Lactobacillus rhamnosus Hsryfm 1301 Fermented Milk Regulates Lipid Metabolism and Inflammatory Response in High-Fat Diet Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Probiotic Bacteria and Fermented Milk Preparation
2.2. Animals and Treatment
2.3. Body Weight and Food Intake
2.4. Blood Samples Handling
2.5. Liver Samples Handling
2.6. Indicator Testing
2.7. Experimental Pathology of Liver
2.8. Statistical Methods
3. Result
3.1. Effect of Lactobacillus rhamnosus Hsryfm 1301 Fermented Milk on the Body Weight of Rats
3.2. Effect of Lactobacillus rhamnosus Hsryfm 1301 Fermented Milk on Serum Biochemical Indexes of Rats
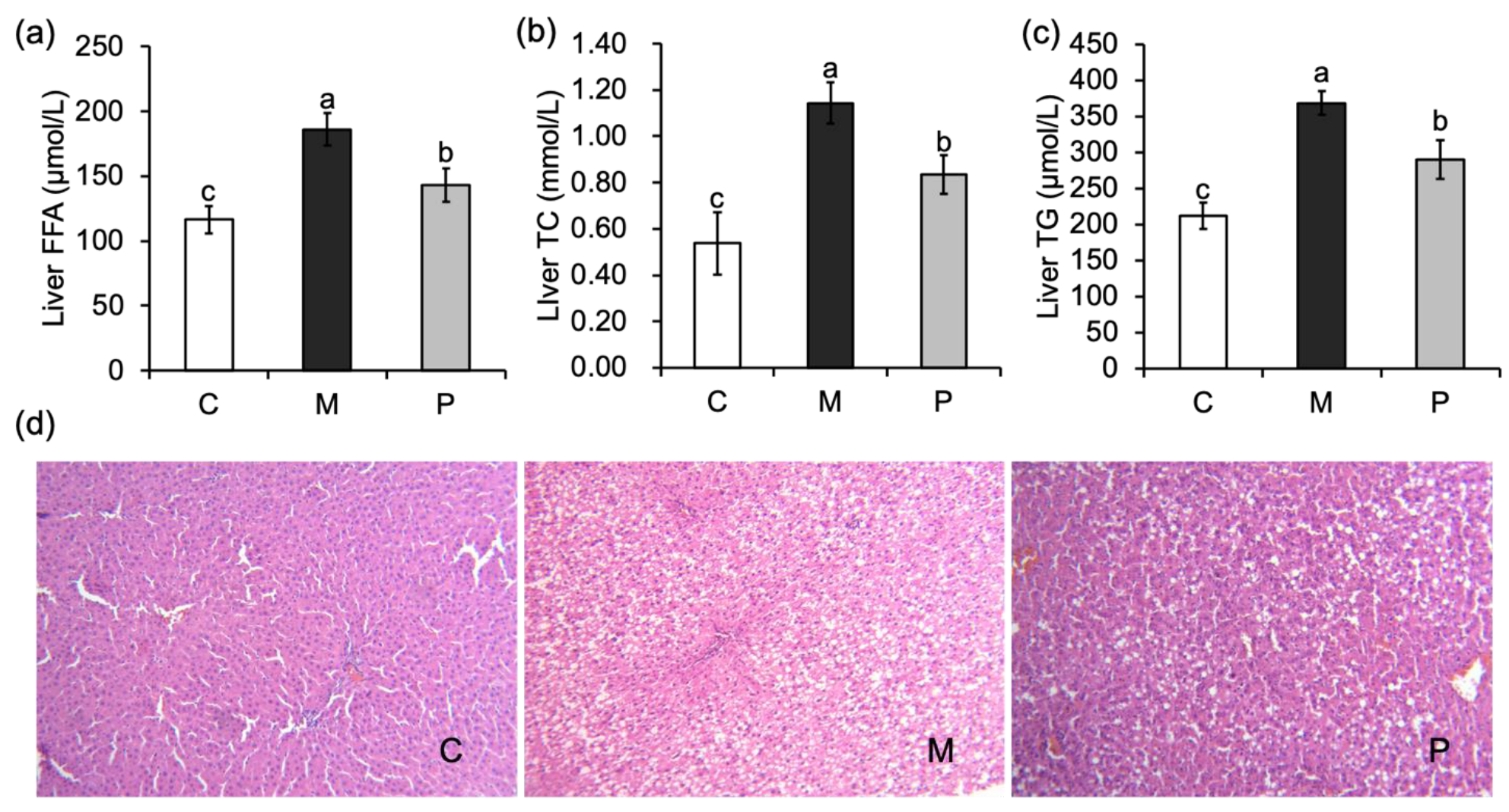
3.3. Effect of Lactobacillus rhamnosus Hsryfm 1301 Fermented Milk on Liver Injury of Rats
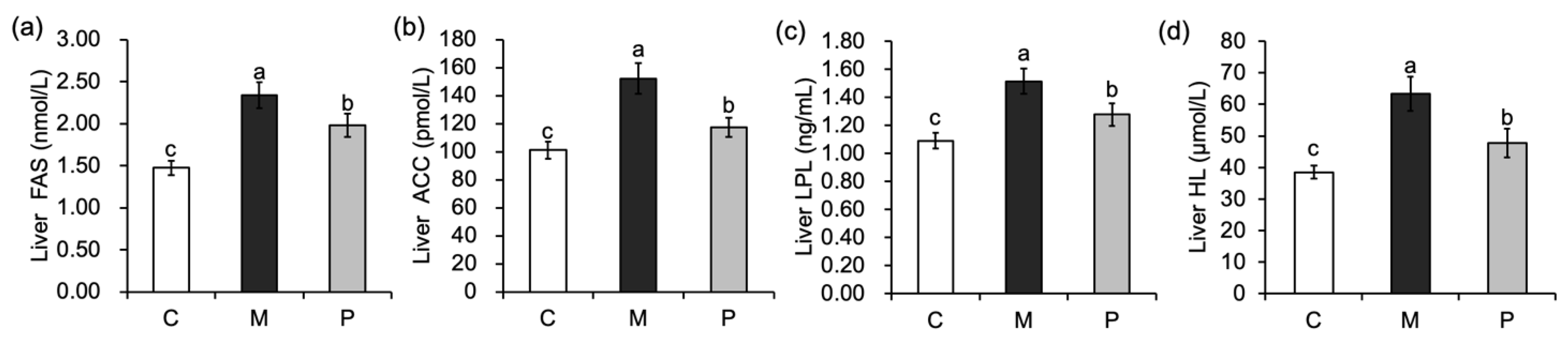
3.4. Effect of Lactobacillus rhamnosus Hsryfm 1301 Fermented Milk on Liver Fatty Acid Metabolism of Rats
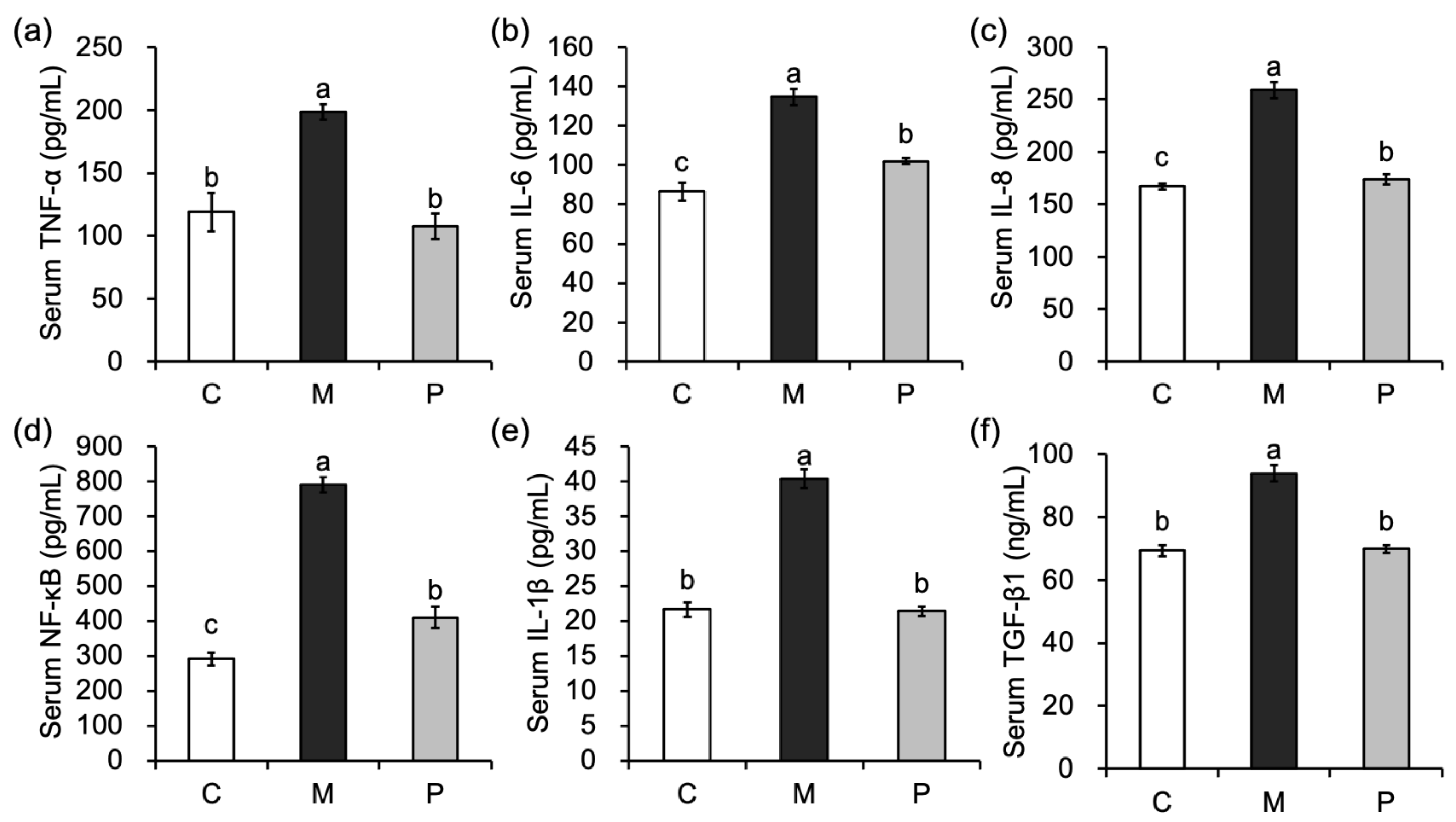
3.5. Effect of Lactobacillus rhamnosus Hsryfm 1301 Fermented Milk on Serum Inflammatory Factors in Rats
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sang, J.; Qu, H.; Gu, R.; Chen, D.; Chen, X.; Yin, B.; Huang, Y.; Xi, W.; Wang, C.; Huang, Y. Proteomics study of the effect of high-fat diet on rat liver. Br. J. Nutr. 2019, 122, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Adachi-Akahane, S. Inter-organ communication in the regulation of lipid metabolism: Focusing on the network between the liver, intestine, and heart. J. Pharmacol. Sci. 2013, 123, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Mirhoseini, M.; Shirzad, H.; Sedighi, M.; Shahinfard, N.; Rafieian-Kopaei, M. A review on promising natural agents effective on hyperlipidemia. J. Evid. Based Integr. Med. 2015, 20, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Shattat, G.F. A review article on hyperlipidemia: Types, treatments and new drug targets. Biomed. Pharmacol. J. 2014, 7, 399–409. [Google Scholar] [CrossRef]
