Valorization of Fruit Peels into Biovanillin and Statistical Optimization of Process Using Enterobacter hormaechei through Solid-State Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection of Substrates
2.3. Estimation of Theferulic Acid Content of Different Fruit Peels
2.4. Microorganism Maintenance and Inoculum Preparation
2.5. Selection of Best Substrate for Biovanillin Production through Solid-State Fermentation
2.6. Optimization of the Fermentation Process for Maximum Production of Biovanillin
2.7. Extraction of Biovanillin and Quantitative Estimation
2.8. Crystallization of Biovanilin from Total Fermented Media
2.9. Identification and Quantitative Assessment of Crystallized Vanillin
3. Results and Discussion
3.1. Estimation of Nutritional Potential and Ferulic Acid Extracted from Fruit Peels
3.2. Screening of Best Substrate for Biovanillin Production
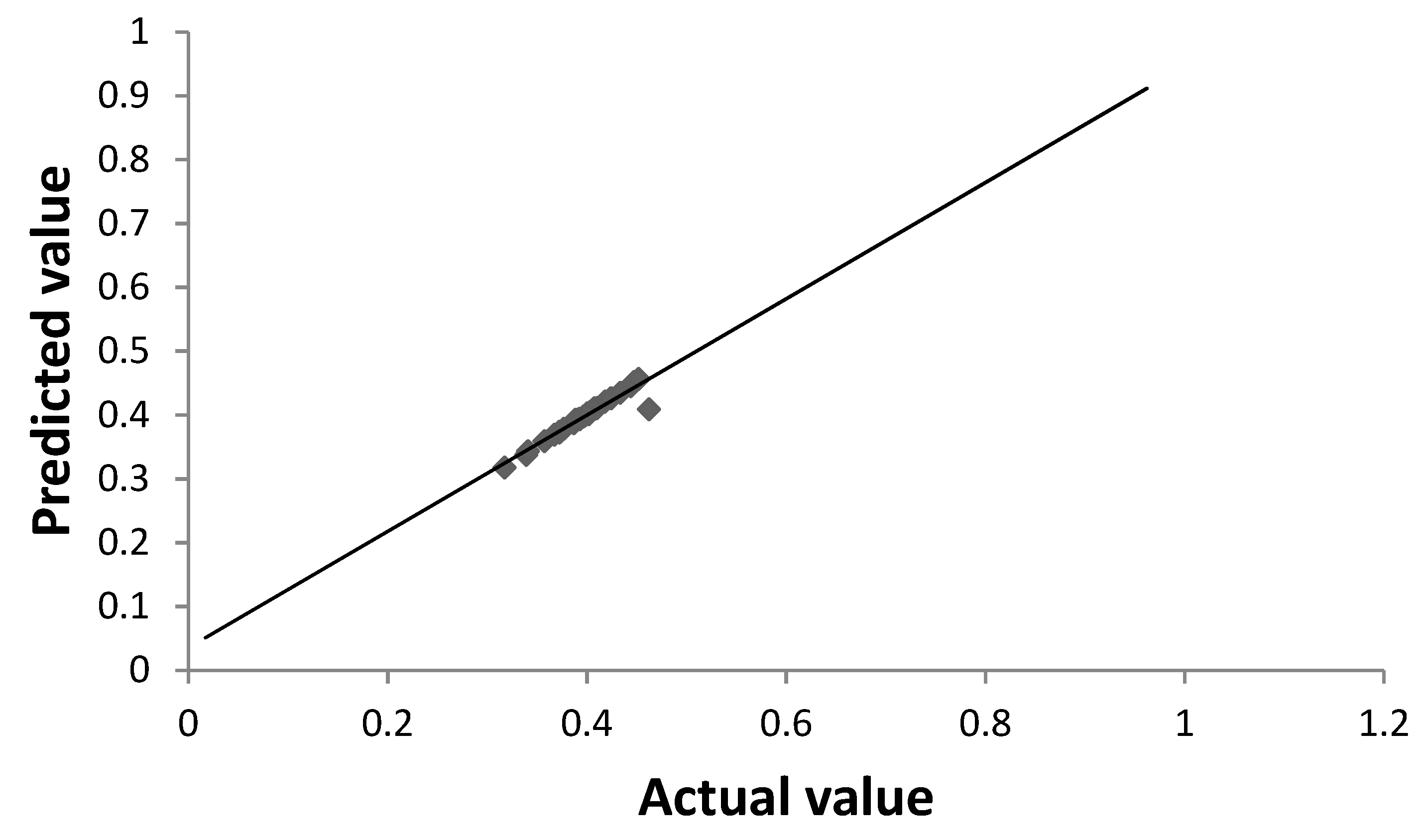
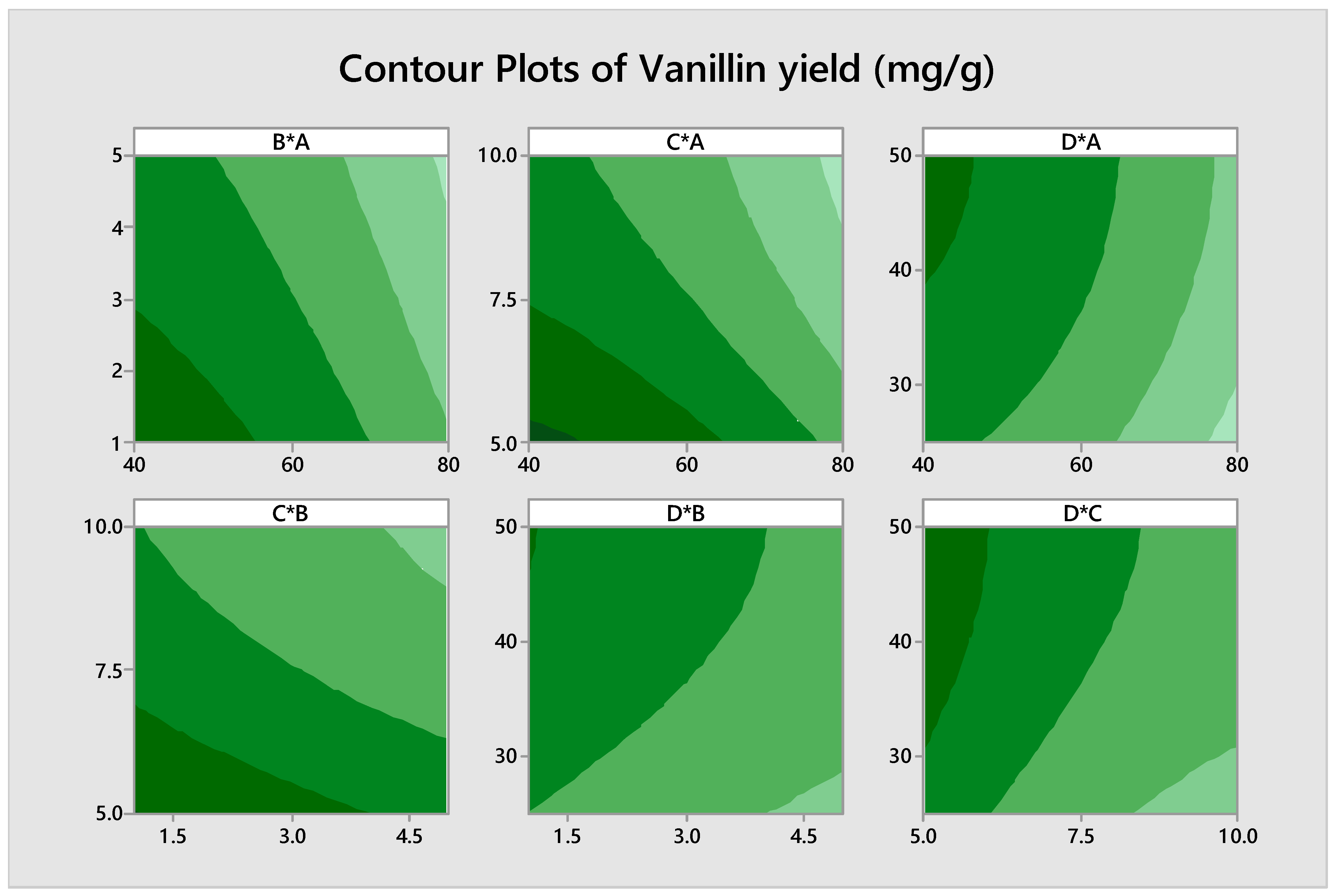
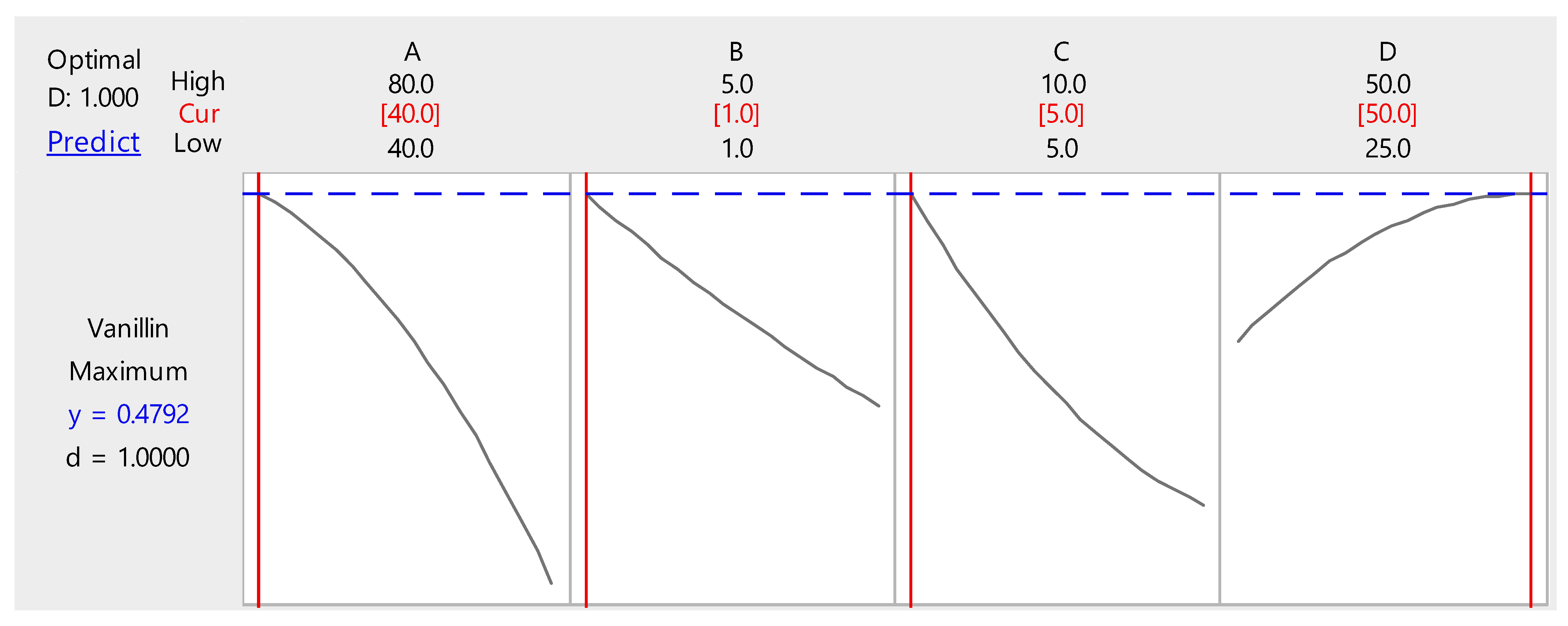
3.3. Optimization of Biovanillin Production Process by RSM
+ 0.00039 B∗B + 0.00119 C∗C − 0.000037 D∗D − 0.000005 A∗B − 0.000000 A∗C
+ 0.000004 A∗D + 0.00006 B∗C − 0.000019 B∗D + 0.000022 C∗D
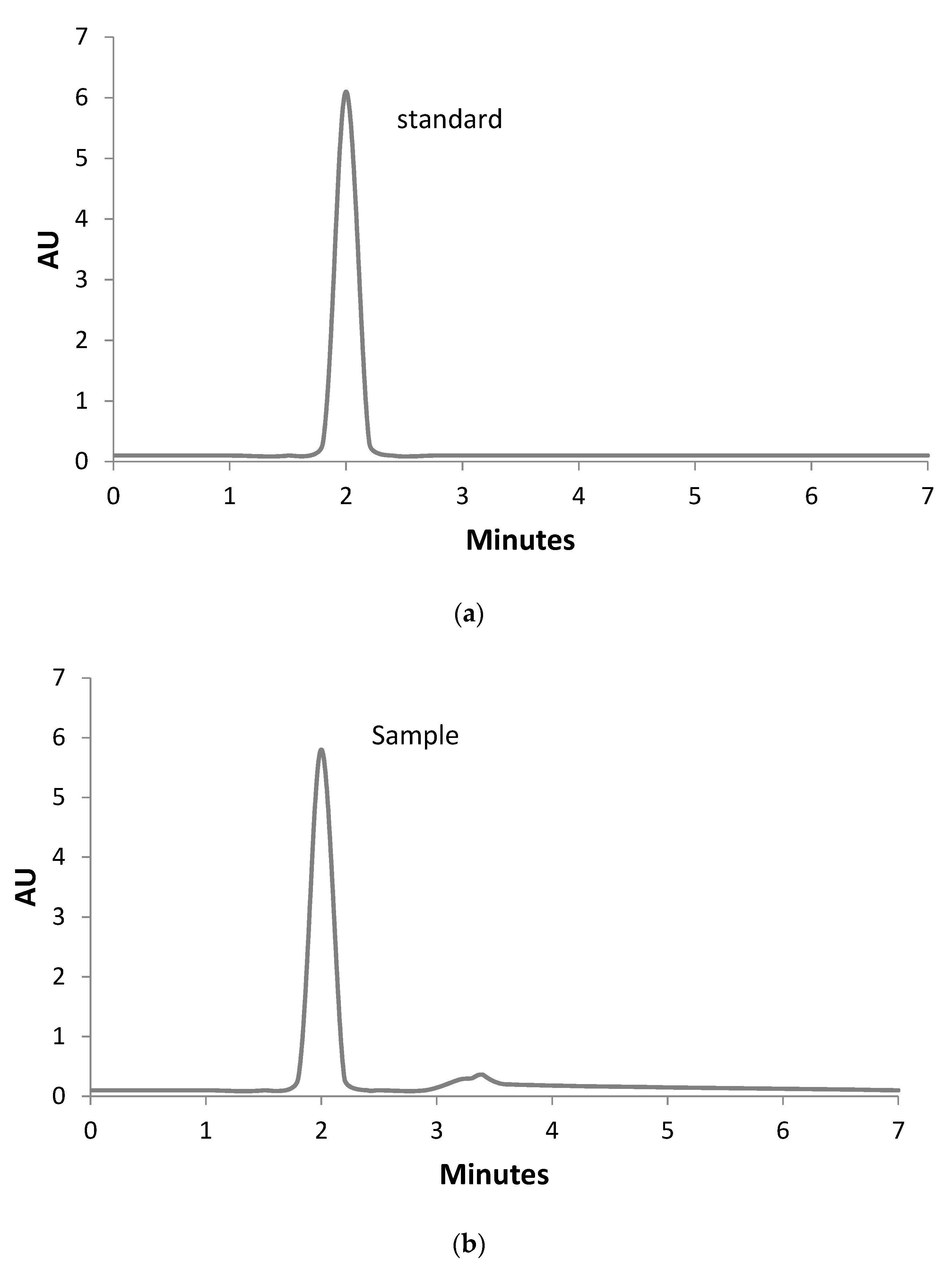
3.4. Identification and Quantitative Assessment of Biovanillin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramachandra Rao, S.; Ravishankar, G.A. Vanilla flavour: Production by conventional and biotechnological routes. J. Sci. Food Agric. 2000, 80, 289–304. [Google Scholar] [CrossRef]
- Sinha, A.K.; Sharma, U.K.; Sharma, N.A. Comprehensive review on vanilla flavor: Extraction, isolation and quantification of vanillin and others constituents. Int. J. Food Sci. Nutr. 2008, 59, 299–326. [Google Scholar] [CrossRef]
