Abstract
The consumption of plant-based dairy alternatives has increased rapidly around the world as a result of numerous positive health effects. Little information is available about the potential use of watermelon seed milk in the manufacture of yoghurt. The present study was undertaken to investigate the remedial action of yoghurt enriched with watermelon seed milk in renal injured hyperuricemic rats. A new yoghurt, substituting cow’s milk with different proportions of watermelon seed milk was prepared, followed by evaluation of its acceptability and functionality. Four different types of yoghurt were prepared from cow’s milk containing 3% fat, with different proportions of blended watermelon seed milk (0.0, 25, 50 and 75%). Sensorial traits, i.e., appearance, flavor, body and texture, and overall acceptability demonstrated that the blended treatment (50% cow’s milk and 50% watermelon seed milk.) was the most acceptable. This blend was then tested as an anti-hyperuricemia agent in rats. In this respect, twenty-four male albino rats were assigned into four groups (n = 6). The first group was solely administered a standard diet, and served as the negative control. The other rats (n = 18) received a basal diet including 20 g/kg dietary potassium oxonate in order to induce hyperuricemia. The hyperuricemic rats were then divided into three groups; the first group did not receive any treatment and served as the positive control, while the second and third groups were administered 10% cow’s milk yoghurt and 10% watermelon seed milk yoghurt, respectively. Interestingly, the results showed that the hyperuricemic group receiving a diet supplemented with 10% watermelon seed milk yoghurt was not significantly different from the negative control in the measured biological parameters, and saw a significant improvement in renal function compared to the positive control. The biologically favorable action of watermelon seed milk yoghurt could be attributed to its potential promotion of antioxidant status via enhancement of the activities of superoxide dismutase, catalase, and glutathione transferase. Collectively, this study concluded that watermelon seed milk can be used in yoghurt manufacturing in proportions of up to 50%, and may improve kidney function as an anti-hyperuricemic agent.
Keywords:
yoghurt; watermelon seeds; liver function; kidney functions; hyperuricemia; kidney tissues 1. Introduction
Hyperuricemia is considered one of the most common metabolic diseases all over the world. Being associated with elevated blood levels of uric acid [1,2], it may lead to health problems such as gout (accumulation of uric acid crystals in the bones of the joints and toes), kidney stones, and kidney failure [3]. Uric acid is the end product of metabolized purine, generally synthesized within all body cells. Externally, food containing nitrogen compounds are a significant outsource of purines, potentially promoting the accumulation of uric acid in tissues and probably forming crystals. These crystals may lead to tissue damage and severe inflammation known as articular cartilage ulceration osteophytes and erosive lesions [4,5]. The reduction of uric acid levels in hyperuricemic rats may occur via the synergistic effect of both xanthine oxidase inhibition and uricosuric activities [6].
It is important to state that inflammation occurs as a result of excessive production of reactive oxygen species (ROS), which leads to tissue injury. Interestingly, the antioxidant activity of polyphenols depends on their functional groups. Plant-based dairy alternatives contain bioactive compounds, phenolic compounds, unsaturated fatty acids and dietary fiber, making them an excellent choice for people with chronic diseases. Taking this into account, the antioxidant activity of polyphenol depends on their functional groups; the number of hydroxyl groups greatly influences metal ion chelation ability and scavenging of radicals, confirming the hypothesis that the antioxidant activity of polyphenols is related to their capacity to scavenge a wide range of ROS [7]. As a result of the multiple health benefits of nutritional plants and their potential clinical applications, the pharmaceutical and nutritional sciences have witnessed a flourishing of scientific literature related to the potential use of these plants as novel medicinal remedies thanks to the presence of pharmacologically active compounds [8,9]. The use of fruit waste (e.g., fruit or vegetable peels) as functional food ingredients has recently gained growing scientific and technological interest due to their significant levels of dietary fiber, bioactive compounds and natural bio-antioxidants as possible substitutes of synthetic antioxidants [7,10,11,12]. Moreover, food waste is available in ample amounts as a byproducts of the manufacture of agricultural commodities [13]. Plant seed proteins generally have many biological activities in their native and chemically modified forms, including antibacterial, antifungal and antiviral actions [14,15,16,17,18,19], and their use in food systems may increase nutritional and biological added value. Watermelon seeds (Citrullus lanatus) are a valuable non-conventional food waste stuff with reportedly valuable antimicrobial, antihypertensive, antioxidative, anticancer and cardio-protective effects. These byproducts of watermelon consumption remain underutilized despite the fact that they show good functional and nutritional properties due to their high content of several nutrients, supporting their use as a dietary supplement or a food additive [20]. Additionally, melon seeds are rich in essential amino acids such as arginine, methionine, tryptophan, vitamins, such as B1, B2 and minerals such as calcium, magnesium, potassium, iron, zinc, sulfur, and phosphorous [21,22]. They contain moderate quantities of minerals and proteins in addition to a high content of lycopene, which has considerable nutritional and health benefits [23]. Watermelon seed oil contains 28.10% total saturated fatty acids (SFA) against 71.9% unsaturated fatty acids, which in turn comprises 14.50% total monounsaturated fatty acids (MUSFA) and 57.40% polyunsaturated fatty acids (PUSFA). Linoleic acid (C18:2) is the predominant fatty acid in watermelon seed oil, and represents 56.90% of the total fatty acids [24]. Increasing polyunsaturated fatty acids reduces oxidative stress and blood cholesterol levels, and prevents coronary heart disease [25]. These nutritional sources can be incorporated into popular food systems, e.g., yoghurt. Yoghurt is considered the most popular fermented dairy product consumed worldwide [26]. It is obtained by fermenting milk with lactic acid bacteria, and is preferred by consumers due to its effective health-improving and immune-boosting effects [27]. Reviewing the available literature, little is known about the potential benefits of adding watermelon seeds to the yoghurt manufacturing process. The present study aimed to investigate the impact on the manufacturing process and on the functional properties of yoghurt of replacing different proportions of cow’s milk with a vegetable milk manufactured from watermelon seeds. Furthermore, the yoghurt manufactured with a high proportion of the vegetable milk was tested for its potential biological action in Albino rats to assess possible use as a functional food in the treatment of hyperuricemia.
2. Materials and Methods
2.1. Materials and Reagents
Watermelon seeds were obtained from a local market at Zagazig, Egypt. The study employed fresh standardized cow’s milk (3% fat) obtained from the Dairy Technology Unit, Food Science Department, Faculty Agriculture, Zagazig University (Egypt). Yoghurt cultures containing Streptococcus salivarius subsp. thermophilus EMCC104 and Lactobacillus delbruekii subsp. bulgaricus EMCC1102 were obtained from the Microbiological Resources Center (MIRCEN), Faculty of Agriculture, Ain Shams University, Egypt. Gallic acid, potassium oxonate and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma (St. Louis, MO, USA). The kits utilized for the biochemical analyses were purchased from Gamma Trade Company, Cairo Egypt. The basal pellet diet consisting of 10% protein, 15% casein, 5% cellulose, 10% fat, and 65% corn starch was obtained from the central animal house of the National Research Center, Dokki, Giza, Egypt. Salt and vitamin mixtures were added at a 4% and 1% ratio, respectively, following AOAC [28]. Water was available ad libitum. Male adult albino rats of the Sprague Dawely strain with a weight of 120 ± 10 g were obtained from the Agricultural Reached Center, Giza, Egypt.
2.2. Preparation of Watermelon Seed Milk (WMS-Milk)
WMS-milk was prepared according to the procedures of Chakrabarti and Gangopadhyay [29]. Seeds (100 gm) were cleaned, dehulled, and soaked in water containing 1% sodium hydroxide at a 1:4 w/v ratio overnight at room temperature. The soaked seeds were blanched in boiling water for 15 min and recovered from the water, then combining with 400 mL water and blended in a grinder. The resulting emulsion was filtered through a double-layer muslin cloth and boiled for 5 min with constant stirring.
