Impact of Calcium and Nitrogen Addition on Bioethanol Production by S. cerevisiae Fermentation from Date By-Products: Physicochemical Characterization and Technical Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Extraction and Preparation of Date Juice for Fermentation
2.3. Microorganisms
2.4. Fermentation and Fractional Distillation
2.4.1. Fermentation Procedure
2.4.2. Fractional Distillation
2.5. Chemicals
2.6. Analysis
3. Results
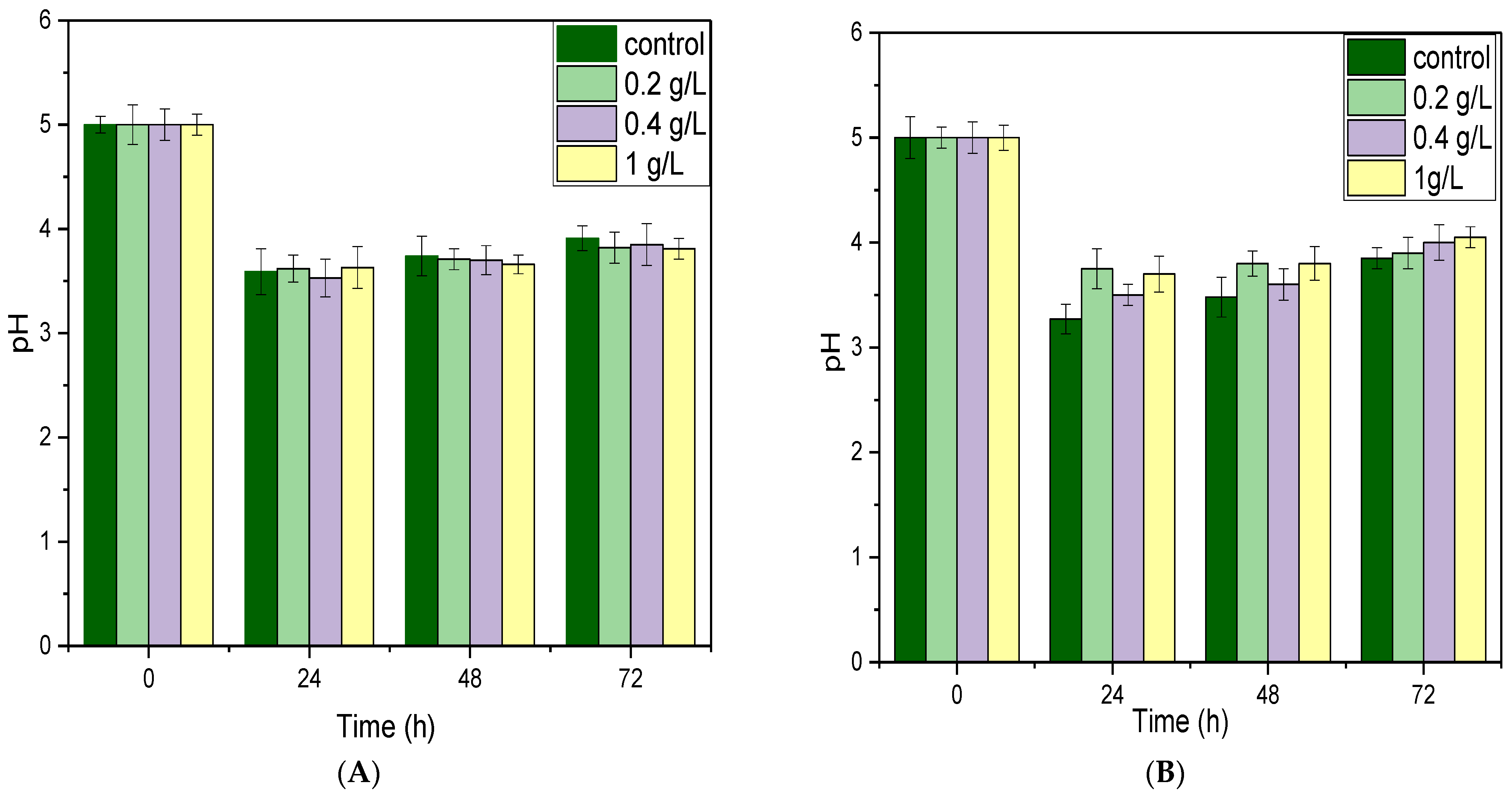
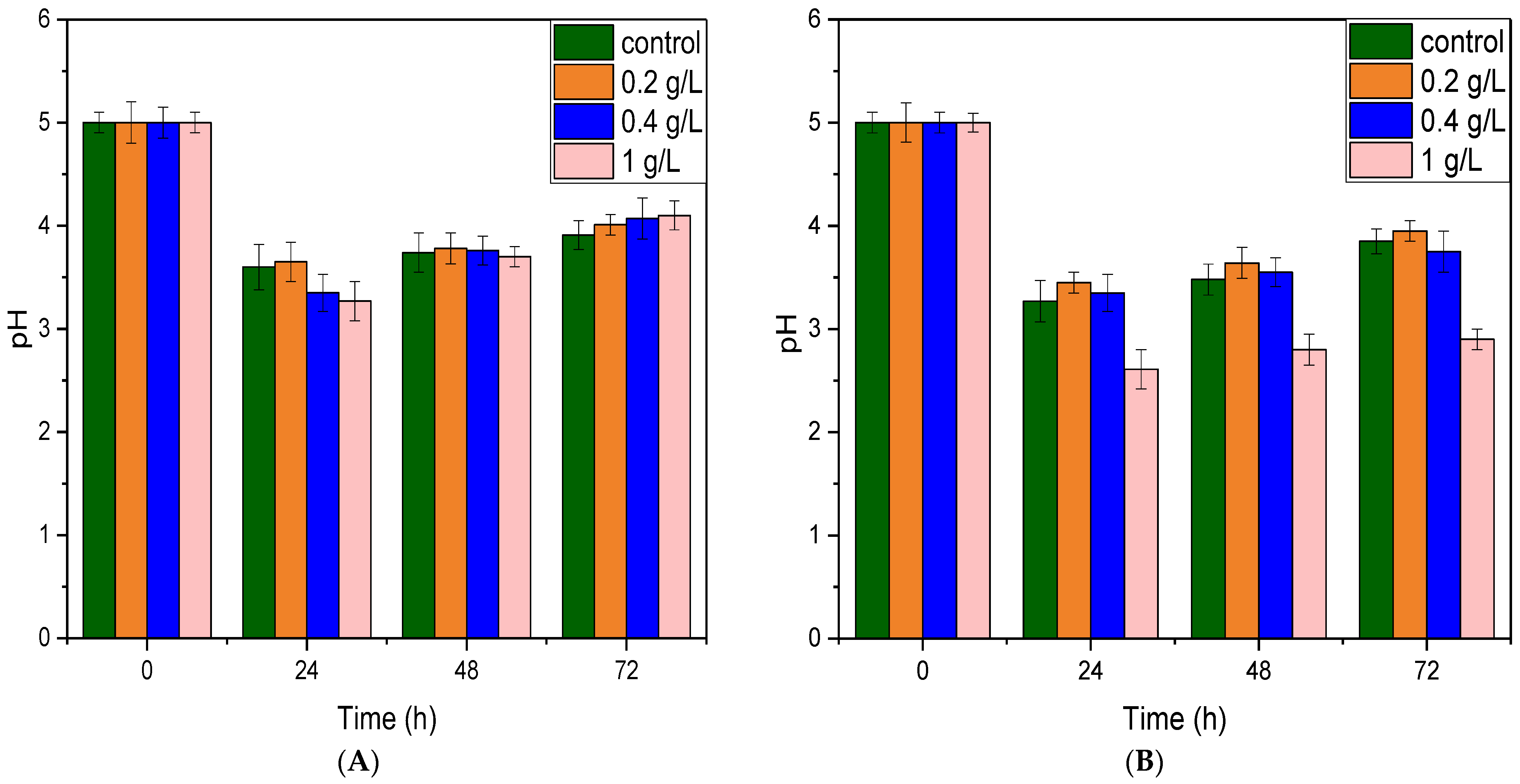
