Abstract
The need for a more sustainable society has prompted the development of bio-based processes to produce fuels, chemicals, and materials in substitution for fossil-based ones. In this context, microorganisms have been employed to convert renewable carbon sources into various products. The methylotrophic yeast Komagataella phaffii has been extensively used in the production of heterologous proteins. More recently, it has been explored as a host organism to produce various chemicals through new metabolic engineering and synthetic biology tools. This review first summarizes Komagataella taxonomy and diversity and then highlights the recent approaches in cell engineering to produce renewable chemicals and proteins. Finally, strategies to optimize and develop new fermentative processes using K. phaffii as a cell factory are presented and discussed. The yeast K. phaffii shows an outstanding performance for renewable chemicals and protein production due to its ability to metabolize different carbon sources and the availability of engineering tools. Indeed, it has been employed in producing alcohols, carboxylic acids, proteins, and other compounds using different carbon sources, including glycerol, glucose, xylose, methanol, and even CO2.
1. Introduction
The urgency for effective strategies to mitigate climate change is evident. Environmental issues caused by deliberate petroleum exploitation to produce chemicals, energy, and materials are a major concern regarding global warming. In this context, the global economy needs to be transformed faster, and the circular bioeconomy seems to be the right way to achieve it. This new form of economy aims to reuse, recycle, and remanufacture biomass (from agroecological systems, forestry, and urban wastes) to generate valuable products, such as fuels, biomaterials, and fine chemicals [1]. The circular bioeconomy benefits from integrated frameworks to utilize biomass and address its uses to human needs. One of the major areas that compose this framework is biotechnology, which has the potential to lead bioeconomy global advances even further [2]. The extensive range of roles that biotechnology plays alongside bioengineering provides new techniques to modify the DNA of several microorganisms and plants. Such modifications can improve the utilization of lignocellulosic biomass as raw material through the heterologous protein expression and the production of renewable chemicals, biofuels, and materials [3].
Lignocellulosic biomass polymeric carbohydrates, cellulose, and hemicellulose are mainly composed of glucose and xylose. Glucose is a C6 sugar easily consumed by most microorganisms and can be converted into an extensive range of different chemicals. Xylose, a C5 sugar, can be naturally consumed by filamentous fungi, yeasts, and bacteria. However, this pentose is more challenging to metabolize with the same efficiency as glucose. Nonetheless, xylose can also be converted into various chemicals, such as xylitol [4] and xylonic acid [5]. Recently, advances in genetic manipulation techniques have opened up the range of microorganisms that can produce fine chemicals from glucose and xylose with higher yields and productivity. The yeast Komagataella phaffii is one example of it. Recent research enabled this yeast to consume these sugars and other substrates and produce various chemicals of industrial interest, as will be discussed in this review.
Komagataella phaffii (previously Pichia pastoris) is a well-known methylotrophic yeast. It has many valuable features such as low nutritional requirement, the ability to reach high cell densities (even in acidic culture media), and is widely employed in biotechnological processes to produce heterologous proteins of commercial interest [6]. Therefore, its ability to use methanol as a carbon and energy source, its non-fermentative utilization of glucose under aerobic conditions, and its efficiency to grow on glycerol are some of the main reasons for the preferential choice of this yeast for bioprocess development [7]. These characteristics transformed K. phaffii into a suitable host for many industrial applications. More recently, this yeast has been called the “biotech yeast” for its broad utilization as a cell factory for synthetic biology and metabolic engineering aiming for valuable chemical compound productions (Figure 1) [7,8].
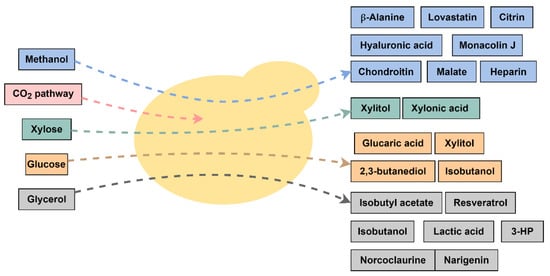
Figure 1.
Representative scheme of the different carbon sources and chemicals produced by engineered K. phaffii strains. 3-HP = 3-hydroxy propionic acid.
K. phaffii has already been used as a successful host for heterologous protein production. However, optimizing K. phaffii to produce chemicals is still necessary, mainly aiming at methanol metabolism, fermentation parameters, and the construction of new genetic tools, such as promoters and plasmids. New methods, platforms, and strategies have been developed to enable more feasible ways to engineer this yeast to address these necessities [9]. Strategies to increase the efficiency rate of target integration genes in the K. phaffii genome through the knockout of homologous genes (ku70 [10] and Dnl4p [11]), and also the utilization of CRISPR/Cas9 to facilitate the genome editing of K. phaffii [12], have already been used. Several examples of the available tools to enhance K. phaffii utilization as a good biotechnology host are summarized in [13].
