The Influence of Delayed Sealing and Repeated Air Ingress during the Storage of Maize Silage on Fermentation Patterns, Yeast Development and Aerobic Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Determination of Dry Matter (DM)
2.3. Chemical Analyses
2.4. Aerobic Stability Measurement
2.5. Microbiological Analyses
2.6. Statistical Analysis
3. Results
3.1. Effects of Delayed Sealing and Repeated Air Ingress on Fermentation Patterns, Water Soluble Carbohydrate Concentration (WSC) and Dry Matter (DM) Losses
3.1.1. Effects of Sealing Time during the Early Phase of Fermentation until Day 16
3.1.2. Effects of Sealing Time and Air Ingress during the Later Phase of Fermentation from Day 32
3.2. Effects of Delayed Sealing and Repeated Air Ingress on Yeast Development and Aerobic Stability (ASTA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | Dry matter |
| VOC | Volatile organic compounds |
| EL | Ethyl lactate |
| EA | Ethyl acetate |
| WSC | Water-soluble carbohydrates |
References
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Marlow, UK, 1991; pp. 122–129. [Google Scholar]
- Gerlach, K.; Ross, F.; Weiss, K.; Büscher, W.; Südekum, K.H. Changes in maize silage fermentation products during aerobic deterioration and effects on dry matter intake by goats. J. Agric. Food Sci. 2013, 22, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Brüning, D.; Gerlach, K.; Weiss, K.; Südekum, K.H. Effect of compaction, delayed sealing and aerobic exposure on maize silage quality and on formation of volatile compounds. Grass Forage Sci. 2018, 73, 53–66. [Google Scholar] [CrossRef]
- Jonsson, A.; Pahlow, G. Systematic classification and biochemical characterization of yeasts growing in grass silages inoculated with Lactobacillus cultures. Anim. Res. Dev. 1984, 20, 7–11. [Google Scholar]
- Kung, L., Jr.; Sheperd, A.C.; Smagala, A.M.; Endres, K.M.; Bessett, C.A.; Ranjit, N.K.; Glancey, J.L. The Effect of Preservatives Based on Propionic Acid on the Fermentation and Aerobic Stability of Corn Silage and a Total Mixed Ration1. J. Dairy Sci. 1998, 81, 1322–1330. [Google Scholar] [CrossRef]
- Tabacco, E.; Righi, F.; Quarantelli, A.; Borreani, G. Dry matter und nutritional losses during aerobic deterioration of corn and sorghum silages as influenced by different lactic acid bacteria inocula. J. Dairy Sci. 2011, 94, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, H.; Theobald, P. Additive Type Affects Fermentation, Aerobic Stability and Mycotoxin Formation during Air Exposure of Early-Cut Rye (Secale cereale L.) Silage. Agronomy 2020, 10, 1432. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Oude-Elferink, S.J.W.E.; Spoelstra, S.F. Microbiology of Ensiling. Silage Science and Technology; American Society of Agronomy, Inc.: Madison, WI, USA, 2003; pp. 31–93. [Google Scholar]
