Enabling Ethanologenesis in Moorella thermoacetica through Construction of a Replicating Shuttle Vector
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Methylation Strain
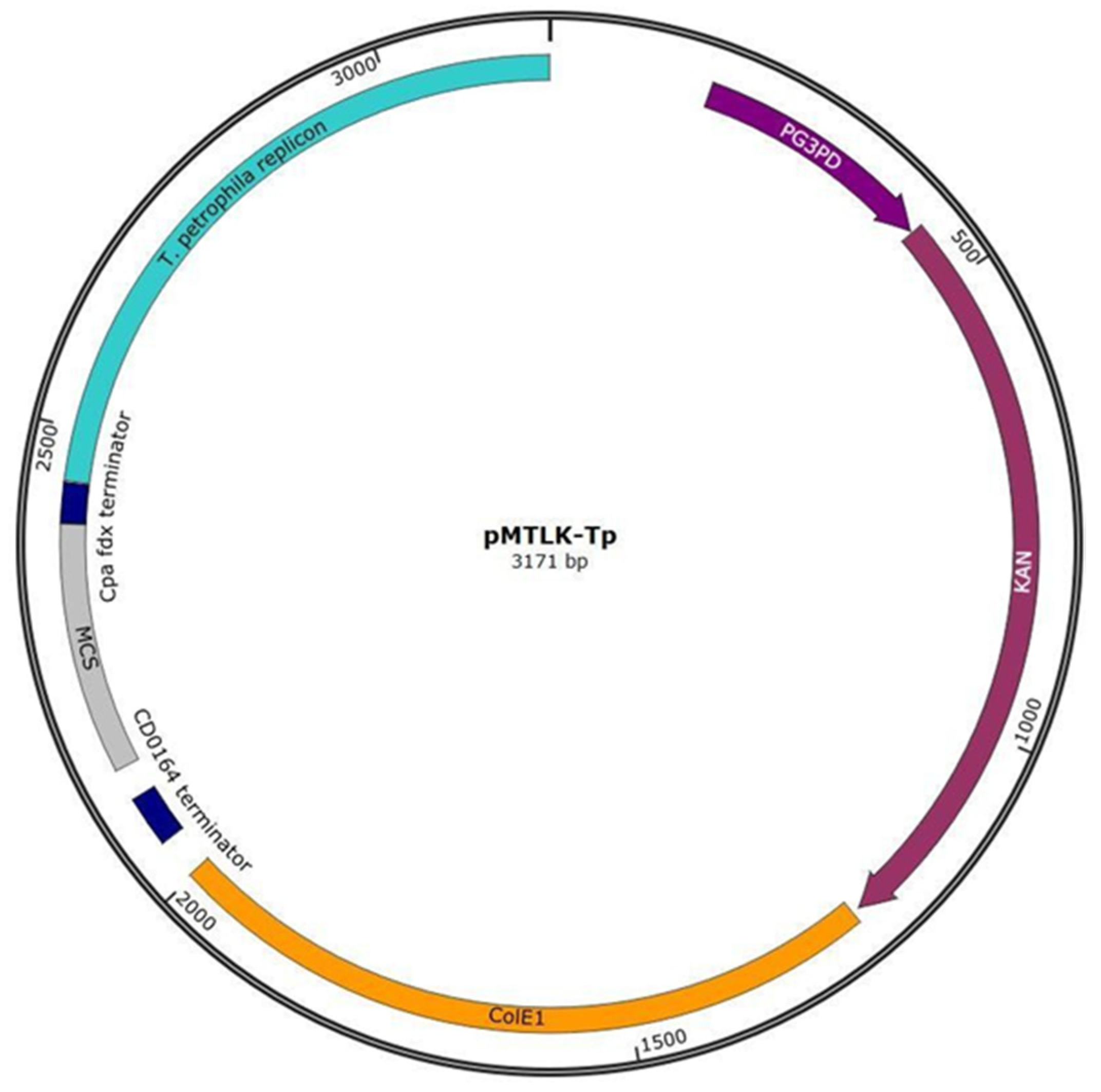
2.3. Shuttle Vector Construction
2.4. Transformation of M. thermoacetica
2.5. Genomic DNA Extraction
2.6. Fluorescence Measurement by qPCR
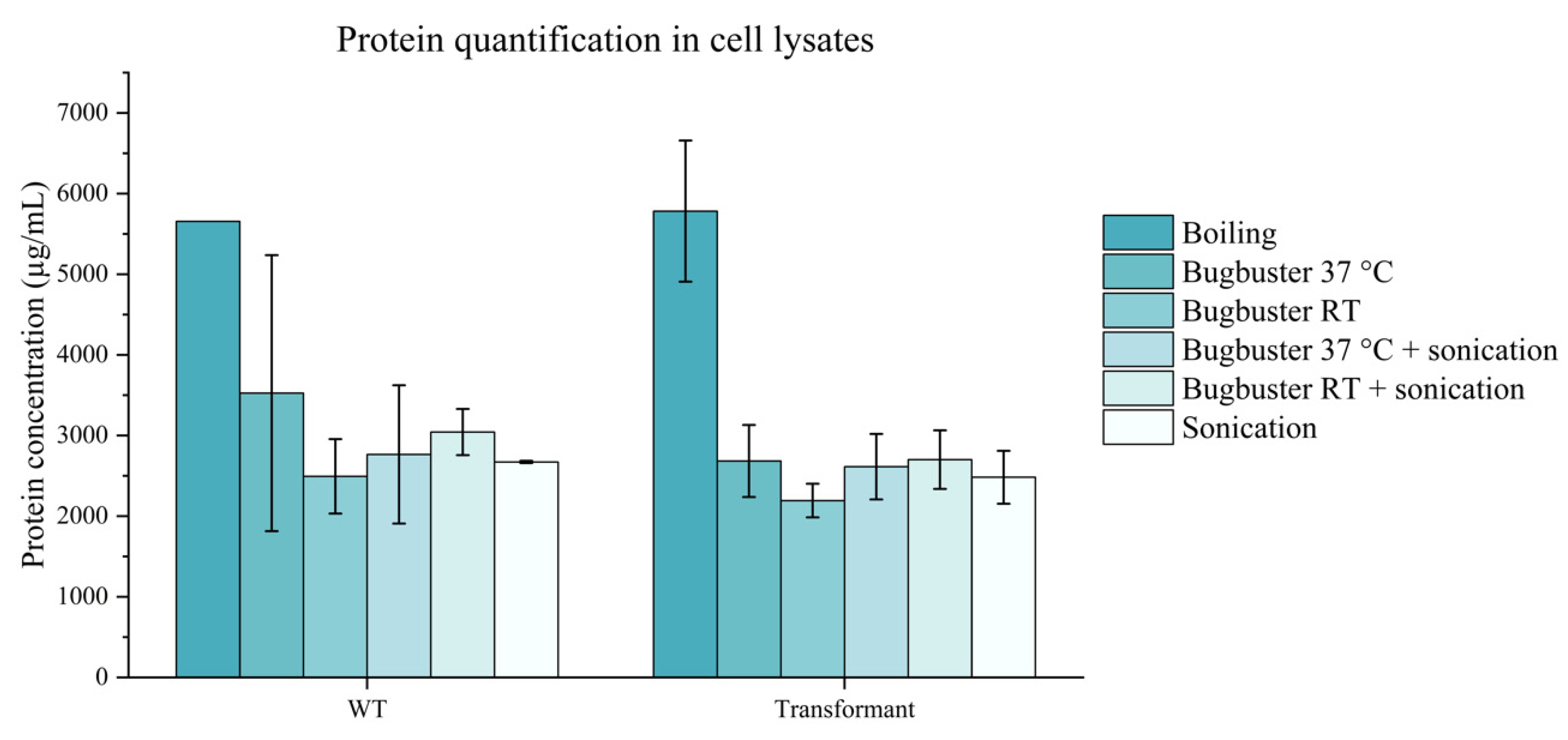
2.7. Protein Quantification
2.8. Ethanol Production
3. Results
3.1. E. coli Methylation Strain for In Vivo Plasmid Pre-Methylation
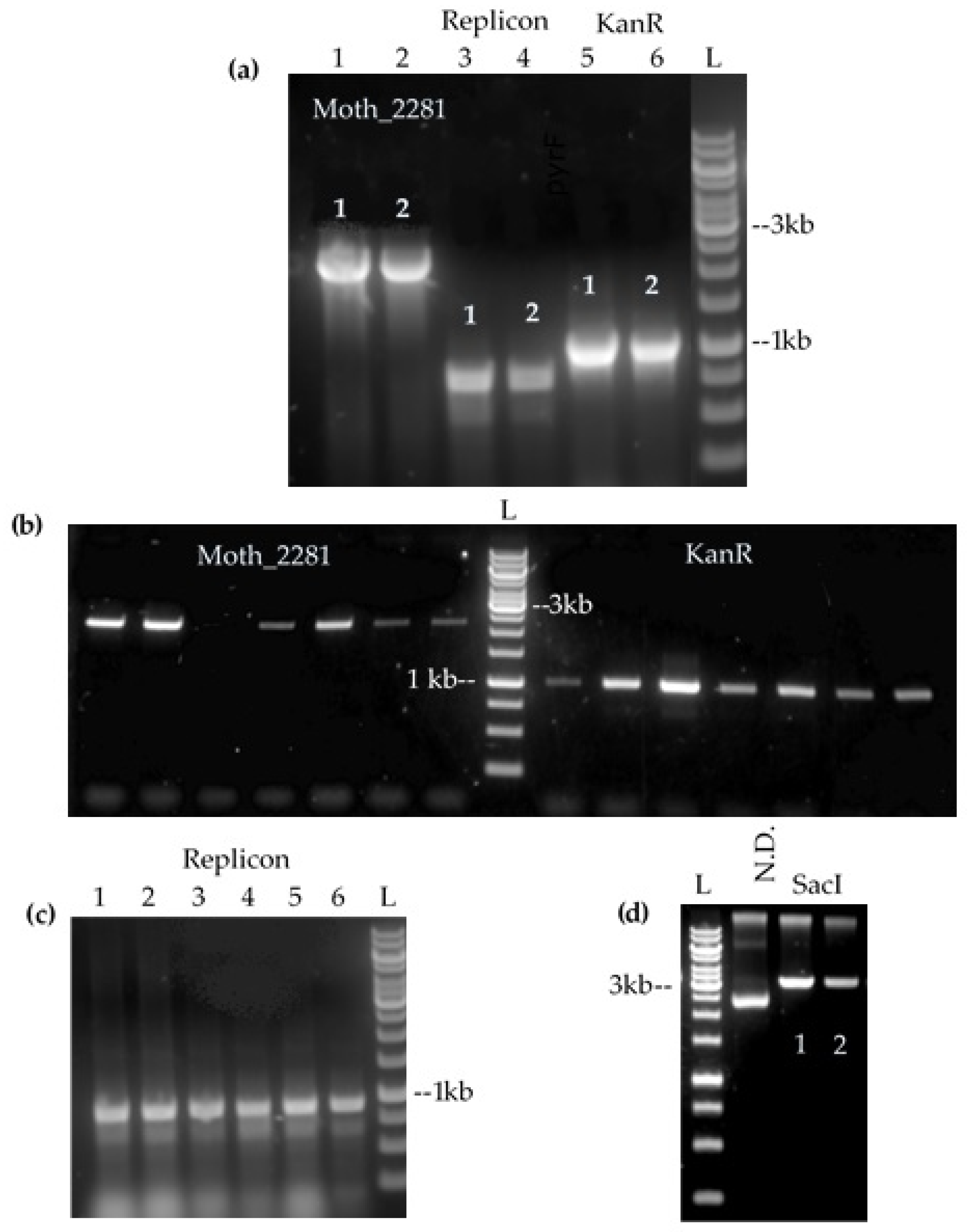
3.2. Transformation of the Candidate Shuttle Vectors into M. thermoacetica
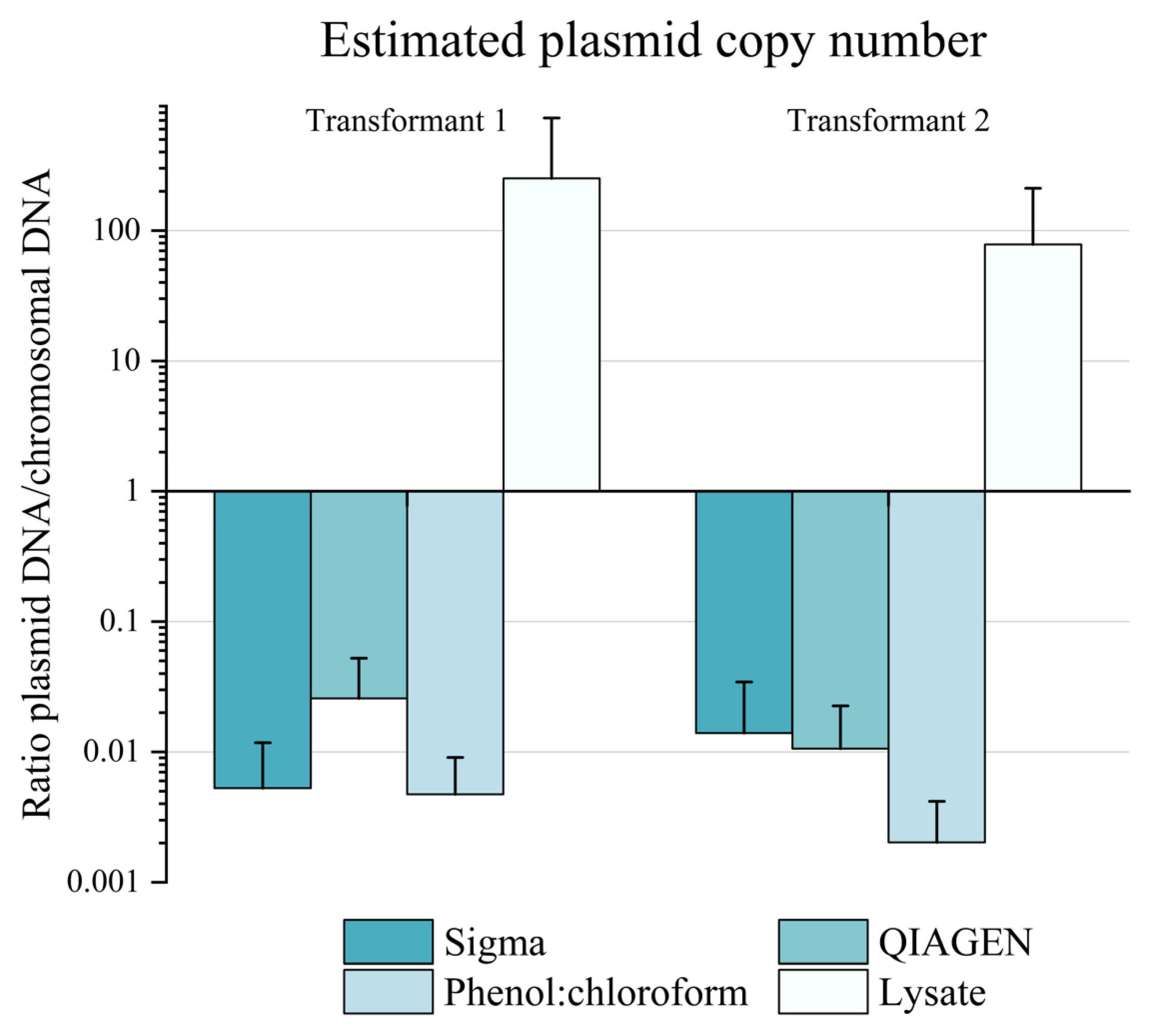
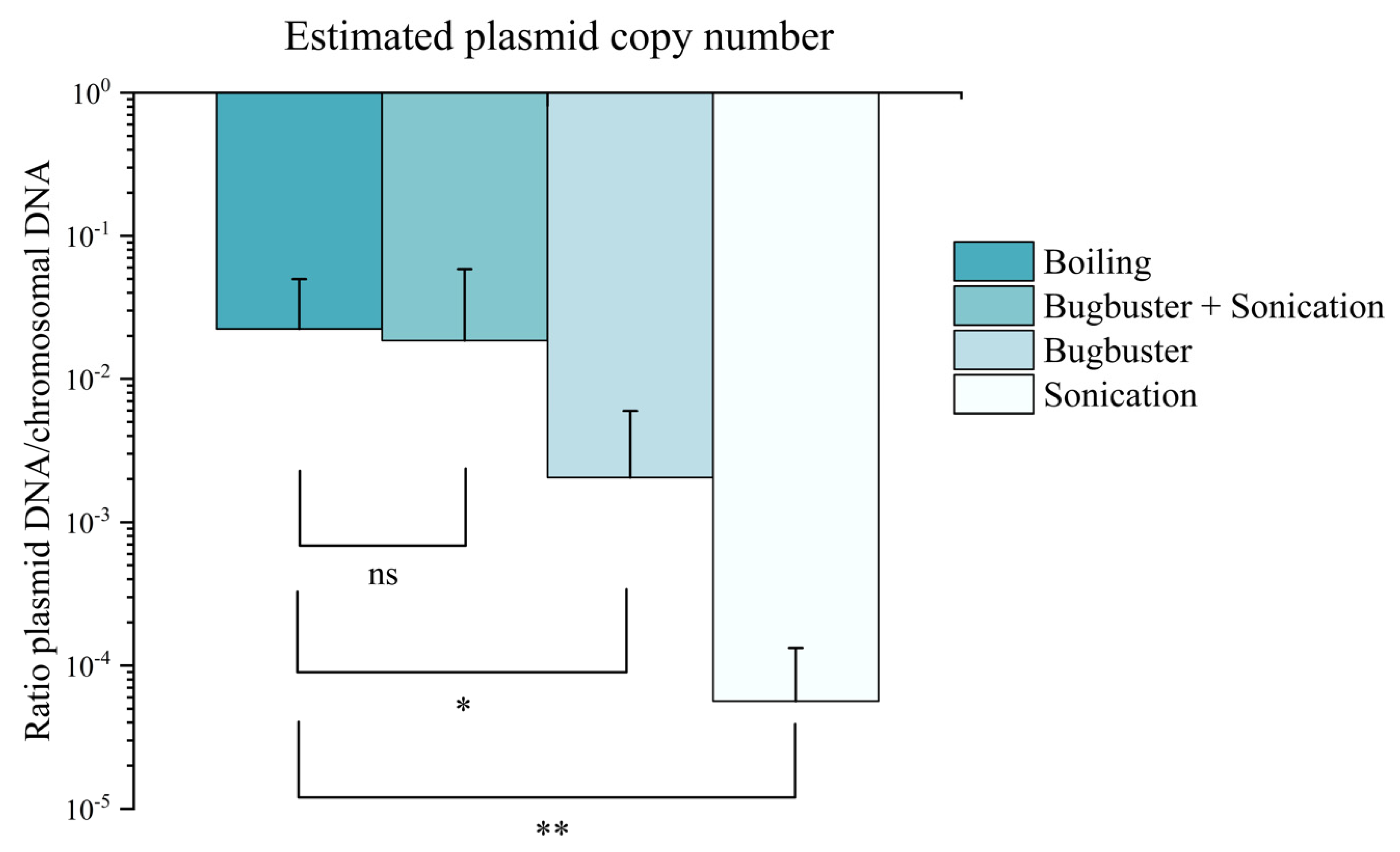
3.3. Characterisation of Plasmid Behaviour
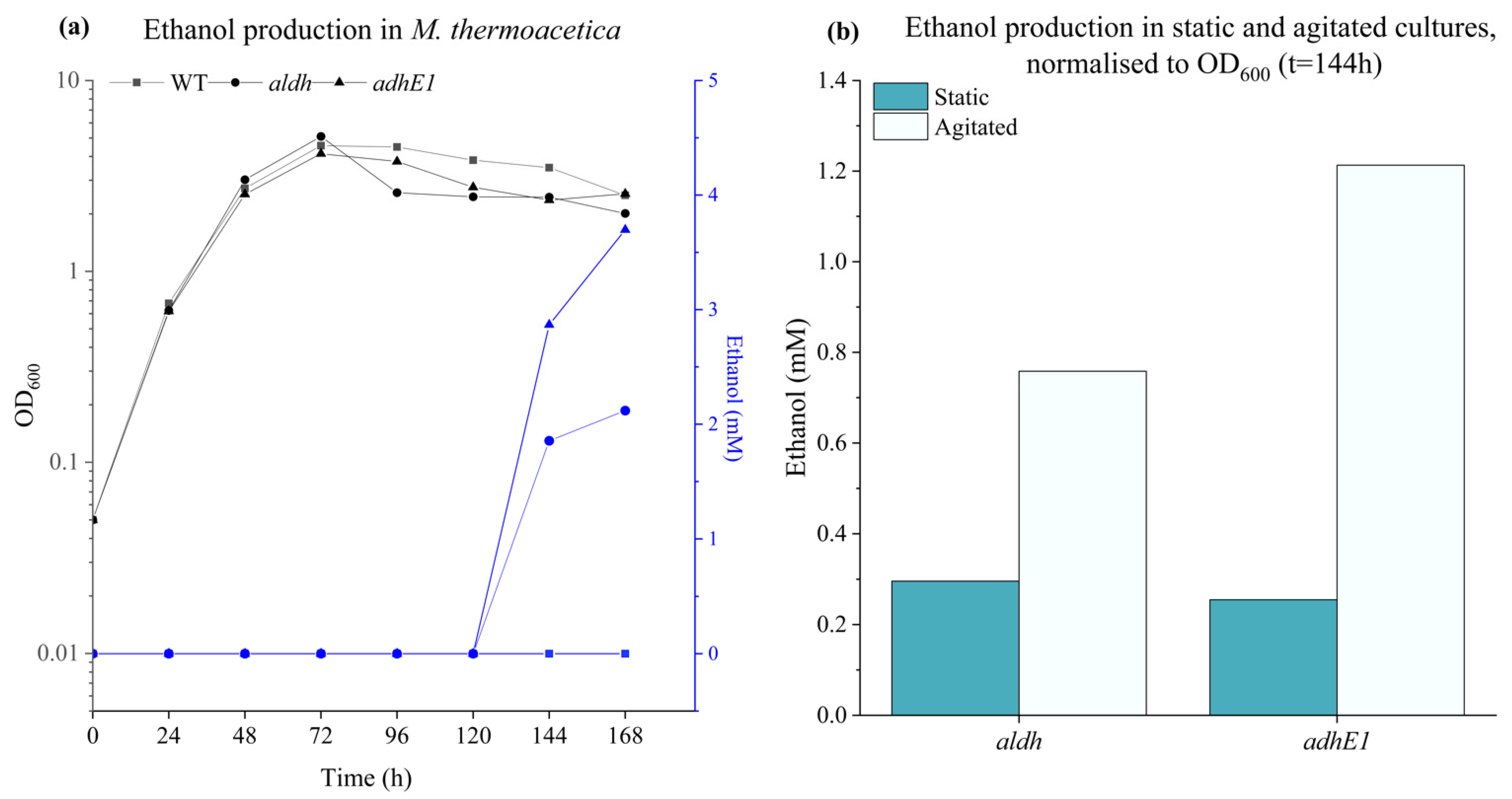
3.4. Ethanol Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drake, H.L.; Gößner, A.S.; Daniel, S.L. Old Acetogens, New Light. Ann. N. Y. Acad. Sci. 2008, 1125, 100–128. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl Pathway of CO2 fixation. Biochim. Biophys. Acta-Proteins Proteom. 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [PubMed]