Previous works have focused on genetic tools and modifications to transform K. phaffii into a robust host aiming at biotechnological approaches [7,14,15]. In this review, we outline the recent advances (last five years) in bioengineering of the yeast K. phaffii for producing renewable chemicals and proteins. The first section briefly explains K. phaffii primordial characteristics and taxonomy. Subsequently, a detailed description of the yeast metabolism of different carbon sources (methanol, glycerol, glucose, and xylose) alongside strategies for the metabolic engineering of the yeast to produce the desired chemicals. The global market size and value (ie., used in this work as the mathematical predictions of the annual volume and value of a product, based on the available data of the past years) of these products is also shown. Additionally, we revise the recent strategies to improve fermentative processes with K. phaffii. Finally, the last sections discuss the challenges and prospects of K. phaffii industrial utilization in producing renewable chemicals.
2. Komagataella Taxonomy and Diversity
The obligate aerobic yeast Komagataella phaffii is a non-pathogenic certified and generally recognized as a safe (GRAS) microorganism. It is classified in the Saccharomycetales order and Saccharomycetaceae family. For being capable of using methanol as the only carbon source, K. phaffii is methylotrophic and was anteriorly known as Pichia pastoris. Phylogenetic studies placed P. pastoris in the genus Komagataella proposed by Yamada et al. (1995) after the analysis of partial sequences of rRNAs subunits (18S and 26S) of the 12 strains of methanol assimilating yeasts [16]. Supporting this previous study, Kurtzman and Robnett (1998) compared gene sequences (D1/D2 LSU rRNA) of about 500 species of Ascomycetous yeasts. The multigenic sequence analysis sustained the phylogenetic position of Pichia pastoris in the Komagataella genus [17].
The first species of P. pastoris (described initially as Zygosaccharomyces pastoris) isolated from a chestnut tree was described in 1919 by Guilliermond [18]. Komagataella genus is currently composed of seven species, with most of them (including K. phaffii) isolated from tree exudates in North America and Europe (Table 1) [19]. All species have spherical to ovoid shapes white/cream colonies, and during asexual reproduction, haploid cells multiply via multilateral budding and do not have pseudohyphae or true hyphae. In sexual reproduction (diploid cells), the ascospore is hat-shaped, ranging from 1 to 4 spores (that can be conjugated or not) [20]. The Komagataella species can grow at high cell densities using methanol, glucose, or glycerol as a carbon source with a doubling time of 2 to 3 h, and ammonium can be used as a nitrogen source during growth [19,20]. Recently, it was demonstrated that some species from Komagataella, including K. phaffii, possess the capacity to grow on xylose [21].

Table 1.
Komagataella species diversity and its characteristics.
Since all species have similar phenotypic and fermentative features, they cannot be distinguished using the conventional taxonomy, for example, morphological tests, that are usually used for yeasts. Therefore, gene sequence analysis (D1/D2 LSU rRNA, ITS, EF-1α) and other gene markers tracking are necessary for the correct species placement and identification [20,22]. Several genome sequencing and transcriptome studies have been conducted throughout the years [23,24,25]. A recent study sequenced the genome of all seven type species of Komagataella and its strains (a total of 25 isolates), identifying strains capable of growing using xylose as the sole carbon source, and which have a higher tolerance to stress conditions, such as alkaline pH [21].
The genomes of the two most studied species of the genus, K. pastoris and K. phaffii (previously both species were classified as P. pastoris), have close genome structures to K. ulmi and K. kurtzmanii, respectively. All species are capable of growing in glucose, glycerol, methanol, and ethanol. All 25 strains could grow at acidic pH (4.0) after 7 days, whereas at pH 9.0, all the species were strongly affected. Only the K. pseudopastoris (anteriorly known as P. pseudopastoris) and K. kurtzmanii CBS 12,817 strains were able to adapt and had better growth when compared to the other species [21]. Commonly, non-engineered yeasts in this genus do not present exponential growth on xylose; however, as indicated in the study of Heistinger et al. (2022), all seven species are capable of using this sugar and grow at slow rates. The species K. pastoris CBS 704 and K. populi CBS 12,362 showed the best growth on xylose [21].
Despite the initial studies on Komagataella diversity, the strains of K. phaffii X33 (prototrophic strain) and GS115 (HIS− phenotype) are the ones most used in biotechnological applications, and are mainly used in studies of heterologous expression [22]. In recent years, there have been impressive methodological developments to model and analyze the metabolism of K. phaffii and engineer its genome and metabolic pathways, including improvements in genome modification with homologous recombination and target-guided gene cloning using CRISPR/Cas9 [14]. Indeed, the information about the genetic and physiological profile of this specie and the availability of genetic tools to manipulate it has been crucial for works on metabolic engineering and recombinant protein production studies. The most recent data will be presented and discussed in the following sections.
