Abstract
The aim of this research was to evaluate the effect of the inclusion of Acacia mearnsii (AM) at different levels of inclusion on ruminal digestion and in vitro gas production. A. mearnsii forage was incorporated in the diet at different levels of 0 (AM0), 20 (AM20), and 40 (AM40) %. In situ degradation of dry matter (DM) and organic matter (OM) showed differences between treatments (P < 0.05), obtaining the highest value of the degradation of soluble fraction (A), insoluble but potentially degradable fraction (B), degradation rate in % per hour (c), potential degradation (A + B), and effective degradation for all passage rates in % h (0.02, 0.05, and 0.08) in AM0 with respect to AM20 and AM40. The in vitro digestibility of DM and OM was higher (P < 0.05) in AM0 with approximately 23.6% and 22.8% of DM and OM, respectively, compared to treatments AM20 and AM40. Cumulative gas production (PG) and gas production asymptote (B) were lower at AM0 and AM20 versus AM40; however, gas production rate (c) and total CH4 production were lower at AM40 with about 40.1 mL CH4/0.500 g fermented DM versus AM0 and AM20. Under the conditions of this study, it is concluded that the incorporation of A. mearnsii (20% and 40%) in the feed of ruminants negatively affected the digestion of nutrients; however, it reduced the production of CH4, which may be associated with the low activity of microorganisms toward the substrate due to the possible tannin/nutrient complex. This shows that in animals with little history of consuming plants rich in tannin, more than 3% of tannin could not be incorporated into the diet.
1. Introduction
Ruminant production systems around the world are influenced by the geographical, agroecological, and socioeconomic conditions of the region in which they are located [1,2,3]. Those systems that implement extensive grazing are limited by the predominant forage species in the meadow and its management, reflecting on the quality of the food ingested by the animal, productive performance, and emission of greenhouse gases (GHG) [4,5].
The production of ruminants under extensive systems is generally characterized by having monocultures of variable forages in their botanical composition and nutritional value [6,7] which, in most cases, predispose to the production of GHG, mainly: Methane (CH4), carbon dioxide (CO2), and nitrous oxide (N2O) [8], in response to the high amount of structural carbohydrates (cellulose, hemicellulose) and low protein intake [6]. These components promote considerable energy losses in the animal (2–12%), reflecting low productive performance [9]. Ruminants will generate approximately 18% of GHG and contribute about 13-19% of CH4 [10,11] and 9% of CO2 worldwide [12]. Although there is evidence that CH4 is the second most abundant GHG after CO2, its polluting potential (21-28 times greater) worries the world population and encourages the search for alternatives to remedy this problem [11,13,14].
Under this background, the use of unconventional food resources, agro-industrial residues, fodder trees and shrubs are proposed as possible solutions due to their high nutritional value, presence of bioactive compounds (tannins, saponins, essential oils, etc.) [15,16], reduction in competition with foods used in human nutrition, substitution of expensive raw materials in the formulation of rations, and reduction in GHG [17,18,19]. Leguminous trees or shrubs rich in bioactive compounds can reduce GHG (CH4), due to the presence of polyphenols [condensed tannins (TC) and hydrolyzed tannins (TH)] [20]. Tannins decrease CH4 biosynthesis directly by inhibiting methanogen microorganisms or indirectly by reducing the population of ruminal protozoa [21,22]. The reduction in CH4 in the rumen is possibly explained by: (1) The indirect formation of tannin/nutrient complexes (carbohydrate, protein, lipid) and subsequent reduction in substrate for microbial degradation, (2) direct action of tannin on methanogenic archaea by binding to the protein adhesin or parts of the cell envelope of the microorganism, which consequently inhibits the growth of methanogen in response to the inefficient transfer of H2 between species (methanogen-protozoan), and (3) reduction in H2 available for the formation of CH4 in response to the presence of degraded TC subunits in the rumen that function as H2 sinks [23]. However, the effects of tannins have not been consistent due to their constant variability, facts related to the source, dose, type, molecular weight, and adaptability of ruminants to the intake [24,25].
Some tree species, such as those found in the Acacia genus, are often abundant and their foliage can provide high levels of protein to ruminant diets. Acacia mearnsii is classified as a legume rich in tannins with the potential to reduce ruminal methanogenesis and improve ammoniacal nitrogen (NH3) in the rumen; however, negative effects have been shown on the digestibility of nutrients that could limit its use as a food source [26]. However, moderate amounts of tannins (20–40 g/kg DM) in ruminant diets may be favorable and inhibit the negative effects [27] attributed to the ability of tannins to form complexes with protein and protect them from degradation in the rumen as well as raise their flow to the duodenum where they will be absorbed more efficiently [28]. Based on this background, the objective of this research was to determine the effect of the addition of Acacia mearnsii at different levels of substitution of the main feed source and its effect on the in situ ruminal degradation kinetics and in vitro rumen fermentation.
