either by thermal degradation [110,111] or enzymatic degradation [112] to TA, which was consequently used as a substrate for PHA fermentation. Currently, the in-depth genome analysis of a potential strain, Pseudomonas umsongensis GO16, using TA for PHA production has been reported [113]. This approach is becoming more promising as an opportunity to promote the circularity of plastic usage.
2.8. Polybutylene Adipate-co-Terephthalate (PBAT)
PBAT is considered a biodegradable polyester and it is also compostable [114]. PBAT has properties comparable to petroleum-based plastics such as PE, PET, and PS with more flexibility and toughness [11]. In addition, it has a higher elongation at break than other biodegradable polymers such as PLA and PBS [115]. Therefore, PBAT is considered highly promising, with potential applications in the medical, industrial, agricultural, and packaging sector.
The research to improve PBAT properties includes reinforcement through composite materials, such as silver oxide [116], nano-chitin [117], and cinnamon oil [114], which are incorporated into PBAT to enhance structural, thermal, mechanical, barrier, and antimicrobial properties, thus expanding its application possibilities.
PBAT can be 100% degraded either enzymatically—by bacteria, fungi, and algae—or thermally/chemically [115]. TA, 1,4-BDO, and adipic acid are the monomers of PBAT, which traditionally have been produced through petroleum-based chemical processes, but the recent advances in biotechnology have led to the microbial production of these building blocks. Clearly, also in this case, TA can also be received from PET depolymerization, enabling the upcycling of conventional plastic waste to renewable polymers. In summary, biodegradability and bio-based production of PBAT have promoted its market uptake (thus, it has risen from the third biggest market of global bioplastic production, in 2020, to the biggest one in 2021) [21,22].
2.9. Polybutylene Succinate (PBS)
PBS is an aliphatic polyester that comprises succinic acid and 1,4-BDO as monomers. The mechanical property of PBS is comparable to PP, thus surpassing those of PLA. Due to its superior processability, good flexibility, and good chemical resistance, it can be used in various applications, e.g., mulching films, garbage bags, textiles, automotive, sports devices [2,11,118]. Traditionally, PBS monomers are produced from fossil-based feedstock [11], but recent biotechnological advances allowed both succinic acid and 1,4-BDO to be produced from renewable feedstock (sugar, starch, glycerol, lignocellulose, and other bio-waste), using non- or recombinant microbial strains [119]. In 2015, a bio-based (50%) PBS production plant was opened in Thailand by a joint venture between PTT Public Company Limited and Mitsubishi Chemical Corporation, under the trade name ‘BioPBS™’. They used bio-based succinic acid from the fermentation of renewable feedstock such as sugarcane, cassava, and corn [120,121]. Fully bio-based PBS is expected to arrive shortly and it could have at least 15–20% lower negative environmental impact than the fossil-based one [122].
Notably, PBS can be blended with other biodegradable polymers, such as starch, PLA, and PHB, to enhance the material performance; for example, a small amount of PBS blended with PLA can significantly increase the elongation to break [123].
3. Fermentation Technology Providing Building Blocks for Renewable Plastics
The current advance in metabolic and bioprocess engineering allows several plastic building blocks to be produced (Figure 4) by microbial fermentation from renewable resources. In Figure 5, an overview of current processes for plastic production from bio-based building blocks is illustrated. A more detailed description is provided in the following sub-section.
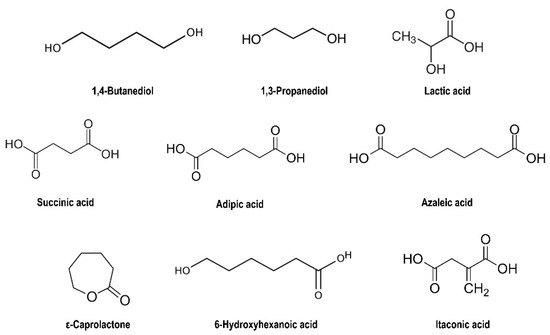
Figure 4.
Chemical structure of plastic building blocks.
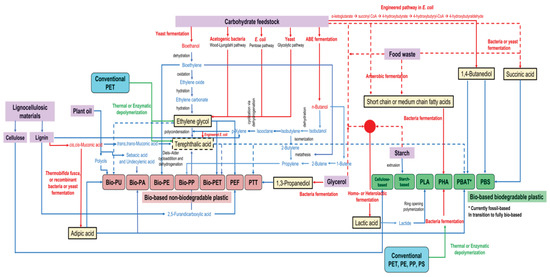
Figure 5.
Overview of the production of renewable plastics from bio-based and upcycled building blocks. The blue lines show chemical conversion, while the red lines show biological conversion/fermentation processes. The green lines show integration of conventional plastic upcycling to bioplastic production. Solid and dashed lines are used to better distinguish the different pathways.
3.1. 1,4-Butanediol (1,4-BDO)
1,4-BDO is a building block of many plastics such as PU, PBAT, PBS, PBT, and poly(butylene furandicarboxylate) (PBF) [18]. It is produced in a large volume from petroleum-based feedstock; over 2.5 million tons annually [20]. 1,4-BDO is expected to have an annual growth rate of 7% during the 2021–2026 period because of its increased demand, especially for renewable plastics [124]. As the trend toward sustainable chemical production, 1,4-BDO has been receiving attention on producing via fermentation of renewable feedstocks to lower energy consumption, greenhouse gas emission, as well as production cost. 1,4-BDO fermentation has been established by an integrated biotechnology platform. Since 1,4-BDO is not a natural product produced by organisms, the development of the 1,4-BDO production pathway involves constructing new biochemical pathways in the bacterial strain and engineering a host strain to have a favorable carbon flux.
The study by Yim et al. [125] reported the first success of direct biocatalytic routes to produce 1,4-BDO from renewable carbohydrate feedstock. They engineered E. coli to synthesize 1,4-BDO from common central metabolites, succinate and -ketoglutarate, by introducing non-native enzymes and blocking natural fermentation products to force 1,4-BDO production. Finally, they were successful in producing 18 g/L of 1,4-BDO from glucose in 5 days. The previous review article described the developed technology for 1,4-BDO fermentation by firstly employing the computational software to identify all potential pathways for 1,4-BDO production and select the most suitable one based on the maximum theoretical yield, minimum pathway length and non-native step, and thermodynamic favorability. The biosynthetic pathway started from converting succinyl-CoA, a TCA cycle intermediate, to 4-hydroxybutyrate (4-HB) by constructing hydrogenase enzymes. Then, 4-HB is converted to 4-hydroxybutyryl-CoA, 4-hydroxybutyraldehyde, and finally 1,4-BDO [126]. The key success of 1,4-BDO fermentation is that the host strain needs to have balanced energy and redox and not produce unwanted by-products [127]. Thus, the anaerobic condition is required for 1,4-BDO production to ensure enough redox for the several reduction steps. Various strategies, including gene knockouts, mutations, and substitutions, were employed to allow E. coli growth and reduce enzyme inhibitory during the high NADH level in oxygen-limited conditions [128]. Currently, successful production of 1,4-BDO with a titer exceeding 120 g/L, with the production rate higher than 3 g/L/h and the yield over 100% of the commercial target, has been reported [126].
The industrial-scale fermentation of 1,4-BDO has succeeded: Genomatica developed commercial bio-based processes for 1,4-BDO production from renewable feedstocks such as sugarcane, sugar beets, and corn. They estimated that the bio-production of 1,4-BDO is expected to save 93% of greenhouse gas emissions, equal to 700,000 tons per year, compared to fossil-based production [129]. With their partners, DuPont Tate & Lyle BioProducts, they successfully showed demo-scale production of 1,4-BDO from sugar, with over 2000 tons in 2012 [130]. Moreover, thanks to the partnership with Genomatica, Novamont opened the first dedicated industrial plant to produce bio-based 1,4-BDO by bacterial fermentation of sugars, with a capacity of 30,000 tons per year [131]. This allowed the company to develop their fourth generation of Mater-Bi, largely obtained from bio-based 1,4-BDO and starch-based polymers, increasing the production capacity of their new Mater-Bi plant in Patrica (Italy) to 150,000 tons per year.
These examples highlight the increasing use of fermentation technology to provide renewable plastics’ building blocks.
3.2. 1,3-Propanediol (1,3-PDO)
1,3-PDO is an important building block for poly(trimethylene terephthalate) (PTT) and polyurethane. The increasing demand of PTT leads to the rise in the 1,3-PDO market, which is estimated to reach 776.3 million USD by 2022 with a compound annual growth rate (CAGR) of 7% from 2021 to 2026 [132,133]. The fermentation of glycerol to 1,3-PDO has been described since the 19th century [134]. It is more advantageous than chemical methods in terms of reducing the use of toxic solvent and energy required and producing greater yield [135]. Despite the demand for it as a bioplastic monomer, the current driving force of 1,3-PDO bioproduction via fermentation is its contribution to utilizing crude glycerol surplus from biodiesel production. This renewable process is gaining more interest as valorizing waste to higher-value products and promoting more sustainable biofuel production.
Many microbes have been reported to ferment 1,3-PDO using glycerol as a feedstock, including bacteria (e.g., Clostridium butyricum, Enterobacter agglomerans, Klebsiella pneumoniae, Lactobacillus brevis, Lactobacillus reuteri, recombinant E. coli) and yeast S. cerevisiae [20,135,136,137,138,139]. The fed-batch fermentation reported by Wang et al. [140] achieved very high titer of 1,3-PDO, 104.8 and 94.2 g/L for refined and crude glycerol, respectively, contributing to the productivity of 3.4 and 3.0 g/L/h. The same study also investigated the potential industrial-scale production using sequential fed-batch fermentation and achieved a production of 1,3-PDO for eight cycles. Even though the average titer is reduced (85.0 g/L), it benefits the up-scale fermentation by reducing time of seed cultivation and improving stability of 1,3-PDO production. The yield of 1,3-PDO production from fed-batch fermentation of glycerol reported to our knowledge mostly ranges from 0.45 to 0.58 g1,3-PDO/gGly, indicating the favorable mode of operation for up-scale production [135,140,141].
