Using Formic Acid to Promote Bacterial Cellulose Production and Analysis of Its Material Properties for Food Packaging Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganisms and Maintenance
2.3. Relative Expressions of QS Genes Using a Quantitative Polymerase Chain Reaction (qPCR)
2.4. Characteristic Analysis of BC
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. X-ray Diffraction (XRD)
2.4.3. Fourier Transform Infrared (FTIR)
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Water Content
2.5. Statistical Analysis
3. Results and Discussion
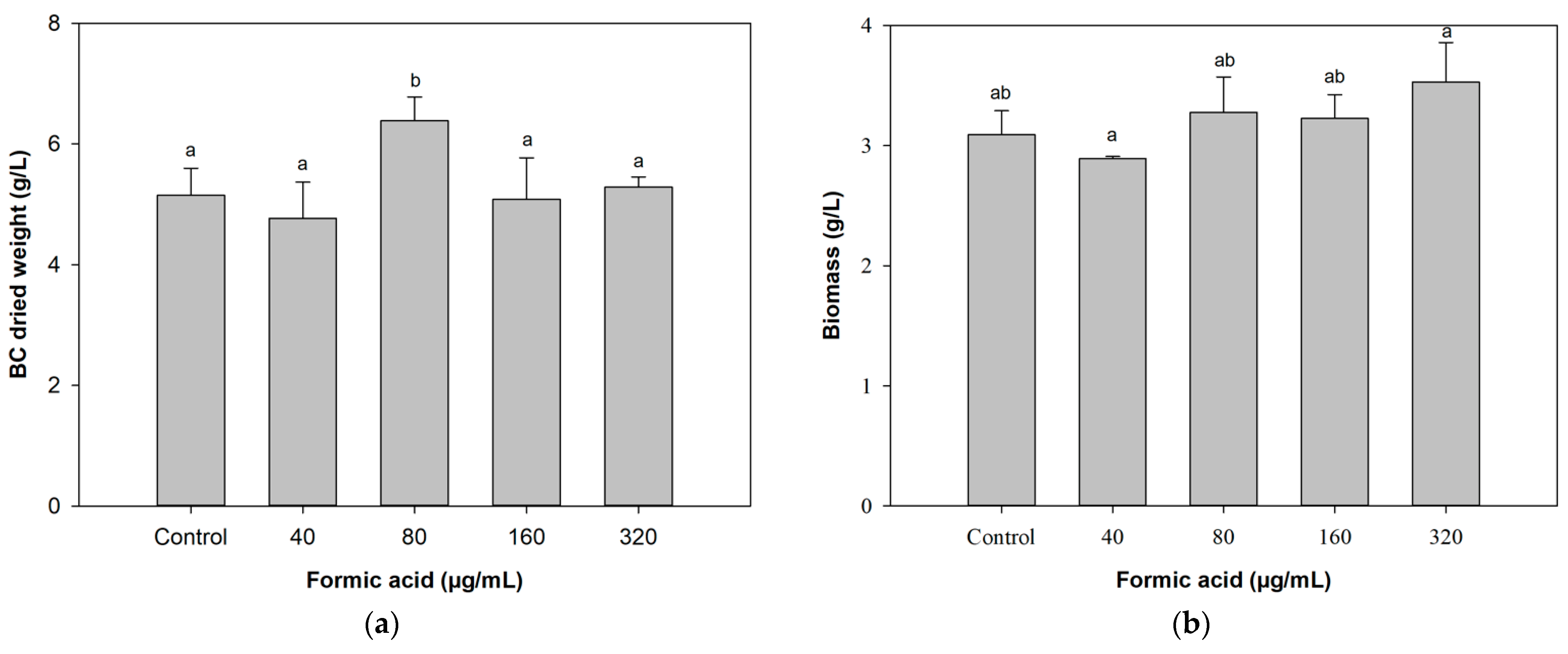
3.1. Effects of Different Concentrations of FA on BC Production
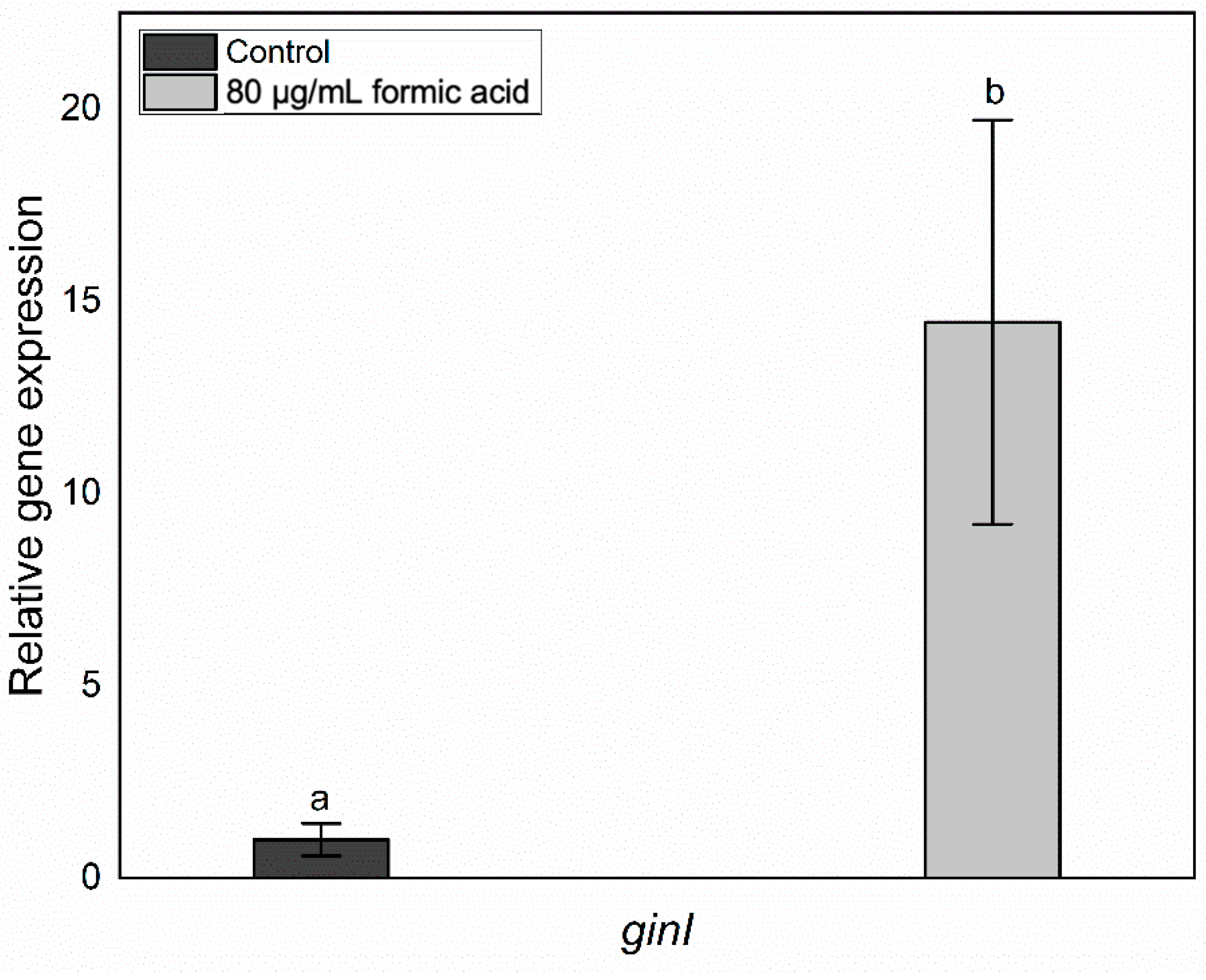
3.2. Effects of FA on QS-Related Genes Involved in BC Production
3.3. Morphology of the Produced BC
3.4. Characterization of the Produced BC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, R.M., Jr. Cellulose structure and biosynthesis: What is in store for the 21st century? J. Polym. Sci. Part A Polym. Chem. 2004, 42, 487–495. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Abd Rahman, N. Overview of bacterial cellulose production and application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Choi, S.M.; Shin, E.J. The nanofication and functionalization of bacterial cellulose and its applications. Nanomaterials 2020, 10, 406. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ning, D.; Xu, D.; Cheng, Y.; Mondal, A.K.; Zou, Q.; Zhu, H.; Huang, F. Preparation and characterization of super hydrophobic aerogels derived from tunicate cellulose nanocrystals. Carbohydr. Res. 2022, 511, 108488. [Google Scholar] [CrossRef] [PubMed]
- Nakagaito, A.; Iwamoto, S.; Yano, H. Bacterial cellulose: The ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl. Phys. A 2005, 80, 93–97. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, T.; Park, J.K. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr. Polym. 2012, 88, 596–603. [Google Scholar] [CrossRef]
- Lin, S.-P.; Calvar, I.L.; Catchmark, J.M.; Liu, J.-R.; Demirci, A.; Cheng, K.-C. Biosynthesis, production and applications of bacterial cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 1–10. [Google Scholar] [CrossRef]