- Wa, Y.; Yin, B.; He, Y.; Xi, W.; Huang, Y.; Wang, C.; Guo, F.; Gu, R. Effects of single probiotic- and combined probiotic-fermented milk on lipid metabolism in hyperlipidemic rats. Front. Microbiol. 2019, 10, 1312. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Yu, H.; Gu, R.; Chen, D.; Chen, X.; Huang, Y.; Xi, W.; Huang, Y. Proteomics for studying the effects of L. rhamnosus LV108 against non-alcoholic fatty liver disease in rats. RSC Adv. 2018, 8, 38517–38528. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, S.E.; Speck, M.L. Deconjugation of bile acids by intestinal lactobacilli. Appl. Environ. Microbiol. 1977, 33, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, Z.; Chen, X.; Huang, Y.; Yin, B.; Guo, F.; Zhao, H.; Zhao, T.; Zhenquan, Y.; Huang, J.; et al. The effect of Lactobacillus rhamnosus hsryfm 1301 on the intestinal microbiota of a hyperlipidemic rat model. BMC Complement. Altern. Med. 2014, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ishibashi, M.; Seimon, T.A.; Lee, M.; Sharma, S.; Fitzgerald, K.; Samokhin, A.O.; Wang, Y.; Sayers, S.; Aikawa, M.; et al. Free cholesterol accumulation in macrophage membranes activates toll-like receptors and p38 mitogen-activated protein kinase and induces cathepsin K. Circ. Res. 2009, 104, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Gora, S.; Maouche, S.; Atout, R.; Wanherdrick, K.; Lambeau, G.; Cambien, F.; Ninio, E.; Karabina, S. Phospholipolyzed LDL induces an inflammatory response in endothelial cells through endoplasmic reticulum stress signaling. FASEB J. 2010, 24, 3284–3297. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.R.; Stuart, L.M.; Wilkinson, K.; Van Gils, J.M.; Deng, J.C.; Halle, A.; Rayner, K.J.; Boyer, L.; Zhong, R.; Frazier, W.A.; et al. CD36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010, 11, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Carpintero, R.; Gruaz, L.; Brandt, K.J.; Scanu, A.; Faille, D.; Combes, V.; Grau, G.E.; Burger, D. HDL interfere with the binding of T cell microparticles to human monocytes to inhibit pro-inflammatory cytokine production. PLoS ONE 2010, 5, e11869. [Google Scholar] [CrossRef] [PubMed]
- Puranik, R.; Bao, S.; Nobecourt, E.; Nicholls, S.; Dusting, G.J.; Barter, P.J.; Celermajer, D.S.; Rye, K.-A. Low dose apolipoprotein A-I rescues carotid arteries from inflammation in vivo. Atherosclerosis 2008, 196, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Wakil, S.J. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 1989, 28, 4523–4530. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Evans, J.L.; Iverson, A.J. Identification of an isozymic form of acetyl-CoA carboxylase. J. Biol. Chem. 1990, 265, 1502–1509. [Google Scholar] [CrossRef]
- Lambert, J.E.; Ramos-Roman, M.A.; Browning, J.D.; Parks, E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014, 146, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Eckel, R.H. Lipoprotein lipase: From gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E271–E288. [Google Scholar] [CrossRef] [PubMed]
- Zambon, A.; Austin, M.A.; Brown, B.G.; Hokanson, J.E.; Brunzell, J.D. Effect of hepatic lipase on LDL in normal men and those with coronary artery disease. Arter. Thromb. A J. Vasc. Biol. 1993, 13, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, V.; Ciccarese, F.; Ciminale, V. Oncogenic pathways and the electron transport chain: A dange ROS liaison. Br. J. Cancer 2019, 122, 168–181. [Google Scholar] [CrossRef] [PubMed]