The open mixed culture (MMC) technology is also receiving attention due to the advantage in non-sterile fermentation, which is favorable for the up-scale production, as it could reduce costs related to sterilization and pure feedstock requirements and enhance tolerance to feedstock and by-product (e.g., organic acid) toxicity [141]. Varrone et al. reported the potential continuous anaerobic fermentation of crude glycerol to 1,3-PDO by enriched anaerobic sludge at pH 5.5 and with a retention time of 12 h. Crude glycerol concentration could be increased to almost 90 g/L, but the maximum substrate conversion rate (94%) was observed at a concentration around 50–60 g/L, using an animal fat-derived crude glycerol. The maximum productivity of 1,3-PDO observed in this study was 37.8 g/L/d [142]. After optimization, the adapted MMC reached a glycerol consumption rate of 137 g/L/d and a predicted 1,3-PDO production rates of 82.6 g/L/d [42].
DuPont Tate & Lyle in collaboration with Genencor patented the engineered E. coli strain transformed with the Klebsiella pneumoniae genes to produce high titer 1,3-PDO (up to 129 g/L) from glucose. They press-released the commercial process for 1,3-PDO from corn sugar in 2007, which consumes 40% less energy and reduces 20% greenhouse gas emissions compared to using petroleum-based feedstock [143,144].
3.3. Lactic Acid
Fermentation of lactic acid has been discovered since around the 18th century, and it has become a very important product for food, chemical, cosmetic, and pharmaceutical industries. Lactic acid is the monomer of biodegradable PLA, which has various applications and relatively high market expansion. Thus, lactic fermentation technology plays a very important role in supporting the renewable plastic market. Microbial fermentation of lactic acid has numerous benefits over chemical synthesis by offering the advantages of low environmental impact, consumption of cost-effective renewable substrates, low-temperature requirements, low energy consumption, and the production of optically pure lactic acid (L(+) or D(−)) instead of a racemic DL-lactic acid mixtures by petrochemical process [84,145]. There are many manufacturers commercializing fermentative lactic acid such as Corbion, Galactic, NatureWorks LLC, Futerro, Henan Jindan Lactic Acid Technology Co., Ltd., BASF SE, Musashino Chemical (China) Co., Ltd., ThyssenKrupp AG, Dow, Cellulac, Jungbunzlauer Suisse AG, Vaishnavi Bio Tech, Teijin Limited, and Danimer Scientific [146].
The fermentation pathways of lactic acid from hexoses or pentoses include homolactic and heterolactic fermentation. Homolactic fermentation converts one mole of glucose to two moles of lactic acid via entering the glycolysis pathway. In contrast, the heterolactic fermentation produces lactic acid with co-product, CO2, ethanol, and/or acetic acid by metabolizing either hexoses or pentoses via pentose phosphate pathway [147]. Lactic acid bacteria (LAB) can be typically found among bacterial genera Lactobacillus, Carnobacterium, Lactococcus, Streptococcus, Enterococcus, Vagococcus, Leuconostoc, Oenococcus, Pediococcus, Tetragonococcus, Aerococcus, and Weissella of the order Lactobacillales [148]. Several Bacillus sp. have also been reported to ferment lactic acid, e.g., Bacillus coagulans, Bacillus subtilis, Bacillus licheniformis, Bacillus thermoamylovorans, and Bacillus stearothermophilus [149,150,151,152,153,154,155].
Current development on lactic fermentation focuses on the use of renewable feedstock and process optimization to reduce production cost. Typical renewable feedstock includes food waste, sugar and starchy waste, lignocellulosic materials, crude glycerol, and microalgae to enhance the economic feasibility of lactic acid production and use the non-competitive substrate for food application [84]. Pediococcus acidilactici, for instance, was engineered to use lignocellulosic biomass (corn stover and wheat straw) by Qiu et al. [156,157], reaching high titers of D-lactic acid and L-lactic acid, with 97.3 and 130.8 g/L, respectively. The recent strategy to minimize contamination risk and facilitate non-sterilization lactic acid fermentation is using alkali- and thermo-tolerance strains [158]. They isolated Enterococcus faecium WH51-1, and successfully produced 44.6 g/L lactic acid with a yield of 0.89 g/g, from corn steep water at high temperature (45 °C) and pH (9.0). Another study by Zhang et al. [155] also succeeded in producing lactic acid with non-sterilized fermentation and reached high D-lactic acid concentration (145.2 g/L).
3.4. Succinic Acid
Succinic acid has been the forefront chemical produced for biorefinery platforms and is the essential monomer for PBS and poly(butylene succinate-co-butylene terephthalate) (PBST). The bio-production of succinic acid has been obtained via fermentation by various strains; for example, Actinobacillus succinogenes, Mannheimia succiniciproducens, Anaerobiospirillum succiniciproducens, Basfia succiniciproducens, and recombinant E. coli [159]. However, the production of succinic acid leads to a drastic drop in pH; therefore, a significant amount of alkali is needed to maintain pH, thus impacting the production cost. Metabolic engineered yeast strains such as S. cerevisiae and Yarrowia lipolytica have been developed for their tolerance to low pH conditions [160,161].
In addition, many renewable carbon sources have been studied for succinic acid production. Chen et al. [162] reported the simultaneous saccharification and fermentation (SSF) process for producing succinic acid from liquefied cassava powder using E. coli strain NZN111. A two-stage culture technique was applied, with a growth phase and fermentation phase in aerobic and anaerobic conditions, respectively. A very high succinic acid production of 106.17 g/L was obtained at 40 °C, which yielded 0.66 g/g cassava powder and reached the productivity of 2.54 g/L/h. Food waste hydrolysate was also studied for succinic acid production by A. succinogenes and E. coli in batch fermentation, reaching a production of 24.1 and 26.4 g/L, respectively. However, A. succinogenes was found to produce a high amount of by-products (13.7 g/L), including acetic, formic, and pyruvic acids [163]. Stylianou et al. [164] evaluated the continuous production of succinic acid from municipal solid waste and showed 21.2 g/L of succinic acid with a yield of 0.47 g/g and productivity of 1.27 g/L/h.
The demand for succinic acid in renewable plastic (PBS and PBST) production was projected to be 82,000 M t, with a 13.7% market share in 2020 [165]. The major key players commercializing succinic acid are LCY Biosciences Inc., Myriant, Reverdia, and Succinity. The first two companies use the technology based on developed E. coli strains. Reverdia uses recombinant S. cerevisiae, which can co-produce succinate with ethanol, so the ATP from ethanol fermentation can support succinic acid synthesis. Finally, Succinity employs B. succiniciproducens as a producing strain [166]. Each company has active plants with capacities exceeding 10,000 M tons in Europe, Asia, and the USA [167].
3.5. Adipic Acid
Adipic acid or hexanedioic acid has been widely used to produce PBAT, polyamide 4-6 (PA 4-6), PA 6-6, and PA 6. The biological approaches to producing adipic acid are either chemo-catalytic conversion of the bio-based precursors cis,cis-muconic acid or D-glucaric acid to adipic acid, or direct fermentation [168]. cis,cis-Muconic can be produced from lignin-derived aromatic compounds such as catechol, protocatechuate, and benzoate. D-glucaric acid can be produced from synthetic pathways in bacteria, but as it could be achieved at a very low titer, it is less favorable than cis,cis-muconic acid as a substrate for adipic acid.
The adipic acid synthesis pathway is usually not a native pathway in microorganisms. However, some have been identified to natively produce adipic acid, e.g., cellulolytic actinobacterium Thermobifida fusca, but the meager yield was found (0.045 g/g glucose) [169]. The successful expression of enoate reductases, the key enzyme for hydrogenation of muconic acid to adipic acid, from Bacillus coagulans in S. cerevisiae was demonstrated by Raj et al. [170]. The researchers were able to produce the final titer of adipic acid of 2.59 mg/L. The interesting method exploiting engineered reversal of the β-oxidation and expression of ω-functionalization enzymes in E. coli showed up to 170 mg/L of adipic acid production from glycerol [171]. Genomatica Inc. has patented several genetically engineered microorganisms producing adipic acid (JP2020174684A). Future research focusing on the improvement of titer is still needed. However, it can be expected that bio-adipic acid will enter the market in the coming years and substitute current petroleum-derived commercial synthesis.
3.6. New Emerging Bioplastic Monomers
3.6.1. Azelaic Acid
Azelaic acid or nonanedioic acid is an -dicarboxylic acid with nine carbons. It has been recently reported as a valuable bio-based monomer for biodegradable polymers; for example, azelaic acid-based polyesters, terpolymer containing azelaic acid, and polyamide containing azelaic acid [172]. An example of an azelaic acid-based polymer is poly(ethylene azelate) which showed to be biodegradable at a comparable rate with poly--caprolactone [173]. The azelaic acid market is predicted to reach 160 million USD by 2023 [172].
The biosynthesis of azelaic acid starts from oleic acid derived from renewable oil, traditionally via ozonolysis (oxidation with ozone). However, it can now be synthesized using microbial fermentation. The direct biotransformation of nonanoic acid and its ester to azelaic acid was recently proposed using Candida tropicalis as a whole-cell biocatalyst. The biotransformation by continuous feeding of pure nonanoic acid, with the addition of inducer (nonane) and glucose, resulted in 30 g/L azelaic acid production with 0.3 g/L-h productivity and 90% molar yield [174].