- Barghini, P.; Di Gioia, D.; Fava, F.; Ruzzi, M. Vanillin production using metabolically engineered Escherichia coli under non-growing conditions. Microb. Cell Factories. 2007, 6, 13. [Google Scholar] [CrossRef]
- Galadima, A.I.; Salleh, M.M.; Hussin, H.; Safri, N.M.; Noor, R.M.; Chong, C.S.; Yahya, A.; Mohamad, S.E.; Abd-Aziz, S.; Yusof, N.N.M. One-step conversion of lemongrass leaves hydrolysate to biovanillin by Phanerochaete chrysosporium ATCC 24725 in batch culture. Waste Biomass Valori. 2020, 11, 4067–4080. [Google Scholar] [CrossRef]
- Gallage, N.J.; Møller, B.L. Vanillin–bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol. Plant. 2015, 8, 40–57. [Google Scholar] [CrossRef]
- Zamzuri, N.A.; Abd-Aziz, S. Biovanillin from agro wastes as an alternative food flavour. J. Sci. Food Agric. 2013, 93, 429–438. [Google Scholar] [CrossRef]
- Converti, A.; Aliakbarian, B.; Domínguez, J.; Vázquez, G.B.; Perego, P. Microbial production of biovanillin. Braz. J. Microbiol. 2010, 41, 519–530. [Google Scholar] [CrossRef]
- Torres, B.R.; Aliakbarian, B.; Torre, P.; Perego, P.; Domínguez, J.M.; Zilli, M.; Converti, A. Vanillin bioproduction from alkaline hydrolyzate of corn cob by Escherichia coli JM109/pBB1. Enzyme Microb. Technol. 2009, 44, 154–158. [Google Scholar] [CrossRef]
- Banerjee, G.; Chattopadhyay, P. Vanillin biotechnology: The perspectives and future. J. Sci. Food Agric. 2019, 99, 499–506. [Google Scholar] [CrossRef]
- Chakraborty, D.; Kaur, B.; Obulisamy, K.; Selvam, A.; Wong, J.W. Agrowaste to vanillin conversion by a natural Pediococcus acidilactici strain BD16. Environ. Technol. 2017, 38, 1823–1834. [Google Scholar] [CrossRef]
- Baqueiro-Peña, I.; Guerrero-Beltrán, J.Á. Vanilla (Vanilla planifolia Andr.), its residues and other industrial by-products for recovering high value flavor molecules: A review. J. Appl. Res. Med. Aromat. Plants. 