Methane Production of Pistia Stratiotes as a Single Substrate and as a Co-Substrate with Dairy Cow Manure
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Batch and Post Digestion Test
2.3. Continuous Experiment
2.4. Inoculum and Substrate
2.5. Analytical Methods
3. Results
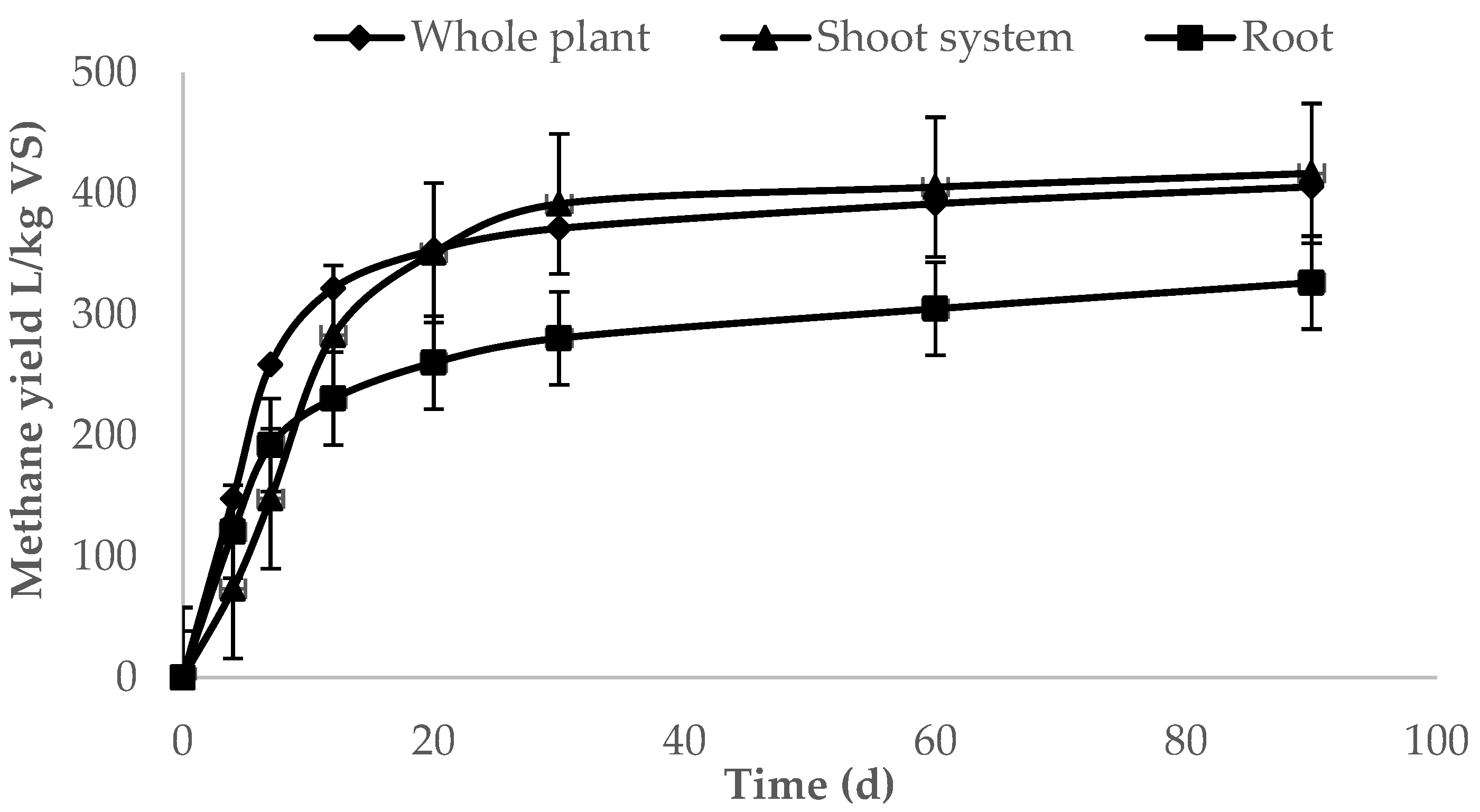
3.1. Batch Digestion Test
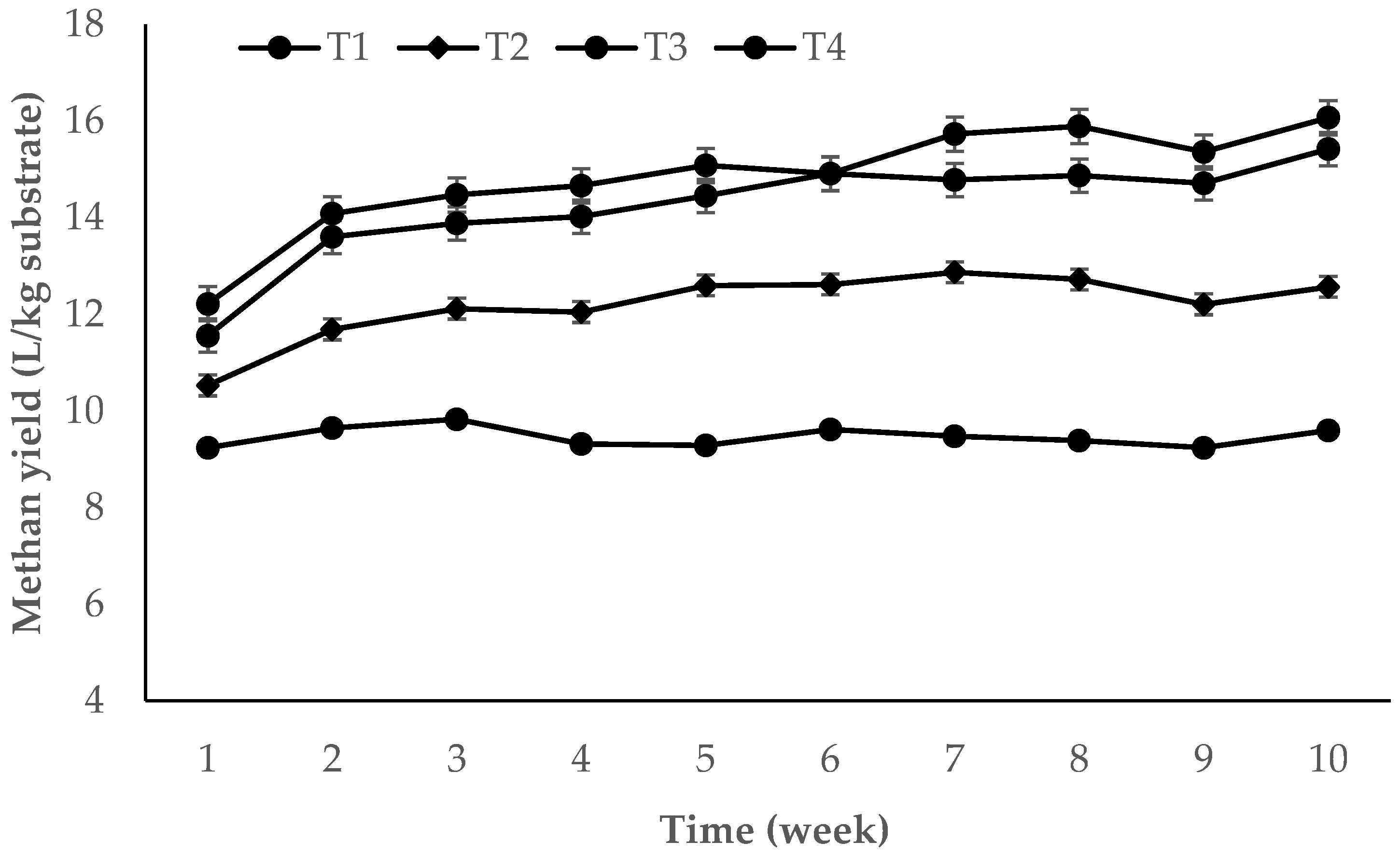
3.2. Continuous Study
3.3. Variables in the Liquid Phase
3.4. Post Digestion Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sutaryo, S.; Ward, A.J.; Møller, H.B. Anaerobic inhibition in thermophilic anaerobic digestion of dairy cattle manure. J. Indonesian Trop. Anim. Agric. 2014, 39, 83–90. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Zhao, J.; Krooneman, J.; Euverink, G.J. Strategies to boost anaerobic digestion performance of cow manure: Laboratory achievements and their full-scale application potential. Sci. Total Environ. 2021, 55, 142940. [Google Scholar] [CrossRef] [PubMed]
- Dębowski, M.; Kazimierowicz, J.; Zieliński, M.; Bartkowska, I. Co-Fermentation of microalgae biomass and miscanthus × giganteus silage—Assessment of the substrate, biogas production and digestate characteristics. Appl. Sci. 2022, 12, 7291. [Google Scholar] [CrossRef]
- Whangchai, K.; Inta, W.; Unpaprom, Y.; Bhuyar, P.; Adoonsok, D.; Ramaraj, R. Comparative analysis of fresh and dry-floating aquatic plant Pistia stratiotes via chemical pretreatment for second-generation (2G) bioethanol. Bioresour. Technol. 2021, 14, 100651. [Google Scholar] [CrossRef]
- Wilkie, A.C.; Evans, J.M. Aquatic plants: An opportunity feedstock in the age of bioenergy. Biofuels 2010, 1, 311–321. [Google Scholar] [CrossRef]
- Sharma, B.M.; Sridhar, M.K.C. The productivity of Pistia stratiotes L. in a eutrophic lake. Environ. Pollut. Ecol. Biol. 1981, 24, 277.e89. [Google Scholar] [CrossRef]
- Nipaney, P.C.; Panholzer, M.B. Influence of temperature on biogas production from Pistia stratiotes. Biol. Wastes 1987, 19, 267–274. [Google Scholar] [CrossRef]
- Tavares, A.; Ziglioli, E.; Remor, P.; Cavaler, J.; Alino, J.; Bastos, J.; Edwiges, T. Comparison of the biochemical methane potential of different organic biomass. Adv. Ecol. Environ. Res. 2020, 5, 210–215. [Google Scholar]