| Group | Initial Weight (g) | Final Weight (g) | Weight Gain (g) | Weekly Food Intake (g) |
|---|---|---|---|---|
| Control group (C) | 216.40 ± 6.03 a | 347.7 ± 13.89 b | 131.30 ± 11.55 b | 1076.13 ± 201.96 a |
| Model group (M) | 218.11 ± 5.15 a | 401.55 ± 18.76 a | 183.44 ± 21.18 a | 1086.25 ± 223.24 a |
| Lactobacillus rhamnosus hsryfm1301 treatment group (P) | 218.93 ± 6.21 a | 357.00 ± 18.14 b | 138.07 ± 19.77 b | 994.13 ± 161.87 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, H.; Zong, L.; Sang, J.; Liang, J.; Wa, Y.; Chen, D.; Huang, Y.; Chen, X.; Gu, R. Lactobacillus rhamnosus Hsryfm 1301 Fermented Milk Regulates Lipid Metabolism and Inflammatory Response in High-Fat Diet Rats. Fermentation 2022, 8, 584. https://doi.org/10.3390/fermentation8110584
Qu H, Zong L, Sang J, Liang J, Wa Y, Chen D, Huang Y, Chen X, Gu R. Lactobacillus rhamnosus Hsryfm 1301 Fermented Milk Regulates Lipid Metabolism and Inflammatory Response in High-Fat Diet Rats. Fermentation. 2022; 8(11):584. https://doi.org/10.3390/fermentation8110584
Chicago/Turabian StyleQu, Hengxian, Lina Zong, Jian Sang, Jiaojiao Liang, Yunchao Wa, Dawei Chen, Yujun Huang, Xia Chen, and Ruixia Gu. 2022. "Lactobacillus rhamnosus Hsryfm 1301 Fermented Milk Regulates Lipid Metabolism and Inflammatory Response in High-Fat Diet Rats" Fermentation 8, no. 11: 584. https://doi.org/10.3390/fermentation8110584
APA StyleQu, H., Zong, L., Sang, J., Liang, J., Wa, Y., Chen, D., Huang, Y., Chen, X., & Gu, R. (2022). Lactobacillus rhamnosus Hsryfm 1301 Fermented Milk Regulates Lipid Metabolism and Inflammatory Response in High-Fat Diet Rats. Fermentation, 8(11), 584. https://doi.org/10.3390/fermentation8110584