- Driehuis, F.; van Wikselaar, P.G. The occurrence and prevention of ethanol fermentation in high-dry-matter grass silage. J. Sci. Food Agric. 2000, 80, 711–718. [Google Scholar] [CrossRef]
- Krooneman, J.; Faber, F.; Alderkamp, A.C.; Oude Elferink, S.J.W.H.; Driehuis, F.; Cleenwerck, L.; Swings, J.; Gottschal, J.C.; Vancanneyt, M. Lactobacillus diolivorans sp. nov., a 1,2-propanediol-degrading bacterium isolated from aerobically stable maize silage. Int. J. Syst. Evol. Microbiol. 2002, 52, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, H.; Theobald, P.; Kroschewski, B.; Weiss, K. Effects of Type and Composition of Additive on Fermentation, Aerobic Stability and Formation of Volatile Organic Compounds (VOC) in Whole-Crop Rye Silage. Agronomy 2020, 10, 1873. [Google Scholar] [CrossRef]
- Kruis, A.; Levisson, M.; Mars, A.E.; van der Ploeg, M.; Daza, F.G.; Ellena, V.; Kengen, S.W.M.; van der Oost, J.; Weusthuis, R.A. Ethyl acetate production by the elusive alcohol acetyltransferase from yeast. Metab. Eng. 2017, 41, 92–101. [Google Scholar] [CrossRef]
- Weiss, K.; Kroschewski, B.; Auerbach, H. Formation of volatile organic compounds during the fermentation of maize as affected by sealing time and silage additive use. Arch. Anim. Nutr. 2020, 74, 150–163. [Google Scholar] [CrossRef]
- Moshtaghi Nia, S.A.; Wittenberg, K.M. Effect of delayed wrapping on preservation and quality of whole crop barley forage ensiled as large bales. Can. J. Anim. Sci. 2000, 80, 145–151. [Google Scholar]
- Mills, J.A.; Kung, L. The Effect of Delayed Ensiling and Application of a Propionic Acid-Based Additive on the Fermentation of Barley Silage1, 2. J. Dairy Sci. 2002, 85, 1969–1975. [Google Scholar] [CrossRef] [Green Version]
- McEniry, J.; Kiely, O.P.; Clipson, N.J.W.; Forristal, P.D.; Doyle, E.M. The relative impacts of wilting, chopping, compaction and air infiltration on the conservation characteristics of ensiled grass. Grass Forage Sci. 2007, 62, 470–484. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Savage, R.M.; da Silva, E.B.; Polukis, S.A.; Smith, M.L.; Johnson, A.C.B.; Miller, M.A. The effects of air stress during storage and low packing density on the fermentation and aerobic stability of corn silage inoculated with Lactobacillus buchneri 40788. J. Dairy Sci. 2021, 104, 4206–4222. [Google Scholar] [CrossRef]