In addition, multiple enzyme processes have been studied for the production of azelaic acid. Otte et al. developed a dual-expression system in E. coli expressing three-enzyme cascade (two plant enzymes; lipoxygenase and hydroperoxide lyase, and an endogenous oxidoreductase) to convert linoleic acid to azelaic acid in one-pot process [175,176]. The production of azelaic acid was 29 mg/L with a 34% conversion yield. Another study reported expressing several enzymes, including hydratase from Stenotrophomonas maltophilia, alcohol dehydrogenase (ADH) from Micrococcus luteus, and Bayer-Villiger monooxygenase from Pseudomonas putida, in E. coli for the transformation of oleic acid and plant oil into 9-hydroxynonanoic acid (an intermediate of azelaic acid) [177]. The compound can be consequently oxidized by ADH from P. putida GPo1 to azelaic acid [178]. They further investigated azelaic acid production using this system on several renewable oils and were able to produce 4.3 mM azelaic acid from 3 g/L olive oil [179]. Although the enzyme catalytic process is interesting for azelaic acid production, further work to increase its titer is still needed.
3.6.2. Lactones
ε-Caprolactone is a cyclic ester with a substantial market for biodegradable plastic. The commercial ε-caprolactone is available as Placcel® M by Daicel Corporation with a purity greater than 99.5%, and it is used as a monomer for ring-opening polymerization with polyols to manufacture poly--caprolactone (PCL) [180]. Biosynthesis of ε-caprolactone has been proposed by several routes. Bornadel et al. employed a bi-enzymatic cascade consisting of a Baeyer–Villiger monooxygenase (BVMO) and an alcohol dehydrogenase (ADH) to convert co-substrate of cyclohexanone and 1,6-hexanediol to ε-caprolactone. They achieved >99% conversion with 20 mM ε-caprolactone production [181]. To solve the low productivity problem from product inhibition, lipase A from Candida antarctica was coupled into the enzymatic cascade for ring-opening oligomerization of in situ formed ε-caprolactone. The oligo-ε-caprolactone is also an easy option for further polymerization to PCL [182].
3.6.3. 6-Hydroxyhexanoic Acid (6HA)
Biodegradable polymer PCL can also be synthesized via polycondensation of 6HA [183]. The bio-production of 6HA has been reported by some recombinant strains. For example, Pseudomonas taiwanensis [184] and Acidovorax sp. [185]. Fermentation of 6HA by recombinant P. taiwanensis in stirred-tank bioreactor with a continuous cyclohexane supply was reported to reach a final concentration of 25 mM (3.3 g/L) [186].
3.6.4. Itaconic Acid
Itaconic acid was mentioned as one of the promising compounds, among other 12 bio-based products from biorefinery carbohydrates, by the US Department of Energy in 2004 [187]. Its application for innovative polymers (e.g., itaconic-derived antimicrobial polymer [188]) is growing, and the compound market is expected to reach USD 117.1 million by 2026 [189]. Itaconic acid is known to be produced from filamentous fungi, Aspergillus terreus [190]. Current advances in itaconic acid fermentation lie in the development of non-filamentous engineered strains, e.g., E. coli, Ustilago maydis, and Y. lipolytica, which tolerate high titers to improve feasibility in large-scale production [191,192].
4. Future Perspectives
Triggered by the negative impact on the environment derived from large amounts of fossil-based plastic production and post-consumer plastic waste, research into renewable plastics has been increasing. More recently, focus has been dedicated to the biodegradable ones, illustrated by more than 20% market growth, compared to three years ago. By far, all types of renewable plastics have shown the possibility to be synthesized from bio-based building blocks, at least on the laboratory scale. Fermentation technology has shown to play an important role in developing bio-based building blocks for renewable and/or plastics. Monomers for PBAT, PBS, PLA, drop-in, PTT, and PEF have been obtained through fermentation by both, natural and engineered strains. Some of these polymers even reached the commercialization stage, e.g., partially bio-based PBS synthesized from renewable bio-based succinic acid or PTT synthesized from bio-based 1,3-PDO by engineered bacterial strains. PHA production has also been developed using both renewable biomass and conventional plastic waste as feedstock. As a next step, efforts toward developing low-cost and high-titer processes are needed to accelerate commercialization.
In this sense, microbial defined mixed cultures or synthetic mixed cultures could contribute in decreasing production costs from 2G feedstock [3]. Moreover, metabolic engineering is going to represent a key driver for the industrialization of renewable plastics, by facilitating the bio-production of commodity chemical building blocks. Previously, E. coli was genetically manipulated to produce biochemicals such as D-lactate [193] and succinate [194]. These benefit the future up-scaled production, as E. coli is a well-characterized species and has simple nutrient requirements. However, new platforms based on non-conventional strains for industrial applications are under development. Omics technologies contribute to advancement in metabolic engineering. They help, for instance, in unravelling the metabolic pathway of unculturable microorganisms or in providing the data on beneficial mutations during adaptive laboratory evolution. Multi-omics approaches with computational system biology, protein engineering, and synthetic biology are the tools for establishing new (or unconventional) metabolic pathways producing desired plastic monomers [195]. For example, a proteomics-guided approach has been used to engineer polyketide synthases for in vitro production of adipic acid [196]. Such technologies also broaden the application of microbes in bio-upcycling; for example, the elucidation of EG [197] and 1,4-BDO [198] metabolism in Pseudomonas putida KT2440 were obtained by genome sequencing and proteomics analysis, which could be beneficial in upcycling plastic monomers. Using a metabolic engineering tool-set is an interesting approach that will allow the exploitation of new bio-based building blocks production routes that help meet the market demand of renewable plastic.
5. Conclusions
The role of plastics in our society and economy is growing every year, but the rate of reuse and recycling is relatively low, showing considerable plastic pollution problems. Renewable plastics are materials of interest, for their potential contribution to alleviating negative environmental impacts seen with conventional fossil-based plastics, even though they are not going to solve the problem of poor waste management or low recycling rates, per se. Bio-based plastics promote carbon-neutral plastic production, using renewable biomass instead of depleting petrochemicals. The benefits would be magnified if they were designed to be biodegradable or compostable, allowing the carbon to re-enter the biogenic cycle, whenever reuse (think about facemasks) or closed-loop recycling (i.e., munch films) is not possible. Moreover, a recent study suggested that increased recycling rates would decrease the cost of recycled bioplastics by almost 50%, which is not the case with fossil-based ones (where recycled plastics are still more expensive than the virgin ones) [33]. The growth rate of global bioplastic production is expected to be more than 200% within the next five years [22], thus reaching a larger market share and become more of general use. The production of renewable and more bio-based plastic polymers, together with the development of new upcycling technologies, can thus provide a significant contribution to more sustainable plastic industry.
Currently, bio-based precursors of renewable plastic are forecasted to have a total growth of 4.5% by 2023 [199]. This will be achieved thanks to a significant contribution of fermentation technology that enables microbial production of various building blocks, such as 1,4-BDO, 1,3-PDO, FDCA, succinic acid, and other new fermentative compounds (azelaic acid, lactones, etc.). The advances in molecular biotechnology and bioprocess engineering have led to the development of superior microbial cell factories for unconventional bioproducts and more effective fermentation processes with increased titers. As an example, we can mention the traditionally petroleum-based biodegradable PBS that currently is synthesized from bio-based succinic acid and 1,4-BDO produced by metabolic engineered bacteria. New emerging plastic building blocks obtained through fermentation are turning toward polymers that can be functionalized with improved properties, or formulated to be recyclable materials, contributing to the solution of the end-of-life issue. Moreover, new bio-based building blocks should lead to polymers with superior functional properties. Future efforts are going to be dedicated to the scale-up of these technologies, to reach industrial scale and decrease production costs, which are still not competitive with conventional fossil-based plastics.
Author Contributions
Conceptualization, C.V.; writing—original draft preparation, P.L.; writing—review and editing, P.L. and C.V.; visualization, P.L.; supervision, C.V.; project administration, C.V.; funding acquisition, C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the H2020 UPLIFT project (Grant Agreement no. 953073).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank the European Commission, under H2020 Grant Agreement no. 953073 (acronym: UPLIFT), and the Department of Chemistry and Bioscience, Aalborg University for the financial support of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ali, S.S.; Elsamahy, T.; Al-Tohamy, R.; Zhu, D.; Mahmoud, Y.A.G.; Koutra, E.; Metwally, M.A.; Kornaros, M.; Sun, J. Plastic wastes biodegradation: Mechanisms, challenges and future prospects. Sci. Total Environ. 2021, 780, 146590. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, N. A brief overview of renewable plastics. Mater. Today Sustain. 2020, 7, 100031. [Google Scholar] [CrossRef]
- Jenkins, S.; Quer, A.M.I.; Fonseca, C.; Varrone, C. Microbial degradation of plastics: New plastic degraders, mixed cultures and engineering strategies. In Soil Microenvironment for Bioremediation and Polymer Production; Jamil, N., Kumar, P., Batool, R., Eds.; Wiley Online Book: Hoboken, NJ, USA, 2019; Chapter 12; pp. 215–238. ISBN 9781119592129. [Google Scholar]
- PlasticsEurope the Facts 2020. Available online: https://www.plasticseurope.org/application/files/3416/2270/7211/Plastics_the_facts-WEB-2020_versionJun21_final.pdf (accessed on 3 September 2021).
- European Environmental Agency. Plastics, the Circular Economy and Europe′s Environment—A Priority for Action; European Environmental Agency: Copenhagen, Denmark, 2021. [Google Scholar]
- Hamilton, L.A.; Feit, S.; Muffett, C.; Kelso, M.; Rubright, S.M.; Bernhardt, C.; Schaeffer, E.; Moon, D.; Morris, J.; Labbé-Bellas, R. Plastic & Climate the Hidden Costs of a Plastic Planet; Center for International Environmental Law (CIEL): Washington, DC, USA, 2019. [Google Scholar]
- Benson, N.U.; Bassey, D.E.; Palanisami, T. COVID pollution: Impact of COVID-19 pandemic on global plastic waste footprint. Heliyon 2021, 7, e06343. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, L.; Slat, B.; Ferrari, F.; Sainte-Rose, B.; Aitken, J.; Marthouse, R.; Hajbane, S.; Cunsolo, S.; Schwarz, A.; Levivier, A.; et al. Evidence that the Great Pacific Garbage Patch is rapidly accumulating plastic. Sci. Rep. 2018, 8, 4666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Environmental Protection Agency Facts and Figures about Materials, Waste and Recycling. Available online: https://www.epa.gov/facts-and-s-about-materials-waste-and-recycling/plastics-material-specific-data (accessed on 20 December 2021).