- Schrecker, S.; Gostomski, P. Determining the water holding capacity of microbial cellulose. Biotechnol. Lett. 2005, 27, 1435–1438. [Google Scholar] [CrossRef]
- Dayal, M.S.; Catchmark, J.M. Mechanical and structural property analysis of bacterial cellulose composites. Carbohydr. Polym. 2016, 144, 447–453. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.; Chen, S.; Li, Z.; Sun, X.; Feng, C.; Wang, H.; Xu, Y. In vitro biodegradability of bacterial cellulose by cellulase in simulated body fluid and compatibility in vivo. Cellulose 2016, 23, 3187–3198. [Google Scholar] [CrossRef]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. Part A 2006, 76, 431–438. [Google Scholar] [CrossRef]
- Lin, S.-P.; Liu, C.-T.; Hsu, K.-D.; Hung, Y.-T.; Shih, T.-Y.; Cheng, K.-C. Production of bacterial cellulose with various additives in a PCS rotating disk bioreactor and its material property analysis. Cellulose 2016, 23, 367–377. [Google Scholar] [CrossRef]
- Santoso, S.P.; Chou, C.-C.; Lin, S.-P.; Soetaredjo, F.E.; Ismadji, S.; Hsieh, C.-W.; Cheng, K.C. Enhanced production of bacterial cellulose by Komactobacter intermedius using statistical modeling. Cellulose 2020, 27, 2497–2509. [Google Scholar] [CrossRef]
- Lin, S.-P.; Huang, Y.-H.; Hsu, K.-D.; Lai, Y.-J.; Chen, Y.-K.; Cheng, K.-C. Isolation and identification of cellulose-producing strain Komagataeibacter intermedius from fermented fruit juice. Carbohydr. Polym. 2016, 151, 827–833. [Google Scholar] [CrossRef]
- Santoso, S.P.; Lin, S.-P.; Wang, T.-Y.; Ting, Y.; Hsieh, C.-W.; Yu, R.-C.; Angkawijaya, A.E.; Soetaredjo, F.E.; Hsu, H.-Y.; Cheng, K.-C. Atmospheric cold plasma-assisted pineapple peel waste hydrolysate detoxification for the production of bacterial cellulose. Int. J. Biol. Macromol. 2021, 175, 526–534. [Google Scholar] [CrossRef]
- Lin, S.-P.; Huang, S.-H.; Ting, Y.; Hsu, H.-Y.; Cheng, K.-C. Evaluation of detoxified sugarcane bagasse hydrolysate by atmospheric cold plasma for bacterial cellulose production. Int. J. Biol. Macromol. 2022, 204, 136–143. [Google Scholar] [CrossRef]