2. Materials and Methods
2.1. Study Location
The present research was carried out at “Querochaca” Experimental Farm and Rumenology Laboratory of the Universidad Técnica de Ambato, Facultad de Ciencias Agropecuarias, Tungurahua, Ecuador, at an altitude of 2890 m above sea level. In the sector, there are maximum temperatures of 20 °C and minimum of 7 °C and an average ambient temperature of 15 °C.
2.2. Animals
Six three-year-old Holstein bulls with an average live weight of 450 ± 21.2 kg, provided with a fistula with a cannula in the rumen (Bar Diamond, Parma, ID, USA) were used. The animals were housed in individual pens with a zinc roof and cement floor and access to a diet based on 20% Medicago sativa and 80% Lolium perenne as well as water ad libitum.
2.3. Fodder Samples and Treatments
The A. mearnsii forage was collected from a two-year-old plantation at the Faculty of Agricultural Sciences-UTA (abbreviation in Spanish) and subjected to a cutting frequency of 90 d. Subsequently, the forage (leaves and young stems) was dehydrated under cover in a greenhouse (50 kg). The dehydrated forage was ground in a hammer mill to a particle size of 2 mm and proceeded to be incorporated in the following treatments (Table 1). Six repetitions were performed for each treatment (n = 6), and prior to mixing the treatments, the forages were separately passed through a 1 mm sieve to homogenize the particle size. The A. mearnsii forage contained (%): 22.4, 91.1, 23.6, 39.1, 18.8, 9.1, and 15.8 of dry matter (DM), organic matter (OM), crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber (ADF), metabolizable energy (ME), and condensed tannins (CT), respectively. Forage of A. mearnsii was included in the diets at different levels of 0 (AM0), 20 (AM20), and 40 (AM40) % prior to the evaluation of diets.

Table 1.
Chemical composition of diets with increasing levels of Acacia mearnsii (AM in % except where otherwise noted).
2.4. Rumen Degradation
In situ rumen degradation of nutrients was estimated following the nylon bag methodology (0.42 µ) in the rumen described by Ørskov et al. [29]. In each bull (n = 6), a bag with 5 g of each diet was incubated at the following times (hours): 3, 6, 12, 24, 48, 72, and 96 h. At the end of 96 h, the bags were removed, washed with running water, and dried at 60 °C. The bags used to measure the loss by washing (0 h), were not incubated in the rumen and were only washed with tap water. The residues were stored in polyethylene bags at -4 °C until their subsequent analysis in the laboratory. Nutrient disappearance was calculated as a ratio of incubated and residual material. The data were fitted to the equation: Y = a + b (1-e-ct) and the effective degradation was fitted using the equation DE = a + [(b*c)/(c + k)] considering a rate of passage (k) of 0.02%, 0.05%, and 0.08% [30], (Prisma 4, GraphPad Software, Inc. of San Diego, CA, USA).
2.5. Gas, CH4 Production, and In Vitro Digestibility
Rumen content (liquid and solid fraction) was obtained separately from each bull (n = 6). The ruminal content was collected before feeding in the morning and stored in plastic containers, transported to the laboratory to be processed within the first hour of collection. The preparation of media rich in nitrogen (artificial saliva) was carried out as described by Menke and Steingass [31]. Gas and CH4 production were established using the methodology described by Theodorou et al. [32], which consists of placing 0.500 g of sample of each one of the treatments AM0, AM20, and AM40 in amber glass bottles with a capacity of 100 mL. About 60 mL of the inoculum (70:30 medium; artificial saliva/inoculum; ruminal content) were incubated in the bottles under a constant flow of CO2. The bottles were incubated between 39–40 °C, and gas pressure and volume measurements were taken manually at the following times 3, 6, 9, 12, 24, 36, 48, 72, and 96 h post-incubation with a pressure transducer (DO 9704, Delta OHM, Casella, Italy) and plastic syringes. CH4 production was quantified with a GAS Detection analyzer, model GX–6000, UK following the methodology described by Elghandour et al. [33]. For each treatment, 6 bottles were used, and three additional bottles were used as blanks. At the end of 96 h, the data were fitted to the monobasic equation mL gas= GV (1 + (B/t)C)−1 described by Groot et al. [34]. Additionally, six more flasks for each treatment were incubated up to 48 h to estimate the in vitro digestibility of DM and OM. Gas data were reported in mL/0.500 g of fermented DM.