- Woolston, B.M.; Emerson, D.F.; Currie, D.H.; Stephanopoulos, G. Rediverting Carbon Flux in Clostridium ljungdahlii Using CRISPR Interference (CRISPRi). Metab. Eng. 2018, 48, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kang, S.; Song, Y.; Jin, S.; Lee, J.S.; Lee, J. Genome Engineering of Eubacterium limosum Using Expanded Genetic Tools and the CRISPR-Cas9 System. ACS Synth. Biol. 2019, 8, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.; Henstra, A.M.; Köpke, M.; Winzer, K.; Simpson, S.D.; Minton, N.P. Metabolic Engineering of Clostridium autoethanogenum for Selective Alcohol Production. Metab. Eng. 2017, 40, 104–114. [Google Scholar] [CrossRef]
- Cheng, C.; Li, W.; Lin, M.; Yang, S. Bioresource Technology Metabolic Engineering of Clostridium carboxidivorans for Enhanced Ethanol and Butanol Production from Syngas and Glucose. Bioresour. Technol. 2019, 284, 415–423. [Google Scholar] [CrossRef]
- Bourgade, B.; Minton, N.P.; Islam, M.A. Genetic and Metabolic Engineering Challenges of C1-Gas Fermenting Acetogenic Chassis Organisms. FEMS Microbiol. Rev. 2021, 45, fuab008. [Google Scholar] [CrossRef] [PubMed]
- Abrini, J.; Naveau, H.; Nyns, E.-J. Clostridium autoethanogenum, Sp. Nov., an Anaerobic Bacterium That Produces Ethanol from Carbon Monoxide. Arch. Microbiol. 1994, 161, 345–351. [Google Scholar] [CrossRef]