- da Silva, E.B.; Savage, R.M.; Biddle, A.S.; Polukis, S.A.; Smith, M.L.; Limin, K., Jr. Effects of a chemical additive on the fermentation, microbial communities, and aerobic stability of corn silage with or without air stress during storage. J. Anim. Sci. 2020, 98, skaa246. [Google Scholar] [CrossRef]
- Weissbach, F.; Kuhla, S. Substance losses in determining the dry matter content of silage and green fodder: Arising errors and possibilities of correction. Übers Tierern. 1995, 23, 189–214. [Google Scholar]
- Weissbach, F.; Strubelt, C. Correcting the dry matter content of maize silages as a substrate for biogas production. Landtechnik 2008, 63, 82–83. [Google Scholar]
- Naumann, C.; Bassler, R. Methodenbuch des VDLUFA: Die Chemische Untersuchung von Futtermitteln (Book of Methods of VDLUFA: The Chemical Analysis of Feeds); Ergänzungslieferung (Supplementary Supply) 2012; VDLUFA Verlag: Darmstadt, Germany, 1976; Volume III. (In Germany) [Google Scholar]
- von Lengerken, J.; Zimmermann, K. Handbuch Futtermittelprüfung (Handbook Feed Evaluation), 1st ed.; Deutscher Landwirtschaftsverlag: Berlin, Germany, 1991; pp. 260–267. (In Germany) [Google Scholar]
- Weiss, K.; Kaiser, E. Milchsäurebestimmung in Silageextrakten mit Hilfe der HPLC [Determination of lactic acid by HPLC]. Das Wirtsch. Futter 1995, 41, 69–80. [Google Scholar]
- Weiss, K.; Sommer, G. Bestimmung von Gärsäuren und Alkoholen in Silageextrakten mittels Gaschromatographie [Determination of fermentation acids and alcohols in silage extracts by gas-chromatography]. VDLUFA-Schriftenreihe 2017, 74, 685–694. [Google Scholar]
- Weiss, K.; Sommer, G. Bestimmung von Estern und anderen flüchtigen organischen Substanzen (VOC) in Silageextrakten mit Hilfe der Gaschromatographie [Determination of esters and other volatile organic compounds (VOC) in silage extracts by gas-chromatography]. VDLUFA-Schriftenreihe 2012, 68, 561–569. [Google Scholar]
- Wolthusen, E.; Weissbach, F.; Derno, M. Fermentation acid content and aerobic stability of silages. In Proceedings of the an International Symposium on Production, Evaluation and Feeding of Silage, Rostock, Germany, 12–16 June 1989; pp. 123–132. [Google Scholar]
- Honig, H. Evaluation of aerobic stability. In Proceedings of the EUROBAC Conference, Uppsala, Sweden, 12–16 August 1986; Lindgren, S., Pettersson, K.L., Eds.; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1990; pp. 76–82. [Google Scholar]
- Milliken, G.A.; Johnson, D.E. Analysis of messy data. In Designed Experiments, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009; Volume 1. [Google Scholar]