2.6. Rumen pH
Under the same procedure mentioned above for gas production and digestibility, 6 amber glass flasks were prepared for each treatment and at each time (4, 8, 12, and 24 h post-incubation) ruminal pH was measured with the help of a pH meter (BANTE-221 Portable pH/ORP Meter, London, UK).
2.7. Chemical Analysis
The dry matter (DM) (# 7007) and ash (# 7009) were determined according to the AOAC [35]. Neutral detergent fiber (NDF) and acid detergent fiber (ADF) were determined using methods 12 and 13, respectively, ANKOM2000 fiber analyzer (ANKOM Technology, Macedon, NY, USA). CP was determined by elemental analysis (N) using a LECO CHN 628 (LECO Corporation, MI, USA). Condensed tannins were determined by vanillin assay (catechin equivalent, Price et al. [36]).
2.8. Experimental Design and Statistical Analysis
A completely randomized design was used, with three treatments and six repetitions. All variables were analyzed according to the design used by means of a simple classification ANOVA [37]. Means were compared using Tukey’s test. All variables were analyzed using the SAS [38] (version 9.2, SAS Institute, Cary, NC, USA).
3. Results
3.1. In Situ Rumen Degradation Kinetics
In situ degradation of DM and OM showed differences between treatments (p < 0.05), with AM0 showing the highest degradation of the soluble fraction (A), insoluble but potentially degradable fraction (B), degradation rate in % per hour (c), potential degradation (A + B), and effective degradation for all passage rates in % h (0.02, 0.05, and 0.08) with respect to AM20 and AM40 (Table 2).

Table 2.
In situ rumen degradation kinetics of DM and OM (%) of diets with increasing levels of Acacia Mearnsii (AM).
3.2. In Vitro Digestibility and Rumen pH
The in vitro digestibility of DM and OM showed differences (p < 0.05) between treatments, showing in AM0 (66.9% and 69.5%, respectively) higher digestibility compared to AM20 (DM: 55.6% and OM: 57.5%) and AM40 (DM: 46.6% and OM: 49.6%). Ruminal pH did not show differences between treatments in any of the evaluated hours (4, 8, 12, and 24 h) (p = 0.9170, 0.8387, 0.5716, and 0.5322, respectively), as shown in Table 3.

Table 3.
Digestibility (%) and ruminal pH of diets with increasing levels of Acacia Mearnsii (AM).
3.3. Gas and CH4 Production
Gas and CH4 production showed differences (p < 0.05) between treatments. Cumulative gas production (GP) and gas production asymptote (B) were lower in AM0 and AM20 compared to AM40. CH4 production was lower in the AM40 treatment with approximately 40.15 mL CH4/0.500 g fermented DM compared to AM0 and AM20. The % CH4 generated with respect to the total gas produced was lower (p = 0.0001) in AM40 (20.9%) compared to AM0 and AM20 (35.9% and 31.5%, respectively).
However, at 48 and 96 h of the AM40 treatment, the lowest (p = 0.0030 and 0.0001, respectively) production of CH4 (84.9 and 92.3 mL CH4/0.500 g fermentable DM, respectively) is observed with respect to AM0 and AM20 (Table 4). Figure 1A shows that from 3 h post-incubation, gas production kinetics began, with a marked rise to 96 h in all treatments. With respect to the CH4 production kinetics, it began at 6 h in AM0, and at 9 h in AM20 and AM40, stabilizing in all treatments at 48 h post-incubation and showing an increase in CH4 production of AM0 and AM20 (Figure 1B).

Table 4.
Gas and CH4 production parameters (mL/0.500 g fermentable DM) of diets with increasing levels of Acacia mearnsii (AM).
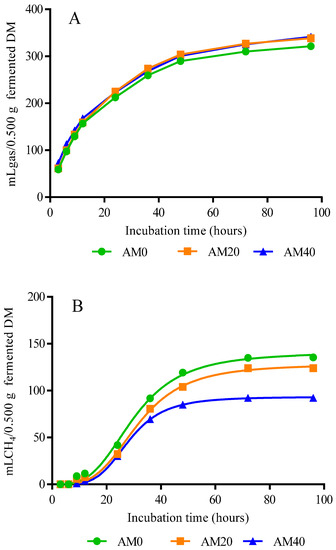
Figure 1.
Gas (A) and CH4 (B) production kinetics of diets with increasing levels of Acacia mearnsii..