3.1. Effect of CaCO3 and CaCl2 on Fermentation
3.2. Effect of Yeast Extract and NH4Cl on Fermentation
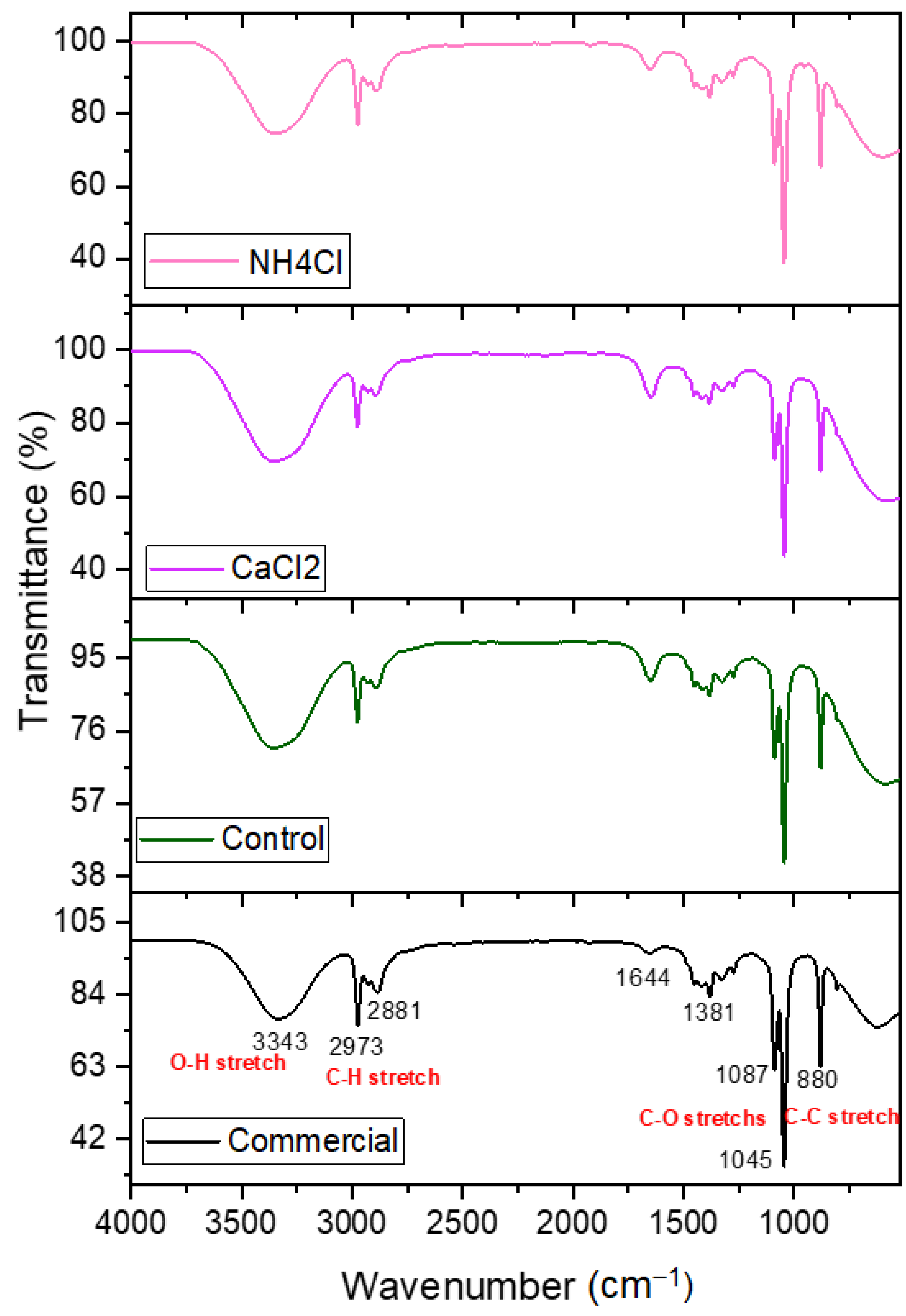
3.3. IR Analyses
3.4. 1 H NMR Analysis
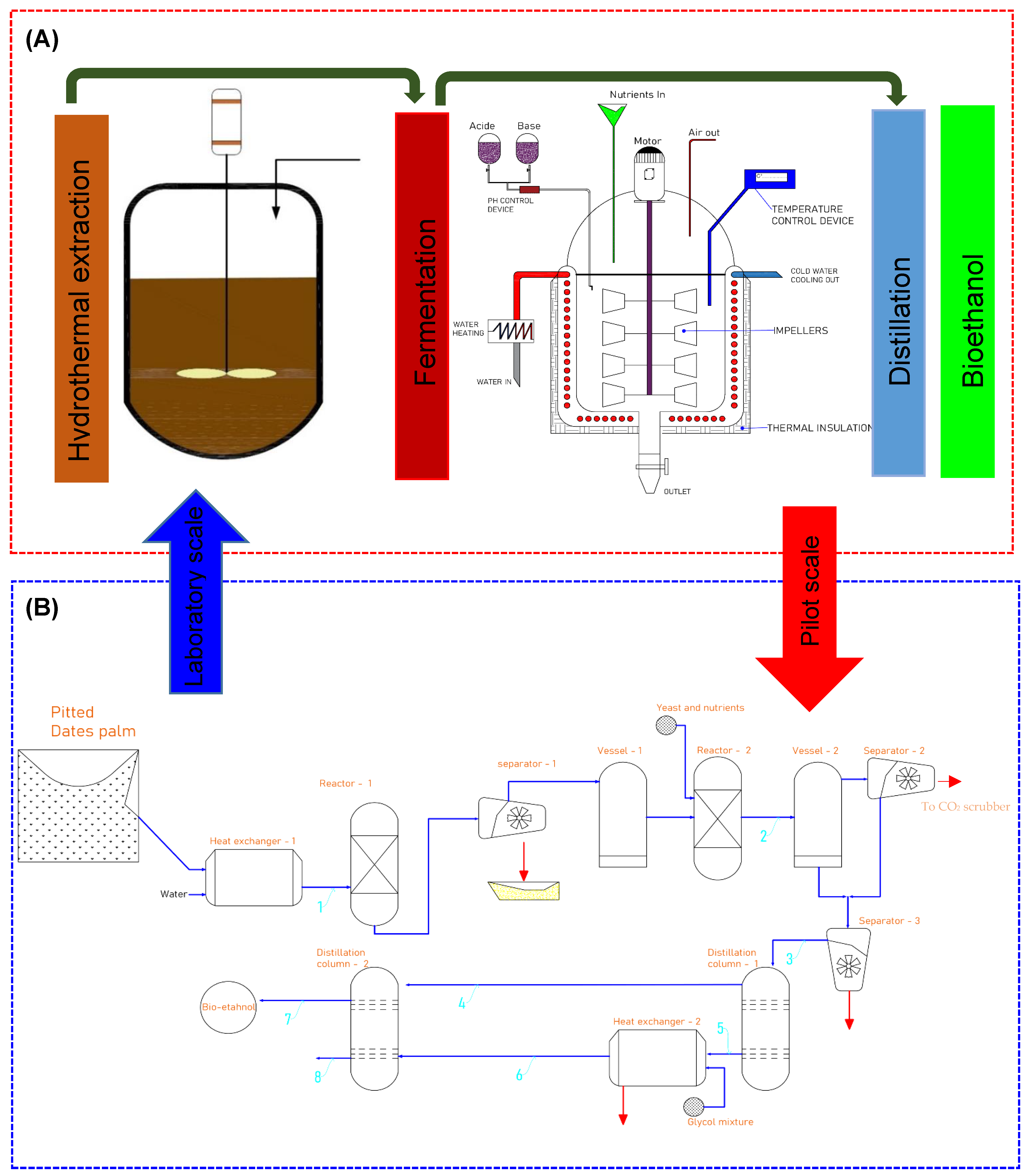
3.5. Technical Design
3.5.1. Hydrothermal Unit
3.5.2. Fermentation Unit
3.5.3. Distillation Unit
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demirbas, A. Progress and recent trends in biofuels. Prog. Energy Combust. Sci. 2007, 33, 1–18. [Google Scholar] [CrossRef]