3. Production of Renewable Chemicals from Glycerol by K. phaffii
3.1. Metabolism of Glycerol
Glycerol is a carbon source widely used by microorganisms through central carbon metabolism [26]. Due to its higher reduction degree than glucose, the primary carbon source for most microorganisms, glycerol can be used to produce reduced fuel and chemicals at higher yields [27]. In addition, as a significant side stream byproduct in biodiesel production, there has been a growing interest in using glycerol to generate value-added products within the global scenario of renewable and environment-friendly energy, waste reduction, and a more sustainable society [26]. However, its industrial utilization by natural microorganisms is usually limited by the production titers, rates, or yields.
K. phaffii utilizes glycerol as a carbon source very efficiently. Glycerol conversion connects to central carbon metabolism in glycolysis via glyceraldehyde-3-phosphate formation (Figure 2). For such, it is phosphorylated to glycerol 3-phosphate by the cytoplasmic glycerol kinase, sequentially glycerol 3-phosphate dehydrogenase oxidates glycerol-3P to form dihydroxy acetone phosphate (DHAP). Finally, DHAP is converted into glyceraldehyde-3-phosphate by triose phosphate isomerase and enters glycolysis (Figure 2) [28]. Despite the oxidative pathway being preferred in glycerol assimilation, the conversion to glyceraldehyde-3-phosphate can also converge to gluconeogenesis by the activity of a cytosolic aldolase (FBA1-2) that catalyzes the reversible reaction of dihydroxyacetone phosphate and glyceraldehyde 3-phosphate into fructose 1,6-bisphosphate (F-1,6BP). The F-1,6BP is converted into Fructose-6P (F-6P) by a phosphofructokinase (FBP1), and the F-6P can enter the pentose phosphate pathway (PPP), an important pathway to regenerate the reducing cofactor NADPH [29].
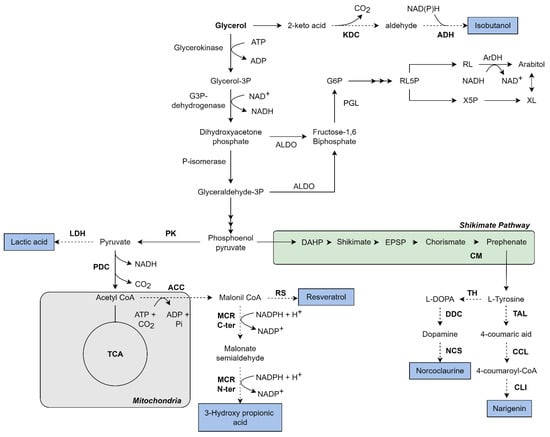
Figure 2.
Metabolic pathway to glycerol assimilation and production of a variety of chemicals by K. phaffii. Black arrows represent the native glycerol metabolism; dotted arrows represent an overexpressed/heterologous pathway. Bold enzyme names represent an overexpressed/heterologous enzyme, and the blue boxes represent the final product. Some reactions are summarized for better comprehension. Additionally, not all cofactors are represented in the figure. G6P: Glucose-6-phosphate; RL5P: Ribulose-5-phosphate; RL: Ribulose; X5P: Xylulose-5-phosphate; XL: Xylulose; DHAP: 3-deoxy-D-arabinoheptulosonate-7-phosphate; EPSP: 5-O-(1-carboxyvinyl)-3-phosphoshikimate; KDC: Keto-acid decarboxylase; ADH: Alcohol dehydrogenase; ArDH: Arabitol dehydrogenase; ALDO: Adolase; PGL: Phosphoglucose isomerase; LDH: Lactate dehydrogenase; PDC: Pyruvate decarboxylase; PK: Pyruvate kinase; ACC: Acetyl-CoA carboxylase; RS: Resveratrol synthase; MCR (C-ter/N-ter): Malonyl CoA reductase; CM: Chorismate isomerase; TH: Tyrosine hydroxylase; DOC: DOPA decarboxylase; NCS: Norcoclaurine synthase; TAL: Tyrosine ammonia lyase; CCL: 4-coumarate CoA ligase; CLI: Chalcone isomerase. The figure of own authorship is based on KEGG pathways (www.genome.jp—accessed on 13 September 2022) and in the literature cited in the text.
Glycerol is regularly used as the primary initial carbon source for K. phaffii cell growth, which allows yeast growth rates similar to those obtained on glucose [30]. In contrast to S. cerevisiae, K. phaffii can maintain its respiratory metabolism and all its related pathways, such as oxidative phosphorylation, electron transport chain, oxidative balance cofactors, and ATP generation, even in the presence of an excess of glucose, and also demonstrates similar biomass production and substrate uptake kinetics when fed with glucose and glycerol [25]. The efficient use of glycerol as a carbon source by K. phaffii is mainly due to the active transport of glycerol via an H+/glycerol carrier system that allows the utilization of an electrochemical gradient of H+ protons across the membrane for the cellular influx of glycerol [31].