4. Discussion
The exploration of unconventional forage resources rich in secondary compounds (tannins, saponins, essential oils, etc.) useful for feeding ruminants in recent years has grown considerably, with the purpose of taking advantage of the beneficial effect of tannin on the use of nutrients (carbohydrates, proteins, and lipids) and the production of CH4 in the rumen [22,39]. However, the beneficial or detrimental effect of tannins will depend on factors such as: Dose, type, molecular weight, and the adaptation of the animals to their consumption [25]. Therefore, in the present study, it was proposed to evaluate the effect of the incorporation of A. mearnsii on the characteristics of ruminal fermentation and CH4 production.
4.1. Rumen Degradation Kinetics and Digestibility
The higher in situ rumen degradation kinetics and in vitro digestibility of DM and OM observed in treatment AM0 (Table 2 and Table 3, respectively) is probably due to the higher use of the protein in the rumen, in response to the inhibition of the formation of tannin-protein complexes due to the absence of tannins in the diet (Table 1), and the subsequent attainment of amino acids, peptides, ammoniacal nitrogen (NH3-N), and volatile fatty acids (VFA) useful for growth and activity of cellulolytic microorganisms that improve their accessibility on the substrate [40,41,42]. However, the lower in situ rumen degradation and in vitro digestibility observed in AM20 and AM40 is probably due to the negative effect of tannins on fiber degradation [43], their toxic effect on ruminal microorganisms, especially in animals that have not been adapted to the consumption of these secondary metabolites [25]. The mechanism by which microbial activity is affected by tannins in the rumen is probably due to the ability of tannin to complex with nutrients (protein, fiber, and lipids) and inhibit microbial enzymatic activity [44,45]. These results are consistent with those reported in [46,47,48].
The existing scientific evidence suggests that the effect of tannin differs according to the type, dose, source, chemical structure, molecular weight, and adaptation of the animals to its consumption [24,25]. High concentrations of tannins in ruminant feed can cause accidental poisoning with high risk to animal health, daily feed intake, and productive performance, due to: (1) Predisposition to intoxication due to the consumption of high levels of tannins (>55 g CT/Kg DM) and the subsequent destruction of the intestinal mucosa, liver, and kidney [25,27,49], (2) decreased palatability of the food in response to the binding of salivary glycoproteins to tannin [25,50], (3) low digestibility and lower rate of passage of the substrate, which implies low food consumption in response to the feeling of satiety caused by the presence of feed in the rumen [25,51], and (4) low intestinal activity of pancreatic enzymes (trypsin and amylase) and decreased synthesis of amino acids [25,52]. These are the reasons why it is important to evaluate nutritional alternatives to improve and preserve animal welfare and its productive capacity.
4.2. Gas and CH4 Production
The lower accumulated gas production shown in AM0 and AM20 (Table 4 and Figure 1A) is probably due to the higher digestibility and better utilization of nutrients (mainly protein), in response to the biological value of the feed components (rich in highly fermentable carbohydrates) [42]. In this context, Blümel et al. [53] proposed that the total volume of gas produced is inversely proportional to substrate digestibility and microbial protein synthesis. As evidenced in the present study in AM40 with the highest total gas production and lower digestibility (Table 3 and Table 4), this is probably due to poor protein utilization and lower microbial protein synthesis in response to the limited access of microorganisms on the fibrous component of the substrate, and the reduced ability of enzymes to access protein (complex tannin/nutrient; protein and fiber) [53]. Barros-Rodríguez et al. [54] found the same trend and showed that the greater production of total gas was associated with a lower synthesis of microbial proteins.
However, the lower CH4 production observed in the present study at AM40 (Table 4 and Figure 1B) is probably attributed to the indirect effect of tannins on fiber digestion and the consequent reduction in H2 generated during the formation of acetic acid from pyruvate, which will later be used as a substrate for the reduction in CO2 to CH4 [55] or directly through the inhibition of methanogenic microorganisms (methanogenic archaea) by binding to the adhesin protein and subsequent inhibition in the formation of methanogen-protozoan complexes that reduce the ability to exchange H2 between species, as well as the growth and activity of methanogens [22,56,57]. These results are consistent with those reported by Vargas-Ortiz et al. [25], Moss et al. [58], and Aragadvay-Yungán et al. [59].
4.3. Rumen pH
The rumen pH evidenced in the present study of Table 3 was not altered by the presence of tannins, which is in an optimal range to promote a balanced microbial cellulolytic and proteolytic activity for the synthesis of microbial protein [60,61]. These results are consistent with those reported by Hariadi and Santoso [62], de Oliveira et al. [63], and Śliwiński et al. [64].