2.3. Determination of Xanthine Oxidase Inhibitory Activity
The xanthine oxidase (XO) inhibitory effect of watermelon seed powder was spectrophotometrically assessed at 290 nm as per Yumita et al. [30] and Sunarni et al. [31], with minor modifications. Formation of uric acid was measured at 295 nm using the UV spectrometer; then, the activity of Xanthine oxidase was determined. The assay mixture contained 300 μL of phosphate buffer (pH 7.5), 100 μL of sample extracts (concentration range 50, 100, 150 and 200 μg/mL) prepared in 1% DMSO, 100 μL XO enzyme solution (0.2 units/mL in phosphate buffer, pH 7.5 at 25 °C), and 100 μL of distilled water. All instances of the solution were freshly prepared. After 15 min of pre-incubation at 37 °C, the reaction was started by adding 200 μL of xanthine substrate solution (0.15 mM) to the mixture. The mixture was incubated at 37 °C for 30 min. before the reaction was stopped by addition of 200 μL of 0.5 M hydrochloric acid. Absorbance was taken at 295 nm, and the xanthine oxidase activity of the assayed samples was expressed as percentage inhibition of XO. Allopurinol was used as a positive control and a blank was prepared in the same way. The inhibition percentage was calculated using the following formula:
where α is XO activity without extract and β is XO activity with extract; the results were expressed in μg/mL. Based on the value inhibition percentage at various concentrations, the IC50 values were determined.
% XO inhibition = (1 − β/α) × 100 (3)
2.4. Determination of Total Phenolic Compounds (TPC)
The total phenolic content (TPC) of the extract of WMS was determined by Folin–Ciocalteu assay using Gallic acid as the standard according to Kaur and Kapoor [32], with minor modifications. Briefly, 100 μL of different concentrations of a test sample was mixed with 1 mL of diluted FC reagent (1:10). After 10 min, 1 mL of 7.5% (w/v) sodium carbonate solution was added to the mixture and incubated in the dark for 90 min. The absorbance was recorded at 725 nm. The phenolic content was calculated from the calibration curve and expressed as gallic acid equivalents (µ GAE/100 g y).
2.5. Determination of Radical Scavenging Activity (RSA)
The method developed by Brand-Williams et al. [33] was used for the measurement of DPPH radical scavenging activity. The absorbance of DPPH purple-colored solution at 517 nm was measured using a spectrophotometer (Thermo Scientific, Wilmington, NC, USA). The scavenging activity was calculated by the following formula:
Scavenging activity (%) = 1 − (Abs sample − Abs blank)/Abs control × 100
2.6. HPLC Identification of Phenolic Acids
HPLC analysis was performed using an Agilent 1200 chromatograph (Agilent, Santa Clara, CA, USA), and chromatographic separations were performed on a LUNA C18 column (5 μm × 250 mm × 4.6 mm, Phenomenex, Torrance, CA, USA). The composition of solvents and gradient elution conditions as described by Mariod et al. [34].
2.7. Yoghurt Manufacture
Yoghurt samples were prepared from standardized cow’s milk (3% fat) as replaced by 0.0, 25, 50, and 75% of WMS-milk and coded as C, T1, T2, and T3, respectively. All milk samples were heated at 85 °C for 10 min and then cooled to 42 ± 1 °C before adding the yoghurt starter culture at a rate of 3%, then incubated at 42 ± 1 °C until obtaining uniform coagulation according to the methods described by Tamime and Robinson [35], with minor modifications. The developed yoghurts were cooled overnight at 5 ± 1 °C and evaluated by the following analyses.
2.8. Methods of Analysis
The total solids, fats, and proteins, crude fiber, ash content, and titratable acidity of the yoghurt samples were analyzed based on the methods of the Association of Official Analytical Chemists [28]. The changes in pH in the yoghurt samples during storage were measured using a laboratory pH meter with a glass electrode (HANNA, Instrument, Portugal).
2.9. Sensory Evaluation
The sensory properties of yoghurt samples were assessed by ten panelists from the Dairy Science Department, Faculty of Agriculture, Zagazig University, for evaluation of color and appearance, flavor, body and texture, consistency, and overall acceptability (nine points in all) as described by Nelson and Trout (1981) [36]. All of the trained panelists were experts in food technology and were subjected to two training sessions in order to study and discuss the various tested sensory descriptors, including the changes in color and appearance, flavor, body and texture, consistency, and overall acceptability of the yoghurt samples. The panelists were instructed to wash their mouths with low sodium spring water (Dasani water) during the sensory evaluation session, and they were encouraged to write down any criticisms on the tested products. Plain and treated yoghurt samples were presented in plastic cups coded with three-digit random codes. Each cup contained 100 mL of yoghurt samples freshly removed from the refrigerator. The sensory evaluation of the different descriptors relied on the pre-selected descriptors: color and appearance (wheying-off, white color, reddish color), consistency (ropy, uniform coagulum), body and texture (absence of curd homogeneity, lumps, bubbles), taste and flavor (sweetness, acidity, bitterness), and overall acceptability (the sum of all the character results). The sensory evaluation was conducted using a comparative test with fresh yoghurt as a reference sample. The data were collected in specially designed ballots.
2.10. The Biological Assay
This study was conducted with the approval of the Faculty of Agriculture, Zagazig, University and the institutional Review Board Number ZU-IACU/3/F/172/2021. After acclimation on a basal diet for seven days, Albino rats were classified into two main sections, with the first (n = 6) receiving only the standard diet and serving as the normal control group while the second (hyperuricemic rats, n = 18) received a basal diet containing 20 g/kg diet potassium oxonate in order to induce hyperuricemia following the protocol described by Mazzali et al. [37]. The Hyperuricemic rats were further allocated into three groups. The first did not receive any treatment and served as the hyperuricemic positive control. The second and third received 10% cow’s milk yoghurt and 10% WMS milk yoghurt (yoghurt manufactured from a blend of 50% cow’s milk and 50% WMS milk), respectively. The serum uric acid level was measured at the start of the experiment and after six weeks of taking potassium oxonate in order to confirm the occurrence of hyperuricemia.
2.11. Blood Sampling and Biochemical Analyses
At the end of the experimental period (five weeks), all rats were fasted overnight before sacrificing. Blood samples were collected from the hepatic portal vein into sterile plain tubes without anticoagulant and allowed to clot. Blood was left in a centrifuge tube at room temperature for 15 min before centrifugation at 2850× g (4000 rpm) for 10 min to recover sera in plastic vials; these were stored at −20 °C for analysis. Kidneys were separated and homogenized in super-cold 0.1 M phosphate buffer at pH 8.0 (1:4 w/v) using a homogenizer. The resulting homogenate was then centrifuged at 2850× g (4000 rpm) for 15 min and the supernatants were collected in order to estimate antioxidants enzyme activities.
2.12. Biochemical Analysis
Kidney function parameters including creatinine, urea, and uric acid were determined according to Bonsens and Taussky [38], Patton and Crouch [39], and Fossati et al., [40], respectively. Total protein, albumin, and globulin were determined as described by Young [41]. Superoxide dismutase (SOD), glutathione peroxidase (GPXs), glutathione transferase (GST), and catalase (CAT) were estimated using the method of Schumann and Klauke [42].
2.13. Histopathological Examination
Five rats from each group were sacrificed from each group six weeks post-feeding on different treatments. The kidneys and livers of the sacrificed animals were isolated and examined macroscopically, then fixed in 10% neutral formalin and embedded in paraffin. Sections with a thickness of five microns were prepared, stained by hematoxylin and eosin as mentioned by Suvarna et al. [43], then examined microscopically.