- Jacob, S.; Banerjee, R. Modeling and optimization of anaerobic codigestion of potato waste and aquatic weed by response surface methodology and artificial neural network coupled genetic algorithm. Bioresour. Technol. 2016, 214, 386–395. [Google Scholar] [CrossRef]
- Madenoğlu, T.G.; Falizi, N.J.; Kabay, N.; Güneşc, A.; Kumara, R.; Pekd, T.; Yüksel, M. Kinetic analysis of methane production from anaerobic digestion of water lettuce (Pistia stratiotes L.) with waste sludge. J. Chem. Technol. Biotechnol. 2019, 94, 1893–1903. [Google Scholar] [CrossRef]
- Wilinska-Lisowska, A.; Ossowska, M.; Czerwionka, K. The influence of co-fermentation of agri-food waste with primary sludge on biogas production and composition of the liquid fraction of digestate. Energies 2021, 14, 1907. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dzienis, L.; Dębowski, M.; Zieliński, M. Optimisation of methane fermentation as a valorisation method for food waste products. Biomass Bioenerg. 2021, 144, 105913. [Google Scholar] [CrossRef]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Methane productivity of manure, straw and solid fraction of manure. Biomass Bioenerg. 2004, 26, 485–495. [Google Scholar] [CrossRef]
- Sutaryo, S.; Adiwinarti, R.; Sudrajad, M.A.; Sari, T.Y.K.; Khayati, L.N.; Ward, A.J.; Purnomoadi, A. Enhancing methane production of dairy cow manure by co-digestion with modified cassava flour waste water. Livest. Res. Rural Dev. 2021, 33, 3377. [Google Scholar]
- Gelegenis, L.; Georgakakis, D.; Angelidaki, I.; Mavris, V. Optimization of biogas production by co-digesting whey with diluted poultry manure. Renew. Energy 2007, 32, 2147–2160. [Google Scholar] [CrossRef]
- Sutaryo, S.; Sempana, A.N.; Lestari, C.M.S.; Ward, A.J. Performance comparison of single and two-phase biogas digesters treating dairy cattle manure at tropical ambient temperature. Trop. Anim. Sci. 2020, 43, 354–359. [Google Scholar] [CrossRef]
- Møller, H.B.; Moset, V.; Brask, M.; Weisbjerg, M.R.; Lund, P. Feces composition and manure derived methane yield from dairy cows: Influence of diet with focus on fat supplement and roughage type. Atmos. Environ. 2014, 94, 36–43. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Waste Water, 19th ed.; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Haug, R.T. The Practical Handbook of Composting Engineering; Lewis Publisher: Chicago, IL, USA, 1993. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Prosedur Statistik Untuk Penelitian Pertanian; Sjamsuddin, E., Bahasjah, J.S., Eds.; UI Press: Jakarta, Indonesia, 2007. [Google Scholar]