- Ghazanfar, M.; Nadeem, M.; Shakir, H.A.; Khan, M.; Ahmad, I.; Franco, M.; Chen, L.; Irfan, M. Valorization of Bombax ceiba Waste into Bioethanol Production through Separate Hydrolysis and Fermentation and Simultaneous Saccharification and Fermentation. Fermentation 2022, 8, 386. [Google Scholar] [CrossRef]
- Ahmad, A.; Summar, A.N.; Muhammad, J.J.; Waseem, M.; Ali, E.; Khan, A.I.; Manzoor, M.F.; Siddeeg, A.; Muhammad-Aadil, R. Efficient utilization of date palm waste for the bioethanol production through Saccharomyces cerevisiae strain. Food Sci. Nutr. 2021, 9, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Safarian, S.; Unnthorsson, R.; Richter, C. Bioethanol Production via Herbaceous and Agricultural Biomass Gasification Inte-grated with Syngas Fermentation. Fermentation 2021, 7, 139. [Google Scholar] [CrossRef]
- Almutawa, A.A. Date production in the Al-Hassa region, Saudi Arabia in the face of climate change. J. Water Clim. Chang. 2022, 13, 2627–2647. [Google Scholar] [CrossRef]
- NationMaster.com. Available online: https://www.nationmaster.com/nmx/ranking/dates-production (accessed on 10 October 2022).