- Liu, M.; Liu, L.; Jia, S.; Li, S.; Zou, Y.; Zhong, C. Complete genome analysis of Gluconacetobacter xylinus CGMCC 2955 for elucidating bacterial cellulose biosynthesis and metabolic regulation. Sci. Rep. 2018, 8, 6266. [Google Scholar] [CrossRef]
- Iida, A.; Ohnishi, Y.; Horinouchi, S. Control of acetic acid fermentation by quorum sensing via N-acylhomoserine lactones in Gluconacetobacter intermedius. J. Bacteriol. 2008, 190, 2546–2555. [Google Scholar] [CrossRef]
- Lin, S.-P.; Kuo, T.-C.; Wang, H.-T.; Ting, Y.; Hsieh, C.-W.; Chen, Y.-K.; Hsu, H.-Y.; Cheng, K.-C. Enhanced bioethanol production using atmospheric cold plasma-assisted detoxification of sugarcane bagasse hydrolysate. Bioresour. Technol. 2020, 313, 123704. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Hsiao, H.-C.; Hou, Y.-C.; Hsieh, C.-W.; Hsu, H.-Y.; Chen, H.-Y.; Lin, S.-P. Improvement in violacein production by utilizing formic acid to induce quorum sensing in Chromobacterium violaceum. Antioxidant 2022, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Andriani, D.; Apriyana, A.Y.; Karina, M. The optimization of bacterial cellulose production and its applications: A review. Cellulose 2020, 27, 6747–6766. [Google Scholar] [CrossRef]
- Iida, A.; Ohnishi, Y.; Horinouchi, S. Identification and characterization of target genes of the GinI/GinR quorum-sensing system in Gluconacetobacter intermedius. Microbiology 2009, 155, 3021–3032. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-Z.; Liu, L.-P.; Ye, L.; Li, W.-C.; Xin, B.; Xie, Y.-Y.; Jia, S.-R.; Wang, T.-F.; Zhong, C. The production of bacterial cellulose in Gluconacetobacter xylinus regulated by luxR overexpression of quorum sensing system. Appl. Microbiol. Biotechnol. 2021, 105, 7801–7811. [Google Scholar] [CrossRef]
- Das, S.; Das, S.; Ghangrekar, M. Bacterial signalling mechanism: An innovative microbial intervention with multifaceted applications in microbial electrochemical technologies: A review. Bioresour. Technol. 2022, 344, 126218. [Google Scholar] [CrossRef]
- Ansaldi, M.; Marolt, D.; Stebe, T.; Mandic-Mulec, I.; Dubnau, D. Specific activation of the Bacillus quorum-sensing systems by isoprenylated pheromone variants. Mol. Microbiol. 2002, 44, 1561–1573. [Google Scholar] [CrossRef]