- Sohn, Y.J.; Kim, H.T.; Baritugo, K.A.; Jo, S.Y.; Song, H.M.; Park, S.Y.; Park, S.K.; Pyo, J.; Cha, H.G.; Kim, H.; et al. Recent Advances in Sustainable Plastic Upcycling and Biopolymers. Biotechnol. J. 2020, 15, 1900489. [Google Scholar] [CrossRef] [PubMed]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef]
- Rennison, A.; Winther, J.R.; Varrone, C. Rational Protein Engineering to Increase the Activity and Stability of IsPETase Using the PROSS Algorithm. Polymers 2021, 13, 3884. [Google Scholar] [CrossRef]
- Eerhart, A.J.J.E.; Faaij, A.P.C.; Patel, M.K. Replacing fossil based PET with biobased PEF; Process analysis, energy and GHG balance. Energy Environ. Sci. 2012, 5, 6407–6422. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain mobility, thermal, and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2021, 1–21. [Google Scholar] [CrossRef]
- Pellis, A.; Malinconico, M.; Guarneri, A.; Gardossi, L. Renewable polymers and plastics: Performance beyond the green. New Biotechnol. 2021, 60, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, P.F.H.; Hackmann, M.M.; Bos, H.L. Green building blocks for bio-based plastics. Biofuels Bioprod. Biorefin. 2014, 8, 306–324. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Yang, Y.-H. Biosynthesis of polyesters and polyamide building blocks using microbial fermentation and biotransformation. Rev. Environ. Sci. Bio/Technol. 2016, 15, 639–663. [Google Scholar] [CrossRef]
- Pellis, A.; Herrero Acero, E.; Gardossi, L.; Ferrario, V.; Guebitz, G.M. Renewable building blocks for sustainable polyesters: New biotechnological routes for greener plastics. Polym. Int. 2016, 65, 861–871. [Google Scholar] [CrossRef]
- European Bioplastics. Nova-Institute Bioplastics Market Data 2020. Available online: https://www.european-bioplastics.org/market/ (accessed on 8 September 2021).
- European Bioplastics. Nova-Institute Bioplastics Market Development Update 2021. Available online: https://www.european-bioplastics.org/global-bioplastics-production-will-more-than-triple-within-the-next-five-years/ (accessed on 12 December 2021).
- European Bioplastics Fact Sheet: What Are Bioplastics? Available online: https://docs.european-bioplastics.org/publications/fs/EuBP_FS_What_are_bioplastics.pdf (accessed on 20 December 2021).
- Naser, A.Z.; Deiab, I.; Darras, B.M. Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: A review. RSC Adv. 2021, 11, 17151–17196. [Google Scholar] [CrossRef]
- European Bioplastics; Nova-Institute Bioplastics Market Data 2018. Available online: https://www.european-bioplastics.org/wp-content/uploads/2016/02/Report_Bioplastics-Market-Data_2018.pdf (accessed on 20 December 2021).
- Loos, K.; Zhang, R.; Pereira, I.; Agostinho, B.; Hu, H.; Maniar, D.; Sbirrazzuoli, N.; Silvestre, A.J.D.; Guigo, N.; Sousa, A.F. A Perspective on PEF Synthesis, Properties, and End-Life. Front. Chem. 2020, 8, 585. [Google Scholar] [CrossRef]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, H.; Du, J.; Liu, K.; Wang, T.; Liu, L. Biocatalytic production of 2,5-furandicarboxylic acid: Recent advances and future perspectives. Appl. Microbiol. Biotechnol. 2019, 104, 527–543. [Google Scholar] [CrossRef]
- Hwang, K.R.; Jeon, W.; Lee, S.Y.; Kim, M.S.; Park, Y.K. Sustainable bioplastics: Recent progress in the production of bio-building blocks for the bio-based next-generation polymer PEF. Chem. Eng. J. 2020, 390, 124636. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Pacific Northwest National Laboratory (PNNL) and National Renewable Energy Laboratory (NREL): Richland, WA, USA, 2004. [CrossRef] [Green Version]
- Avantium Chemicals BV Sustainable and Carbon-Efficient Mono-Ethylene Glycol Generation in Demonstration PLANT. Available online: https://cordis.europa.eu/project/id/822956 (accessed on 12 December 2021).
- Scott, A. Avantium Starts Producing Biobased Ethylene Glycol. Available online: https://cen.acs.org/business/biobased-chemicals/Avantium-starts-producing-biobased-ethylene/97/i45 (accessed on 12 December 2021).
- Roux, M.; Varrone, C. Assessing the Economic Viability of the Plastic Biorefinery Concept and Its Contribution to a More Circular Plastic Sector. Polymers 2021, 13, 3883. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, S.; Haernvall, K.; Scaini, D.; Ghazaryan, G.; Zumstein, M.T.; Sander, M.; Pellis, A.; Guebitz, G.M. Enzymatic surface hydrolysis of poly(ethylene furanoate) thin films of various crystallinities. Green Chem. 2017, 19, 5381–5384. [Google Scholar] [CrossRef]
- Matos, M.; Sousa, A.F.; Fonseca, A.C.; Freire, C.S.R.; Coelho, J.F.J.; Silvestre, A.J.D.; Matos, M.; Sousa, A.F.; Freire, C.S.R.; Silvestre, A.J.D.; et al. A New Generation of Furanic Copolyesters with Enhanced Degradability: Poly(ethylene 2,5-furandicarboxylate)-co-poly(lactic acid) Copolyesters. Macromol. Chem. Phys. 2014, 215, 2175–2184. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, J.; Cao, F.; Wen, B.; Zhu, X.; Wei, P. Chemosynthesis and characterization of fully biomass-based copolymers of ethylene glycol, 2,5-furandicarboxylic acid, and succinic acid. J. Appl. Polym. Sci. 2013, 130, 1415–1420. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Magaziotis, A.; Nerantzaki, M.; Terzopoulou, Z.; Papageorgiou, G.Z.; Bikiaris, D.N. Synthesis and characterization of novel poly(ethylene furanoate-co-adipate) random copolyesters with enhanced biodegradability. Polym. Degrad. Stab. 2018, 156, 32–42. [Google Scholar] [CrossRef]
- Polymer Properties Database Polytrimethylene Terephthalate (PTT). Available online: https://polymerdatabase.com/Polymer%20Brands/PTT.html (accessed on 12 December 2021).
- Zhang, J. Study of Poly(Trimethylene Terephthalate) as an Engineering Thermoplastics Material. J. Appl. Polym. Sci. 2004, 91, 1657–1666. [Google Scholar] [CrossRef]
- Patel, M.; Angerer, G.; Crank, M.; Schleich, J.; Marscheider-Weidemann, F.; Wolf, O.; Hüsing, B. Techno-Economic Feasibility of Large-Scale Production of Bio-Based Polymers in Europe; Wolf, O., Ed.; Techncial Report EUR 22103 EN; European Communities: Sevilla, Spain, 2005; ISBN 9279012304. [Google Scholar]
- Sauer, M.; Marx, H.; Mattanovich, D. Microbial Production of 1,3-Propanediol. Recent Pat. Biotechnol. 2008, 2, 191–197. [Google Scholar] [CrossRef]
- Varrone, C.; Skiadas, I.V.; Gavala, H.N. Effect of hydraulic retention time on the modelling and optimization of joint 1,3 PDO and BuA production from 2G glycerol in a chemostat process. Chem. Eng. J. 2018, 347, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.H.; Bhoi, P.R. An overview of non-biodegradable bioplastics. J. Clean. Prod. 2021, 294, 126218. [Google Scholar] [CrossRef]
- He, Y.; Luo, Y.; Yang, M.; Zhang, Y.; Zhu, L.; Fan, M.; Li, Q. Selective catalytic synthesis of bio-based terephthalic acid from lignocellulose biomass. Appl. Catal. A Gen. 2021, 630, 118440. [Google Scholar] [CrossRef]
- Luo, Z.W.; Lee, S.Y. Biotransformation of p-xylene into terephthalic acid by engineered Escherichia coli. Nat. Commun. 2017, 8, 15689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Nguyen, V.; Frost, J.W. Synthesis of terephthalic acid from methane. ACS Sustain. Chem. Eng. 2016, 4, 5998–6001. [Google Scholar] [CrossRef]
- Polymer Properties Database Biopolyamides. Available online: https://polymerdatabase.com/Polymer%20Brands/Biopolyamides.html (accessed on 12 October 2021).
- Bioplastics News Bio-Based Polyamides. Available online: https://bioplasticsnews.com/2016/11/21/bio-based-polyamides/ (accessed on 12 October 2021).
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(ethylene terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef]
- Salvador, M.; Abdulmutalib, U.; Gonzalez, J.; Kim, J.; Smith, A.A.; Faulon, J.L.; Wei, R.; Zimmermann, W.; Jimenez, J.I. Microbial Genes for a Circular and Sustainable Bio-PET Economy. Genes 2019, 10, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- The Coca-Cola Company Coca-Cola Collaborates with Tech Partners to Create Bottle Prototype Made from 100% Plant-Based Sources. Available online: https://www.coca-colacompany.com/news/100-percent-plant-based-plastic-bottle (accessed on 19 December 2021).