- Anukam, A.; Mohamadi, A.; Naqvi, M.; Granström, K. A review of the chemistry of anaerobic digestion: Methods of accelerating and optimizing process efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef]
- Abraham, A.; Mathewa, A.M.; Parka, H.; Choia, O.; Sindhub, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sangan, B. Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef]
- Orangun, A.; Kaur, H.; Kommalapati, R.R. Batch anaerobic co-digestion and biochemical methane potential analysis of goat manure and food waste. Energies 2021, 14, 1952. [Google Scholar] [CrossRef]
- Dong, L.; Cao, G.; Guo, X.; Liu, T.; Wu, J.; Ren, N. Efficient biogas production from cattle manure in a plug flow reactor: A large scale long term study. Bioresour. Technol. 2019, 278, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Ellegaard, L.; Ahring, B.K. Applications of the anaerobic digestion process. Adv. Biochem. Eng. Biotechnol. 2003, 82, 1–33. [Google Scholar] [PubMed]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sust. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Chow, W.L.; Chong, S.; Lim, J.W.; Chan, Y.J.; Chong, M.F.; Tiong, T.J.; Chin, J.K.; Pan, G. Anaerobic co-digestion of wastewater sludge: A review of potential co-substrates and operating factors for improved methane yield. Processes 2020, 8, 39. [Google Scholar] [CrossRef]
- Karki, R.; Chuenchart, W.; Surendra, K.C.; Shrestha, S.; Raskin, L.; Sung, S.; Hashimoto, A.; Khanal, S.K. Anaerobic co-digestion: Current status and perspectives. Bioresour. Technol. 2021, 330, 125001. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sust. Energy Rev. 2015, 4, 540–555. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Emebu, S.; Pecha, J.; Janáčová, D. Review on anaerobic digestion models: Model classification & elaboration of process phe-nomena. Renew. Sust. Energy Rev. 2022, 160, 112288. [Google Scholar]
- Thygesen, O.; Sommer, S.G.; Shin, S.G.; Triolo, J.M. Residual biochemical methane potential (BMP) of concentrated digestate from full-scale biogas plants. Fuel 2014, 132, 44–46. [Google Scholar] [CrossRef]