2.14. Statistical Analysis
Data were were expressed as mean ± SD and compared among treatment groups using one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. Statistics version 9 (http://www.statistix.com/freetrial.html; accessed on 5 December 2021) was used for analysis of the data [44]. The differences between the means of the treatments were considered significant when LSD was greater than 5%. Each treatment included three replicates.
3. Results and Discussion
Hyperuricemia is considered one of the most common metabolic diseases worldwide. This study investigated the remedial action of yoghurt enriched with WMS milk on renal injured hyperuricemic rats. In this respect, yoghurt manufacture with blended milk (50% cow’s milk and 50% WMS milk) was tested as an anti-hyperuricemic agent in rats.
3.1. Proximate Composition of Cow’s Milk and Watermelon Seed Milk
Table 1 presents the approximate chemical composition of the vegetable milk derived from watermelon seeds (WMS milk) compared to the cow’s milk. WMS milk has a relatively slightly lower protein content (2.96% versus 3.12% in cow’s milk), while a higher lipid content (8.8%) was observed in WMS milk than in cow’s milk (3.68%). The vegetable milk is characterized by the unique presence of fibers and much higher levels of total phenolic (mg GAE/100 g) and DPPH inhibition % activity than cow’s milk, i.e., 1240 mg GAE/100 g and 52.6% for WMS milk compared to 3.9 mg GAE/100 g and 4.8% for cow milk, respectively. These results are in line with those reported by Bisla et al. [45], who found that the moisture, protein, fat and ash contents of WMS milk were 81.33, 2.80, 8.50 and 0.88 g/100 g, respectively. The low content of total phenolic compounds in cow’s milk was in accordance with Vázquez et al. [46], who reported a level of 49.00 mg GAE/L, while the high phenol content in WMS milk agrees with the results of Tabiri et al. [21], who mentioned that the phenol content ranged from 1494 to 5416 mg GAE/100 g. The WMS milk exhibited 53% Xanthine oxidase (XO) % inhibition activity, versus only 1.4% for cow’s milk. These results are in line with those reported by Hameed et al. [6].

Table 1.
Approximate chemical composition, total phenol, % DPPH inhibition and xanthine oxidase inhibitory activity % of cow’s milk and WMS milk *.
3.2. Identification of Phenolic Acids
The typical chromatographic profile of phenolic compounds extracted from watermelon seeds using the HPLC method is shown in in the Supplementary Materials (Figure S1). Seven phenolic compounds, namely, gallic acid, 4-hydroxy benzoic, sinapic acid, caffeic acid, syringic acid, vanilic acid, p-coumaric acid and ferrulic acid were identified by comparing their UV–vis spectra and HPLC retention times with those of authentic standards analyzed under identical conditions. The results in Table 2 show the phenolic compound content in watermelon seed extract. As shown in this table, the phenolic compounds in WMSE range from 3.765 ± 0.46 to 138.410 ± 9.82 μg/100 g, and the predominant compound in WMSE is sinapic acid (138.410 ± 9.82 μg/100 g). Similar results were obtained by Fadimu et al. [47], who indicated that the methanolic extract of watermelon seeds had high content of sinapic acid and other phenolic compounds.

Table 2.
Chromatographic quantification of phenolic acids in watermelon seed extract.
3.3. Chemical Composition of Yoghurt Made from Cow’s Milk and Watermelon Seed Milk
As depicted in Table 3, the control yoghurt recorded the lowest relative content of total solids (TS), ash, and fiber and had the highest protein content compared to WMS milk yoghurt. On the other hand, the TS, ash and fiber contents of yoghurt made from cow’s milk with partial replacement with WMS milk gradually increased (p ≤ 0.05) as a function of the replacement ratio, which could be attributed to the higher contents in the original WMS milk. These result agree with Bisla et al. [45], who found that partial replacement of cow’s milk with WMS milk increased the TS, ash and fiber content in the resultant ice cream. Ismail et al. [48] stated that partial replacement of cow’s milk with soy milk affects the chemical composition of resultant yoghurt.

Table 3.
Chemical composition (%) of different yoghurts prepared from cow’s milk substituted with different ratios of WMS milk at the first day of storage.
3.4. Physicochemical Properties of WMS Milk Yoghurt
The data in Table 4 indicate that the control yoghurt’s titratable acidity (TA) level recorded the highest value. Replacing cow’s milk with WMS milk resulted in a significant (p ≤ 0.05) decrease in titratable acidity in a concentration-dependent manner. This reduction in titratable acidity may be due to antimicrobial activity associated with vegetable seed protein [14,16,49,50], which might inhibit starter activity and acid production [51,52]. In line with these findings, the pH values of all treatments showed a reverse trend to TA, expressing the level of alkalinity. Similar results were obtained by Uzuner et al. [53], who found that partial replacement of cow’s milk with rice milk decreased the TA values of the resultant yoghurt. The total phenolic content (TPC) of yoghurt supplemented with WMS milk significantly (p ≤ 0.05) increased when increasing the replacement ratio compared to control yoghurt (Table 4). For example, the TPC of the yoghurt manufactured from cow’s milk replaced with 25, 50, and 75% WMS milk was 7, 14, and 20 times that of the control cow milk yoghurt, respectively. These relatively higher TPC levels are attributed to the high total phenolic content of watermelon seeds [54]. As a result, the respective radical scavenging activity (RSA) of the WMS milk-manufactured yoghurt was 5, 6, and 7 times that of the cow’s milk yoghurt when the substitution level was 25, 50, and 75%. These results are in line with those reported by Atwaa et al. [55], who reported increases in total phenolic content and radical scavenging activity when fermented camel’s milk was fortified with oat milk.

Table 4.
Titratable acidity, pH values, total phenolic content (TPC) and radical scavenging activity (RSA) of different yoghurt prepared from cow’s milk substituted with different ratios of WMS milk at the first day of storage.
3.5. Sensory Properties of Watermelon Seed Milk Yoghurt
The data presented in Table 5 show that the partial replacement of cow’s milk with WMS milk significantly (p ≤ 0.05) decreased the sensory attributes of the resultant yoghurt, especially its flavor and its body and texture, when compared with the control yoghurt; this decrement was proportional to the replacement ratios. However, all treatments gave acceptable sensory properties except when exceeding the ratio of WMS milk substitution up to 75% (T3). These results agree with those reported by Ismail et al. [48], who found that partial replacement of cow or buffalo milk with soy milk significantly decreased the sensory attributes of the resultant yoghurt. The deterioration in the sensorial properties when replacing cow’s milk with WMS milk might be attributed to the hypothesis that people are already used to the traditional yoghurt taste, and any change may negatively affect the sensorial score. However, by increasing consumer awareness of the health and nutritional aspects, this might influence consumption behavior to be based on the nutritional and health facts rather than mere sensorial attributes. Clearly, new versions of yoghurt with excellent health and nutritional characteristics may gradually convince the consumers to develop their eating preferences. The facts presented in this article may contribute to this trend.

Table 5.
Sensory properties of different yoghurts prepared from cow’s milk substituted with different ratios of WMS milk at the first day of storage.