5. Conclusions
Under the conditions of this study, it is concluded that the incorporation of A. mearnsii (20% and 40%) in the feed of ruminants negatively affected the digestion of nutrients; however, it reduced the production of CH4, which may be associated with the low activity of microorganisms toward the substrate due to the possible tannin/nutrient complex. This shows that in animals with little history of consuming plants rich in tannin, more than 3% of tannin could not be incorporated into the diet.
Author Contributions
Conceptualization, L.V.-O., D.C.-G. and M.B.-R.; data tabulation, M.B.-R., R.L.-O. and A.Z.M.S.; writing—review and editing, M.B.-R., E.M.-R., V.A.-Y. and C.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the financial support from the Research and Development Direction (DIDE, acronym in Spanish) of the Technical University of Ambato, Ecuador, project (PFCAGP19).
Institutional Review Board Statement
All the procedures followed the recommendations of the ARRIVE Guidelines [Kilkenny et al. 2010] and were approved by the Ethical Committee of the Scientific Council of the Faculty of Agricultural Sciences at Universidad Central “Marta Abreu” de Las Villas (agreement 17/2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this paper are available on request from the corresponding author.
Acknowledgments
The authors thank the “Universidad Estatal Peninsula de Santa Elena” for facilitating the laboratories and equipment to carry out this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fusaro, I.; Cavallini, D.; Giammarco, M.; Manetta, A.C.; Martuscelli, M.; Mammi, L.M.E.; Lanzoni, L.; Formigoni, A.; Vignola, G. Oxidative Status of Marchigiana Beef Enriched in n-3 Fatty Acids and Vitamin E, Treated With a Blend of Oregano and Rosemary Essential Oils. Front. Vet. Sci. 2021, 8, 662079. [Google Scholar] [CrossRef]
- Buonaiuto, G.; Palmonari, A.; Ghiaccio, F.; Visentin, G.; Cavallini, D.; Campidonico, L.; Formigoni, A.; Mammi, L.M.E. Effects of complete replacement of corn flour with sorghum flour in dairy cows fed Parmigiano Reggiano dry hay-based ration. Ital. J. Anim. Sci. 2021, 20, 826–833. [Google Scholar] [CrossRef]
- Cavallini, D.; Mammi, L.M.E.; Palmonari, A.; García-González, R.; Chapman, J.D.; McLean, D.J.; Formigoni, A. Effect of an Immunomodulatory Feed Additive in Mitigating the Stress Responses in Lactating Dairy Cows to a High Concentrate Diet Challenge. Animals 2022, 12, 2129. [Google Scholar] [CrossRef]
- Zubieta, Á.S.; Savian, J.V.; de Souza Filho, W.; Wallau, M.O.; Gómez, A.M.; Bindelle, J.; François Bonnet, O.J.; de Faccio Carvalho, P.C. Does grazing management provide opportunities to mitigate methane emissions by ruminants in pastoral ecosystems? Sci. Total Environ. 2021, 754, 142029. [Google Scholar] [CrossRef]
- Buonaiuto, G.; Cavallini, D.; Mammi, L.M.E.; Ghiaccio, F.; Palmonari, A.; Formigoni, A.; Visentin, G. The accuracy of NIRS in predicting chemical composition and fibre digestibility of hay-based total mixed rations. Ital. J. Anim. Sci. 2021, 20, 1730–1739. [Google Scholar] [CrossRef]
- Barros-Rodríguez, M.; Oña-Rodríguez, J.; Mera-Andrade, R.; Artieda-Rojas, J.; Curay-Quispe, S.; Avilés-Esquivel, D.; Guishca-Cunuhay, C. Degradación ruminal de dietas a base de biomasa pos-cosecha de Amaranthus cruentus: Efecto sobre los protozoos del rumen y producción de gas in vitro. Rev. Investig. Vet. Perú 2017, 28, 812–821. [Google Scholar]
- Núñez Torres, O.P.; Barros Rodríguez, M.; Sanchez, D.; Guishca-Cunuhay, C. Comportamiento productivo, degradación ruminal y producción de gas in vitro en ovinos alimentados con dietas a base de residuos pos-cosecha de Chenopodium quinoa. Rev. Investig. Vet. Perú 2018, 9, 765–773. [Google Scholar]