- Uludag-Demirer, S.; Demirer, G.N. Post-Anaerobic treatability and residual biogas potential of digestate. Biomass Convers. Biorefin. 2022, 12, 1695–1702. [Google Scholar] [CrossRef]
| Substrate | Digester Type | Level of Combination | Duration of Study | Temperature Evaluation | Gas Production | Ref. |
|---|---|---|---|---|---|---|
| PS solely | Batch | - | 30 d | 29.5–37.5 °C | 533–707 L biogas/kg VS | [7] |
| PS solely | Batch | - | 30 d | 37 °C | 43 L CH4/kg VS | [8] |
| Potato waste and PS | Batch | 1:1 | 51 d | 37 °C | 447.4 L CH4/kg VS | [9] |
| PS solely | Batch | - | 62.5 d | 37 °C | 321 L biogas/kg VS | [10] |
| TS (%) | VS (%) | Crude Protein (%) | Proportion of PS (%) | pH | C/N Ratio | |
|---|---|---|---|---|---|---|
| T1 | 6.43 ± 0.22 | 5.68 ± 0.36 | 0.59 ± 0.28 | - | 7.25 ± 0.16 | 33.43 |
| T2 | 7.54 ± 0.15 | 6.24 ± 0.00 | 0.70 ± 0.16 | 7.99 | 7.15 ± 0.08 | 30.95 |
| T3 | 8.64 ± 0.21 | 7.44 ± 0.57 | 0.80 ± 0.10 | 14.91 | 7.11 ± 0.08 | 32.29 |
| T4 | 9.60 ± 0.15 | 7.90 ± 0.32 | 0.93 ± 0.23 | 20.94 | 7.10 ± 0.09 | 29.50 |
| Component (%) | Shoot System | Root | Whole |
|---|---|---|---|
| Mass Balance (% of fresh) Moisture Dry matter | 72.25 ± 0.22 9.05 ± 0.20 90.95 ± 2.06 | 27.25 ± 0.22 11.04 ± 0.20 88.96 ± 2.06 | 100 9.73 ± 1.37 90.27 ± 1.7 |
| Volatile solids | 65.90 ± 0.69 | 61.59 ± 0.69 | 65.36 ± 3.43 |
| Ash | 25.05 ± 0.17 | 27.66 ± 2.51 | 24.91 ± 4.64 |
| Crude protein * | 11.05 | 9.17 | 10.52 |
| Extract ether | 1.49 ± 0.07 | 1.40 ± 0.06 | 1.49 ± 0.07 |
| Crude fibre | 20.50 ± 1.33 | 21.14 ± 2.26 | 29.40 ± 0.59 |
| Extract free nitrogen | 32.86 ± 1.39 | 29.59 ± 2.20 | 23.95 ± 0.53 |
| Neutral detergent fibre | 75.47 ± 2.36 | 77.70 ± 0.69 | 78.41 ± 1.95 |
| Acid detergent fibre | 35.39 ± 0.63 | 46.09 ± 2.55 | 53.03 ± 3.37 |
| Lignin | 28.96 ± 0.57 | 26.31 ± 0.46 | 34.85 ± 0.62 |
| Hemicellulose | 40.09 ± 1.74 | 31.61 ± 3.24 | 25.38 ± 1.42 |
| Cellulose | 6.43 ± 1.20 | 19.78 ± 3.01 | 18.18 ± 3.98 |
| C/N ratio | 20.71 | 23.32 | 21.57 |
| Cumulative Methane Yield at 30 d (B0: 30 d) | Cumulative Methane Yield at 60 d (B0: 60 d) | Cumulative Methane Yield at 90 d (B0: 90 d) | |
|---|---|---|---|
| (L/kg VS) | |||
| Whole plant | 371.29 ± 12.17 a | 391.67 ± 17.52 a | 405.68 ± 17.27 a |