- Knicky, M.; Spörndly, R.; Eide, F.; Gertzell, B. Different experimental designs in testing of silage additives. In Proceedings of the Nordic Feed Science Conference, Uppsala, Sweden, 14–15 June 2016; Department of Animal Nutrition and Management, SLU: Uppsala, Sweden, 2016; pp. 21–25. [Google Scholar]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Bolsen, K.K.; Dickerson, J.T.; Brent, B.E., Jr.; Sonon, R.N.; Dalke, B.S.; Lin, C.; Boyer, J.E. Rate and extent of top spoilage losses in horizontal silos. J. Dairy Sci. 1993, 76, 2940–2962. [Google Scholar] [CrossRef]
- Pettersson, K. Ensiling of Forages. Factors Affecting Silage Fermentation and Quality; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1988. [Google Scholar]
- Rooke, J.A.; Hatfield, R.D. Biochemistry of ensiling. In Silage Science and Technology; Agronomy Monographs; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 2003; Volume 42, pp. 95–139. [Google Scholar]
- Condon, M. Responses of lactic acid bacteria to oxygen. FEMS Microbiol. Rev. 1987, 46, 269–280. [Google Scholar] [CrossRef]
- Eklund, T. The antimicrobial effect of dissociated and undissociated acid at different pH levels. J. Appled Bacteriol. 1983, 54, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Noda, F.; Hayashi, K.; Mizunuma, T. Influence of pH on inhibitory activity of acetic acid on osmophilic yeasts used in brine fermentation of soy sauce. Appl. Environ. Microbiol. 1982, 43, 245–246. [Google Scholar] [CrossRef] [Green Version]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic acid increases stability of silage under aerobic conditions. Appl. Environ. Microbiol. 2003, 69, 562–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.C.; Adesogan, A.T. Influence of ensiling temperature, simulated rainfall, and delayed sealing on fermentation characteristics and aerobic stability of corn silage. J. Dairy Sci. 2006, 89, 3122–3132. [Google Scholar] [CrossRef]
- Comino, L.; Tabacco, E.; Righi, F.; Revello-Chion, A.; Quarantelli, A.; Borreani, G. Effects of an inoculant containing a Lactobacillus buchneri that produces ferulate-esterase on fermentation products, aerobic stability, and fibre digestibility of maize silage harvested at different stages of maturity. Anim. Feed. Sci. Technol. 2014, 198, 94–106. [Google Scholar] [CrossRef]
- Auerbach, H.; Weiss, K.; Theobald, P. Effects of inoculant type and composition on fermentation, aerobic stability and volatile organic compounds in grass silage. In Proceedings of the V International Symposium on Forage Quality and Conservation, Piracicaba, Brazil, 16–18 July 2017; Nussio, L.G., Sousa, D.O., Gritti, V.C., Salvati, G.G.S., Santos, W.P., Salvo, P.A.R., Eds.; ESALQ: Piracicaba, Brazil, 2017. [Google Scholar]