- Haque, S.; Ahmad, F.; Dar, S.A.; Jawed, A.; Mandal, R.K.; Wahid, M.; Lohani, M.; Khan, S.; Singh, V.; Akhter, N. Developments in strategies for Quorum Sensing virulence factor inhibition to combat bacterial drug resistance. Microb. Pathog. 2018, 121, 293–302. [Google Scholar] [CrossRef]
- Kim, E.-M.; Woo, H.M.; Tian, T.; Yilmaz, S.; Javidpour, P.; Keasling, J.D.; Lee, T.S. Autonomous control of metabolic state by a quorum sensing (QS)-mediated regulator for bisabolene production in engineered E. coli. Metab. Eng. 2017, 44, 325–336. [Google Scholar] [CrossRef]
- Shen, Y.-P.; Fong, L.S.; Yan, Z.-B.; Liu, J.-Z. Combining directed evolution of pathway enzymes and dynamic pathway regulation using a quorum-sensing circuit to improve the production of 4-hydroxyphenylacetic acid in Escherichia coli. Biotechnol. Biofuels 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Morohoshi, T.; Kato, M.; Fukamachi, K.; Kato, N.; Ikeda, T. N-acylhomoserine lactone regulates violacein production in Chromobacterium violaceum type strain ATCC 12472. FEMS Microbiol. Lett. 2008, 279, 124–130. [Google Scholar] [CrossRef]
- Wang, H.; Wu, P.; Zheng, D.; Deng, L.; Wang, W. N-Acyl-homoserine lactone (AHL)-mediated microalgal–bacterial communication driving chlorella-activated sludge bacterial biofloc formation. Environ. Sci. Technol. 2022, 56, 12645–12655. [Google Scholar] [CrossRef]
- Ruhs, P.A.; Malollari, K.G.; Binelli, M.R.; Crockett, R.; Balkenende, D.W.; Studart, A.R.; Messersmith, P.B. Conformal bacterial cellulose coatings as lubricious surfaces. ACS Nano 2020, 14, 3885–3895. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Lionetto, F.; Nisi, R.; Stoppa, M.; Licciulli, A. Sustainable production of stiff and crystalline bacterial cellulose from orange peel extract. Sustainability 2022, 14, 2247. [Google Scholar] [CrossRef]