- Barecka, M.H.; Skiborowski, M.; Górak, A. Process Intensification in Practice: Ethylene Glycol Case Study. In Practical Aspects of Chemical Engineering; Ochowiak, M., Doligalski, M., Woziwodzki, S., Mitkowski, P.T., Eds.; Springer: Cham, Switzerland, 2018; pp. 17–34. ISBN 978-3-319-73978-6. [Google Scholar]
- Cabulong, R.B.; Valdehuesa, K.N.G.; Ramos, K.R.M.; Nisola, G.M.; Lee, W.K.; Lee, C.R.; Chung, W.J. Enhanced yield of ethylene glycol production from d-xylose by pathway optimization in Escherichia coli. Enzym. Microb. Technol. 2017, 97, 11–20. [Google Scholar] [CrossRef]
- Wang, Y.; Xian, M.; Feng, X.; Liu, M.; Zhao, G. Biosynthesis of ethylene glycol from d-xylose in recombinant Escherichia coli. Bioengineered 2018, 9, 233–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, B.; Zhang, H.; De Mey, M.; Lim, C.G.; Li, Z.J.; Stephanopoulos, G. Engineering a novel biosynthetic pathway in Escherichia coli for production of renewable ethylene glycol. Biotechnol. Bioeng. 2016, 113, 376–383. [Google Scholar] [CrossRef]
- Islam, M.A.; Hadadi, N.; Ataman, M.; Hatzimanikatis, V.; Stephanopoulos, G. Exploring biochemical pathways for mono-ethylene glycol (MEG) synthesis from synthesis gas. Metab. Eng. 2017, 41, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Uranukul, B.; Woolston, B.M.; Fink, G.R.; Stephanopoulos, G. Biosynthesis of monoethylene glycol in Saccharomyces cerevisiae utilizing native glycolytic enzymes. Metab. Eng. 2019, 51, 20–31. [Google Scholar] [CrossRef]
- Gevo An Overview of Gevo’s Biobased Isobutananol Production Process. Available online: https://gevo.com/wp-content/uploads/2019/11/Gevo-WP_Isobutanol.1.pdf (accessed on 15 October 2021).
- Carraher, J.M.; Pfennig, T.; Rao, R.G.; Shanks, B.H.; Tessonnier, J.P. cis,cis-Muconic acid isomerization and catalytic conversion to biobased cyclic-C6-1,4-diacid monomers. Green Chem. 2017, 19, 3042–3050. [Google Scholar] [CrossRef] [Green Version]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A review. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Shiramizu, M.; Toste, F.D. On the Diels-Alder Approach to Solely Biomass-Derived Polyethylene Terephthalate (PET): Conversion of 2,5-Dimethylfuran and Acrolein into p-Xylene. Chem. Eur. J. 2011, 17, 12452. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, Y.; Kimura, S.; Kasuya, K.I. Synthesis and Verification of Biobased Terephthalic Acid from Furfural. Sci. Rep. 2015, 5, 8249. [Google Scholar] [CrossRef]
- Chen, G.Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef] [Green Version]
- Gama, N.; Ferreira, A.; Materials, A. Polyurethane foams: Past, present, and future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef] [Green Version]
- Utomo, R.N.C.; Li, W.J.; Tiso, T.; Eberlein, C.; Doeker, M.; Heipieper, H.J.; Jupke, A.; Wierckx, N.; Blank, L.M. Defined Microbial Mixed Culture for Utilization of Polyurethane Monomers. ACS Sustain. Chem. Eng. 2020, 8, 17466–17474. [Google Scholar] [CrossRef]
- Heinrich, L.A. Future opportunities for bio-based adhesives—Advantages beyond renewability. Green Chem. 2019, 21, 1866–1888. [Google Scholar] [CrossRef] [Green Version]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Zahoor, A.F. Bio-based polyurethane: An efficient and environment friendly coating systems: A review. Prog. Org. Coat. 2016, 91, 25–32. [Google Scholar] [CrossRef]
- Sahoo, S.; Kalita, H.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of vegetable oil based polyurethane derived from low viscous bio aliphatic isocyanate: Adhesion strength to wood-wood substrate bonding. Macromol. Res. 2017, 25, 772–778. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Reis, K.C.; Pereira, J.; Smith, A.C.; Carvalho, C.W.P.; Wellner, N.; Yakimets, I. Characterization of polyhydroxybutyrate-hydroxyvalerate (PHB-HV)/maize starch blend films. J. Food Eng. 2008, 89, 361–369. [Google Scholar] [CrossRef]
- Singh, R.P.; Pandey, J.K.; Rutot, D.; Degée, P.; Dubois, P. Biodegradation of poly(ε-caprolactone)/starch blends and composites in composting and culture environments: The effect of compatibilization on the inherent biodegradability of the host polymer. Carbohydr. Res. 2003, 338, 1759–1769. [Google Scholar] [CrossRef]
- Marichelvam, M.K.; Jawaid, M.; Asim, M. Corn and Rice Starch-Based Bio-Plastics as Alternative Packaging Materials. Fibers 2019, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Mathiot, C.; Ponge, P.; Gallard, B.; Sassi, J.F.; Delrue, F.; Le Moigne, N. Microalgae starch-based bioplastics: Screening of ten strains and plasticization of unfractionated microalgae by extrusion. Carbohydr. Polym. 2019, 208, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, S.N.; Canejo, J.P.; Echeverria, C.; Godinho, M.H. Functional materials from liquid crystalline cellulose derivatives: Synthetic routes, characterization and applications. In Liquid Crystalline Polymers; Springer: Cham, Switzerland, 2015; pp. 339–368. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M. Cellulose Plastics. In Brydson’s Plastics Materials: Eighth Edition; Gilbert, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 617–630. ISBN 9780323358248. [Google Scholar]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Mubarak, N.M.; Hallad, S.; Hugar, S.; Fayaz, H. Biodegradable carboxymethyl cellulose based material for sustainable packaging application. Sci. Rep. 2020, 10, 21960. [Google Scholar] [CrossRef]
- Zhang, W.; Jing, Z.; Shan, Y.; Ge, X.; Mu, X.; Jiang, Y.; Li, H.; Wu, P. Paper reinforced with regenerated cellulose: A sustainable and fascinating material with good mechanical performance, barrier properties and shape retention in water. J. Mater. Chem. A 2016, 4, 17483–17490. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, C.; Liang, Z.; He, S.; Kuang, Y.; Song, J.; Mi, R.; Chen, G.; Jiao, M.; Hu, L.; et al. Lignin as a wood-inspired binder enabled strong, water stable, and biodegradable paper for plastic replacement. Wiley Online Libr. 2019, 30, 1906307. [Google Scholar] [CrossRef]
- Su, Z.; Huang, S.; Wang, Y.; Ling, H.; Yang, X.; Jin, Y.; Wang, X.; Zhang, W. Robust, high-barrier, and fully recyclable cellulose-based plastic replacement enabled by a dynamic imine polymer. J. Mater. Chem. A 2020, 8, 14082–14090. [Google Scholar] [CrossRef]
- Emergen Research Cellulose-Based Plastics Market. Available online: https://www.emergenresearch.com/industry-report/cellulose-based-plastics-market (accessed on 15 December 2021).