3.6. Effects of WMS Milk Yoghurt on Renal Function and Serum Proteins
Table 6 illustrates the effects of feeding hyperuricemic rats a diet including yoghurt prepared from cow’s milk partially substituted with WMS milk on the levels of urea, creatinine, uric acid, total protein (TP), albumin, and globulin. The hyperuricemic control group (the positive control) showed significantly (p ≤ 0.05) higher uric acid, urea, and creatinine levels than the normal rat group (negative control) or other treated groups. The lowest mean values of urea, uric acid, and creatinine (34.72 ± 2.54, 3.44 ± 0.42 and 0.72 ± 0.02 mg/dL) were recorded by the negative control (group 1), while the maximum levels (70.30 ± 5.32, 8.96 ± 1.04 and 1.84 ± 0.04 mg/dL, respectively) were recorded with the positive control (group 2). As depicted in our present findings, administering of cow’s milk yoghurt and WMS milk yoghurt to the hyperuricemic rats induced a significant reduction in the markers of renal function compared to the positive control (hyperuricemic rats). Hyperuricemic rats exhibited significantly (p ≤ 0.05) reduced total serum protein (TP), albumin, and globulin levels compared to the normal rats (negative control). Administertion of cow’s milk yoghurt and WMS milk yoghurt to the hyperuricemic rats resulted in a significant (p ≤ 0.05) increase in these serum protein levels which was close to those of the normal rats. The group receiving WMS milk yoghurt showed only a small significant (p < 0.05) difference from the normal rats (negative control). This conspicuous change may be partially due to the richness of watermelon seeds in enzymatic activities, being considered a diuretic and beneficial agent in chronic or acute eczema [45]. Furthermore, watermelon seeds contain many bioactive components such as phenolic compounds, flavonoids, minerals, and vitamins [54] that may indirectly reduce uric acid levels and therefore keep the kidney safe from the damage potentially caused by oxidative stress. These bioactive components act as superoxide scavengers, resulting in the suppression of reactive oxygen species (ROS) and uric acid formation [56].

Table 6.
Urea, creatinine, uric acid, total protein (TP), albumin, and globulin in hyperuricemia rats receiving watermelon seed milk yoghurt (WMS-milk yoghurt) compared to rats fed on cow’s milk yoghurt.
It is noteworthy to state that WMS milk may exert anti-hyperuricemic action in potassium oxonate-induced hyperuricemic rats. The underlying mechanisms of this action might be through the inhibition of xanthine oxidase activity and uricosuric activity, enhancing uric acid excretion in urine, which is supported by the natural action of watermelon seeds as antioxidants [21]. Natural antioxidants can reduce uric acid in the blood through direct uricosuric potential or through enhancing the glomerular filtration rate. Moreover, as a potent antioxidant, WMS milk can diminish oxidative stress and inflammation in cells, thereby reducing the synthesis and ultimately the blood level of uric acid [57,58]. Another probable mechanism is that any material can reduce uric acid in the blood by inhibiting the activity of the xanthine oxide enzyme, which reduces uric acid production. WMS milk inhibits on xanthine oxidase activity t a level about 38 times that of cow’s milk (Table 1). This distinguished action is probably due to the high content of bioactive constituents such as polyphenols in watermelon seed, which have been reported to have good inhibitory activity against xanthine oxidase [59]. Using WMS milk as vegetable milk at the expense of animal milk may protect against chronic kidney disease (CKD) [60]. Chiu et al. [61], studied the relationship between the cumulative impact of plant-based nutrition and serum uric acid level and found that vegetarians showed a lower mean uric acid serum concentration than non-vegetarians. Animal protein intake is significantly associated with a higher risk of developing CKD; therefore, substitution of red meat with plant protein may decrease the risk of CKD. In this respect, a higher intake of plant protein was significantly associated with a lower risk of prevalent CKD, as a 20 g increase in plant protein intake reduced the risk of developing CKD by 16% [62]. Taking this into account, purine-rich foods of meat origin are related to the risk of gout much more than plant proteins [63,64]. The current data align with previous reports [65,66] that watermelon seeds function as anti-hyperuricemic agents.
3.7. Effect of WMS Milk Yoghurt on Antioxidant Enzymatic Activities in Kidney of Hyperuricemia Rats
The data presented in Table 7 show that the hyperuricemic rats had significantly (p ≤ 0.05) lower values of SOD, CAT, GPXs, and GST than the normal rats. However, hyperuricemia in rat groups treated with cow’s milk yoghurt and WMS milk yoghurt showed significant (p ≤ 0.05) increases in SOD, GPXs, GST, and CAT activities compared with the hyperuricemic control. Watermelon seeds have bioactive components such as phenolic compounds, flavonoids, minerals, and vitamins which improve their biological functions [67]. Therefore, oxidative stress in body tissues can be removed by balancing antioxidants and free radicals. The rats treated with WMS milk yoghurt showed an increase in the activity of these enzymes, demonstrating their practical ability to prevent the harmful effects of free radicals; it should be stressed that caffeic acid is amongst the main antioxidant components of watermelon seeds [47]. These results are in harmony with previous studies elsewhere [68,69] which found that rats’ feed-induced oxidative stress was reversed by feeding on watermelon seeds which exhibited increased SOD, GPXs, GST, and CAT.

Table 7.
The levels of the antioxidant enzymes SOD, GPX, GST and CAT in the kidney of hyperuricemic rats receiving watermelon seed milk yoghurt compared to rats receiving cow’s milk yoghurt.
3.8. Kidney Histopathological Examination
The histopathological examination of kidneys in normal rats (negative control group) showed normal appearance of renal tubules and glomeruli, as depicted in Figure 1A. Figure 1B shows the renal tissue of the positive control rats (hyperuricemic group) throughout the experiment period, which showed venous fibrosis with increased hemorrhage and increased cellular space in the cortical region (the positive control group). On the other hand, while the histopathological examination of hyperuricemic rat kidneys treated with normal yoghurt showed restoration the corticomedullary junction structures there was still mild capillary congestion (arrows) and increased intertubuler cellularity (Figure 1C). The histopathological examination of hyperuricemic rat kidneys treated with WMS milk (50%) yogurt showed a significant improvement in the renal tubules and an increase in the cellular structure, as shown in Figure 1D. Reviewing the available literature, Ghalehkandi et al. [70] reported that hyperuricemic rats exhibited congestion or inflammatory cellular infiltration degeneration of kidney and altered kidney vessels vacillations due to toxin accumulation. It seems that the richness of watermelon seeds in antioxidants and dietary fiber can lower the level of free radicals. Several previous studies have reported the beneficial effects of these bioactive components and their role in prevention of ethanol-induced hepatotoxicity [71,72]. Additionally, watermelon seeds contain cucurbocitrin, which might play an important role in depressing of blood pressure and improving kidney function [47].