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Chen, P.; Yan, L.; Wu, Z.; Li, S.; Bai, Z.; Yan, X.; Wang, N.; Liang, N.; Li, H. A microbial transformation using Bacillus subtilis B7-S to produce natural vanillin from ferulic acid. Sci. Rep. 2016, 6, 20400. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, E.G.; Das, A.; Lee, S.H.; Li, C.; Ryu, H.K.; Choi, M.S.; Seo, W.T.; Kim, S.W. Enhanced vanillin production from recombinant E. coli using NTG mutagenesis and adsorbent resin. Biotechnol. Prog. 2007, 23, 1143–1148. [Google Scholar] [CrossRef]
- Galadima, A.I.; Salleh, M.M.; Hussin, H.; Shiong, C.C.; Yahaya, A.; Mohamad, S.E.; Aziz, S.A.; Yusof, N.N.M.; Al-Junid, A.F.M. Improvement of biovanillin production with two-stage pH control strategy from lemongrass leaves hydrolysates using Phanerochaete chrysosporium ATCC 24725 in batch culture. Biomass Convers. Biorefin. 2020, 1–10. [Google Scholar] [CrossRef]
- Yan, L.; Chen, P.; Zhang, S.; Li, S.; Yan, X.; Wang, N.; Liang, N.; Li, H. Biotransformation of ferulic acid to vanillin in the packed bed-stirred fermentors. Sci. Rep. 2016, 6, 34644. [Google Scholar] [CrossRef]
- Buranov, A.U.; Mazza, G. Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents. Food Chem. 2009, 115, 1542–1548. [Google Scholar] [CrossRef]
- Tilay, A.; Bule, M.; Kishenkumar, J.; Annapure, U. Preparation of ferulic acid from agricultural wastes: Its improved extraction and purification. J. Agric. Food Chem. 2008, 56, 7644–7648. [Google Scholar] [CrossRef]
- Kaur, B.; Chakraborty, D. Biotechnological and molecular approaches for vanillin production: A review. Appl. Biochem. Biotechnol. 2013, 169, 1353–1372. [Google Scholar] [CrossRef]
- Zaki, S.A.; Abdelatif, S.H.; Abdelmohsen, N.R.; Ismail, F.A. Phenolic compounds and antioxidant activities of pomegranate peels. Int. J. Food Eng. 2015, 1, 73–76. [Google Scholar] [CrossRef]