- Gerber, P.J.; Hristov, A.N.; Henderson, B.; Makkar, H.; Oh, J.; Lee, C.; Meinen, R.; Montes, F.; Ott, T.; Firkins, J.; et al. Technical options for the mitigation of direct methane and nitrous oxide emissions from livestock: A review. Animal 2013, 7, 220–234. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Ani. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Haque, M.N. Dietary manipulation: A sustainable way to mitigate methane emissions from ruminants. J. Ani. Sci. Techno. 2018, 60, 15. [Google Scholar] [CrossRef]
- Sun, K.; Liu, H.; Fan, H. Research progress on the application of feed additives in ruminal methane emission reduction: A review. PeerJ 2021, 9, e11151. [Google Scholar] [CrossRef]
- Vallejo-Hernández, L.H.; Elghandour, M.M.; Greiner, R.; Anele, U.Y.; Rivas-Cáceres, R.R.; Barros-Rodríguez, M.; Salem, A.Z. Environmental impact of yeast and exogenous xylanase on mitigating carbon dioxide and enteric methane production in ruminants. J. Clean. Prod. 2018, 189, 40–46. [Google Scholar] [CrossRef]
- Slade, E.M.; Riutta, T.; Roslin, T.; Tuomisto, H.L. The role of dung beetles in reducing greenhouse gas emissions from cattle farming. Sci. Rep. 2016, 6, 18140. [Google Scholar] [CrossRef]
- Membrive, C.M.B. Anatomy and Physiology of the Rumen. In Rumenology; Millen, E.D., De Beni Arrigoni, M., Lauritano Pacheco, R., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–38. [Google Scholar] [CrossRef]
- Buenaño-Sanchez, M.; Barros-Rodriguez, M.; Zurita-Vasquez, H.; Hidalgo, L.; Perez-Aldas, L.; Arias-Toro, D. Effect of drying temperature of passiflora edulis residues on rumen degradation kinetics and enteric CH4 and CO2 production. Trop. Subtrop. Agroecosyst. 2019, 22. Available online: https://www.revista.ccba.uady.mx/ojs/index.php/TSA/article/view/3081/1338 (accessed on 11 October 2022).
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial By-Products Rich in Polyphenols be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef]
- Pacheco, D.; Waghorn, G.; Janssen, P.H. Decreasing methane emissions from ruminants grazing forages: A fit with productive and financial realities? Anim. Prod. Sci. 2014, 54, 1141–1154. [Google Scholar] [CrossRef]
- Choudhary, S.; Santra, A.; Muwel, N.; Sarkar, S.; Mandal, A.; Das, S.K. Screening of forest tree leaves from North Eastern Himalayan region as feed additives for modulating in vitro rumen fermentation and methanogenesis from total mixed ration. Agroforest Syst. 2022, 96, 359–374. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I. A review on the use of agro-industrial Co-products in animals’ diets. Ital. J. Anim. Sci. 2022, 21, 577–594. [Google Scholar] [CrossRef]
- Lagrange, S.; Beauchemin, K.A.; MacAdam, J.; Villalba, J.J. Grazing diverse combinations of tanniferous and non-tanniferous legumes: Implications for beef cattle performance and environmental impact. Sci. Total Environ. 2020, 746, 140788. [Google Scholar] [CrossRef]
- Boussaada, A.; Arhab, R.; Calabrò, S.; Grazioli, R.; Ferrara, M.; Musco, N.; Thlidjane, M.; Cutrignelli, M.I. Effect of Eucalyptus globulus leaves extracts on in vitro rumen fermentation, methanogenesis, degradability and protozoa population. Ann. Anim. Sci. 2018, 18, 753–767. [Google Scholar] [CrossRef]
- Ku-Vera, J.C.; Jiménez-Ocampo, R.; Valencia-Salazar, S.S.; Montoya-Flores, M.D.; Molina-Botero, I.C.; Arango, J.; Solorio-Sánchez, F.J. Role of secondary plant metabolites on enteric methane mitigation in ruminants. Front. Vet. Sci. 2020, 584. [Google Scholar] [CrossRef]
- Tedeschi, L.O.; Muir, J.P.; Naumann, H.D.; Norris, A.B.; Ramírez-Restrepo, C.A.; Mertens-Talcott, S.U. Nutritional aspects of ecologically relevant phytochemicals in ruminant production. Front. Vet. Sci. 2021, 8, 155. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Beauchemin, K.A. Potential of molecular weight and structure of tannins to reduce methane emissions from ruminants: A review. Animals 2019, 9, 856. [Google Scholar] [CrossRef]
- Vargas-Ortiz, L.; Andrade-Yucailla, V.; Barros-Rodríguez, M.; Lima-Orozco, R.; Macías-Rodríguez, E.; Contreras-Barros, K.; Guishca-Cunuhay, C. Influence of Acacia mearnsii Fodder on Rumen Digestion and Mitigation of Greenhouse Gas Production. Animals 2022, 12, 2250. [Google Scholar] [CrossRef]