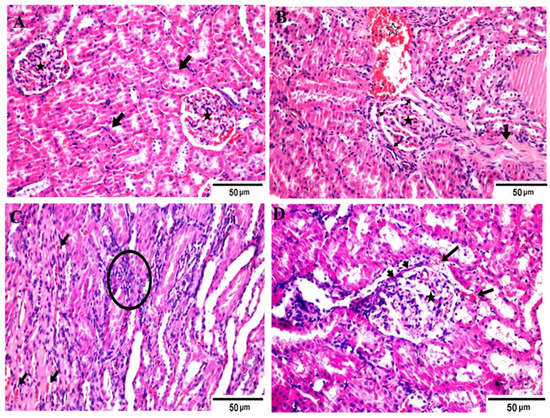
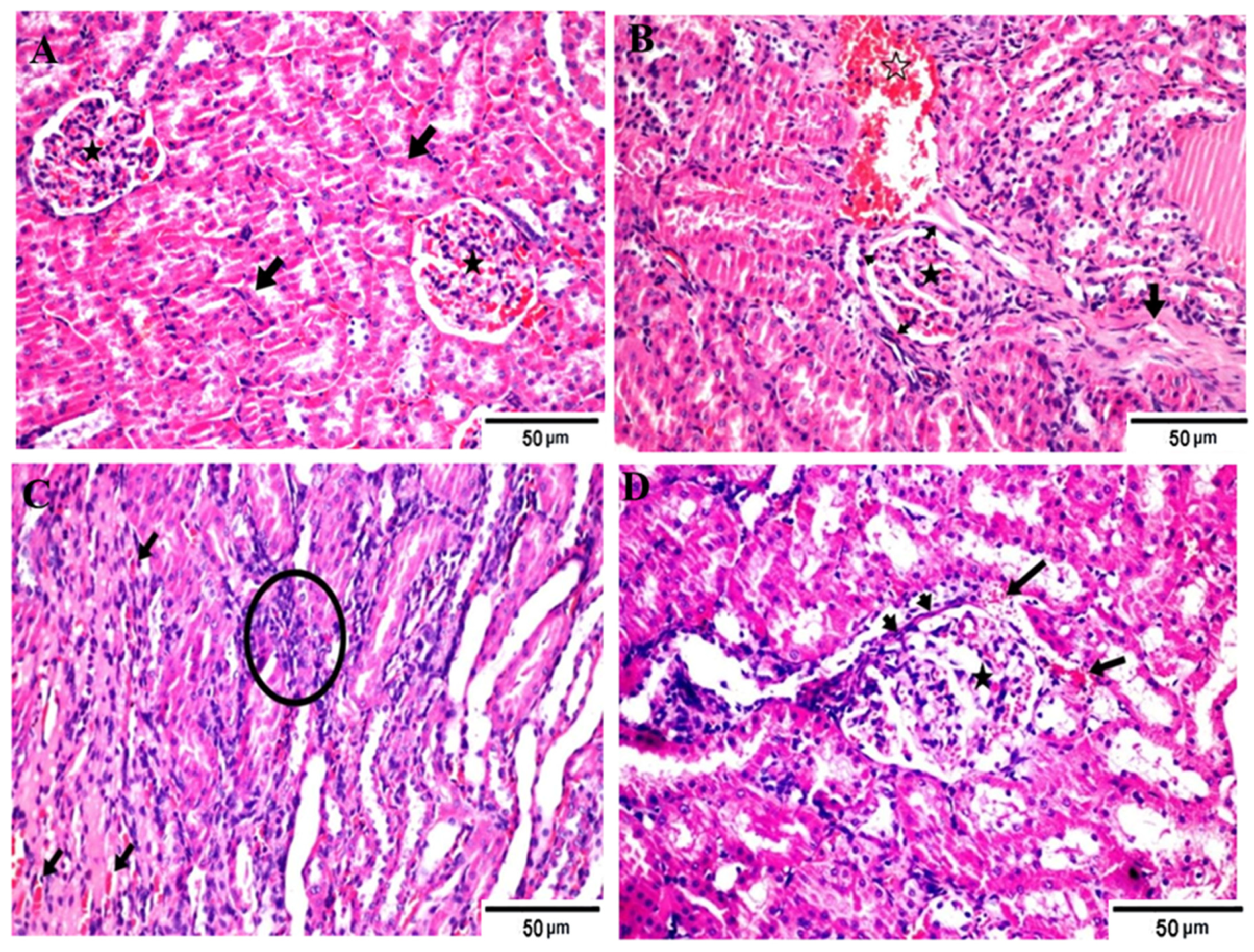
Figure 1.
Photomicrographs of different H&E-stained kidney sections of different groups. (A) Negative control group showing apparently normal renal tubules (arrows) and glomeruli (stars); (B) positive control (hyperuricemic rats) showing necrotic glomerular tufts (star), thickening basement membrane (heads) and periglomerular fibrous bands (arrow), associated with interstitial hemorrhages (open star); (C) hyperuricemic rats treated with normal yoghurt (C) showing restoration of the corticomedullary junction structures other than mildly congested capillaries (arrows) and increased intertubuler cellularity (star); (D) hyperuricemic rats treated with WMS milk (50%) yogurt showing apparently normal renal structures except for lobulated glomerular tufts (star), partial thickening of basement membrane (heads), and periglomerular-extravasated erythrocytes (arrows). Hematoxylin and eosin stain, Magnification × 400.
3.9. Liver Histopathological Examination
The histopathological examination of the liver in normal rats (negative control group) showed the normal appearance of hepatocytes, presented in Figure 2A. Liver of the positive control rats (hyperuricemic: second group) showed an accumulation of hepatocytes with the spread of several infections in the central vein area (Figure 2B). The histopathological examination of the liver in hyperuricemic rats treated with normal yoghurt showed restorion of the normal hepatic cords, with marked diplocytes and sinusoids and mild active kupffer cells beside a slightly dilated and congested center vein (Figure 2C). On the other hand, the livers of hyperuricemic rats treated with WMS milk (50%) yogurt showed a significant improvement in hepatocytes, with the appearance of mild infiltration and edema in the inflammatory cells of the central vein combined with the appearance of kupffer cells and a decrease in necrosis and fatty changes (Figure 2D). The high content of antioxidants and dietary fiber in watermelon seeds may help to reduce the level of free radicals [47]. Furthermore, watermelon seeds have been reported to improve liver function based on their high level of bioactive components [73].
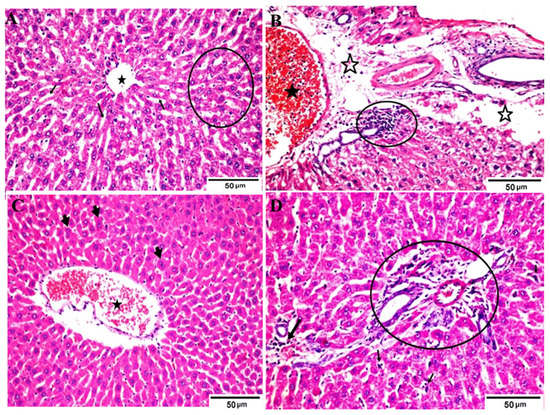
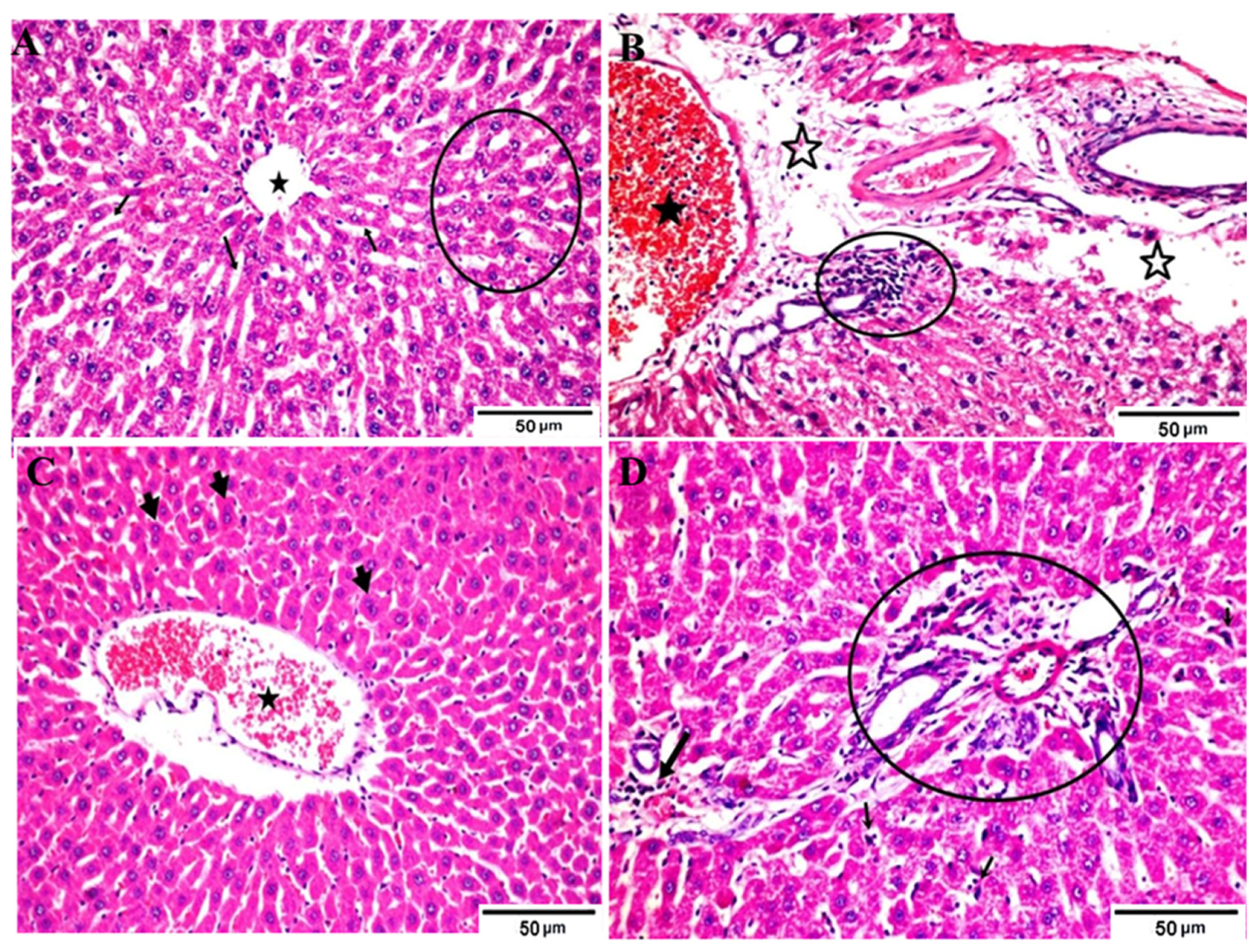
Figure 2.