- Nikolić, S.; Mojović, L.; Rakin, M.; Pejin, D. Bioethanol production from corn meal by simultaneous enzymatic saccharification and fermentation with immobilized cells of Saccharomyces cerevisiae var. ellipsoideus. Fuel 2009, 88, 1602–1607. [Google Scholar] [CrossRef]
- Pejin, J.D.; Mojović, L.V.; Pejin, D.J.; Kocić-Tanackov, S.D.; Savić, D.S.; Nikolić, S.B.; Djukić-Vuković, A.P. Bioethanol production from triticale by simultaneous saccharification and fermentation with magnesium or calcium ions addition. Fuel 2015, 142, 58–64. [Google Scholar] [CrossRef]
- Azam, M.M.; Ezeji, T.C.; Qureshi, N. Novel Technologies for Enhanced Production of Ethanol: Impact of High Productivity on Process Economics. Eur. Chem. Bull. 2014, 3, 904–910. [Google Scholar]
- Nabais, R.C.; Sá-Correia, I.; Viegas, C.; Novais, J.M. Influence of Calcium Ion on Ethanol Tolerance of Saccharomyces bayanus and Alcoholic Fermentation by Yeasts. Appl. Environ. Microbiol. 1988, 54, 2439–2446. [Google Scholar] [CrossRef]
- Discipline, S. Biotechnology letters. Biotechnol. Lett. 1979, 1, 102. [Google Scholar]
- Su, Y.; Heras, J.M.; Gamero, A.; Querol, A.; Guillamón, J.M. Impact of Nitrogen Addition on Wine Fermentation by S. cere-visiae Strains with Different Nitrogen Requirements. J. Agric. Food Chem. 2021, 69, 6022–6031. [Google Scholar] [CrossRef]
- Yue, G.; Yu, J.; Zhang, X.; Tan, T. The influence of nitrogen sources on ethanol production by yeast from concentrated sweet sorghum juice. Biomass Bioenergy 2012, 39, 48–52. [Google Scholar] [CrossRef]
- Joshi, J. Enhanced Production of Ethanol from Red Potatoes Grown in Hilly Regions of Nepal Using Various Nitrogen Sources. Int. J. Appl. Sci. Biotechnol. 2014, 2, 41–44. [Google Scholar] [CrossRef]
- Joginder, S.D.; Ashok, K.; Sunil, K.T. Bioethanol production from starchy part of tuberous plant (potato) using Saccharomyces cerevisiae MTCC-170. Afr. J. Microbiol. Res. 2013, 7, 5253–5260. [Google Scholar] [CrossRef]
- Tsunatu, D.; Atiku, K.; Samuel, T.; Hamidu, B.; Dahutu, D. Production of bioethanol from rice straw using yeast extracts peptone dextrose. Niger. J. Technol. 2016, 36, 296–301. [Google Scholar] [CrossRef]
- Sheikh, R.A.; Al-Bar, O.A.; Soliman, Y.M.A. Biochemical studies on the production of biofuel (bioethanol) from potato peels wastes by Saccharomyces cerevisiae: Effects of fermentation periods and nitrogen source concentration. Biotechnol. Biotechnol. Equip. 2016, 30, 497–505. [Google Scholar] [CrossRef]
- Tareen, A.K.; Danbamrongtrakool, N.; Sultan, I.N.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksati, P. Utilization of urea as a nitrogen source for ethanol production from oil palm trunk using simultaneous saccharification and fermentation. Agric. Nat. Resour. 2021, 55, 448–455. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Hamden, Z.; El-Ghoul, Y.; Alminderej, F.M.; Saleh, S.M.; Majdoub, H. High-Quality Bioethanol and Vinegar Production from Saudi Arabia Dates: Characterization and Evaluation of Their Value and Antioxidant Efficiency. Antioxidants 2022, 11, 1155. [Google Scholar] [CrossRef]
- Bajpai, P.K.; Margaritis, A. Effect of calcium chloride on the growth and ethanol production by free cells of Zymomonas mobilis. Biotechnol. Lett. 1984, 6, 673–676. [Google Scholar] [CrossRef]