- Ibrahim, S.L.; Hassen, A. Effects of Graded Levels of Mimosa (Acacia mearnsii) Tannin Purified with Organic Solvents on Gas, Methane, and In Vitro Organic Matter Digestibility of Eragrostis curvula Hay. Animals 2022, 12, 562. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Tech. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Piñeiro-Vázquez, A.T.; Jiménez-Ferrer, G.; Alayon-Gamboa, J.A.; Chay-Canul, A.J.; Ayala-Burgos, A.J.; Aguilar-Pérez, C.F.; Ku-Vera, J.C. Effects of quebracho tannin extract on intake, digestibility, rumen fermentation, and methane production in crossbred heifers fed low-quality tropical grass. Trop. Anim. Health Pro. 2018, 50, 29–36. [Google Scholar] [CrossRef]
- Ørskov, E.R.; Hovell, D.; Mould, F. The use of the nylon bag technique for the evaluation of feedstuffs. Trop. Anim. Pro. 1980, 5, 195–213. [Google Scholar]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; Mcallan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminants feeds. Anim. Feed Sci. Tech. 1994, 48, 185–197. [Google Scholar]
- Elghandour, M.M.Y.; Rodríguez-Ocampo, I.; Parra-Garcia, A.; Salem, A.Z.M.; Greiner, R.; Marquez-Molina, O.; Barros-Rodriguez, M.; Barbabosa-Pilego, A. Biogas production from prickly pear cactus containing diets supplemented with Moringa oleifera leaf extract for a cleaner environmental livestock production. J. Clean. Prod. 2018, 185, 547–553. [Google Scholar] [CrossRef]
- Groot, J.C.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed Sci. Tech. 1996, 64, 77–89. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A critical evaluation of the vanillin reaction assay for tannin in sorghum grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics, A Biometrical Approach, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1997. [Google Scholar]
- SAS. Institute Inc. SAS/STAT® 9.2 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Soltan, Y.A.; Patra, A.K. Ruminal Microbiome Manipulation to Improve Fermentation Efficiency in Ruminants. In Animal Feed Science and Nutrition-Production, Health and Environment; Patra, A., Ed.; IntechOpen: London, UK, 2021; Available online: https://www.intechopen.com/chapters/79866 (accessed on 11 October 2022).
- Hoover, W.H.; Stokes, S.R. Balancing carbohydrates and proteins for optimum rumen microbial yield. J. Dairy Sci. 1991, 74, 3630–3644. [Google Scholar] [CrossRef]
- Barros-Rodríguez, M.A.; Solorio-Sánchez, F.J.; Sandoval-Castro, C.A.; Ahmed, A.M.M.; Rojas-Herrera, R.; Briceño-Poot, E.G.; Ku-Vera, J.C. Effect of intake of diets containing tannins and saponins on in vitro gas production and sheep performance. Anim. Prod. Sci. 2014, 54, 1486–1489. [Google Scholar]
- Krause, D.O.; Denman, S.E.; Mackie, R.I.; Morrison, M.; Rae, A.L.; Attwood, G.T.; McSweeney, C.S. Opportunities to improve fibre degradation in the rumen: Microbiology, ecology and genomics. FEMS Microbiol. Rev. 2003, 27, 663–693. [Google Scholar] [CrossRef]
- Hervás, G.; Frutos, P.; Giraldes, F.J.; Mantecon, A.R.; Álvarez del Pino, M.C. Effect of different doses of quebracho tannins extract on rumen fermentation in ewes. Anim. Feed Sci. Tech. 2003, 109, 65–78. [Google Scholar] [CrossRef]
- Jones, G.A.; McAllister, T.A.; Muir, A.D.; Cheng, K.J. Effects of sainfoin (Onobrichys viciifolia Scop.) condensed tannins on growth and proteolysis by four strains of ruminal bacteria. Appl. Environ. Microbiol. 1994, 60, 1374–1378. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P. Methane mitigation from ruminants using tannins and saponins. Trop. Anim. Health Pro. 2012, 44, 729–739. [Google Scholar] [CrossRef]
- Monforte-Briceño, G.E.; Sandoval-Castro, C.A.; Ramírez-Avilés, L.; Leal, C.M.C. Defaunating capacity of tropical fodder trees: Effects of polyethylene glycol and its relationship to in vitro gas production. Anim. Feed Sci. Tech. 2005, 123, 313–327. [Google Scholar] [CrossRef]