3.2. K. phaffii Engineering for Renewable Chemicals from Glycerol
Lignocellulosic valorization through chemical production from glucose and xylose has been widely evaluated and applied. Similarly, using crude glycerol, a byproduct of biodiesel production, to produce platform and fine chemicals is attractive [26]. Its valorization is an exciting option in developing a glycerol-based integrated biorefinery concept due to the high production volumes of crude glycerol in the biodiesel production chain [26,32]. The chemical composition of crude glycerol is variable and can contain 60–80% glycerol, 10–20% of soap or other organic compounds, and 10–20% methanol [33]. Methanol is toxic for most microbes, except for methylotrophic microorganisms. Thus, as K. phaffii is able to grow in methanol as the sole carbon source, this yeast is a very well-fit microorganism for utilizing crude glycerol as a primary carbon source for the production of renewables chemicals [14]. Indeed, new metabolic pathway designs and engineering tools for K. phaffii increased in the last few years, mainly towards producing complex (secondary) metabolites from glycerol [14]. K. phaffii engineered strains for the production of different chemicals from glycerol, including lactic acid, 3-hydroxypropionic acid (3-HP), isobutanol, isobutyl acetate, resveratrol, norcoclaurine, and narigenin, have been constructed. The details are presented and discussed in the following sections.
3.2.1. Lactic Acid Production from Glycerol by K. phaffii
Lactic acid (2-hydroxypropanoic acid) is one of the most important industrially used acids. A wide range of organisms can produce lactic acid, including the lactic acid bacteria (LAB) (e.g., strains from Lactobacillus genus), filamentous fungi (e.g., genus Rhizopus), yeasts (Kluyveromyces lactis and Saccharomyces cerevisiae), and also by microalgae (e.g., Scenedesmus obliquus) [34,35]. More information about lactic acid production by different microorganisms is revised in [35]. Moreover, lactic acid can also be obtained by chemical synthesis [36].
Lactic acid has several applications, such as in the food industry, primarily as an acidulant and preservative chemical agent; and in the pharmaceutical and chemical industries, mainly as a pH regulator, humectant, and solvent. In addition, its monomeric form can be used to produce biodegradable poly-lactic acid (PLA). The PLA has several industrial applications, such as automobile, packaging, and cosmetic industries [34]. The manufacturing of biodegradable PLA materials, ecologically feasible alternatives to the consistent use of petroleum for plastic production, has contributed immensely to the global attention to the biotechnological production of lactic acid. Indeed, the lactic acid market in 2021 was valued at USD 2.9 billion, and the annual growth expectation is of 8.0% rate until 2030 (www.grandviewresearch.com—accessed on 14 September 2022).
Lactic acid production through the biological route is advantageous compared to the chemical one. The main advantage regarding the biotechnological production of lactic acid is that the microorganisms can be engineered to produce only one isomer of lactic acid (L-lactic acid), which facilitates the subsequent production of PLA [37]. K. phaffii is one candidate organism for its production using glycerol as substrate [36,37]. As K. phaffii does not produce lactic acid naturally, a biotechnological route for glycerol utilization in fermentative production was proposed (Figure 2). The gene ldh that codifies for the enzyme lactate dehydrogenase (LDH) was cloned into the yeast through homologous recombination. Additionally, a new homologous lactate transporter (PAS) was identified and overexpressed to enhance lactic acid production in the LDH-expressing strains. The strains were evaluated by their abilities to produce lactic acid in different oxygen concentrations using glycerol as the sole carbon source. The results demonstrated that a higher lactic acid production could be achieved in limited oxygen conditions, though it can guide the yeast through a fermentative metabolism. Under the best conditions, the engineered strain (GLS) was able to produce lactic acid with a yield of 0.7 g/g (Table 2) when fed with pure glycerol (40 g/L). This was the first reported work concerning K. phaffii genetic modification to produce lactic acid from glycerol [36].

Table 2.
Production of chemicals from different carbon sources by engineered K. phaffii strains.
A subsequent study approached a different strategy to increase the lactic acid production by K. phaffii. As the pyruvate leads to lactic acid production (Figure 2), the authors performed an overexpression of LDH enzymes in combination with the pyruvate decarboxylase (PDC) deletion. This strategy aimed to guide the pyruvate towards lactic acid production and decrease the formation of by-products (e.g., acetic acid). The best strain produced lactic acid with a yield of 0.65 g/g under oxygen-limited condition. Although the acetic acid production was reduced by 20%, the metabolic flux was guided into arabitol formation. The NADH regeneration necessity can explain the flux deviation to arabitol under oxygen-limited conditions. This cofactor unbalance can lead the glycerol metabolism towards the reoxidation of NADH by the reduction of xylulose or ribulose, producing arabitol as a resulting metabolite (Figure 2) [37].