- Ahmed, B.; Mabrouk, K.; Cherif, K.; Boudjemaa, B. Bioethanol production from date palm fruit waste fermentation using solar energy. Afr. J. Biotechnol. 2016, 15, 1621–1627. [Google Scholar] [CrossRef]
- Tesfaw, A.; Assefa, F. Current Trends in Bioethanol Production by Saccharomyces cerevisiae: Substrate, Inhibitor Reduction, Growth Variables, Coculture, and Immobilization. Int. Sch. Res. Not. 2014, 2014, 532852. [Google Scholar] [CrossRef] [PubMed]
- Kalil, M.S.; Alshiyab, H.S.; Yusoff, W.M.W. Effect of Nitrogen Source and Carbon to Nitrogen Ratio on Hydrogen Production using C. acetobutylicum. Am. J. Biochem. Biotechnol. 2008, 4, 393–401. [Google Scholar] [CrossRef][Green Version]
- Turhan, I.; Bialka, K.L.; Demirci, A.; Karhan, M. Ethanol production from carob extract by using Saccharomyces cerevisiae. Bioresour. Technol. 2010, 101, 5290–5296. [Google Scholar] [CrossRef]
- Chniti, S.; Jemni, M.; Rejeb, Z.B.; Chaabane, H.; Hassouna, M. Effect of the Nitrogen Source on Bioethanol Production from Syrup Dates by Saccharomyces cerevisiae. Int. J. Agric. Innov. Res. 2015, 4, 530–535. [Google Scholar]
- Jiménez-Martí, E.; Aranda, A.; Mendes-Ferreira, A.; Mendes-Faia, A.; del Olmo, M.L. The nature of the nitrogen source added to nitrogen depleted vinifications conducted by a Saccharomyces cerevisiae strain in synthetic must affects gene expression and the levels of several volatile compounds. Antonie Leeuwenhoek 2007, 92, 61–75. [Google Scholar] [CrossRef]
- ter Schure, E.G.; van Riel, N.A.; Verrips, C.T. The role of ammonia metabolism in nitrogen catabolite repression in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2000, 24, 67–83. [Google Scholar] [CrossRef]
- Shafaghat, H.; Najafpour, G.D.; Rezaei, P.S.; Sharifzadeh, M. Optimizacija rasta Saccharomyces cerevisiae (ptcc 24860) na pretretiranoj melasi za proizvodnju etanola primenom metode odzivne površine. Chem. Ind. Chem. Eng. Q. 2010, 16, 199–206. [Google Scholar] [CrossRef]
- Martınez-Moreno, R.; Morales, P.; Gonzalez, R.; Mas, A.; Beltran, G. Biomasse production and alcoholic fermentation per-formance of Saccharomyces cerevisiaeas a function of nitrogen source. FEMS Yeast Res. 2012, 12, 477–485. [Google Scholar] [CrossRef]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fer-mentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Ferchichi, M.; Crabbe, E.; Hintz, W.; Gil, G.-H.; Almadidy, A. Influence of Culture Parameters on Biological Hydrogen Production by Clostridium saccharoperbutylacetonicum ATCC 27021. World J. Microbiol. Biotechnol. 2005, 21, 855–862. [Google Scholar] [CrossRef]
- de Kok, S.; Kozak, B.U.; Pronk, J.T.; van Maris, A.J. Energy coupling in Saccharomyces cerevisiae: Selected opportunities for metabolic engineering. FEMS Yeast Res. 2012, 12, 387–397. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Huang, J.; Man, D.; Guo, M. Comparisons of urea or ammonium on growth and fermentative metabolism of Saccharomyces cerevisiae in ethanol fermentation. World J. Microbiol. Biotechnol. 2021, 37, 98. [Google Scholar] [CrossRef]
- Louhichi, B.; Belgaib, J.; Benamor, H.; Hajji, N. Production of bio-ethanol from three varieties of dates. Renew. Energy 2013, 51, 170–174. [Google Scholar] [CrossRef]