- Balch, W.E.; Schoberth, S.; Tanner, R.S.; Wolfe, R.S. Acetobacterium, a New Genus of Hydrogen-Oxidizing, Carbon Dioxide-Reducing, Anaerobic Bacteria. Int. J. Syst. Bacteriol. 1977, 27, 355–361. [Google Scholar] [CrossRef]
- Nagaraju, S.; Davies, N.K.; Walker, D.J.F.; Köpke, M.; Simpson, S.D. Genome Editing of Clostridium autoethanogenum Using CRISPR/Cas9. Biotechnol. Biofuels 2016, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Annan, F.J.; Al-sinawi, B.; Humphreys, C.M.; Norman, R.; Winzer, K.; Köpke, M.; Simpson, S.D.; Minton, N.P.; Henstra, A.M. Engineering of Vitamin Prototrophy in Clostridium Ljungdahlii and Clostridium autoethanogenum. Appl. Microbiol. Biotechnol. 2019, 103, 4633–4648. [Google Scholar] [CrossRef]
- Beck, M.H.; Flaiz, M.; Bengelsdorf, F.R.; Dürre, P. Induced Heterologous Expression of the Arginine Deiminase Pathway Promotes Growth Advantages in the Strict Anaerobe Acetobacterium woodii. Appl. Microbiol. Biotechnol. 2020, 104, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.; Ko, H.; Kim, D.; Choi, D.G.; Park, S.; Kim, S.; Chang, I.S.; Choi, I. Complete Genome Sequence of a Carbon Monoxide-Utilizing, Eubacterium limosum KIST612. J. Bacteriol. 