To further investigate glycerol metabolism and the production of lactic acid and arabitol in K. phaffii strains, the lactic acid producer strain GLp was extensively investigated under different growth conditions. Once more, lactic acid production was proven better (5-folds higher) in conditions of oxygen limitation, although the arabitol formation is also better in the same conditions. To overcome this issue, a putative arabitol dehydrogenase gene (ArDH) was disrupted. As expected, the gene deletion decreased the arabitol production (50% less), leaving only residual concentrations, and increased the lactic acid production yield by 20% (when compared to previous study (Table 2) [37,38].
3.2.2. 3-Hydroxy-Propionic Acid (3-HP) Production from Glycerol in K. phaffii
3-Hydroxy propionic acid or 3-hydroxypropanoate (3-HP) is a three-carbon organic compound and is a lactic acid isomer (2-hydroxypropanoic acid). 3-HP is an important building block, mostly used in producing petroleum-based chemicals such as acrylic acid and biopolymers (poly 3-HP) [39,54]. The 3-HP is a versatile molecule for the production of platform chemicals due to its compositional particularity of having a carboxyl and a hydroxyl group. Besides acrylic acid and poly 3-HP, it can be used to produce a wide range of chemicals (e.g., methyl acrylate, ethyl 3-HP, acrylamide, 1,3-propanediol and malonic acid) [54]. Such a wide range of possibilities involving 3-HP utilization increased its global market value, and in 2021 it was valued at USD 111.74 million, with an annual growth expectation of 4.71%, and possibly reaching USD 147.24 million in 6 years (www.marketwatch.com—accessed on 1 September 2022).
Despite the diverse applications, no biological processes are commercially available to produce 3-HP; on the other hand, several patents have been claimed in the past few years, mostly regarding the conversion of 3-HP into acrylic acid in one step reaction. Some microorganisms can produce 3-HP naturally, including Lactobacillus sp., and several microorganisms have been modified to enhance the 3-HP production, although the titers, rates and yields (TRY), are not optimum yet [55]. For a more comprehensive view on 3-HP biological production, see [54], and for process engineering for 3-HP production from glycerol through the last few years, see [55].
Nonetheless, the strain engineering of microorganisms aiming to enhance the 3-HP TRY is still necessary to develop a feasible application of the industrial 3-HP biological process [54]. In this context, K. phaffii is a suitable candidate to produce 3-HP from glycerol, as this yeast has a naturally well-established glycerol metabolism. To this end, 3-HP was produced in a strain of P. pastoris expressing the genes leads to the 3-HP production through the malonyl-CoA pathway (Figure 2). Three heterologous genes were expressed in K. phafffii, encoding for malonyl-CoA reductase from Chloroflexus aurantiacus (mcrCa), an acetyl-CoA carboxylase from Yarrowia lipolytica (ACCYl), and a cytosolic NADH kinase from S. cerevisiae (cPOSSc). As a result, the recombinant strain of K. phaffii was able to produce 24.75 g/L of 3-HP, with a yield of 0.13 Cmol/Cmol, and 0.54 g/L/h in a fed-batch fermentation. The authors also detected arabitol as a byproduct at the end of the fermentation [39].
3.2.3. Isobutanol and Isobutyl Acetate Production from Glycerol in K. phaffii
The four-carbon aliphatic alcohol isobutanol is a valuable platform compound extensively used in various industries such as food, chemicals, and pharmaceuticals [56]. The production of this compound through fermentation by microorganisms is considered a possibility as a second-generation biofuel. When set side by side with ethanol, the prevailing biofuel produced (first generation), isobutanol, presents elevated ignition power, less corrosive, and lower aqueous miscibility [57]. Furthermore, isobutanol (due to its chemical and physical characteristics) is conformable with the procedures and processes commonly applied in the gasoline industry. Consequently, there is no need for profound changes in infrastructure [58].
The Crabtree-negative methylotrophic yeast, K. phaffii, was metabolically engineered to produce isobutanol and isobutyl acetate from glucose and glycerol (Table 2) [40]. For the production of isobutanol, genes of the native amino acid biosynthetic pathway were overexpressed, guiding the carbon flux into the 2-keto acids degradation pathway (commonly known as the Ehrlich pathway). In this route, the enzyme 2-keto acid decarboxylase (KDC) decarboxylated the 2-keto acid into an aldehyde that is reduced into alcohol by an alcohol dehydrogenase (ADH) (Figure 2). The first strategy to increase alcohol production was through the Ehrlich pathway, employing new KDC and ADH enzymes selected to be expressed in K. phaffii. For the KDC, two enzymes were screened, one from L. lactis (ARO10) and the other from S. cerevisiae (TH13). Two enzymes (both from S. cerevisiae) were selected for ADH, ADH6, and ADH7. Additionally, aiming to enhance the isobutanol production, the media was supplemented with 2-ketoisovalerate, a precursor of the Ehrlich pathway. The heterologous expression of KDC and ADH with supplemented media resulted in the production of 284 mg/L of isobutanol [40].