- Chniti, S.; Djelal, H.; Hassouna, M.; Amrane, A. Residue of dates from the food industry as a new cheap feedstock for ethanol production. Biomass Bioenergy 2014, 69, 66–70. [Google Scholar] [CrossRef]
- Hashem, M.; Alamri, S.A.; Alrumman, S.A.; Qahtani, M.S.A. Enhancement of Bio-Ethanol Production from Date Molasses by Non-Conventional Yeasts. Res. J. Microbiol. 2015, 10, 114–125. [Google Scholar] [CrossRef]
- Zeinelabdeen, M.A.; Abasaeed, A.E.; Gaily, M.H.; Sulieman, A.K.; Putra, M.D. Coproduction of Fructose and Ethanol from Dates by S. cerevisiae ATCC 36859. Int. J. Chem. Mol. Eng. 2013, 7, 758–761. [Google Scholar]
- Terwisscha Van Scheltinga, J.; Ligterink, N.F.W.; Boogert, A.C.A.; Van Dishoeck, E.F.; Linnartz, H. Infrared spectra of complex organic molecules in astronomically relevant ice matrices: I. Acetaldehyde, ethanol, and dimethyl ether. Astron. Astrophys. 2018, 611, A35. [Google Scholar] [CrossRef]
- Zeinalipour-Yazdi, C.D.; Loizidou, E.Z. An experimental FTIR-ATR and computational study of H-bonding in ethanol/water mixtures. Chem. Phys. 2021, 550, 111295. [Google Scholar] [CrossRef]
- Zuriarrain, A.; Zuriarrain, J.; Villar, M.; Berregi, I. Quantitative determination of ethanol in cider by 1H NMR spectrometry. Food Control 2015, 50, 758–762. [Google Scholar] [CrossRef]
- Gaikwad, V.; Panghal, A.; Jadhav, S.; Sharma, P.; Bagal, A.; Jadhav, A.; Chhikara, N. Designing of Fermenter and its utilization in food industries. Preprints 2018, 1–24. [Google Scholar] [CrossRef]
- Villadsen, J.; Nielsen, J.; Lidén, G. Principles, Bioreaction Engineering, 2nd ed.; Springer: New York, NY, USA, 2011; pp. 339–409. [Google Scholar]
- Park, J.-W.; Park, K.-G.; Lee, N.-Y.; Lee, J.-H.; Lee, J.-W. Enhanced extraction of reducing sugars from fruit of Hovenia dulcis with treatment of cellulase and sequential production of ethanol and acetic acid containing ampelopsin from extracted reducing sugars. Ind. Crop. Prod. 2019, 139, 111522. [Google Scholar] [CrossRef]
- Ali Abbas, R.; Flayeh, H.M. Bioethanol (Biofuel) Production from Low Grade Dates. Iraqi J. Chem. Pet. Eng. 2019, 20, 41–47. [Google Scholar] [CrossRef]
- Taghizadeh-Alisaraei, A.; Motevali, A.; Ghobadian, B. Ethanol production from date wastes: Adapted technologies, challenges, and global potential. Renew. Energy 2019, 143, 1094–1110. [Google Scholar] [CrossRef]
- Salikandi, M.; Salehi, S.; Ashoori, S.; Soleimani, M. Techno-economic study of bioethanol production from olive wastes cake (OWC) in Iran. Clean Technol. Environ. Policy 2021, 23, 1851–1862. [Google Scholar] [CrossRef]
- Matthew Rendleman, C.; Shapouri, H. New technologies in ethanol production. AgEcon Search 2007, 1–25. [Google Scholar]
- Aditiya, H.; Mahlia, T.; Chong, W.; Nur, H.; Sebayang, A. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Gil-Chaves, I.-D.; García, L.C.; Rodríguez, G. Simulation of ethanol extractive distillation with mixed glycols as separating agent. Braz. J. Chem. Eng. 2014, 31, 259–270. [Google Scholar] [CrossRef]





| Calcium Source | ||||
|---|---|---|---|---|
| CaCO3 | ||||
| Amounts (g/L) | pHi | pHf | Bioethanol concentration (g/L) | Ethanol productivity (g/L/h) |
| 0.0 (Control) | 5 ± 0.2 | 3.91 ± 0.1 | 31.6 ± 0.5 | 0.4 |