- De Lima Fontes, M.; Meneguin, A.B.; Tercjak, A.; Gutierrez, J.; Cury, B.S.F.; Dos Santos, A.M.; Ribeiro, S.J.; Barud, H.S. Effect of in situ modification of bacterial cellulose with carboxymethylcellulose on its nano/microstructure and methotrexate release properties. Carbohydr. Polym. 2018, 179, 126–134. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Subhan, F.; Islam, S.U.; Khan, S.; Shah, N.; Manan, S.; Ullah, M.W.; Yang, G. Development of three-dimensional bacterial cellulose/chitosan scaffolds: Analysis of cell-scaffold interaction for potential application in the diagnosis of ovarian cancer. Int. J. Biol. Macromol. 2019, 137, 1050–1059. [Google Scholar] [CrossRef]
- Lin, S.-P.; Hsieh, S.-C.; Chen, K.-I.; Demirci, A.; Cheng, K.-C. Semi-continuous bacterial cellulose production in a rotating disk bioreactor and its materials properties analysis. Cellulose 2014, 21, 835–844. [Google Scholar] [CrossRef]
- Ciecholewska-Juśko, D.; Broda, M.; Żywicka, A.; Styburski, D.; Sobolewski, P.; Gorący, K.; Migdał, P.; Junka, A.; Fijałkowski, K. Potato juice, a starch industry waste, as a cost-effective medium for the biosynthesis of bacterial cellulose. Int. J. Mol. Sci. 2021, 22, 10807. [Google Scholar] [CrossRef]
- Adepu, S.; Khandelwal, M. Drug release behaviour and mechanism from unmodified and in situ modified bacterial cellulose. Proc. Indian Natl. Sci. Acad. 2021, 87, 110–120. [Google Scholar] [CrossRef]
- Gorgieva, S. Bacterial cellulose as a versatile platform for research and development of biomedical materials. Processes 2020, 8, 624. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Quero, F.; Blaker, J.J.; Hill, C.A.; Eichhorn, S.J.; Bismarck, A. Surface only modification of bacterial cellulose nanofibres with organic acids. Cellulose 2011, 18, 595–605. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Q.; Han, Z.; Li, J.; Liao, B.; Hu, L.; Huang, J.; Zou, C.; Jia, C.; Huang, J. Comparison of bacterial nanocellulose produced by different strains under static and agitated culture conditions. Carbohydr. Polym. 2020, 227, 115323. [Google Scholar] [CrossRef] [PubMed]
- Molina-Ramírez, C.; Enciso, C.; Torres-Taborda, M.; Zuluaga, R.; Gañán, P.; Rojas, O.J.; Castro, C. Effects of alternative energy sources on bacterial cellulose characteristics produced by Komagataeibacter medellinensis. Int. J. Biol. Macromol. 2018, 117, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T.; Kait, C.F.; Murugesan, T. A “Fourier Transformed infrared” compound study of lignin recovered from a formic acid process. Procedia Eng. 2016, 148, 1312–1319. [Google Scholar] [CrossRef]