Aiming to enhance isobutanol production, and considering cost-effective approaches, another strategy to improve the level of this compound was applied. Hereupon, the overexpression of part of the L-valine biosynthetic pathway was performed in the engineered strain, eliminating the supplementation of the costly 2-ketoisovalerate [40]. When this approach was applied in S. cerevisiae, the levels of isobutanol generation were not elevated, but in contrast, in K. phaffii, the production of isobutanol reached 0.89 g/L. Further, the fine-tuning expression of critical enzymes boosted the isobutanol production to 2.22 g/L, which represents a 43-fold enhancement compared to the first results. Additionally, to explore the versatility of this yeast, a gene (ATF1 from S. cerevisiae) that codes for an alcohol O-acyltransferase was heterologously expressed in K. phaffii to produce different volatile esters, which resulted in the production of 51 mg/L of isobutyl acetate ester and 24 mg/L isopentyl acetate ester [40].
3.2.4. Aromatic Secondary Metabolite Production from Glycerol in K. phaffii
Plant phenolic compounds, such as stilbenoids, flavonoids, and benzylisoquinoline alkaloids (BIAs), are mostly generated from the amino acid L-tyrosine (L-Tyr). These metabolites have a valuable application in the pharmaceutical and food industries. Considering the commercial interest in these aromatic compounds, producing them at high levels by microorganisms is of great interest to [31].
In that way, engineering yeasts have been studied for the overproduction of the amino acid L-Tyr. An engineered K. phaffii L-Tyr strain was obtained to enhance the production of phenolic compounds such as resveratrol, naringenin, norcoclaurine, and reticuline (Figure 2) (Table 2). The overexpression of a phosphate synthase (ARO4K229L) and chorismate mutase (ARO7G141S), both from S. cerevisiae, improved the L-Tyr concentration using glycerol as a carbon source in K. phaffii [31]. The ARO4K229L is a phosphate mutase, an enzyme involved in the shikimate pathway, which is a route that synthesizes the amino acids phenylalanine, tyrosine, and tryptophan. The shikimate pathway is shown in Figure 2, where the enzyme chorismate mutase (CM) modifies chorismate into prephenate, the precursor for aromatic amino acids, such as L-tyrosine.
With the enhancement of tyrosine production by the engineered K. phaffii strain, the resveratrol production reached 451 mg/L, whereas naringenin was 306 mg/L. When a fed-batch approach was performed with glycerol as a carbon source, the amount of these aromatic metabolites was even more significant, reaching 1825 mg/L for the resveratrol and 1067 mg/L for naringenin. These values are the highest reported in the literature for the two compounds [31]. This work mentioned above was the first to evaluate the potential of K. phaffii to produce phenolic metabolites using crude glycerol, confirming the importance and versatility of this yeast to generate a range of compounds of interest.
3.3. Glycerol Co-Utilization by K. phaffii
Among the major characteristics of K. phaffii, the ability to use glucose and glycerol as carbon sources in fast growth rates, resulting in high cell densities, turns attention to the use of this yeast in different applications [7]. One of the main challenges to producing high amounts of a compound of interest is the carbon distribution, which can go for cell growth and synthesis of the product itself. When a mixed carbon source is utilized, it is possible that the cell metabolism could be reasonably split, redirecting the substrates in pathways for the growth and production of aimed compounds. A new study reported an engineered strain of K. phaffii, GS115, that was able to co-utilize glucose and glycerol, redirecting the consumption of glycerol for growth, whereas maintaining the glucose for product generation [59].
For this purpose, the identification of K. phaffii potential genes involved in the carbon catabolite repression was performed. In this search, the deletion of the glucose sensor (gss1) eliminated the glucose-induced suppression of glycerol use in media containing glucose and glycerol. When the transcriptome of the control (non-deleted strain) and the deleted mutant was compared, it was possible to identify that the genes related to glycerol metabolism were under glucose regulation. Then, the overexpression of these genes related to glycerol utilization pathways (gt1, gut1, and gut2) made it feasible for the mutated K. phaffii to co-utilize both substrates. Moreover, the knockout of targeted genes lowered the carbon flux from glucose onto major cell pathways, such as glycolysis [60]. Through the metabolic engineering of K. phaffii, the new strain was able to utilize glycerol for cell growth, whereas glucose was used for product formation [60]. The co-utilization of different substrates is an interesting and versatile strategy, highlighting K. phaffii as an alternative microbial platform for producing value-added products.
4. Renewables from Sugars and Lignocellulosic Hydrolysates
Lignocellulose is one of the most abundant and promising renewable resources, with a global annual yield of around 1.3 billion tons [61]. It can be obtained from agricultural residues after crop processing, forest residues, energy feedstock residues, municipalities, and others [62]. Lignocellulose consists of a complex tridimensional polymer that composes the cell wall matrix of plant biomass, which is usually a remnant material after the extraction of sugars for first-generation bioethanol production or other processes [63]. It is composed of cellulose (38–50% on average), hemicellulose (23–32%), lignin (15–25%), and small amounts of pectin, nitrogen compounds, and inorganic compounds. This ratio may vary depending on plant biomass source, such as herbs, softwood, or hardwood (Table 3) [64].