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Handbook of Biopolymers and Biodegradable Plastics: Properties, Processing and Applications; Ebnesajjad, S., Ed.; Elsevier Inc.: Chadds Ford, PA, USA, 2012; ISBN 9781455730032. [Google Scholar]
- Shah, S.; Matkawala, F.; Garg, S.; Nighojkar, S.; Nighojkar, A.; Kumar, A. Emerging Trend of Bio-plastics and Its Impact on Society. Biotechnol. J. Int. 2020, 24, 1–10. [Google Scholar] [CrossRef]
- Prados, E.; Maicas, S. Bacterial Production of Hydroxyalkanoates (PHA). Univers. J. Microbiol. Res. 2016, 4, 23–30. [Google Scholar] [CrossRef]
- Bartels, M.; Gutschmann, B.; Widmer, T.; Grimm, T.; Neubauer, P.; Riedel, S.L. Recovery of the PHA Copolymer P(HB-co-HHx) with Non-halogenated Solvents: Influences on Molecular Weight and HHx-Content. Front. Bioeng. Biotechnol. 2020, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Rhu, D.H.; Lee, W.H.; Kim, J.Y.; Choi, E. Polyhydroxyalkanoate (PHA) production from waste. Water Sci. Technol. 2003, 48, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, R. Production of Polyhydroxyalkanoates (PHA) by Haloferax mediterranei from Food Waste Derived Nutrients for Biodegradable Plastic Applications. J. Microbiol. Biotechnol. 2021, 31, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Panda, A.N.; Ray, L. An investigation for recovery of polyhydroxyalkanoates (PHA) from Bacillus sp. BPPI-14 and Bacillus sp. BPPI-19 isolated from plastic waste landfill. Int. J. Biol. Macromol. 2019, 134, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zain, N.F.; Paramasivam, M.; Tan, J.S.; Lim, V.; Lee, C.K. Response surface methodology optimization of polyhydroxyalkanoate production by Burkholderia cepacia BPT1213 using waste glycerol from palm oil-based biodiesel production. Biotechnol. Prog. 2021, 37, e3077. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Tabatabaei, M.; Ghazali, F.M.; Abd-Aziz, S.; Shirai, Y.; Hassan, M.A. Polyhydroxyalkanoate production from anaerobically treated palm oil mill effluent by new bacterial strain Comamonas sp. EB172. World J. Microbiol. Biotechnol. 2010, 26, 767–774. [Google Scholar] [CrossRef]
- Arumugam, A.; Senthamizhan, S.G.; Ponnusami, V.; Sudalai, S. Production and optimization of polyhydroxyalkanoates from non-edible Calophyllum inophyllum oil using Cupriavidus necator. Int. J. Biol. Macromol. 2018, 112, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Jung, H.R.; Yang, S.Y.; Moon, Y.M.; Song, H.S.; Jeon, J.M.; Choi, K.Y.; Yang, Y.H. Bioconversion of plant biomass hydrolysate into bioplastic (polyhydroxyalkanoates) using Ralstonia eutropha 5119. Bioresour. Technol. 2019, 271, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Prajapati, V.; Patel, K.; Trivedi, U. Optimization and characterization of PHA from isolate Pannonibacter phragmitetus ERC8 using glycerol waste. Int. J. Biol. Macromol. 2016, 86, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ling, C.; Hajnal, I.; Wu, Q.; Chen, G.Q. Construction of Halomonas bluephagenesis capable of high cell density growth for efficient PHA production. Appl. Microbiol. Biotechnol. 2018, 102, 4499–4510. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, M.; Thakur, I.S. Analysis and optimization of process parameters for production of polyhydroxyalkanoates along with wastewater treatment by Serratia sp. ISTVKR1. Bioresour. Technol. 2017, 242, 55–59. [Google Scholar] [CrossRef]
- Kanavaki, I.; Drakonaki, A.; Geladas, E.D.; Spyros, A.; Xie, H.; Tsiotis, G. Polyhydroxyalkanoate (PHA) Production in Pseudomonas sp. phDV1 Strain Grown on Phenol as Carbon Sources. Microorganisms 2021, 9, 1636. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yoon, J.J.; Kim, H.J.; Hong, J.W.; Gi Hong, Y.; Song, H.S.; Moon, Y.M.; Jeon, J.M.; Kim, Y.G.; Yang, Y.H. Engineering of artificial microbial consortia of Ralstonia eutropha and Bacillus subtilis for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production from sugarcane sugar without precursor feeding. Bioresour. Technol. 2018, 257, 92–101. [Google Scholar] [CrossRef]
- Pereira, J.; Queirós, D.; Lemos, P.C.; Rossetti, S.; Serafim, L.S. Enrichment of a mixed microbial culture of PHA-storing microorganisms by using fermented hardwood spent sulfite liquor. New Biotechnol. 2020, 56, 79–86. [Google Scholar] [CrossRef]
- Akdoğan, M.; Çelik, E. Enhanced production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biopolymer by recombinant Bacillus megaterium in fed-batch bioreactors. Bioprocess Biosyst. Eng. 2021, 44, 403–416. [Google Scholar] [CrossRef]
- Jung, H.R.; Yang, S.Y.; Moon, Y.M.; Choi, T.R.; Song, H.S.; Bhatia, S.K.; Gurav, R.; Kim, E.J.; Kim, B.G.; Yang, Y.H. Construction of Efficient Platform Escherichia coli Strains for Polyhydroxyalkanoate Production by Engineering Branched Pathway. Polymers 2019, 11, 509. [Google Scholar] [CrossRef] [Green Version]
- Madadi, R.; Maljaee, H.; Serafim, L.S.; Ventura, S.P.M. Microalgae as Contributors to Produce Biopolymers. Mar. Drugs 2021, 19, 466. [Google Scholar] [CrossRef] [PubMed]
- Abdo, S.M.; Ali, G.H. Analysis of polyhydroxybutrate and bioplastic production from microalgae. Bull. Natl. Res. Cent. 2019, 43, 97. [Google Scholar] [CrossRef]
- Das, S.K.; Sathish, A.; Stanley, J. Production of Biofuel and Bioplastic from Chlorella Pyrenoidosa. Mater. Today Proc. 2018, 5, 16774–16781. [Google Scholar] [CrossRef]
- Costa, S.S.; Miranda, A.L.; Andrade, B.B.; de Jesus Assis, D.; Souza, C.O.; de Morais, M.G.; Costa, J.A.V.; Druzian, J.I. Influence of nitrogen on growth, biomass composition, production, and properties of polyhydroxyalkanoates (PHAs) by microalgae. Int. J. Biol. Macromol. 2018, 116, 552–562. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Ganesh Saratale, R.; Cho, S.K.; Dattatraya Saratale, G.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Naresh Bharagava, R.; Kumar, G.; Su Kim, D.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef]
- Kenny, S.T.; Runic, J.N.; Kaminsky, W.; Woods, T.; Babu, R.P.; Keely, C.M.; Blau, W.; O’Connor, K.E. Up-Cycling of PET (Polyethylene Terephthalate) to the Biodegradable Plastic PHA (Polyhydroxyalkanoate). Environ. Sci. Technol. 2008, 42, 7696–7701. [Google Scholar] [CrossRef]
- Kenny, S.T.; Runic, J.N.; Kaminsky, W.; Woods, T.; Babu, R.P.; O’Connor, K.E. Development of a bioprocess to convert PET derived terephthalic acid and biodiesel derived glycerol to medium chain length polyhydroxyalkanoate. Appl. Microbiol. Biotechnol. 2012, 95, 623–633. [Google Scholar] [CrossRef]
- Tiso, T.; Narancic, T.; Wei, R.; Pollet, E.; Beagan, N.; Schröder, K.; Honak, A.; Jiang, M.; Kenny, S.T.; Wierckx, N.; et al. Towards bio-upcycling of polyethylene terephthalate. Metab. Eng. 2021, 66, 167–178. [Google Scholar] [CrossRef]
- Narancic, T.; Salvador, M.; Hughes, G.M.; Beagan, N.; Abdulmutalib, U.; Kenny, S.T.; Wu, H.; Saccomanno, M.; Um, J.; O’Connor, K.E.; et al. Genome analysis of the metabolically versatile Pseudomonas umsongensis GO16: The genetic basis for PET monomer upcycling into polyhydroxyalkanoates. Microb. Biotechnol. 2021, 14, 2463–2480. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Black-Solís, J.D.; Ortega-Gudiño, P.; Sabino-Gutiérrez, M.A.; Benítez-Jiménez, J.J.; Barajas-Cervantes, A.; Bautista-Baños, S.; Hurtado-Colmenares, L.B. Preparation and Characterization of Bio-Based PLA/PBAT and Cinnamon Essential Oil Polymer Fibers and Life-Cycle Assessment from Hydrolytic Degradation. Polymers 2019, 12, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, F.V.; Cividanes, L.S.; Gouveia, R.F.; Lona, L.M.F. An overview on properties and applications of poly(butylene adipate-co-terephthalate)–PBAT based composites. Polym. Eng. Sci. 2019, 59, E7–E15. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, R.; Rajeswari, N.; Tamilselvi, A. Antimicrobial, mechanical, barrier, and thermal properties of bio-based poly (butylene adipate-co-terephthalate) (PBAT)/Ag2O nanocomposite films for packaging application. Polym. Adv. Technol. 2018, 29, 61–68. [Google Scholar] [CrossRef]
- Meng, D.; Xie, J.; Waterhouse, G.I.N.; Zhang, K.; Zhao, Q.; Wang, S.; Qiu, S.; Chen, K.; Li, J.; Ma, C.; et al. Biodegradable Poly(butylene adipate-co-terephthalate) composites reinforced with bio-based nanochitin: Preparation, enhanced mechanical and thermal properties. J. Appl. Polym. Sci. 2020, 137, 48485. [Google Scholar] [CrossRef]
- Chen, S.; Lin, S.; Hu, Y.; Ma, M.; Shi, Y.; Liu, J.; Zhu, F.; Wang, X. A lignin-based flame retardant for improving fire behavior and biodegradation performance of polybutylene succinate. Polym. Adv. Technol. 2018, 29, 3142–3150. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Singh, P.; Alsanie, W.F.; Grammatikos, S.A.; Thakur, V.K. Synthesis of Bio-based monomers and polymers using microbes for a sustainable bioeconomy. Bioresour. Technol. 2022, 344, 126156. [Google Scholar] [CrossRef] [PubMed]
- Mitsubishi Chemical Coporation BIOPBSTM. Available online: https://www.mcpp-global.com/en/mcpp-asia/products/brand/biopbsTM/ (accessed on 12 January 2022).
- PTT MCC Biochem Co., Ltd. What’s BioPBS. Available online: https://www.pttmcc.com/what-is-biopbs (accessed on 13 January 2022).
- Platnieks, O.; Gaidukovs, S.; Kumar Thakur, V.; Barkane, A.; Beluns, S. Bio-based poly (butylene succinate): Recent progress, challenges and future opportunities. Eur. Polym. J. 2021, 161, 110855. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H. Poly(butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Mordor Intelligence 1,4 Butanediol Market—Growth, Trends, COVID-19 Impact, and Forecast (2021–2026). Available online: https://www.mordorintelligence.com/industry-reports/1-4-butanediol-market (accessed on 28 September 2021).
- Yim, H.; Haselbeck, R.; Niu, W.; Pujol-Baxley, C.; Burgard, A.; Boldt, J.; Khandurina, J.; Trawick, J.D.; Osterhout, R.E.; Stephen, R.; et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Chem. Biol. 2011, 7, 445–452. [Google Scholar] [CrossRef]
- Barton, N.R.; Burgard, A.P.; Burk, M.J.; Crater, J.S.; Osterhout, R.E.; Pharkya, P.; Steer, B.A.; Sun, J.; Trawick, J.D.; Van Dien, S.J.; et al. An integrated biotechnology platform for developing sustainable chemical processes. J. Ind. Microbiol. Biotechnol. 2015, 42, 349–360. [Google Scholar] [CrossRef]
- Burgard, A.; Burk, M.J.; Osterhout, R.; Van Dien, S.; Yim, H. Development of a commercial scale process for production of 1,4-butanediol from sugar. Curr. Opin. Biotechnol. 2016, 42, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Sung, L.Y.; Li, H.; Huang, C.H.; Hu, Y.C. Combining CRISPR and CRISPRi Systems for Metabolic Engineering of E. coli and 1,4-BDO Biosynthesis. ACS Synth. Biol. 2017, 6, 2350–2361. [Google Scholar] [CrossRef] [PubMed]
- Genomatica GENO BDOTM Process: Commercial Scale; Proven; Available to License. Available online: https://www.genomatica.com/products/#bdo (accessed on 28 September 2021).