- Singh, J.P.; Kaur, A.; Shevkani, K.; Singh, N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J. Food Sci. Technol. 2016, 53, 4056–4066. [Google Scholar] [CrossRef]
- Mazhar, B.; Jahan, N.; Ali, N.M.; Andleeb, S.; Ali, S. Production of vanillin by a novel bacterium from waste residues of rice bran oil. Punjab Univ. J. Zool. 2017, 32, 137–142. [Google Scholar]
- Saeed, S.; Baig, U.U.R.; Tayyab, M.; Altaf, I.; Irfan, M.; Raza, S.Q.; Nadeem, F.; Mehmood, T. Valorization of banana peels waste into biovanillin and optimization of process parameters using submerged fermentation. Biocatal. Agric. Biotechnol. 2021, 36, 102154. [Google Scholar] [CrossRef]
- Salleh, N.H.M.; Daud, M.Z.M.; Arbain, D.; Ahmad, M.S.; Ismail, K.S.K. Optimization of alkaline hydrolysis of paddy straw for ferulic acid extraction. Ind. Crops Prod. 2011, 34, 1635–1640. [Google Scholar] [CrossRef]
- Nurika, I.; Suhartini, S.; Azizah, N.; Barker, G.C. Extraction of Vanillin Following Bioconversion of Rice Straw and Its Optimization by Response Surface Methodology. Molecules 2020, 25, 6031. [Google Scholar] [CrossRef] [PubMed]
- Aarabi, A.; Mizani, M.; Honarvar, M.; Faghihian, H.; Gerami, A. Extraction of ferulic acid from sugar beet pulp by alkaline hydrolysis and organic solvent methods. J. Food Meas. Charact. 2016, 10, 42–47. [Google Scholar] [CrossRef]
- Barberousse, H.; Roiseux, O.; Robert, C.; Paquot, M.; Deroanne, C.; Blecker, C. Analytical methodologies for quantification of ferulic acid and its oligomers. J. Sci. Food Agric. 2008, 88, 1494–1511. [Google Scholar] [CrossRef]
- Ma, X.-K.; Daugulis, A.J. Effect of bioconversion conditions on vanillin production by Amycolatopsis sp. ATCC 39116 through an analysis of competing by-product formation. Bioprocess Biosyst Eng. 2014, 37, 891–899. [Google Scholar] [CrossRef]