| 0.2 | 5 ± 0.2 | 3.91 ± 0.1 | 32.4 ± 0.8 | 0.41 |
| 0.4 | 5 ± 0.1 | 4 ± 0.1 | 36.5 ± 1 | 0.46 |
| 1 | 5 ± 0.15 | 4.05 ± 0.1 | 38 ± 0.9 | 0.48 |
| CaCl2 | ||||
| 0.2 | 5 ± 0.2 | 3.81 ± 0.11 | 33.4 ± 0.8 | 0.42 |
| 0.4 | 5 ± 0.15 | 3.84 ± 0.15 | 41.5 ± 0.85 | 0.53 |
| 1 | 5 ± 0.2 | 3.81 ± 0.15 | 39.9 ± 0.9 | 0.511 |
| Nitrogen Source | ||||
| Yeast extract | ||||
| 0.2 | 5 ± 0.2 | 4.01 ± 0.1 | 39.4 ± 0.9 | 0.41 |
| 0.4 | 5 ± 0.1 | 4.07 ± 0.1 | 47.4 ± 1 | 0.60 |
| 1 | 5 ± 0.1 | 4.08 ± 0.2 | 55.3 ± 1 | 0.70 |
| NH4Cl | ||||
| 0.2 | 5 ± 0.2 | 3.95 ± 0.1 | 47.4 ± 0.9 | 0.60 |
| 0.4 | 5 ± 0.1 | 3.75 ± 0.1 | 50.9 ± 0.8 | 0.65 |
| 1 | 5 ± 0.1 | 2.9 ± 0.2 | 65.3 ± 1 | 0.83 |
| Bioethanol Production Conditions | Supplementation Medium | |||||||
|---|---|---|---|---|---|---|---|---|
| Date by-products (varieties) | Time (h) | pH | Yeast | Without | With * | Ethanol concentration (g/L) | Increase (%) | Reference |
| Date juice (Kunta, Eguoua, and Bouhatem) | 72 | 6 | S. cerevisiae | ˣ | 50 | - | [36] | |
| Date syrup (Deglet-Nour) | 72 | 6 | S. cerevisiae | ˣ | 63 | - | [37] | |
| Z. rouxii | ˣ | 33 | - | |||||
| Date molasses (commercial molasses from Saudi) | 96 | 4 | H. guilliermondii | ˣ | 11 | - | [38] | |
| H.uvarum | ˣ | 10 | - | |||||
| Syrup dates (Deglet-Nour) | 72 | S. cerevisiae | ˣ | N (yeast extract) | 24.8 41.9 | - 68.9 | [27] | |
| Syrup dates (Ruzaiz variety) | 48 | - | S. cerevisiae | ˣ | 48.9 | [39] | ||
| Date juice (Khodhari) | 72 | 5 | S. cerevisiae | ˣ | N (NH4Cl) N (yeast extract) Ca (CaCO3) Ca (CaCl2) | 31.6 65.3 55.3 38 41.5 | - 106.6 75 20.25 31.3 | This work |
| Operating Parameters | Values |
|---|---|
| Temperature of alcoholic wine | 75 °C |
| Feed/solvent ratio | 1.25 |
| Reflux ratio | 1.1 |
| Number of theoretical steps | 16 |
| Feed stage | 7 |
| Column pressure | 1 atm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alminderej, F.M.; Hamden, Z.; El-Ghoul, Y.; Hammami, B.; Saleh, S.M.; Majdoub, H. Impact of Calcium and Nitrogen Addition on Bioethanol Production by S. cerevisiae Fermentation from Date By-Products: Physicochemical Characterization and Technical Design. Fermentation 2022, 8, 583. https://doi.org/10.3390/fermentation8110583
Alminderej FM, Hamden Z, El-Ghoul Y, Hammami B, Saleh SM, Majdoub H. Impact of Calcium and Nitrogen Addition on Bioethanol Production by S. cerevisiae Fermentation from Date By-Products: Physicochemical Characterization and Technical Design. Fermentation. 2022; 8(11):583. https://doi.org/10.3390/fermentation8110583
Chicago/Turabian StyleAlminderej, Fahad M., Zeineb Hamden, Yassine El-Ghoul, Bechir Hammami, Sayed M. Saleh, and Hatem Majdoub. 2022. "Impact of Calcium and Nitrogen Addition on Bioethanol Production by S. cerevisiae Fermentation from Date By-Products: Physicochemical Characterization and Technical Design" Fermentation 8, no. 11: 583. https://doi.org/10.3390/fermentation8110583
APA StyleAlminderej, F. M., Hamden, Z., El-Ghoul, Y., Hammami, B., Saleh, S. M., & Majdoub, H. (2022). Impact of Calcium and Nitrogen Addition on Bioethanol Production by S. cerevisiae Fermentation from Date By-Products: Physicochemical Characterization and Technical Design. Fermentation, 8(11), 583. https://doi.org/10.3390/fermentation8110583