- Auerbach, H.; Weiss, K.; Theobald, P.; Nadeau, E. Effects of inoculant type on dry matter losses, fermentation pattern, yeast count and aerobic stability of green rye silages. In Proceedings of the 12. BOKU-Symposium Tierernährung, Vienna, Austria, 11 April 2013; Mair, C., Kraft, M., Wetscherek, W., Schedle, K., Eds.; University of Natural Resources and Life Sciences: Vienna, Austria, 2013; pp. 179–185. [Google Scholar]
- Wilkinson, J.M.; Davis, D.R. The aerobic stability of silage: Key findings and recent developments. Grass Forage Sci. 2012, 68, 1–19. [Google Scholar] [CrossRef]
- Zimmer, E. Efficient silage systems. In Forage Conservation in the 80s-Occasional Symposium; British Grassland Society Conference: Brighton, UK, 1980; pp. 176–194. [Google Scholar]
- Brüning, D.; Gerlach, K.; Weiß, K.; Südekum, K.-H. Effect of compaction, delayed sealing and aerobic exposure on forage choice and short-term intake of maize silage by goats. Grass Forage Sci. 2018, 73, 392–405. [Google Scholar] [CrossRef]
- Schink, B.; Zeikus, J.G. Microbial Methanol Formation: A Major End Product of Pectin Metabolism. Curr. Microbiol. 1980, 4, 387–389. [Google Scholar] [CrossRef]
- Prade, R.A.; Zhan, D.; Ayoubi, P.; Mort, A.J. Pectins, pectinases and plantmicrobe interactions. In Biotechnology and Genetic Engineering Reviews; Intercept Limited: Hampshire, UK, 1999; Volume 16, pp. 361–391. [Google Scholar]
- Sieiro, C.; Garcia-Fragna, B.; Lopez-Seijas, J. Microbial pectic enzymes in the food and wine industry. In Food industrial Processes—Methods and Equipment; Valdez, B., Ed.; Intech: London, UK, 2012; pp. 201–218. [Google Scholar]
- Ohimain, E.I. Methanol contamination in traditionally fermented alcoholic beverages: The microbial dimension. SpringerPlus 2016, 5, 1607. [Google Scholar] [CrossRef] [Green Version]
- Bilska-Kos, A.; Solecka, D.; Dziewulska, A.; Ochodzki, P.; Jończyk, M.; Bilski, H.; Sowiński, P. Low temperature caused modifications in the arrangement of cell wall pectins due to changes of osmotic potential of cells of maize leaves (Zea mays L.). Protoplasma 2017, 254, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Hafner, S.D.; Howard, C.R.; Muck, R.E.; Franco, B.; Montes, F.; Green, P.G.; Mitloehner, F.; Trabue, S.L.; Rotz, C.A. Emission of volatile organic compounds from silage: Compounds, sources, and implications. Atmos. Environ. 2013, 77, 827–839. [Google Scholar] [CrossRef]
- Weiss, K. Volatile organic compounds in silages–effects of management factors on their formation: A review. Slovak J. Anim. Sci. 2017, 50, 55–67. [Google Scholar]