- Chakraborty, D.; Selvam, A.; Kaur, B.; Wong, J.W.C.; Karthikeyan, O.P. Application of recombinant Pediococcus acidilactici BD16 (fcs+/ech+) for bioconversion of agrowaste to vanillin. Appl. Microbiol. Biotechnol. 2017, 101, 5615–5626. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Bioconversions of ferulic acid, an hydroxycinnamic acid. Crit. Rev. Microbiol. 2006, 32, 115–125. [Google Scholar] [CrossRef]
- Gasson, M.J.; Kitamura, Y.; McLauchlan, W.R.; Narbad, A.; Parr, A.J.; Parsons, E.L.H.; Payne, J.; Rhodes, M.J.; Walton, N.J. Metabolism of ferulic acid to vanillin: A bacterial gene of the enoyl-SCoA hydratase/isomerase superfamily encodes an enzyme for the hydration and cleavage of a hydroxycinnamic acid SCoA thioester. J. Biol. Chem. 1998, 273, 4163–4170. [Google Scholar] [CrossRef]
- Bloem, A.; Bertrand, A.; Lonvaud-Funel, A.; De Revel, G. Vanillin production from simple phenols by wine-associated lactic acid bacteria. Lett. Appl. Microbiol. 2007, 44, 62–67. [Google Scholar] [CrossRef]
- Dos Santos Barbosa, E.; Perrone, D.; do Amaral Vendramini, A.L.; Leite, S.G.F. Vanillin production by Phanerochaete chrysosporium grown on green coconut agro-industrial husk in solid state fermentation. BioResources 2008, 3, 1042–1050. [Google Scholar] [CrossRef]
- Hussin, H.; Salleh, M.M.; Siong, C.C.; Naser, M.A.; Abd-Aziz, S.; Al-Junid, A.F.M. Optimization of biovanillin production of lemongrass leaves hydrolysates through Phanerochaete chrysosporium. J. Teknol. 2015, 77, 31. [Google Scholar] [CrossRef][Green Version]
- Saeed, S.; Hashmi, A.S.; Tayyab, M.; Awan, A.R.; Anjum, A.A.; Firyal, S. Hyperproduction of alginate by mutated strain of Azotobacter vinelandii through submerged fermentation. Pak. J. Zool. 2016, 48, 1585–1589. [Google Scholar]
- Saeed, S.; Tayyab, M.; Mehmood, T.; Awan, A.R.; Firyal, S.; Nadeem, F.; Irfan, M. Valorization of potato peel for production of alginate and optimization of the process through response surface methodology (RSM) by using Azotobacter nigricans. Biomass Convers. Biorefin. 2021, 1–9. [Google Scholar] [CrossRef]