2011, 193, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.S.C.; Balkwill, D.L.; Drake, G.R.; Tanner, R.S. Clostridium carboxidivorans Sp. Nov., a Solvent-Producing Clostridium Isolated from an Agricultural Settling Lagoon, and Reclassification of the Acetogen Clostridium scatologenes Strain SL1 as Clostridium Drakei Sp. Nov. Int. J. Syst. Evol. Microbiol. 2005, 55, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Basen, M.; Geiger, I.; Henke, L. A Genetic System for the Thermophilic Acetogenic Bacterium Thermoanaerobacterium kivui. Appl. Environ. Microbiol. 2018, 84, e02210-17. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chai, C.; Li, N.; Rowe, P.; Minton, N.P.; Yang, S.; Jiang, W.; Gu, Y. CRISPR/Cas9-Based Efficient Genome Editing in Clostridium ljungdahlii, an Autotrophic Gas-Fermenting Bacterium. ACS Synth. Biol. 2016, 5, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, F.E.; Peterson, W.H.; McCoy, E.; Johnson, M.J.; Ritter, G.J. A New Type of Glucose Fermentation by Clostridium thermoaceticum. J. Bacteriol. 1942, 43, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Kita, A.; Iwasaki, Y.; Sakai, S.; Okuto, S.; Takaoka, K.; Suzuki, T.; Yano, S.; Sawayama, S.; Tajima, T.; Kato, J.; et al. Development of Genetic Transformation and Heterologous Expression System in Carboxydotrophic Thermophilic Acetogen Moorella thermoacetica. J. Biosci. Bioeng. 