Photomicrographs of different H&E-stained liver sections of different groups. (A) Negative control (A) showing normal hepatic parenchymal structures of the central vein (star), hepatic cords (circle) and sinusoids (arrows) containing kupffer cells; (B) positive control (hyperuricemic rats) showing engorged portal vein by blood and lymphocytosis (star) surrounded by edema (open star) and inflammatory cell aggregations (circle); (C) hyperuricemic rats treated with normal yoghurt showing restoration of the normal hepatic cords with marked * diplocytes (arrows) and sinusoids, mild active kupffer cells, and a slightly dilated and congested center vein; (D) Hyperuricemic rats treated with WMS milk (50%) yogurt showing apparently normal hepatic portal trades (circle) including vein, artery and bile duct, with slight congestion and inflammatory cells still present (thick arrow) as well as mild kupffer cell hyperplasia (small arrows). Hematoxylin and eosin stain, Magnification × 400.
4. Conclusions
Given the above information, watermelon seed milk could be considered a new vegetable milk which may have good health protective effects due to its high antioxidant components and plant protein nature. This vegetable milk can replace animal milk in some vital food products such as yoghurt. Substitution of cow’s milk with WMS milk at a ratio up to 50% during the manufacture of yoghurt slightly affected the total sensorial preference of the product, while adding numerous health benefits based on its high content of the total phenolic compounds (410 mg/100 g). WMS milk (50%) yoghurt proved to confer effective protection against hyperuricemia in Albino rats. The bioactive components of WMS milk were able to prevent hyperuricemia by lowering the concentrations of parameters relevant to kidney dysfunction and severe injury of kidney tissues, as confirmed by rat histopathological examination. Clearly, WMS milk can be recommended as a partial substitute for cow’s milk (up to 50%) in the manufacture of functional yoghurts.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fermentation8020041/s1, Figure S1: The chromatogram of watermelon seed extract phenolics. Peak numbers: (2) gallic acid; (3) vanillic acid; (4) p-coumaric acid; (5) caffeic acid; (6) 4-hydroxy benzoic; (7) sinapic acid; (8) Syringic acid.
Author Contributions
M.R.S., E.S.H.A., K.M.E.-Z., A.A.E. and M.Z.S. involved in the conception of the research idea and methodology design, supervision, performed data analysis and interpretation. H.H.A.H., A.A. and E.K.E., involved in methodology, drafted and prepared the manuscript for publication and revision. The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted with the approval of the approval of the Faculty of Agriculture, Zagazig, University and the Institutional Review Board Number ZU-IACU/3/F/172/2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
The authors thank the Taif University Researchers Supporting Program (Project number: TURSP-2020/151), Taif University, Saudi Arabia for support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, J.-H.; Chuang, S.-Y.; Chen, H.-J.; Yeh, W.-T.; Pan, W.-H. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: A chinese cohort study. Arthritis Care Res. 2009, 61, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Sun, L.; Guo, W. Roles of hyperuricemia in metabolic syndrome and cardiac-kidney-vascular system diseases. Am. J. Transl. Res. 2018, 10, 2749–2763. [Google Scholar]
- Kaneko, K.; Aoyagi, Y.; Fukuuchi, T.; Inazawa, K.; Yamaoka, N. Total Purine and Purine Base Content of Common Foodstuffs for Facilitating Nutritional Therapy for Gout and Hyperuricemia. Biol. Pharm. Bull. 2014, 37, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Corrado, A.; D’Onofrio, F.; Santoro, N.; Melillo, N.; Cantatore, F.P. Pathogenesis, clinical findings and management of acute and chronic gout. Minerva Med. 2006, 97, 495–509. [Google Scholar] [PubMed]
- El Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef]
- Hameed, B.J.; Shari, F.H.; Ramadhan, U.H. Anti-hyperuricemic, Uricosuric and Xanthine-oxidase Inhibitory Activities of Watermelon Powder in a Rat Gout Model. J. Biol. Sci. 2018, 18, 468–474. [Google Scholar] [CrossRef][Green Version]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Lozano, R.; Gómez-Serranillos, M.P. Current uses and knowledge of medicinal plants in the Autonomous Community of Madrid (Spain): A descriptive cross-sectional study. BMC Complement. Med. Ther. 2020, 20, 1–13. [Google Scholar] [CrossRef]
- Ramalingum, N.; Mahomoodally, M.F. The Therapeutic Potential of Medicinal Foods. Adv. Pharmacol. Sci. 2014, 2014, 1–18. [Google Scholar] [CrossRef]
- Chaouch, M.A.; Benvenuti, S. The Role of Fruit by-Products as Bioactive Compounds for Intestinal Health. Foods 2020, 9, 1716. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Swelam, S.; Zommara, M.A.; El-Aziz, A.E.-A.M.A.; Elgammal, N.A.; Baty, R.S.; Elmahallawy, E.K. Insights into Chufa Milk Frozen Yoghurt as Cheap Functional Frozen Yoghurt with High Nutritional Value. Fermentation 2021, 7, 255. [Google Scholar] [CrossRef]
- Badawi, A.M.; Motawee, M.M. Potential Protective Effect of Fortified Camel Milk Products with Chromium on Alloxan Induced Hyperglycemia in Rats. Int. J. Sci. Res. 2016, 5, 2319–7064. [Google Scholar]
- Sitohy, M.; Osman, A. Antimicrobial activity of native and esterified legume proteins against Gram-negative and Gram-positive bacteria. Food Chem. 2010, 120, 66–73. [Google Scholar] [CrossRef]
- Sitohy, M.Z.; Osman, A.O. Enhancing Milk Preservation with Esterified Legume Proteins. Probiotics Antimicrob. Proteins 2011, 3, 48–56. [Google Scholar] [CrossRef]
- Osman, A.; Abbas, E.; Mahgoub, S.; Sitohy, M. Inhibition of Penicillium digitatum in vitro and in postharvest orange fruit by a soy protein fraction containing mainly β-conglycinin. J. Gen. Plant Pathol. 2016, 82, 293–301. [Google Scholar] [CrossRef]
- Abbas, E.; Osman, A.; Sitohy, M. Biochemical control of Alternaria tenuissima infecting post-harvest fig fruit by chickpea vicilin. J. Sci. Food Agric. 2020, 100, 2889–2897. [Google Scholar] [CrossRef]