- Saeed, S.; Mehmood, T.; Irfan, M. Statistical optimization of cultural parameters for the optimized production of alginic acid using apple (Malus domestica) peels through solid-state fermentation. Biomass Convers. Biorefin. 2020, 1–9. [Google Scholar] [CrossRef]
- Vermani, M.; Kelkar, S.; Kamat, M. Studies in polysaccharide production and growth of Azotobactervinelandii MTCC 2459, a plant rhizosphere isolate. Lett. Appl. Microbiol. 1997, 24, 379–383. [Google Scholar] [CrossRef]
- Rashid, J.; Samat, N.; Mohtar, W.; Yusoff, W. Optimization of temperature, moisture content and inoculum size in solid state fermentation to enhance mannanase production by Aspergillus terreus SUK-1 using RSM. Pak. J. Biol. Sci. PJBS 2011, 14, 533–539. [Google Scholar] [CrossRef]
- Nema, A.; Patnala, S.H.; Mandari, V.; Kota, S.; Devarai, S.K. Production and optimization of lipase using Aspergillus niger MTCC 872 by solid-state fermentation. Bull. Natl. Res. Cent. 2019, 43, 82. [Google Scholar] [CrossRef]


| Codes | Independent Parameters | Unit | Low-Level | High Level |
|---|---|---|---|---|
| A | Moisture content | % | 40 | 80 |
| B | Inoculum size | mL | 1 | 5 |
| C | pH | - | 5 | 10 |
| D | Temperature | °C | 25 | 50 |
| Sr. No. | A | B | C | D | Biovanillin Yield (mg/g) | Predicted Y | Residuals |
|---|---|---|---|---|---|---|---|
| 1 | 80 | 5 | 10 | 50 | 0.338 | 0.3373 | 0.0015 |
| 2 | 80 | 4 | 10 | 25 | 0.317 | 0.3175 | −0.0004 |
| 3 | 60 | 3 | 7.5 | 35 | 0.3923 | 0.3940 | −0.0017 |
| 4 | 40 | 5 | 5 | 45 | 0.443 | 0.4451 | −0.0012 |
| 5 | 80 | 5 | 5 | 25 | 0.357 | 0.3588 | −0.0016 |
| 6 | 60 | 3 | 6 | 50 | 0.424 | 0.4259 | −0.0015 |
| 7 | 60 | 1 | 7 | 30 | 0.4068 | 0.41030 | −0.0034 |