Table 3.
Composition of different lignocellulosic biomasses and hydrolysates.
Cellulose is a linear polymer of glucose monomers linked by β-(1-4) glycosidic bonds that form long and stable glucan microfibrils, providing support to the plant cell wall. Hemicellulose is a mixture of linear and branched polysaccharides connected by acetyl and methyl groups creating an amorphous structure. Xyloglucan, formed by glucose core chain, and heteroxylan, formed by xylose core chain, are the major components of hemicelluloses in plants. These polymers often present heterogeneous side chains of different hexoses (glucose, galactose, mannose, and fucose), pentoses (xylose and arabinose), and uronic acids (glucuronic acid and galacturonic acid). There may also be side branches of methyl, feruloyl, and acetyl groups [66]. Lignin is a heterogeneous aromatic polymer formed by coupling monomers of monolignols such as coniferyl alcohol, sinapyl alcohol, and p-coumaryl alcohol, in a complex structure linked by either carbon-carbon bond connections or ether linkages. A detailed description of lignocellulose is given at [67].
Because of the high degree of polymerization, the production of bioproducts from lignocellulosic biomass requires pretreatment processes to separate or solubilize complex components and release fermentable sugars, e.g., glucose and xylose. Primary, pretreatment methods are applied towards the degradation of the lignin matrix and the removal of hemicellulose fraction. This also reduces cellulose crystallization and increases its surface to later enzymatic and microbial digestion [68].
Pretreatment processes are generally classified as physical, chemical, physicochemical, or biological, and their combinations. Over the last three decades, several pretreatment methods have been developed to provide fast degradation of lignin and high sugar availability with the efficient hydrolysis of cellulose and hemicellulose., including chemical (organic solvents like acid, alkaline, and others), physical (grinding and milling), physio-chemical (hot water, ammonia fiber explosion, and steam explosion), and biological (enzymatic digestion). For detailed data about pretreatments and their applications, refer to [65,68].
During lignocellulosic biomass pretreatment and hydrolysis processes, the cellulose and hemicellulose breakdown, and the sugars are released into the fermentation media, called hydrolysate. In addition, several bioactive compounds are also released or formed during these processes, which can be grouped as organic acids (acetic acid, formic acid, levulinic acid), furaldehydes (furfural and 5-hydroxymethylfurfural), and phenolic compounds (ferulic acid, coumaric acid, vanillin, syringaldehyde, coniferyl alcohol, and others) [69]. In general, lignocellulose inhibitors damage cell membranes and inhibit key metabolic enzymes, affecting the fermentative bioprocess by inhibiting microbial metabolism [69]. Aldehydes can also generate reactive oxygen species (ROS) and lead to their accumulation in the cytoplasm. These toxic molecules inhibit the central carbon metabolism enzymes, such as alcohol dehydrogenase, pyruvate dehydrogenase, and aldehyde dehydrogenase. A more detailed view of lignocellulose-derived inhibitors formation and their effects are described in [69,70]. Apart from biomass constitution, the type and conditions of pretreatments also determine the nature and abundance of the inhibitors released [65]. Some examples of the composition of lignocellulose hydrolysates are presented in the table (Table 3).
To overcome the negative effects of lignocellulose-derived inhibitors, the cells developed strategies to detoxify these compounds and repair damages. Yeast response to lignocellulose inhibitors involves cellular modifications to transcriptional and proteomic levels, resulting in changes in metabolic fluxes and cell reorganization to increase detoxification mechanisms and cell membrane composition [70,71]. For instance, the detoxification of furaldehydes occurs through the compound’s NADH- and NADPH-dependent reduction, which affects the yeast’s redox balance and fermentation pathways. In K. phaffii and S. cerevisiae, the presence of furfural and HMF leads to the increased expression of several classes of genes and also of oxidoreductases encoding genes that reduce aldehydes into less toxic corresponding alcohols [71].
K. phaffii shows a dose-dependent response to furaldehydes with a relatively high tolerance to these lignocellulose-derived molecules, maintaining cell viability in the presence of 1.25 g/L furfural and 0.25 g/L HMF. In the presence of these furaldehydes, K. phaffii transcriptome analysis revealed the up-regulation of genes involved in oxidoreduction processes and transmembrane transport [71]. Transcriptional profiling of K. phaffii in the presence of acetic acid revealed up-regulation of genes involved in nucleic acid processing, methylation, and Rho protein signal transduction regulation, while the presence of furaldehyde revealed up-regulation of genes involved in oxidoreduction and membrane transport [71]. In addition, K. phaffii has been shown to sustain growth in the presence of up to 6 g/L of acetic acid in a defined medium. Moreover, the up regulation of genes involved in transmembrane transport, RNA processing, and regulation of metabolic processes was also reported in K. phaffii in the presence of 10% diluted acid hydrolysate. In contrast, cellular viability was completely inhibited in the presence of 30% diluted acid hydrolysate [71].