- DuPont Tate & Lyle BioProducts Genomatica and DuPont Tate & Lyle Bio Products Successfully Produce 1,4-Butanediol (BDO) on Commercial Scale. Available online: https://duponttateandlyle.com/genomatica-and-dupont-tate-lyle-bio-products-successfully-produce-14-butanediol-bdo-on-commercial-scale/ (accessed on 28 September 2021).
- Novamont Opening of The World’s First Industrial Scale Plant for the Production of Butanediol via Fermentation of Renewable Raw Materials. Available online: https://www.novamont.com/eng/read-press-release/mater-biotech/ (accessed on 28 September 2021).
- Mordor Intelligence 1,3-Propanediol (PDO) Market—Growth, Trends, COVID-19 Impact, and Forecasts (2021–2026). Available online: https://www.mordorintelligence.com/industry-reports/1-3-propanediol-pdo-market (accessed on 28 September 2021).
- PRNewswire 1,3-Propanediol (PDO) Market to be Driven by Rising Infiltration of Polyurethane & High Demand for Polyesters Till 2022. Available online: https://www.prnewswire.com/news-releases/1-3-propanediol-pdo-market-to-be-driven-by-rising-infiltration-of-polyurethane--high-demand-for-polyesters-till-2022--million-insights-300984122.html (accessed on 3 October 2021).
- Biebl, H.; Menzel, K.; Zeng, A.P.; Deckwer, W.D. Microbial production of 1,3-propanediol. Appl. Microbiol. Biotechnol. 1999, 52, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Tabah, B.; Varvak, A.; Pulidindi, I.N.; Foran, E.; Banin, E.; Gedanken, A. Production of 1,3-propanediol from glycerol via fermentation by Saccharomyces cerevisiae. Green Chem. 2016, 18, 4657–4666. [Google Scholar] [CrossRef]
- Durgapal, M.; Kumar, V.; Yang, T.H.; Lee, H.J.; Seung, D.; Park, S. Production of 1,3-propanediol from glycerol using the newly isolated Klebsiella pneumoniae J2B. Bioresour. Technol. 2014, 159, 223–231. [Google Scholar] [CrossRef]
- Ju, J.H.; Wang, D.; Heo, S.Y.; Kim, M.S.; Seo, J.W.; Kim, Y.M.; Kim, D.H.; Kang, S.A.; Kim, C.H.; Oh, B.R. Enhancement of 1,3-propanediol production from industrial by-product by Lactobacillus reuteri CH53. Microb. Cell Fact. 2020, 19, 6. [Google Scholar] [CrossRef] [Green Version]
- Kongjan, P.; Jariyaboon, R.; Reungsang, A.; Sittijunda, S. Co-fermentation of 1,3-propanediol and 2,3-butanediol from crude glycerol derived from the biodiesel production process by newly isolated Enterobacter sp.: Optimization factors affecting. Bioresour. Technol. Rep. 2021, 13, 100616. [Google Scholar] [CrossRef]
- Przystałowska, H.; Zeyland, J.; Szymanowska-Powałowska, D.; Szalata, M.; Słomski, R.; Lipiński, D. 1,3-Propanediol production by new recombinant Escherichia coli containing genes from pathogenic bacteria. Microbiol. Res. 2015, 171, 1–7. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhou, J.J.; Shen, J.T.; Zheng, Y.F.; Sun, Y.Q.; Xiu, Z.L. Sequential fed-batch fermentation of 1,3-propanediol from glycerol by Clostridium butyricum DL07. Appl. Microbiol. Biotechnol. 2020, 104, 9179–9191. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Gao, H.; Wang, H.; Wan, Z.; Jiang, Y.; Xin, F.; Zhang, W.; Jiang, M. Current advances in microbial production of 1,3-propanediol. Biofuels Bioprod. Biorefin. 2021, 15, 1566–1583. [Google Scholar] [CrossRef]
- Varrone, C.; Floriotis, G.; Heggeset, T.M.B.; Le, S.B.; Markussen, S.; Skiadas, I.V.; Gavala, H.N. Continuous fermentation and kinetic experiments for the conversion of crude glycerol derived from second-generation biodiesel into 1,3 propanediol and butyric acid. Biochem. Eng. J. 2017, 128, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Emptage, M.; Haynie, S.L.; Laffend, L.A.; Pucci, J.P.; Whited, G. Process for the Biological Production of 1,3-Propanediol with High Titer. U.S. Patent No. 6514733, 4 February 2003. [Google Scholar]
- Nova-Institute USA. World’s First Propanediol Production from Corn Sugar Opened. Available online: https://renewable-carbon.eu/news/u-s-a-worlds-first-propanediol-production-from-corn-sugar-opened/ (accessed on 5 October 2021).
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research Lactic Acid Market Share, Industry Report, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/lactic-acid-and-poly-lactic-acid-market (accessed on 13 October 2021).
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; de Souza Oliveira, R.P. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Florou-Paneri, P.; Christaki, E.; Bonos, E. Lactic Acid Bacteria as Source of Functional Ingredients. In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes; Kongo, M., Ed.; IntechOpen: London, UK, 2013; pp. 589–614. ISBN 978-953-51-5349-8. [Google Scholar]
- Awasthi, D.; Wang, L.; Rhee, M.S.; Wang, Q.; Chauliac, D.; Ingram, L.O.; Shanmugam, K.T. Metabolic engineering of Bacillus subtilis for production of D-lactic acid. Biotechnol. Bioeng. 2018, 115, 453–463. [Google Scholar] [CrossRef]
- Danner, H.; Neureiter, M.; Madzingaidzo, L.; Gartner, M.; Braun, R. Bacillus stearothermophilus for thermophilic production of L-lactic acid. Appl. Biochem. Biotechnol. 1998, 70–72, 895–903. [Google Scholar] [CrossRef]
- Gao, T.; Wong, Y.; Ng, C.; Ho, K. L-lactic acid production by Bacillus subtilis MUR1. Bioresour. Technol. 2012, 121, 105–110. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, P.; Tao, F. L-Lactic acid production by Bacillus coagulans through simultaneous saccharification and fermentation of lignocellulosic corncob residue. Bioresour. Technol. Rep. 2019, 6, 131–137. [Google Scholar] [CrossRef]
- Patel, M.; Ou, M.; Ingram, L.O.; Shanmugam, K.T. Fermentation of sugar cane bagasse hemicellulose hydrolysate to l(+)-lactic acid by a thermotolerant acidophilic Bacillus sp. Biotechnol. Lett. 2004, 26, 865–868. [Google Scholar] [CrossRef]
- Sakai, K.; Yamanami, T. Thermotolerant Bacillus licheniformis TY7 produces optically active l-lactic acid from kitchen refuse under open condition. J. Biosci. Bioeng. 2006, 102, 132–134. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, C.; Assavasirijinda, N.; Yu, B.; Wang, L.; Ma, Y. Non-sterilized fermentation of high optically pure d-lactic acid by a genetically modified thermophilic Bacillus coagulans strain. Microb. Cell Fact. 2017, 16, 213. [Google Scholar] [CrossRef]
- Qiu, Z.; Gao, Q.; Bao, J. Constructing xylose-assimilating pathways in Pediococcus acidilactici for high titer d-lactic acid fermentation from corn stover feedstock. Bioresour. Technol. 2017, 245, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Gao, Q.; Bao, J. Engineering Pediococcus acidilactici with xylose assimilation pathway for high titer cellulosic l-lactic acid fermentation. Bioresour. Technol. 2018, 249, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.T.; Salem, S.S.; Fouda, A.; El-Gamal, M.S.; Abdel-Rahman, M.A. Use of Corn-Steep Water Effluent as a Promising Substrate for Lactic Acid Production by Enterococcus faecium Strain WH51-1. Fermentation 2021, 7, 111. [Google Scholar] [CrossRef]
- Ferone, M.; Raganati, F.; Olivieri, G.; Salatino, P.; Marzocchella, A. Continuous Succinic Acid Fermentation by Actinobacillus Succinogenes: Assessment of Growth and Succinic Acid Production Kinetics. Appl. Biochem. Biotechnol. 2019, 187, 782–799. [Google Scholar] [CrossRef]
- Yan, D.; Wang, C.; Zhou, J.; Liu, Y.; Yang, M.; Xing, J. Construction of reductive pathway in Saccharomyces cerevisiae for effective succinic acid fermentation at low pH value. Bioresour. Technol. 2014, 156, 232–239. [Google Scholar] [CrossRef]
- Yuzbashev, T.V.; Yuzbasheva, E.Y.; Sobolevskaya, T.I.; Laptev, I.A.; Vybornaya, T.V.; Larina, A.S.; Matsui, K.; Fukui, K.; Sineoky, S.P. Production of succinic acid at low pH by a recombinant strain of the aerobic yeast Yarrowia lipolytica. Biotechnol. Bioeng. 2010, 107, 673–682. [Google Scholar] [CrossRef]
- Chen, C.; Ding, S.; Wang, D.; Li, Z.; Ye, Q. Simultaneous saccharification and fermentation of cassava to succinic acid by Escherichia coli NZN111. Bioresour. Technol. 2014, 163, 100–105. [Google Scholar] [CrossRef]
- Sun, Z.; Li, M.; Qi, Q.; Gao, C.; Lin, C.S.K. Mixed Food Waste as Renewable Feedstock in Succinic Acid Fermentation. Appl. Biochem. Biotechnol. 2014, 174, 1822–1833. [Google Scholar] [CrossRef]