2013, 115, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Takemura, K.; Kato, S.; Fujii, T.; Wada, K.; Iwasaki, Y.; Aoi, Y.; Matsushika, A.; Murakami, K.; Nakashimada, Y. Metabolic Engineering of Moorella Thermoacetica for Thermophilic Bioconversion of Gaseous Substrates to a Volatile Chemical. AMB Express 2021, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, Y.; Kita, A.; Sakai, S.; Takaoka, K.; Yano, S.; Tajima, T.; Kato, J.; Nishio, N.; Murakami, K.; Nakashimada, Y. Engineering of a Functional Thermostable Kanamycin Resistance Marker for Use in Moorella thermoacetica ATCC39073. FEMS Microbiol. Lett. 2013, 343, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, S.; Gerdom, M.; Bengelsdorf, F.R.; Linder, S.; Flüchter, S.; Öztürk, H.; Blümke, W.; May, A.; Fischer, R.J.; Bahl, H.; et al. Acetone Production with Metabolically Engineered Strains of Acetobacterium woodii. Metab. Eng. 2016, 36, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Guzman, L.M.; Weiss, D.S.; Beckwith, J. Tight Regulation, Modulation, and High-Level Expression by Vectors Containing the Arabinose PBAD Promoter. J. Bacteriol. 1995, 177, 4121–4130. [Google Scholar] [CrossRef] [PubMed]