- Gerlach, K.; Weiß, K.; Südekum, K.H. Effects of ethyl ester supplementation to forage on short-term dry matter intake and preference by goats. Arch. Anim. Nutr. 2019, 73, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Daniel JL, P.; Weiss, K.; Custódio, L.; Sá Neto, A.; Santos, M.C.; Zopollatto, M.; Nussio, L.G. Occurrence of volatile organic compounds in sugarcane silage. Anim. Feed Sci. Technol. 2013, 185, 101–105. [Google Scholar] [CrossRef]
- Raun, B.M.L.; Kristensen, N.B. Metabolic effects of feeding ethanol or propanol to postpartum transition Holstein cows. J. Dairy Sci. 2011, 94, 2566–2580. [Google Scholar] [CrossRef] [Green Version]
- Raun, B.M.L.; Kristensen, N.B. Propanol in maize silage at Danish dairy farms. Acta Agric. Scand Sect. A 2010, 60, 53–59. [Google Scholar] [CrossRef]
- Weiss, K.; Kroschewski, B.; Auerbach, H. Effects of air exposure, temperature and additives on fermentation characteristics, yeast count, aerobic stability and volatile organic compounds in corn silage. J. Dairy Sci. 2016, 99, 8053–8069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auerbach, H.; Weiss, K. The effect of different types of silage additives on dry matter losses, fermentation pattern, volatile organic compounds and aerobic stability of sorghum silage. In Proceedings of the XVI International Silage Conference, Hämeenlinna, Finland, 2–4 July 2012; Kuoppala, K., Rinne, M., Vanhatalo, A., Eds.; MTT Agrifood Research Finland, Universiy of Helsinki: Helsinki, Finland, 2012; pp. 418–419. [Google Scholar]
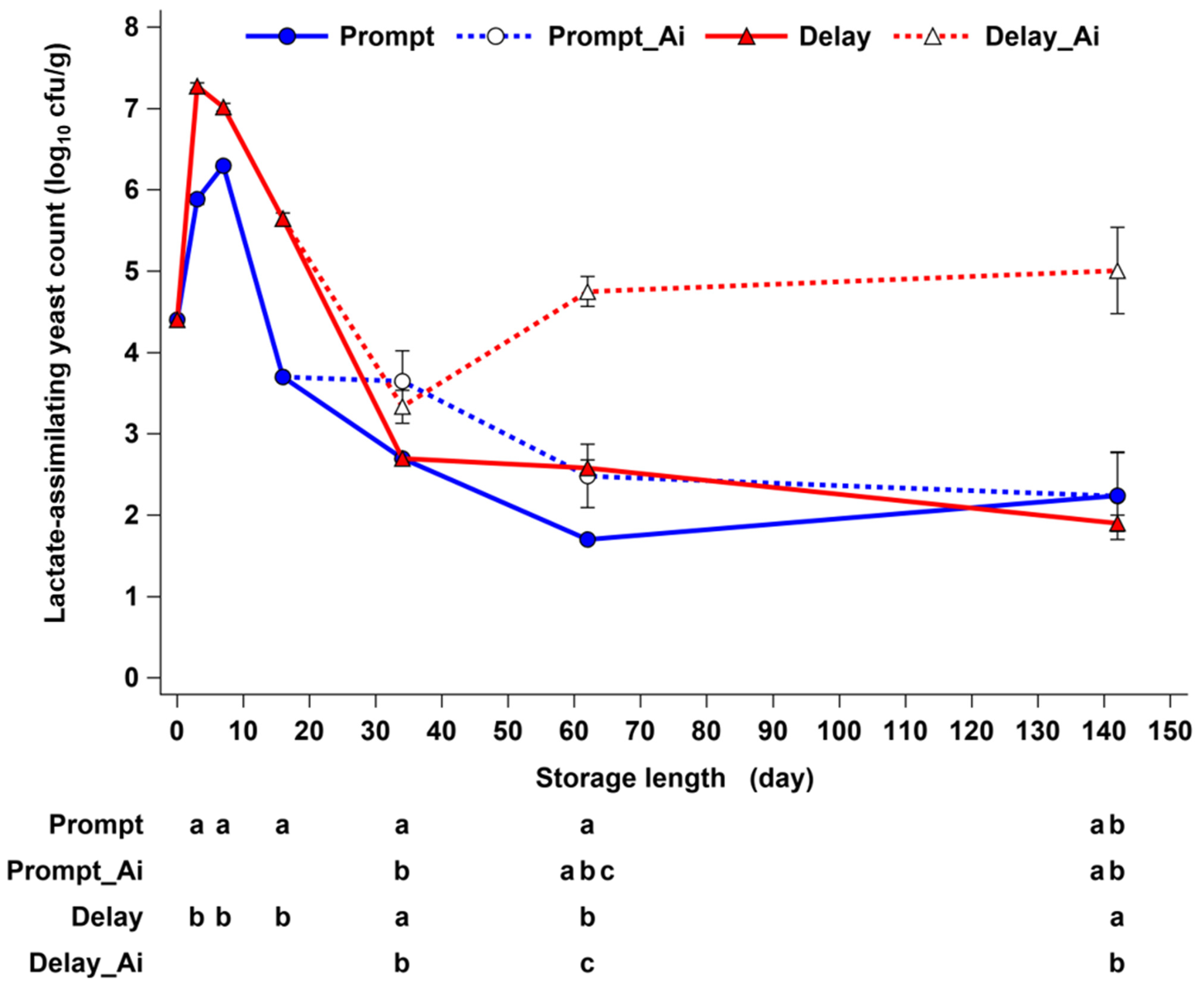
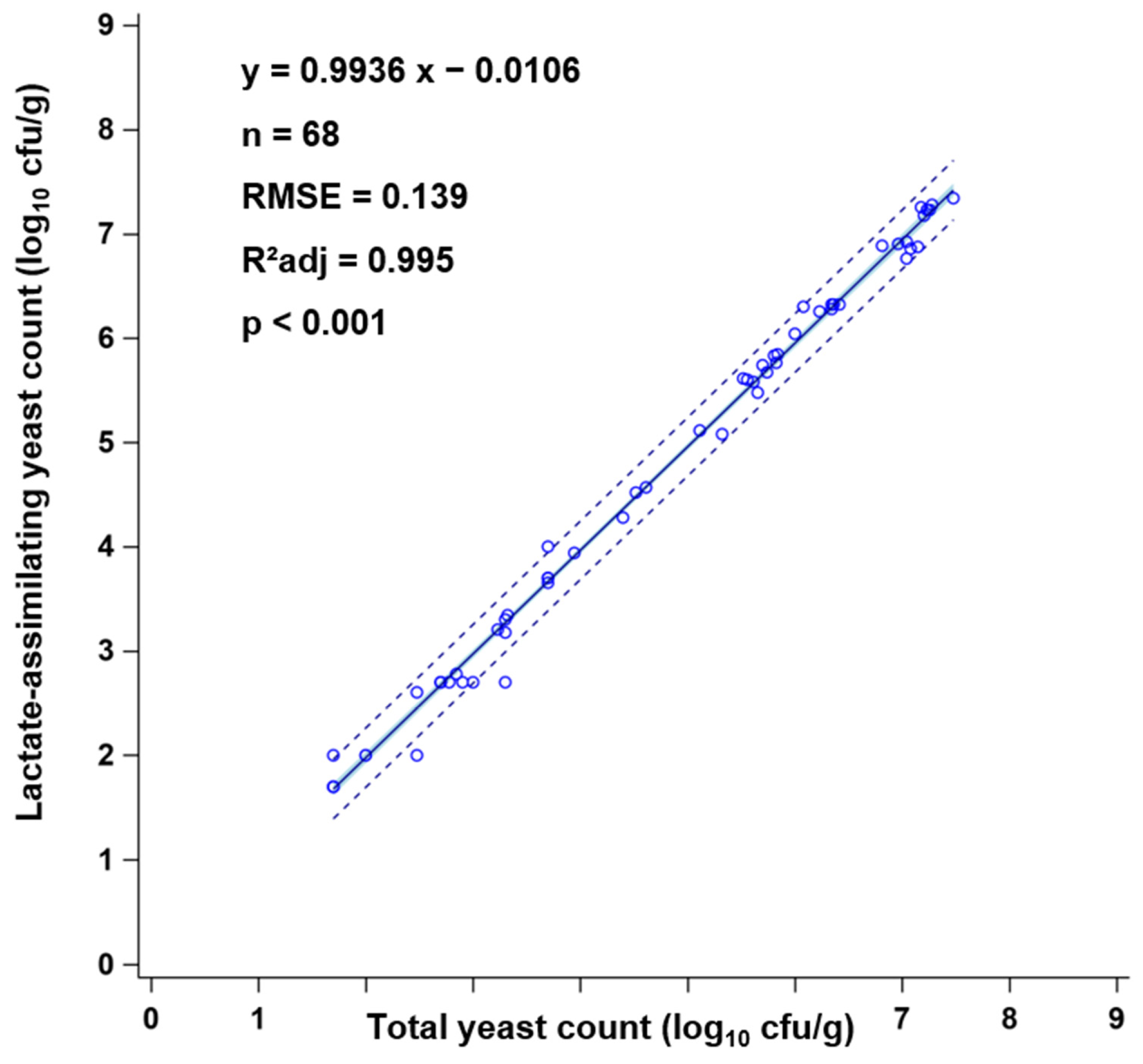

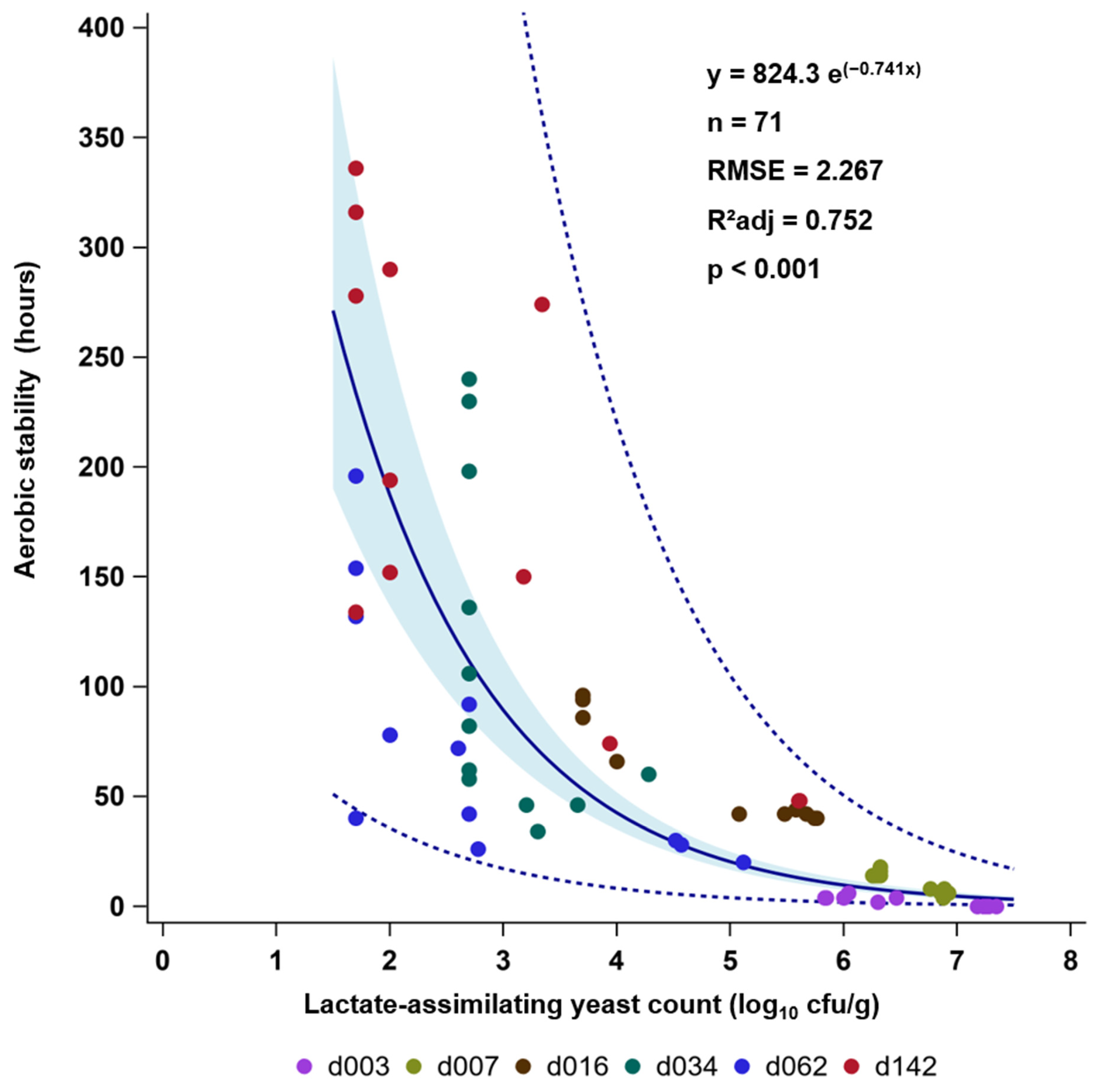
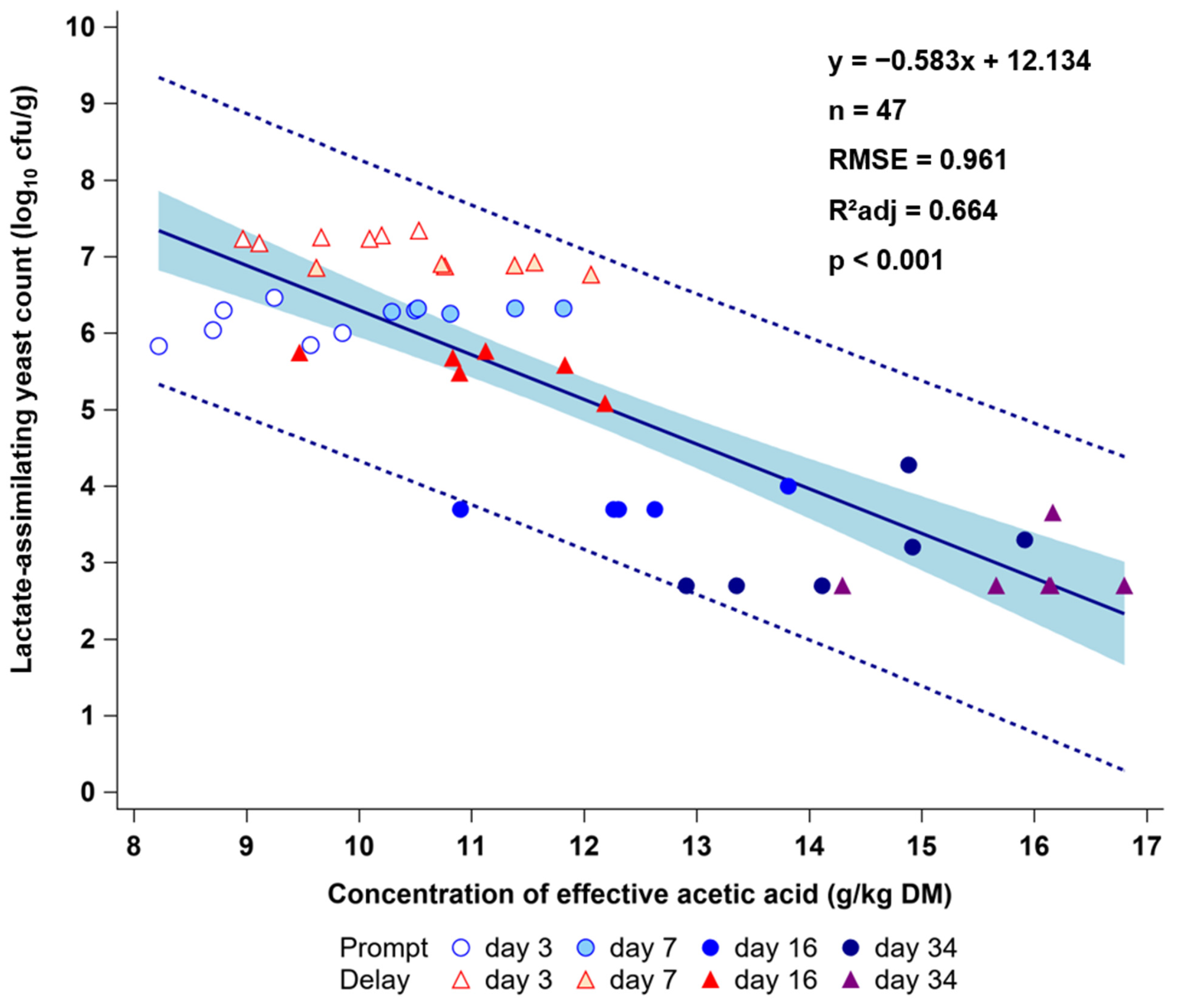
| Parameter | n | Mean | Range |
|---|---|---|---|
| Dry matter (g/kg) | 3 | 26.8 | 26.6–27.0 |
| Crude protein | 2 | 79.0 | 77.7–80.3 |
| Ether extract | 2 | 25.6 | 24.7–26.4 |
| Crude fibre | 2 | 213 | 21.2–21.4 |
| Crude ash | 2 | 45.9 | 45.4–46.5 |
| NDFom * | 2 | 453 | 44.9–45.7 |
| ADFom # | 2 | 241 | 24.1–24.1 |
| ADL + | 2 | 31.6 | 31.4–31.9 |
| Water-soluble carbohydrates | 2 | 158 | 157–160 |
| Buffering capacity † | 2 | 27.4 | 26.6–28.2 |
| Nitrate | 2 | 1.6 | 1.6–1.6 |
| Total yeast count (log10 cfu/g) | 6 | 4.4 | 4.3–4.5 |
| Mould count (log10 cfu/g) | 6 | 5.5 | 5.3–5.8 |
| Storage Length | ST | Ai | pH | Lactate | Acetate | Ethanol | WSC | DM Loss |
|---|---|---|---|---|---|---|---|---|
| Length (Days) | (g/kg DM) | (g/kg DM) | (g/kg DM) | (g/kg DM) | (%) | |||
| 3 | Prompt | - | 4.02 a | 13.9 b | 10.7 a | 6.8 a | 48.9 b | 3.6 a |
| Delay | - | 4.22 b | 11.0 a | 12.6 b | 19.7 b | 13.4 a | 10.5 b | |
| SEM | 0.01 | 0.41 | 0.29 | 0.59 | 0.57 | 0.06 | ||
| Effects † | ST | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 7 | Prompt | - | 3.79 a | 24.2 b | 12.1 a | 17.9 a | 11.7 b | 5.0 a |