- Stylianou, E.; Pateraki, C.; Ladakis, D.; Cruz-Fernández, M.; Latorre-Sánchez, M.; Coll, C.; Koutinas, A. Evaluation of organic fractions of municipal solid waste as renewable feedstock for succinic acid production. Biotechnol. Biofuels 2020, 13, 72. [Google Scholar] [CrossRef] [Green Version]
- Bio-Conversion and Separation Technology. WP 8.1.Determination of Market Potentialfor Selected Platform Chemicals—Itaconic Acid, Succinic Acid, 2,5-Furandicarboxylic Acid; WEASTRA s.r.o.: Bratislava, Slovakia, 2013. [Google Scholar]
- Nghiem, N.P.; Kleff, S.; Schwegmann, S. Succinic Acid: Technology Development and Commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Jiang, M.; Ma, J.; Wu, M.; Liu, R.; Liang, L.; Xin, F.; Zhang, W.; Jia, H.; Dong, W. Progress of succinic acid production from renewable resources: Metabolic and fermentative strategies. Bioresour. Technol. 2017, 245, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ma, L.; Mao, Y. Biological production of adipic acid from renewable substrates: Current and future methods. Biochem. Eng. J. 2016, 105, 16–26. [Google Scholar] [CrossRef]
- Deng, Y.; Mao, Y. Production of adipic acid by the native-occurring pathway in Thermobifida fusca B6. Wiley Online Libr. 2015, 119, 1057–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, K.; Partow, S.; Correia, K.; Khusnutdinova, A.N.; Yakunin, A.F.; Mahadevan, R. Biocatalytic production of adipic acid from glucose using engineered Saccharomyces cerevisiae. Metab. Eng. Commun. 2018, 6, 28–32. [Google Scholar] [CrossRef]
- Clomburg, J.M.; Blankschien, M.D.; Vick, J.E.; Chou, A.; Kim, S.; Gonzalez, R. Integrated engineering of β-oxidation reversal and ω-oxidation pathways for the synthesis of medium chain ω-functionalized carboxylic acids. Metab. Eng. 2015, 28, 202–212. [Google Scholar] [CrossRef]
- Todea, A.; Deganutti, C.; Spennato, M.; Asaro, F.; Zingone, G.; Milizia, T.; Gardossi, L.; Pellis, A. Azelaic Acid: A Bio-Based Building Block for Biodegradable Polymers. Polymers 2021, 13, 4091. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Bikiaris, D.N.; Achilias, D.S.; Karagiannidis, N. Synthesis, Crystallization, and Enzymatic Degradation of the Biodegradable Polyester Poly(ethylene azelate). Macromol. Chem. Phys. 2010, 211, 2585–2595. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jun, M.W.; Seong, Y.J.; Park, H.; Ahn, J.; Park, Y.C. Direct Biotransformation of Nonanoic Acid and Its Esters to Azelaic Acid by Whole Cell Biocatalyst of Candida tropicalis. ACS Sustain. Chem. Eng. 2019, 7, 17958–17966. [Google Scholar] [CrossRef]
- Otte, K.B.; Kirtz, M.; Nestl, B.M.; Hauer, B. Synthesis of 9-Oxononanoic Acid, a Precursor for Biopolymers. ChemSusChem 2013, 6, 2149–2156. [Google Scholar] [CrossRef]
- Otte, K.B.; Kittelberger, J.; Kirtz, M.; Nestl, B.M.; Hauer, B. Whole-Cell One-Pot Biosynthesis of Azelaic Acid. ChemCatChem 2014, 6, 1003–1009. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Seo, J.H.; Kang, W.R.; Kim, M.J.; Lee, J.H.; Oh, D.K.; Park, J.B. Simultaneous enzyme/Whole-Cell biotransformation of plant oils into C9 carboxylic acids. ACS Catal. 2016, 6, 7547–7553. [Google Scholar] [CrossRef]
- Song, J.W.; Lee, J.H.; Bornscheuer, U.T.; Park, J.B. Microbial Synthesis of Medium-Chain α,ω-Dicarboxylic Acids and ω-Aminocarboxylic Acids from Renewable Long-Chain Fatty Acids. Adv. Synth. Catal. 2014, 356, 1782–1788. [Google Scholar] [CrossRef]
- Seo, E.J.; Yeon, Y.J.; Seo, J.H.; Lee, J.H.; Boñgol, J.P.; Oh, Y.; Park, J.M.; Lim, S.M.; Lee, C.G.; Park, J.B. Enzyme/whole-cell biotransformation of plant oils, yeast derived oils, and microalgae fatty acid methyl esters into n-nonanoic acid, 9-hydroxynonanoic acid, and 1,9-nonanedioic acid. Bioresour. Technol. 2018, 251, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Gantrade Corporation Caprolactone Monomer: A Gateway Building Block for Advanced Performance Intermediates. Available online: https://www.gantrade.com/blog/caprolactone-monomer-a-gateway-building-block-for-advanced-performance-intermediates (accessed on 16 December 2021).
- Bornadel, A.; Hatti-Kaul, R.; Hollmann, F.; Kara, S. A Bi-enzymatic Convergent Cascade for ε-Caprolactone Synthesis Employing 1,6-Hexanediol as a ‘Double-Smart Cosubstrate. ChemCatChem 2015, 7, 2442–2445. [Google Scholar] [CrossRef]
- Schmidt, S.; Scherkus, C.; Muschiol, J.; Menyes, U.; Winkler, T.; Hummel, W.; Grçger, H.; Liese, A.; Herz, H.-G.; Bornscheuer, U.T.; et al. An Enzyme Cascade Synthesis of ε-Caprolactone and its Oligomers. Angew. Chem. Int. Ed. 2015, 54, 2784–2787. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Schäfer, L.; Karande, R.; Bühler, B. Maximizing Biocatalytic Cyclohexane Hydroxylation by Modulating Cytochrome P450 Monooxygenase Expression in P. taiwanensis VLB120. Front. Bioeng. Biotechnol. 2020, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Salamanca, D.; Bühler, K.; Engesser, K.H.; Schmid, A.; Karande, R. Whole-cell biocatalysis using the Acidovorax sp. CHX100 Δ6HX for the production of ω-hydroxycarboxylic acids from cycloalkanes. New Biotechnol. 2021, 60, 200–206. [Google Scholar] [CrossRef]
- Bretschneider, L.; Heuschkel, I.; Wegner, M.; Lindmeyer, M.; Bühler, K.; Karande, R.; Bühler, B. Conversion of Cyclohexane to 6-Hydroxyhexanoic Acid Using Recombinant Pseudomonas taiwanensis in a Stirred-Tank Bioreactor. Front. Catal. 2021, 1, 683248. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Chiloeches, A.; Funes, A.; Cuervo-Rodríguez, R.; López-Fabal, F.; Fernández-García, M.; Echeverría, C.; Muñoz-Bonilla, A. Biobased polymers derived from itaconic acid bearing clickable groups with potent antibacterial activity and negligible hemolytic activity. Polym. Chem. 2021, 12, 3190–3200. [Google Scholar] [CrossRef]
- Verified Market Research Itaconic Acid Market Size and Forecast. Available online: https://www.verifiedmarketresearch.com/product/itaconic-acid-market/ (accessed on 18 December 2021).
- Cunha da Cruz, J.; Machado de Castro, A.; Camporese Sérvulo, E.F. World market and biotechnological production of itaconic acid. 3 Biotech 2018, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Gopaliya, D.; Kumar, V.; Khare, S.K. Recent advances in itaconic acid production from microbial cell factories. Biocatal. Agric. Biotechnol. 2021, 36, 102130. [Google Scholar] [CrossRef]
- Teleky, B.E.; Vodnar, D.C. Recent Advances in Biotechnological Itaconic Acid Production, and Application for a Sustainable Approach. Polymers 2021, 13, 3574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zuo, Z.R.; Chen, X.Z.; Niu, D.D.; Tian, K.M.; Prior, B.A.; Shen, W.; Shi, G.Y.; Singh, S.; Wang, Z.X. Evaluation of genetic manipulation strategies on d-lactate production by Escherichia coli. Curr. Microbiol. 2011, 62, 981–989. [Google Scholar] [CrossRef]
- Jantama, K.; Zhang, X.; Moore, J.C.; Shanmugam, K.T.; Svoronos, S.A.; Ingram, L.O. Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C. Biotechnol. Bioeng. 2008, 101, 881–893. [Google Scholar] [CrossRef]
- Chudasama, A. Exploiting Technologies in the Emerging Bioeconomy. Sugar Sugar Deriv. Chang. Consum. Prefer. 2020, 25–38. [Google Scholar] [CrossRef]
- Hagen, A.; Poust, S.; De Rond, T.; Fortman, J.L.; Katz, L.; Petzold, C.J.; Keasling, J.D. Engineering a Polyketide Synthase for in Vitro Production of Adipic Acid. ACS Synth. Biol. 2016, 5, 21–27. [Google Scholar] [CrossRef]
- Li, W.J.; Jayakody, L.N.; Franden, M.A.; Wehrmann, M.; Daun, T.; Hauer, B.; Blank, L.M.; Beckham, G.T.; Klebensberger, J.; Wierckx, N. Laboratory evolution reveals the metabolic and regulatory basis of ethylene glycol metabolism by Pseudomonas putida KT2440. Environ. Microbiol. 2019, 21, 3669–3682. [Google Scholar] [CrossRef]
- Li, W.J.; Narancic, T.; Kenny, S.T.; Niehoff, P.J.; O’Connor, K.; Blank, L.M.; Wierckx, N. Unraveling 1,4-Butanediol Metabolism in Pseudomonas putida KT2440. Front. Microbiol. 2020, 11, 382. [Google Scholar] [CrossRef] [Green Version]
- Chinthapalli, R.; Skoczinski, P.; Carus, M.; Baltus, W.; De Guzman, D.; Käb, H.; Raschka, A.; Ravenstijn, J. Biobased Building Blocks and Polymers—Global Capacities, Production and Trends, 2018–2023. Ind. Biotechnol. 2019, 15, 237–241. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).