- Sitohy, M.; Taha, S.; Abdel-Hamid, M.; Abdelbacki, A.; Hamed, A.; Osman, A. Protecting potato plants against PVX and PVY viral infections by the application of native and chemically modified legume proteins. J. Plant Dis. Prot. 2021, 128, 1101–1114. [Google Scholar] [CrossRef]
- Oseni, O.; Okoye, V. Studies of Phytochemical and Antioxidant properties of the fruit of watermelon (Citrullus lanatus). (Thunb.). J. Pharm. Biomed. Sci. 2013, 27, 508–514. [Google Scholar]
- Otutu, O.; Seidu, K.; Muibi, B.; Oladokun, F.; Oyalowo, M. Potential food value of watermelon (Citrullus lanatus) seed constituents. Int. J. Sci. Technol. 2015, 3, 222. [Google Scholar]
- Tabiri, B.; Agbenorhevi, J.K.; Wireko-Manu, F.D.; Ompouma, E.I. Watermelon Seeds as Food: Nutrient Composition, Phytochemicals and Antioxidant Activity. Int. J. Nutr. Food Sci. 2016, 5, 139. [Google Scholar] [CrossRef]
- Vinhas, A.S.; Silva, C.S.; Matos, C.; Moutinho, C.; Ferreira da Vinha, A. Valorization of watermelon fruit (Citrullus lanatus) byproducts: Phytochemical and biofunctional properties with emphasis on recent trends and advances. World J. Adv. Healthc. Res. 2021, 5, 302–309. [Google Scholar]
- Finbarrs-Bello, E.; Nto, N.J.; Ikele, I.T.; Sani, M.I.; Atuadu, V. Haematopoietic Enhancing Effect of Ethanolic Seed Extract of Citrullus lanatus (Watermelon) on Bone Marrow of Wistar Rats. Eur. J. Med. Plants 2016, 17, 1–7. [Google Scholar] [CrossRef]
- Oluba, O.; Ogunlowo, Y.; Ojieh, G.; Adebisi, K.; Eidangbe, G.; Isiosio, I. Physicochemical Properties and Fatty Acid Composition of Citrullus lanatus (Egusi Melon) Seed Oil. J. Biol. Sci. 2008, 8, 814–817. [Google Scholar] [CrossRef]
- Berger, S.; Raman, G.; Vishwanathan, R.; Jacques, P.F.; Johnson, E.J. Dietary cholesterol and cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2015, 102, 276–294. [Google Scholar] [CrossRef] [PubMed]
- Savaiano, D.A.; Hutkins, R.W. Yogurt, cultured fermented milk, and health: A systematic review. Nutr. Rev. 2021, 79, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Hudson, J.; Korpela, R.; Reyes-Gavilán, C.G.D.L. Impact on Human Health of Microorganisms Present in Fermented Dairy Products: An Overview. BioMed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Williams, S. Official Methods of Analysis; Association of Official Analytical Chemists: Rockville, MD, USA, 1984. [Google Scholar]
- Chakrabarti, S.; Gangopadhyay, S. Innovation of technology for preparation of Rasogolla analogue from soy milk. J. Food Sci. Technol. (Mysore) 1990, 27, 242–243. [Google Scholar]
- Yumita, A.; Suganda, A.G.; Sukandar, E.Y. Xanthine oxidase inhibitory activity of some Indonesian medicinal plants and active fraction of selected plants. Int. J. Pharm. Pharm. Sci. 2013, 5, 293–296. [Google Scholar]
- Sunarni, T.; Fidrianny, I.; Iwo, M.I.; Wirasutisna, K.R. Constituent and Antihyperuricemic Activity of Stelechocarpus Burahol Leaves Subfractions. Asian J. Pharm. Clin. Res. 2017, 10, 435. [Google Scholar] [CrossRef][Green Version]
- Kaur, C.; Kapoor, H.C. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mariod, A.A.; Ibrahim, R.M.; Ismail, M.; Ismail, N. Antioxidant activities of phenolic rich fractions (PRFs) obtained from black mahlab (Monechma ciliatum) and white mahlab (Prunus mahaleb) seedcakes. Food Chem. 2010, 118, 120–127. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Yoghurt: Science and Technology; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Bodyfelt, F.; Tobias, J.; Trout, G. Sensory evaluation of cultured milk products. In The Sensory Evaluation of Dairy Products; Van Nostrand Reinhold: New York, NY, USA, 1988. [Google Scholar]
- Mazzali, M.; Hughes, J.; Kim, Y.-G.; Jefferson, J.A.; Kang, D.-H.; Gordon, K.L.; Lan, H.Y.; Kivlighn, S.; Johnson, R. Elevated Uric Acid Increases Blood Pressure in the Rat by a Novel Crystal-Independent Mechanism. Hypertension 2001, 38, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Bonsens, K.; Taussky, D. Determination of serum creatinine. J. Chem. Inv. 1984, 27, 648–660. [Google Scholar]
- Patton, C.; Crouch, S. Enzymatic colorimetric method to determine urea in serum. Anal. Chem. 1977, 49, 464–469. [Google Scholar] [CrossRef]
- Fossati, P.; Prencipe, L.; Berti, G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin. Chem. 1980, 26, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Young, D. Effects of Disease on Clinical Lab. Tests, 4th ed.; AACC: Washington, DC, USA, 2001. [Google Scholar]
- Schumann, G.; Klauke, R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: Preliminary upper reference limits obtained in hospitalized subjects. Clin. Chim. Acta 2003, 327, 69–79. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Boyd, C.; Petersen, S.; Gilbert, W.; Rodgers, R.; Fuhlendorf, S.; Larsen, R.; Wolfe, D.; Jensen, K.; Gonzales, P.; Nenneman, M. Analytical Software. 2009. Statistix 9. Tallahassee, Florida, USA. Evaluation of Methods Used to Improve Grasslands as Ring-Necked Pheasant (Phasianus colchicus) Brood Habitat. Ph.D. Thesis, South Dakota State University, Brookings, SD, USA, 2018. [Google Scholar]
- Bisla, G.; Archana, P.V.; Sharma, S. Development of ice creams from Soybean milk & Watermelon seeds milk and Evaluation of their acceptability and Nourishing potential. Adv. Appl. Sci. Res. 2012, 3, 371–376. [Google Scholar]
- Vázquez, C.V.; Rojas, M.G.V.; Ramírez, C.A.; Chávez-Servín, J.L.; García-Gasca, T.; Ferriz Martínez, R.A.; García, O.P.; Rosado, J.L.; López-Sabater, C.M.; Castellote, A.I.; et al. Total phenolic compounds in milk from different species. Design of an extraction technique for quantification using the Folin–Ciocalteu method. Food Chem. 2015, 176, 480–486. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Ghafoor, K.; Babiker, E.E.; Al-Juhaimi, F.; Abdulraheem, R.A.; Adenekan, M.K. Ultrasound-assisted process for optimal recovery of phenolic compounds from watermelon (Citrullus lanatus) seed and peel. J. Food Meas. Charact. 2020, 14, 1784–1793. [Google Scholar] [CrossRef]