| Delay | - | 4.05 b | 19.5 a | 13.2 b | 20.5 b | 7.5 a | 10.8 b | |
| SEM | 0.01 | 0.48 | 0.34 | 0.50 | 0.21 | 0.11 | ||
| Effects † | ST | <0.001 | <0.001 | 0.042 | 0.004 | <0.001 | <0.001 | |
| 16 | Prompt | - | 3.77 a | 27.5 a | 13.7 a | 19.4 a | 7.7 b | 5.3 a |
| Delay | - | 3.92 b | 26.2 a | 12.7 a | 21.1 a | 6.0 a | 11.2 b | |
| SEM | 0.01; 0.01 | 0.87; 0.79 | 0.53; 0.48 | 0.72; 0.66 | 0.39; 0.35 | 0.09; 0.08 | ||
| Effects † | ST | <0.001 | 0.286 | 0.203 | 0.113 | 0.009 | <0.001 | |
| 34 | Prompt | - | 3.76 a | 31.6 a | 14.8 a | 20.4 a | 6.8 a | 5.3 a |
| + | 3.76 a | 28.8 a | 16.8 b | 21.1 a | 6.5 a | 5.8 b | ||
| Delay | - | 3.89 c | 31.4 a | 17.4 b | 27.9 b | 6.0 a | 11.6 c | |
| + | 3.83 b | 30.9 a | 18.3 b | 29.0 b | 6.3 a | 12.1 d | ||
| SEM | 0.004 | 0.86 | 0.43 | 0.68 | 0.25 | 0.07 | ||
| Effects † | ST | <0.001 | 0.299 | 0.001 | <0.001 | 0.076 | <0.001 | |
| Ai | <0.001 | 0.092 | 0.011 | 0.205 | 0.994 | <0.001 | ||
| ST × Ai | <0.001 | 0.211 | 0.231 | 0.800 | 0.254 | 0.991 | ||
| 62 | Prompt | - | 3.74 a | 34.0 b | 16.9 a | 20.7 a | 6.7 a | 5.4 a |
| + | 3.74 a | 28.7 a | 18.5 a | 21.5 a | 6.8 a | 6.0 b | ||
| Delay | - | 3.83 c | 33.6 b | 20.9 b | 27.3 b | 6.1 a | 11.8 c | |
| + | 3.79 b | 31.9 ab | 22.0 b | 26.7 b | 6.8 a | 12.2 d | ||
| SEM | 0.002 | 1.04 | 0.42 | 0.69 | 0.16 | 0.07 | ||
| Effects † | ST | <0.001 | 0.205 | <0.001 | <0.001 | 0.138 | <0.001 | |
| Ai | <0.001 | 0.010 | 0.014 | 0.895 | 0.028 | <0.001 | ||
| ST × Ai | <0.001 | 0.114 | 0.610 | 0.330 | 0.147 | 0.129 | ||
| 142 | Prompt | - | 3.86 a | 34.8 b | 25.2 a | 20.8 a | 6.6 ab | 6.0 a |
| + | 3.87 a | 28.0 a | 28.4 a | 21.0 a | 7.5 b | 6.9 a | ||
| Delay | - | 3.88 a | 33.4 b | 27.9 a | 32.3 b | 5.8 a | 11.9 b | |
| + | 3.88 a | 31.8 ab | 28.7 a | 31.6 b | 6.2 a | 12.8 b | ||
| SEM | 0.01 | 1.03 | 1.14 | 0.85 | 0.22 | 0.21 | ||
| Effects † | ST | 0.120 | 0.293 | 0.233 | <0.001 | 0.002 | <0.001 | |
| Ai | 0.737 | 0.003 | 0.113 | 0.782 | 0.017 | 0.003 | ||
| ST × Ai | 0.737 | 0.037 | 0.349 | 0.601 | 0.236 | 0.958 | ||
| Storage | ST | Ai | Methanol | n-Propanol | 2-Butanol | Ethyl Acetate | Ethyl Lactate |
|---|---|---|---|---|---|---|---|
| Length (Days) | (mg/kg DM) | ||||||
| 3 | Prompt | - | 141 a | 0 a | 0 a | 736 a | 27 a |
| Delay | - | 107 a | 4 a | 0 a | 2850 b | 76 b | |
| SEM | 24.5 | 0;4.3 | 0 | 126.9 | 11.6 | ||
| Effects † | ST | 0.353 | 0.363 | <0.001 | 0.013 | ||
| 7 | Prompt | - | 107 a | 0 a | 0 a | 815 a | 157 a |
| Delay | - | 96 a | 0 a | 0 a | 2689 b | 217 b | |
| SEM | 17.6 | 0 | 0 | 108.6 | 10.4 | ||
| Effects † | ST | 0.666 | <0.001 | 0.002 | |||
| 16 | Prompt | - | 165 b | 0 a | 0 a | 1520 a | 217 a |