- Heap, J.T.; Pennington, O.J.; Cartman, S.T.; Minton, N.P. A Modular System for Clostridium Shuttle Plasmids. J. Microbiol. Methods 2009, 78, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Roberts, R.J.; Belfort, M.; Bestor, T.; Bhagwat, A.S.; Bickle, T.A.; Bitinaite, J.; Blumenthal, R.M.; Degtyarev, S.K.; Dryden, D.T.F.; Dybvig, K.; et al. A Nomenclature for Restriction Enzymes, DNA Methyltransferases, Homing Endonucleases and Their Genes. Nucleic Acids Res. 2003, 31, 1805–1812. [Google Scholar] [CrossRef]
- Pingoud, A.; Fuxreiter, M.; Pingoud, V.; Wende, W. Type II Restriction Endonucleases: Structure and Mechanism. Cell. Mol. Life Sci. 2005, 62, 685–707. [Google Scholar] [CrossRef]
- Vasu, K.; Nagaraja, V. Diverse Functions of Restriction-Modification Systems in Addition To Cellular Defense. Microbiol. Mol. Biol. Rev. 2013, 77, 53–72. [Google Scholar] [CrossRef]
- Roberts, R.J.; Vincze, T.; Posfai, J.; Macelis, D. REBASE-a Database for DNA Restriction and Modification: Enzymes, Genes and Genomes. Nucleic Acids Res. 2015, 43, D298–D299. [Google Scholar] [CrossRef]
- Woolston, B.M. Enabling C1-Based Bioconversion with Metabolic Engineering. Ph.D. Thesis, Massachusetts Institute of Technology, Department of Chemical Engineering, Cambridge, MA, USA, 2017. Available online: https://dspace.mit.edu/handle/1721.1/127713 (accessed on 4 October 2022).