| 8 | 40 | 5 | 7.5 | 25 | 0.3964 | 0.3977 | −0.0013 |
| 9 | 80 | 1 | 5 | 25 | 0.3885 | 0.3927 | −0.0041 |
| 10 | 60 | 4 | 7.5 | 42 | 0.3927 | 0.3935 | −0.0007 |
| 11 | 80 | 1 | 10 | 25 | 0.3406 | 0.3429 | −0.0023 |
| 12 | 80 | 1 | 5 | 25 | 0.3885 | 0.3927 | −0.0041 |
| 13 | 80 | 1 | 5 | 50 | 0.4180 | 0.4209 | −0.0028 |
| 14 | 50 | 2 | 10 | 50 | 0.4097 | 0.4105 | −0.0007 |
| 15 | 40 | 5 | 10 | 25 | 0.3724 | 0.3728 | −0.0003 |
| 16 | 60 | 3 | 7 | 40 | 0.4030 | 0.4046 | −0.0016 |
| 17 | 60 | 3 | 7.5 | 50 | 0.4100 | 0.4109 | −0.0009 |
| 18 | 60 | 3 | 7.5 | 45 | 0.4041 | 0.4053 | −0.0012 |
| 19 | 70 | 3 | 7.5 | 27 | 0.3670 | 0.3691 | −0.0020 |
| 20 | 40 | 3 | 7.5 | 35 | 0.4238 | 0.4259 | −0.0020 |
| 21 | 40 | 1 | 5.5 | 25 | 0.4468 | 0.4514 | −0.0045 |
| 22 | 60 | 3 | 7.5 | 40 | 0.3982 | 0.3997 | −0.0014 |
| 23 | 40 | 5 | 10 | 50 | 0.4020 | 0.4010 | 0.0009 |
| 24 | 50 | 3 | 7.5 | 35 | 0.4080 | 0.4099 | −0.0019 |
| 25 | 60 | 3 | 8.5 | 26 | 0.3720 | 0.3739 | −0.0018 |
| 26 | 60 | 2 | 6.5 | 32 | 0.462 | 0.4090 | 0.0529 |
| 27 | 40 | 1 | 5 | 25 | 0.4516 | 0.4564 | −0.0047 |
| 28 | 80 | 5 | 5 | 50 | 0.3867 | 0.3871 | −0.0003 |
| 29 | 60 | 2 | 7.5 | 35 | 0.4001 | 0.4025 | −0.0023 |
| 30 | 40 | 1 | 10 | 50 | 0.4333 | 0.4348 | −0.0015 |
| 31 | 70 | 3 | 7.5 | 35 | 0.3765 | 0.3781 | −0.0016 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 14 | 0.030442 | 0.002174 | 12.94 | 0.000 |
| Linear | 4 | 0.020579 | 0.005145 | 30.63 | 0.000 |
| A | 1 | 0.011084 | 0.011084 | 65.98 | 0.000 |
| B | 1 | 0.003211 | 0.003211 | 19.11 | 0.000 |
| C | 1 | 0.006520 | 0.006520 | 38.81 | 0.000 |
| D | 1 | 0.002039 | 0.002039 | 12.14 | 0.003 |
| Square | 4 | 0.000265 | 0.000066 | 0.39 | 0.810 |
| A∗A | 1 | 0.000133 | 0.000133 | 0.79 | 0.387 |
| B∗B | 1 | 0.000003 | 0.000003 | 0.02 | 0.889 |
| C∗C | 1 | 0.000061 | 0.000061 | 0.36 | 0.555 |