| Delay | - | 93 a | 8 a | 151 b | 3115 b | 289 b | |
| SEM | 23.3; 21.2 | 0;8.0 | 0; 32.9 | 266.8; 243.6 | 10.0; 9.1 | ||
| Effects † | ST | 0.048 | 0.363 | <0.001 | 0.002 | <0.001 | |
| 34 | Prompt | - | 170 a | 0 a | 0 a | 1127 a | 242 a |
| + | 240 a | 0 a | 0 a | 1344 a | 246 a | ||
| Delay | - | 219 a | 0 a | 320 b | 4556 b | 405 b | |
| + | 267 a | 0 a | 125 ab | 3895 b | 406 b | ||
| SEM | 41.4 | 0 | 0–15.3 | 161.2 | 5.6 | ||
| Effects † | ST | 0.387 | <0.001 | <0.001 | <0.001 | ||
| Ai | 0.192 | 0.021 | 0.205 | 0.6850.990 | |||
| ST × Ai | 0.796 | 0.021 | 0.026 | 0.803 | |||
| 62 | Prompt | - | 222 a | 0 a | 0 a | 732 a | 275 a |
| + | 269 b | 12 ab | 0 a | 764 a | 284 a | ||
| Delay | - | 277 b | 159 b | 352 b | 3750 c | 401 b | |
| + | 266 b | 0 a | 144 ab | 2678 b | 414 b | ||
| SEM | 9.1 | 0–12.4 | 0–25.1 | 181.0 | 12.8 | ||
| Effects † | ST | 0.021 | 0.065 | <0.001 | <0.001 | <0.001 | |
| Ai | 0.084 | 0.065 | 0.021 | 0.021 | 0.422 | ||
| ST × Ai | 0.013 | 0.022 | 0.021 | 0.016 | 0.904 | ||
| 142 | Prompt | - | 245 a | 1889 a | 24 a | 268 a | 334 a |
| + | 240 a | 2321 a | 33 ab | 284 a | 358 a | ||
| Delay | - | 369 b | 835 a | 362 b | 1507 b | 508 b | |
| + | 358 ab | 868 a | 231 b | 1374 b | 503 b | ||
| SEM | 37.6 | 30.2–1026.9 | 4.3–39.2 | 88.3 | 24.9 | ||
| Effects † | ST | 0.012 | 0.430 | 0.001 | <0.001 | <0.001 | |
| Ai | 0.838 | 0.786 | 0.387 | 0.524 | 0.713 | ||
| ST × Ai | 0.938 | 0.892 | 0.059 | 0.423 | 0.576 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiß, K.; Kroschewski, B.; Auerbach, H.U. The Influence of Delayed Sealing and Repeated Air Ingress during the Storage of Maize Silage on Fermentation Patterns, Yeast Development and Aerobic Stability. Fermentation 2022, 8, 48. https://doi.org/10.3390/fermentation8020048
Weiß K, Kroschewski B, Auerbach HU. The Influence of Delayed Sealing and Repeated Air Ingress during the Storage of Maize Silage on Fermentation Patterns, Yeast Development and Aerobic Stability. Fermentation. 2022; 8(2):48. https://doi.org/10.3390/fermentation8020048
Chicago/Turabian StyleWeiß, Kirsten, Bärbel Kroschewski, and Horst Uwe Auerbach. 2022. "The Influence of Delayed Sealing and Repeated Air Ingress during the Storage of Maize Silage on Fermentation Patterns, Yeast Development and Aerobic Stability" Fermentation 8, no. 2: 48. https://doi.org/10.3390/fermentation8020048
APA StyleWeiß, K., Kroschewski, B., & Auerbach, H. U. (2022). The Influence of Delayed Sealing and Repeated Air Ingress during the Storage of Maize Silage on Fermentation Patterns, Yeast Development and Aerobic Stability. Fermentation, 8(2), 48. https://doi.org/10.3390/fermentation8020048