- Jebel, F.S.; Almasi, H. Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films. Carbohydr. Polym. 2016, 149, 8–19. [Google Scholar] [CrossRef]
- Wang, L.-F.; Rhim, J.-W. Preparation and application of agar/alginate/collagen ternary blend functional food packaging films. Int. J. Biol. Macromol. 2015, 80, 460–468. [Google Scholar] [CrossRef]
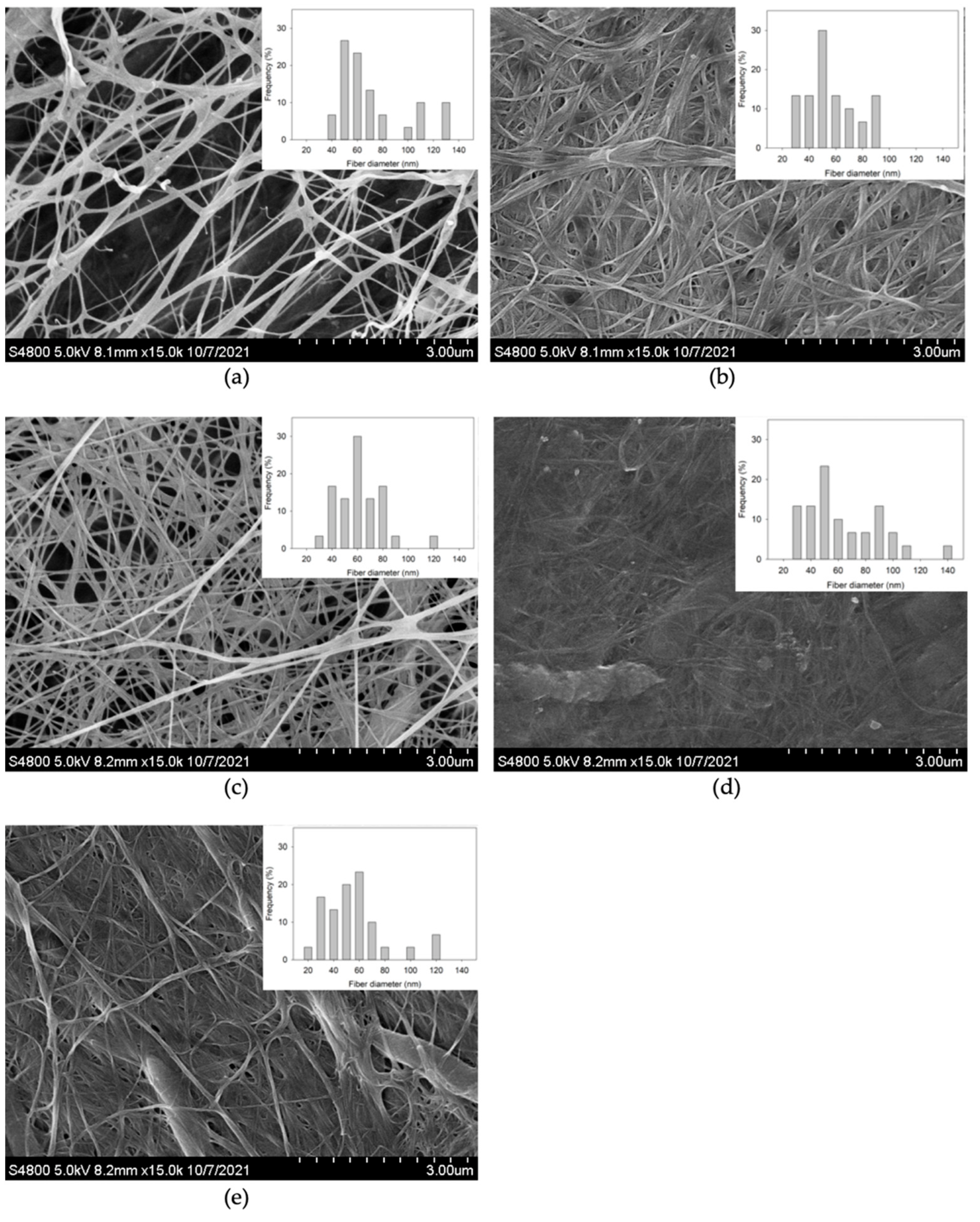

| Addition of FA (µg/mL) | Water Content (%) | Crystallinity (%) | Major Tpeak (°C) | Fiber Size Average (nm) |
|---|---|---|---|---|
| Control | 98.47 ± 0.07 a | 83.1 ± 4 a | 339.5 | 68 ± 27 a |
| 40 | 98.58 ± 0.09 a | 80.9 ± 3.1 a | 346.6 | 52 ± 17.9 a |
| 80 | 98.41 ± 0.08 a | 80.4 ± 2.7 a | 361.1 | 57 ± 18.4 a |
| 160 | 98.57 ± 0.01 a | 84 ± 1.2 a | 359.3 | 60.1 ± 25.7 a |
| 320 | 98.53 ± 0.07 a | 81.6 ± 1.8 a | 354.7 | 52 ± 23.6 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-Y.; Santoso, S.P.; Lin, S.-P. Using Formic Acid to Promote Bacterial Cellulose Production and Analysis of Its Material Properties for Food Packaging Applications. Fermentation 2022, 8, 608. https://doi.org/10.3390/fermentation8110608
Chen T-Y, Santoso SP, Lin S-P. Using Formic Acid to Promote Bacterial Cellulose Production and Analysis of Its Material Properties for Food Packaging Applications. Fermentation. 2022; 8(11):608. https://doi.org/10.3390/fermentation8110608
Chicago/Turabian StyleChen, Tzu-Yu, Shella Permatasari Santoso, and Shin-Ping Lin. 2022. "Using Formic Acid to Promote Bacterial Cellulose Production and Analysis of Its Material Properties for Food Packaging Applications" Fermentation 8, no. 11: 608. https://doi.org/10.3390/fermentation8110608
APA StyleChen, T.-Y., Santoso, S. P., & Lin, S.-P. (2022). Using Formic Acid to Promote Bacterial Cellulose Production and Analysis of Its Material Properties for Food Packaging Applications. Fermentation, 8(11), 608. https://doi.org/10.3390/fermentation8110608