- Trieu-cuot, P.; Courvalin, P. Nucleotide Sequence of the Streptococcus faecalis Plasmid Gene Encoding the 3′5″-Aminoglycoside Phosphotransferase Type III. Gene 1983, 23, 331–341. [Google Scholar] [CrossRef]
- Liu, B.; Wang, C.; Yang, H.; Tan, H. Establishment of a Genetic Transformation System and Its Application in Thermoanaerobacter tengcongensis. J. Genet. Genom. 2012, 39, 561–570. [Google Scholar] [CrossRef]
- Sheng, L.; Kovács, K.; Winzer, K.; Zhang, Y.; Minton, N.P. Development and Implementation of Rapid Metabolic Engineering Tools for Chemical and Fuel Production in Geobacillus thermoglucosidasius NCIMB 11955. Biotechnol. Biofuels 2017, 10, 5. [Google Scholar] [CrossRef]
- Han, D.; Norris, S.M.; Xu, Z. Construction and Transformation of a Thermotoga-E. Coli Shuttle Vector. BMC Biotechnol. 2012, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.G.; Maloney, M.; Lanahan, A.A.; Hon, S.; Hauser, L.J.; Lynd, L.R. Identifying Promoters for Gene Expression in Clostridium thermocellum. Metab. Eng. Commun. 2015, 2, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.P.; Saéz-Saéz, J.; Jensen, S.I.; Nielsen, A.T.; Minton, N.P. A Clean In-Frame Knockout System for Gene Deletion in Acetobacterium woodii. J. Biotechnol. 2022, 353, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Noll, K.M. Plasmid pRQ7 from the Hyperthermophilic Bacterium Thermotoga Species Strain RQ7 Replicates by the Rolling-Circle Mechanism. J. Bacteriol. 1997, 179, 7161–7164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jin, S.G.; Mann, J.R. Synthetic Neomycin-Kanamycin Phosphotransferase, Type II Coding Sequence for Gene Targeting in Mammalian Cells. Genesis 2005, 42, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Guglielmini, J.; Van Melderen, L. Bacterial Toxin-Antitoxin Systems: Translation Inhibitors Everywhere. Mob. Genet. Elements 2011, 1, 283–306. [Google Scholar] [CrossRef]
- Unterholzner, S.J.; Poppenberger, B.; Rozhon, W. Toxin–Antitoxin Systems. Mob. Genet. Elements 2013, 3, e26219. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and Its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Nevin, K.P.; Hensley, S.A.; Franks, A.E.; Summers, Z.M.; Ou, J.; Woodard, T.L.; Snoeyenbos-West, O.L.; Lovley, D.R. Electrosynthesis of Organic Compounds from Carbon Dioxide Is Catalyzed by a Diversity of Acetogenic Microorganisms. Appl. Environ. Microbiol. 2011, 77, 2882–2886. [Google Scholar] [CrossRef]
- Tremblay, P.L.; Angenent, L.T.; Zhang, T. Extracellular Electron Uptake: Among Autotrophs and Mediated by Surfaces. Trends Biotechnol. 2017, 35, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Anindyajati, A.; Anita Artarini, A.; Riani, C.; Retnoningrum, D.S. Plasmid Copy Number Determination by Quantitative Polymerase Chain Reaction. Sci. Pharm. 2016, 84, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Škulj, M.; Okršlar, V.; Jalen, Š.; Jevševar, S.; Slanc, P.; Štrukelj, B.; Menart, V. Improved Determination of Plasmid Copy Number Using Quantitative Real-Time PCR for Monitoring Fermentation Processes. Microb. Cell Fact. 2008, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Sissolak, B.; Zabik, C.; Saric, N.; Sommeregger, W.; Vorauer-Uhl, K.; Striedner, G. Application of the Bradford Assay for Cell Lysis Quantification: Residual Protein Content in Cell Culture Supernatants. Biotechnol. J. 2019, 14, e1800714. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, F.; Kawai, Y.; Iwasaki, Y.; Yoshida, K.; Kita, A.; Tajima, T.; Kato, J.; Murakami, K.; Hoshino, T.; Nakashimada, Y. Thermophilic Ethanol Fermentation from Lignocellulose Hydrolysate by Genetically Engineered Moorella thermoacetica. Bioresour. Technol. 2017, 245, 1393–1399. [Google Scholar] [CrossRef]
- Jaishankar, J.; Srivastava, P. Molecular Basis of Stationary Phase Survival and Applications. Front. Microbiol. 2017, 8, 2000. [Google Scholar] [CrossRef]
- Frock, A.D.; Montero, C.I.; Blumer-Schuette, S.E.; Kelly, R.M. Stationary Phase and Nutrient Levels Trigger Transcription of a Genomic Locus Containing a Novel Peptide (TM1316) in the Hyperthermophilic Bacterium Thermotoga maritima. Appl. Environ. Microbiol. 2013, 79, 6637–6646. [Google Scholar] [CrossRef][Green Version]
- Jahn, M.; Vorpahl, C.; Türkowsky, D.; Lindmeyer, M.; Bühler, B.; Harms, H.; Müller, S. Accurate Determination of Plasmid Copy Number of Flow-Sorted Cells using Droplet Digital PCR. Anal. Chem. 2014, 86, 5969–5976. [Google Scholar] [CrossRef]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The Ultimate QPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef]