| Shoot system | 391.56 ± 17.13 a | 405.45 ± 15.18 a | 416.82 ± 18.80 a |
| Root | 280.47 ± 4.74 b | 304.81 ± 8.52 b | 326.43 ± 10.80 b |
| Methane Yield | TAN | Total VFA | VS Reduction | pH | |||
|---|---|---|---|---|---|---|---|
| (L/kg Substrate) | (L/L Digester Volume) | (L/kg VS Added) | (mg/L) | (mM) | (%) | ||
| T1 | 9.46 ± 0.47 a | 0.43 ± 0.02 a | 166.51 ± 8.24 a | 72.60 ± 14.42 | 166 ± 23.19 | 33.42 ± 2.13 a | 6.94 ± 0.12 |
| T2 | 12.17 ± 0.95 b | 0.55 ± 0.04 b | 195.11 ± 15.23 b | 75.40 ± 17.02 | 171 ± 21.32 | 35.26 ± 3.76 ab | 6.99 ± 0.13 |
| T3 | 14.15 ± 1.37 c | 0.64 ± 0.06 c | 190.16 ± 18.35 bc | 75.40 ± 15.81 | 169 ± 28.85 | 37.32 ± 1.59 bc | 7.03 ± 0.16 |
| T4 | 14.85 ± 1.43 d | 0.67 ± 0.06 d | 187.99 ± 18.08 cd | 79.60 ± 12.32 | 188 ± 19.32 | 38.87 ± 1.18 c | 7.09 ± 0.12 |
| Biogas Yield (L/Kg Digested Slurry) | |
|---|---|
| T1 | 3.68 ± 0.19 a |
| T2 | 4.23 ± 0.19 b |
| T3 | 4.50 ± 0.86 c |
| T4 | 4.86 ± 0.04 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutaryo, S.; Sempana, A.N.; Mulya, R.M.; Sulistyaningrum, D.; Ali, M.S.; Damarjati, R.I.; Purbowati, E.; Adiwinarti, R.; Purnomoadi, A. Methane Production of Pistia Stratiotes as a Single Substrate and as a Co-Substrate with Dairy Cow Manure. Fermentation 2022, 8, 736. https://doi.org/10.3390/fermentation8120736
Sutaryo S, Sempana AN, Mulya RM, Sulistyaningrum D, Ali MS, Damarjati RI, Purbowati E, Adiwinarti R, Purnomoadi A. Methane Production of Pistia Stratiotes as a Single Substrate and as a Co-Substrate with Dairy Cow Manure. Fermentation. 2022; 8(12):736. https://doi.org/10.3390/fermentation8120736
Chicago/Turabian StyleSutaryo, Sutaryo, Aldila Nugrahaini Sempana, Rifo Martio Mulya, Dian Sulistyaningrum, Mochamad Sofyan Ali, Rafi Ihsa Damarjati, Endang Purbowati, Retno Adiwinarti, and Agung Purnomoadi. 2022. "Methane Production of Pistia Stratiotes as a Single Substrate and as a Co-Substrate with Dairy Cow Manure" Fermentation 8, no. 12: 736. https://doi.org/10.3390/fermentation8120736
APA StyleSutaryo, S., Sempana, A. N., Mulya, R. M., Sulistyaningrum, D., Ali, M. S., Damarjati, R. I., Purbowati, E., Adiwinarti, R., & Purnomoadi, A. (2022). Methane Production of Pistia Stratiotes as a Single Substrate and as a Co-Substrate with Dairy Cow Manure. Fermentation, 8(12), 736. https://doi.org/10.3390/fermentation8120736