| D∗D | 1 | 0.000075 | 0.000075 | 0.45 | 0.514 |
| 2-Way Interaction | 6 | 0.000017 | 0.000003 | 0.02 | 1.000 |
| A∗B | 1 | 0.000000 | 0.000000 | 0.00 | 0.971 |
| A∗C | 1 | 0.000000 | 0.000000 | 0.00 | 0.997 |
| A∗D | 1 | 0.000006 | 0.000006 | 0.03 | 0.859 |
| B∗C | 1 | 0.000000 | 0.000000 | 0.00 | 0.961 |
| B∗D | 1 | 0.000002 | 0.000002 | 0.01 | 0.920 |
| C∗D | 1 | 0.000002 | 0.000002 | 0.01 | 0.905 |
| Error | 16 | 0.002688 | 0.000168 | ||
| Lack-of-Fit | 15 | 0.002688 | 0.000179 | ||
| Pure Error | 1 | 0.000000 | 0.000000 | ||
| Total | 30 | 0.033130 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehmood, T.; Ahmed, S.; Waseem, R.; Saeed, S.; Ahmed, W.; Irfan, M.; Ullah, A. Valorization of Fruit Peels into Biovanillin and Statistical Optimization of Process Using Enterobacter hormaechei through Solid-State Fermentation. Fermentation 2022, 8, 40. https://doi.org/10.3390/fermentation8020040
Mehmood T, Ahmed S, Waseem R, Saeed S, Ahmed W, Irfan M, Ullah A. Valorization of Fruit Peels into Biovanillin and Statistical Optimization of Process Using Enterobacter hormaechei through Solid-State Fermentation. Fermentation. 2022; 8(2):40. https://doi.org/10.3390/fermentation8020040
Chicago/Turabian StyleMehmood, Tahir, Sibtain Ahmed, Rida Waseem, Shagufta Saeed, Waqas Ahmed, Muhammad Irfan, and Azmat Ullah. 2022. "Valorization of Fruit Peels into Biovanillin and Statistical Optimization of Process Using Enterobacter hormaechei through Solid-State Fermentation" Fermentation 8, no. 2: 40. https://doi.org/10.3390/fermentation8020040
APA StyleMehmood, T., Ahmed, S., Waseem, R., Saeed, S., Ahmed, W., Irfan, M., & Ullah, A. (2022). Valorization of Fruit Peels into Biovanillin and Statistical Optimization of Process Using Enterobacter hormaechei through Solid-State Fermentation. Fermentation, 8(2), 40. https://doi.org/10.3390/fermentation8020040