- Plotka, M.; Wozniak, M.; Kaczorowski, T. Quantification of Plasmid Copy Number with Single Colour Droplet Digital PCR. PLoS ONE 2017, 12, e0169846. [Google Scholar] [CrossRef]
- Rocha, D.J.P.; Santos, C.S.; Pacheco, L.G.C. Bacterial Reference Genes for Gene Expression Studies by RT-QPCR: Survey and Analysis. Antonie van Leeuwenhoek 2015, 108, 685–693. [Google Scholar] [CrossRef] [PubMed]
| Primer | 5′-to-3′ Sequence |
|---|---|
| 1671_Fwd | AAGTTGGGTTTCGGAGGC |
| 1671_Rev | ATTACATGGGAAATGGGTTTTGTACTGCG |
| 1672_Fwd | AAACCCATTTCCCATGTAATAACGGAGG |
| 1672_Rev | CCTGCCTGTTTCCTTCCTTCACCTCATTAAC |
| 2281_Fwd | ATATATGAGCTCAACAGGCAGGTAACCAATAGAATG |
| 2281_Rev | ATATATCTCGAGAAAACTACCCCCACATAG |
| KAN_Fwd | ATATATggccggccGGACGGTTGCCAAGTAC |
| KAN_Rev | ATATATgtttaaacACTAAAACAATTCATCCAGTAAAA |
| T. pet_Fwd | ATATATggcgcgccGAATGTGGTTAGTGTGATTAG |
| T. pet_Rev | ATATATggccggccTTAACCATATCCCACTAGTTC |
| C. bes_Fwd | ATATATggcgcgccACCGTGAGCATTCTGGACAGGT |
| C. bes_Rev | ATATATggccggccATTCCCATGAGCCCACGAACAGT |
| T. therm_Fwd | ATATATggcgcgccCTGCAGTAAATTAATTAACAGTTTT |
| T. therm_Rev | ATATATggccggccGGTCTCTCAGGAGCC |
| N. therm_Fwd | ATATATggcgcgccAGGCGTTTCCTCCCAC |
| N. therm_Rev | ATATATggccggccTTATTTAACACAATTATCATCCTCCAAG |
| Ori_Fwd | ATATATggcgcgccTTTTCCCTCCTACATAAAAATATCT |
| Ori_Rev | ATATATggccggccCCGTAGATAAATAGCGGATT |
| Pffh_Fwd | ATATATggccggccAATAAACAAGCCGCAGGTTAC |
| Pffh_Rev | CATTTTAGCCATATGCCTGTTACACCCCCGGTTT |
| KAN_Fwd_Pffh | CGGGGGTGTAACAGGCATATGGCTAAAATGAGAAT |
| aldh_Fwd | GTGGACAAGGTAAAAGTGGCTG |
| aldh_Rev | TCATGCTACCCCTTCCTTTACCTG |
| adhE1_Fwd | ATGAAAGTTACAAACGTAGAA |
| adheE1_Rev | TTACTTTTCTTCATCTTCTACA |
| Plasmid | Gram +ve Replicon | Gram – ve Replicon | Selection Marker | Additional Feature |
|---|---|---|---|---|
| 1 | Replicon from pUB110 | ColE1 | PG3PD-kanR | - |
| 2 (pMTLK-Tp) | Replicon from pRKU1 | ColE1 | PG3PD-kanR | - |
| 3 | Replicon from pBAS2 | ColE1 | PG3PD-kanR | - |
| 4 | Replicon from pNB2 | ColE1 | PG3PD-kanR | - |
| 5 | Replicon from pNTHE02 | ColE1 | PG3PD-kanR | - |
| 6 | M. thermoacetica chromosomal origin | ColE1 | PG3PD-kanR | - |
| 7 | Replicon from pRKU1 | ColE1 | PG3PD-kanR | P. thermoglucosidasius toxin-antitoxin system |
| 8 (pMTLKffh) | Replicon from pRKU1 | ColE1 | Pffh-kanR | - |
| 9 | Replicon from pRKU1 | ColE1 | Pffh-kanR | PG3PD-aldh |
| 10 | Replicon from pRKU1 | ColE1 | Pffh-kanR | PG3PD-adhE1 |
| Cycle Step and Description | Temperature (°C) | Duration | Program Cycle | Data Capture |
|---|---|---|---|---|
| Initial denaturation | 95 | 10 min | - | - |
| Denaturation | 95 | 20 s | - | - |
| Annealing | 55 | 20 s | - | - |
| Extension | 72 | 20 s | Return to step 2 (x 50) | Single |
| Melting | 72 | - | - | Continuous |
| Hold | 30 | - | - | - |
| Primer | 5′-to-3′ Sequence |
|---|---|
| rpoB_Fwd | GAAGACCTCCTGTTCCTTAACC |
| rpoB_Rev | CAAAGAGCGGGATATGACCTAC |
| gyrA_Fwd | AACCATCACCGCTGTTATCC |
| gyrA_Rev | GCCCAGTGAGGTCTTCTTTAC |
| Replicon_Fwd | GCAAGTGTGGAAGTGGTTATG |
| Replicon_Rev | CCTCTGGCGGTATCTCAATAAT |
| Kan_Fwd | CTGGGAAGAAGACACTCCATTTA |
| Kan_Rev | GCCACTTACTTTGCCATCTTTC |
| Species | Plasmid | References/Accession Numbers |
|---|---|---|
| Staphylococcus aureus | pUB110 | Geobacillus thermoglucosidasius [32] |
| Thermotoga petrophila | pRKU1 | Thermotoga sp. [33] |
| Caldicellulosiruptor bescii | pBAS2 | Clostridium thermocellum [34] |
| Thermoanaerobacterium thermosaccharolyticum | pNB2 | Accession number: NC_004979.1 |
| Natranaerobus thermophilus | pNTHE02 | Accession number: CP001036.1 |
| M. thermoacetica chromosomal origin | - | Accession number: CP012369 REGION: 2527442.1545 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourgade, B.; Millard, J.; Humphreys, C.M.; Minton, N.P.; Islam, M.A. Enabling Ethanologenesis in Moorella thermoacetica through Construction of a Replicating Shuttle Vector. Fermentation 2022, 8, 585. https://doi.org/10.3390/fermentation8110585
Bourgade B, Millard J, Humphreys CM, Minton NP, Islam MA. Enabling Ethanologenesis in Moorella thermoacetica through Construction of a Replicating Shuttle Vector. Fermentation. 2022; 8(11):585. https://doi.org/10.3390/fermentation8110585
Chicago/Turabian StyleBourgade, Barbara, James Millard, Christopher M. Humphreys, Nigel P. Minton, and M. Ahsanul Islam. 2022. "Enabling Ethanologenesis in Moorella thermoacetica through Construction of a Replicating Shuttle Vector" Fermentation 8, no. 11: 585. https://doi.org/10.3390/fermentation8110585
APA StyleBourgade, B., Millard, J., Humphreys, C. M., Minton, N. P., & Islam, M. A. (2022). Enabling Ethanologenesis in Moorella thermoacetica through Construction of a Replicating Shuttle Vector. Fermentation, 8(11), 585. https://doi.org/10.3390/fermentation8110585