- Bhatta, R.; Krishnamoorthy, U.; Mohammed, F. Effect of feeding tamarind (Tamarindus indica) seed husk as a source of tannin on dry matter intake, digestibility of nutrients and production performance of crossbred dairy cows in mid-lactation. Anim. Feed Sci. Tech. 2000, 83, 67–74. [Google Scholar] [CrossRef]
- McSweeney, C.S.; Palmer, B.; McNeill, D.M.; Krause, D.O. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Tech. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Reed, J.D. Nutritional toxicology of tannins and related polyphenols in forage legumes. J. Ani. Sci. 1995, 73, 1516–1528. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81, 330S–335S. [Google Scholar] [CrossRef]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production-Progress and challenges. Anim. Feed Sci. Tech. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Silanikove, N.; Perevolotsky, A.; Provenza, F.D. Use of tannin-binding chemicals to assay for tannins and their negative postingestive effects in ruminants. Anim. Feed Sci. Tech. 2001, 91, 69–81. [Google Scholar] [CrossRef]
- Blümmel, M.; Makkar, H.P.S.; Becker, K. In vitro gas production: A technique revisited. J. Anim. Physiol. Anim. Nutr. 1997, 77, 24–34. [Google Scholar] [CrossRef]
- Barros-Rodríguez, M.A.; Solorio-Sánchez, F.J.; Sandoval-Castro, C.A.; Klieve, A.; Rojas-Herrera, R.A.; Briceño-Poot, E.G.; Ku-Vera, J.C. Rumen function in vivo and in vitro in sheep fed Leucaena leucocephala. Trop. Anim. Health Pro. 2015, 47, 757–764. [Google Scholar] [CrossRef]
- Tavendale, M.H.; Meagher, L.P.; Pacheco, D.; Walker, N.; Attwood, G.T.; Sivakumaran, S. Methane production from in vitro rumen incubations with Lotus pedunculatus and Medicago sativa, and effects of extractable condensed tannin fractions on methanogenesis. Anim. Feed Sci. Tech. 2005, 123, 403–419. [Google Scholar] [CrossRef]
- Choudhury, P.K.; Salem, A.Z.M.; Singh, R.; Puniya, A.K. Rumen microbiology: An overview. In Rumen Microbiology: From Evolution to Revolution; Puniya, A.K., Singh, R., Kamra, D.N., Eds.; Springer: Delhi, India, 2015; pp. 3–16. [Google Scholar] [CrossRef]
- Formato, M.; Piccolella, S.; Zidorn, C.; Vastolo, A.; Calabrò, S.; Cutrignelli, M.I.; Pacifico, S. UHPLC-ESI-QqTOF Analysis and In Vitro Rumen Fermentation for Exploiting Fagus sylvatica Leaf in Ruminant Diet. Molecules 2022, 27, 2217. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Aragadvay-Yungán, R.G.; Barros-Rodríguez, M.; Ortiz, L.; Carro, M.D.; Navarro Marcos, C.; Elghandour, M.M.M.Y.; Salem, A.Z.M. Mitigation of ruminal methane production with enhancing the fermentation by supplementation of different tropical forage legumes. Environ. Sci. Pollut. Res. 2021, 29, 3438–3445. [Google Scholar] [CrossRef]
- Russell, J.B.; O’Connor, J.D.; Fox, D.G.; Van Soest, P.J.; Sniffen, C.J. A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. J. Anim. Sci. 1992, 70, 3551–3561. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Comstock Pub., Cornel University: Ithaca, NY, USA, 1994; ISBN 0-8014-2772-X. [Google Scholar]
- Hariadi, B.T.; Santoso, B. Evaluation of tropical plants containing tannin on in vitro methanogenesis and fermentation parameters using rumen fluid. J. Sci. Food Agric. 2010, 90, 456–461. [Google Scholar] [CrossRef]
- De Oliveira, S.G.; Berchielli, T.T.; dos Santos Pedreira, M.; Primavesi, O.; Frighetto, R.; Lima, M.A. Effect of tannin levels in sorghum silage and concentrate supplementation on apparent digestibility and methane emission in beef cattle. Anim. Feed Sci. Tech. 2007, 135, 236–248. [Google Scholar] [CrossRef]
- Śliwiński, B.J.; Kreuzer, M.; Wettstein, H.R.; Machmüller, A. Rumen fermentation and nitrogen balance of lambs fed diets containing plant extracts rich in tannins and saponins, and associated emissions of nitrogen and methane. Arch. Anim. Nutr. 2002, 56, 379–392. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).