- Ismail, M.M.; Tabekha, M.M.; Ghoniem, G.A.; Boraey, N.A.E.; Elashrey, H.F.A. Chemical Composition, Microbial Properties and Sensory Evaluation of Yoghurt Made from Admixture of Buffalo, Cow and Soy Milk. J. Food Dairy Sci. 2016, 7, 299–306. [Google Scholar] [CrossRef]
- Sitohy, M.; Mahgoub, S.; Osman, A. Controlling psychrotrophic bacteria in raw buffalo milk preserved at 4 °C with esterified legume proteins. LWT 2011, 44, 1697–1702. [Google Scholar] [CrossRef]
- Osman, A.; Mahgoub, S.; El-Masry, R.; Al-Gaby, A.; Sitohy, M. Extending the technological validity of R aw Buffalo M ilk at room temperature by esterified legume proteins. J. Food Processing Preserv. 2014, 38, 223–231. [Google Scholar] [CrossRef]
- Braide, W.; Odiong, I.; Oranusi, S. Phytochemical and Antibacterial properties of the seed of watermelon (Citrullus lanatus). Prime J. Microbiol. Res. (PJMR) 2012, 2, 99–104. [Google Scholar]
- Adelani-Akande, T.A.; Ajiba, L.C.; Dahunsi, S.O.; Oluyori, A.P.; Chidimma, A.L.; Olatunde, D.S.; Peter, O.A.; Adunola, A.-A.T. Antibacterial activity of watermelon (Citrullus lanatus) seed against selected microorganisms. Afr. J. Biotechnol. 2015, 14, 1224–1229. [Google Scholar] [CrossRef]
- Uzuner, A.E.; Kınık, Ö.; Korel, F.; Yıldız, G.; Yerlikaya, O. Usage of rice milk in probiotic yoghurt production. Carpath. J. Food Sci. Technol. 2016, 8, 5–25. [Google Scholar]
- Seidu, K.T.; Otutu, O.L. Phytochemical composition and radical scavenging activities of watermelon (Citrullus lanatus) seed constituents. Croat. J. Food Sci. Technol. 2016, 8, 83–89. [Google Scholar] [CrossRef]
- Atwaa, E.; Sayed-Ahmed, A.; Eman, T.; Hassan, M. Physicochemical, Microbiological and Sensory Properties of Low Fat Probiotic Yoghurt Fortified with Mango Pulp Fiber Waste as Source of Dietary Fiber. J. Food Dairy Sci. 2020, 11, 271–276. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J.; Gong, D. Dietary Flavonoids as Xanthine Oxidase Inhibitors: Structure–Affinity and Structure–Activity Relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Fong, W.P.; Cheng, C.H.K. The Dual Actions of Morin (3,5,7,2′,4′-Pentahydroxyflavone) as a Hypouricemic Agent: Uricosuric Effect and Xanthine Oxidase Inhibitory Activity. J. Pharmacol. Exp. Ther. 2005, 316, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kensarah, O.A.; Azzeh, F.S. Implementing high vitamin C treatments to decrease blood uric acid levels in hyperuricemic Saudi patients. J. Am. Sci. 2012, 8, 462–467. [Google Scholar]
- Siddiqui, W.A.; Shahzad, M.; Shabbir, A.; Ahmad, A. Evaluation of anti-urolithiatic and diuretic activities of watermelon (Citrullus lanatus) using in vivo and in vitro experiments. Biomed. Pharmacother. 2018, 97, 1212–1221. [Google Scholar] [CrossRef]
- Alvirdizadeh, S.; Yuzbashian, E.; Mirmiran, P.; Eghtesadi, S.; Azizi, F. A prospective study on total protein, plant protein and animal protein in relation to the risk of incident chronic kidney disease. BMC Nephrol. 2020, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.H.T.; Liu, C.-H.; Chang, C.-C.; Lin, M.-N.; Lin, C.-L. Vegetarian diet and risk of gout in two separate prospective cohort studies. Clin. Nutr. 2020, 39, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Yuzbashian, E.; Asghari, G.; Mirmiran, P.; Hosseini, F.-S.; Azizi, F. Associations of dietary macronutrients with glomerular filtration rate and kidney dysfunction: Tehran lipid and glucose study. J. Nephrol. 2014, 28, 173–180. [Google Scholar] [CrossRef]
- Villegas, R.; Xiang, Y.-B.; Elasy, T.; Xu, W.; Cai, H.; Cai, Q.; Linton, M.; Fazio, S.; Zheng, W.; Shu, X.-O. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: The Shanghai Men’s Health Study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Aihemaitijiang, S.; Zhang, Y.; Zhang, L.; Yang, J.; Ye, C.; Halimulati, M.; Zhang, W.; Zhang, Z. The Association between Purine-Rich Food Intake and Hyperuricemia: A Cross-Sectional Study in Chinese Adult Residents. Nutrients 2020, 12, 3835. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Yap, D.Y.; Li, C.W.; Cheng, H.W.B.; Au, H.Y. Excessive watermelon consumption causing hyperkalaemia and increased symptom burden of an end stage renal disease patient. Nephrology 2016, 21, 721. [Google Scholar] [CrossRef] [PubMed]
- Owheruo, J.O.; Oluwajuyitan, T.D.; Ifesan, B.O.; Bolade, M.K. Extruded breakfast meal from malted finger millet (Eleusine coracana) and watermelon (Citrullus lanatus) seed flour: In-Vivo nutritional qualities study. Bull. Natl. Res. Cent. 2021, 45, 1–9. [Google Scholar] [CrossRef]
- Mogotlane, E.A.; Mokwala, P.W.; Mangena, P. Comparative analysis of the chemical compositions of indigenous watermelon (Citrullus lanatus) seeds from two districts in Limpopo Province, South Africa. Afr. J. Biotechnol. 2018, 17, 1001–1006. [Google Scholar]
- Ikpeme, E.; Udensi, O.; Ekerette, E.; Okon, U. Potential of Ginger (Zingiber officinale) Rhizome and Watermelon (Citrullus lanatus) Seeds in Mitigating Aspartame-Induced Oxidative Stress in Rat Model. Res. J. Med. Plant 2016, 10, 55–66. [Google Scholar] [CrossRef]
- Pinheiro, D.T.; Silva, A.L.d.; Silva, L.J.d.; Sekita, M.C.; Dias, D.C.F.d.S. Germination and antioxidant action in melon seeds exposed to salt stress. Pesqui. Agropecu. Trop. 2016, 46, 336–342. [Google Scholar] [CrossRef]
- Ghalehkandi, J.G.; Ebrahimnezhad, Y.; Nobar, R.S. Effect of garlic (Allium sativum) aqueous extract on serum values of urea, uric-acid and creatinine compared with chromium chloride in male rats. Ann. Biol. Res. 2012, 3, 4485–4490. [Google Scholar]
- Singh Gill, N.; Sood, S.; Muthuraman, A.; Bali, M.; Dev Sharma, P. Evaluation of antioxidant and anti-ulcerative potential of Citrullus lanatus seed extract in rats. Lat. Am. J. Pharm. 2011, 30, 429–434. [Google Scholar]
- Artana, I.W. Watermelon, Kalium, and Kidney Health: A Review Literature. Syst. Rev. Pharm. 2020, 11, 1001–1007. [Google Scholar]
- Monday, N.; Bazabang, S.A.; Adebisi, S.S.; Makena, W.; Iliya, I.A. Hepatoprotective Effects of Aqueous Extract of Watermelon (Citrullus lanatus) Seeds on Ethanol-Induced Oxidative Damage in Wister Rats. Sub-Saharan Afr. J. Med. 2018, 5, 129. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).