4.1. Metabolic Engineering of K. phaffii to Produce Renewables Chemicals from Glucose
To produce high value-added products from lignocellulose-derived sugars, it requires K. phaffii to incorporate metabolic adaptations to effectively utilize its most abundant C6 and C5 sugars: glucose and xylose [72]. Bio-based chemicals such as ethanol, butanol, and organic acids, showed a compound annual growth rate (i.e., mean annual growth rate of an investment over a period) of 16.16% (USD 6474 million) in 2017. It is expected to reach around USD 23 million by 2025, with biofuels accounting for the highest revenue [64]. In this context, valorizing renewable feedstock into value-added renewable chemicals is an urgent goal.
Several groups have been working in the metabolic engineering of K. phaffii to produce renewable chemicals from glucose and xylose in the last couple of years (Table 2). The strategies employed are presented below.
4.1.1. Isobutanol Production from Glucose in K. phaffii
As mentioned above, isobutanol (C4H10O) is a branched chain alcohol with the potential as biofuel in substitution of gasoline for high-performance petrol engines [73]. The production of isobutanol creates the opportunity for a range of downstream products. It can be converted into para-xylene via isobutylene and oxidized to terephthalic acid for the production of PET [74]. Bioproduction has been attained in S. cerevisiae; however, its natural susceptibility to the Crabtree effect drives to low isobutanol yield against the preference for ethanol production. In K. phaffii, isobutanol production can be achieved using glycerol and glucose as substrates (Table 2). Different to with glycerol, the production of isobutanol in K. phaffii using glucose was achieved by exploiting the yeast’s endogenous L-valine pathway through overexpression of PpIlv6, PpIlv2, PpIlv5, and PpIlv3, under GAP promoter in two integrative expression plasmids and diverting the intermediates into the 2-keto acid degradation pathway through the heterologous expression of keto acid decarboxylase (KDC) from Lactococcus lactis (LlkivD) and ADH7 from S. cerevisiae (ScADH7). The highest yield of 22.21 mg/g of isobutanol was obtained directly from glucose (Figure 3). Previous work on engineered S. cerevisiae has reached up to 8.49 g/L of isobutanol [75]. In a different approach, the production of isobutanol in S. cerevisiae was regulated with an optogenetic system called OptoEXP and OptoINVRT. These tools enabled the gene regulation through light pulses (using as a model the blue light-activated EL222 [76]) and the production of three different chemicals of industrial interest, lactate, 2-methyl-1-butanol and isobutanol, the latter with titers of 8.49 ± 0.31 g/L [75]. More information about the microbial production of isobutanol and its future perspectives can be found in [77].
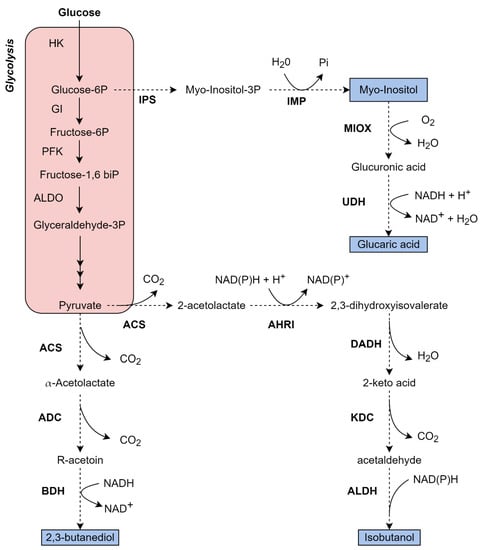
Figure 3.
Metabolic pathway to glucose assimilation and production of a variety of chemicals by K. phaffii. Black arrows in the red boxes summarize the native glucose metabolism; dotted arrows represent an overexpressed/heterologous pathway. Bold enzyme names represent an overexpressed/heterologous enzyme, and the blue boxes represent the product formation. Some reactions are summarized for better comprehension, also, not all cofactors are represented in the figure. HK: Hexokinase; GI: Glucose isomerase; PFK: Phosphofructokinase; ALDO: Fructose-1,6-biphosphato aldolase; ACS: Acetolactate synthase; ADC: Acetolactate decarboxylase; BDH: Butanediol dehydrogenase; AHRI: Acetohydroxy acid reductor isomerase; DADH: Dihydroxy acid dehydratase; KDC: Keto-acid decarboxylase; ALDH: Aldehyde dehydrogenase; IPS: Inositol phosphate synthase; IMP: Inositol monophosphatase; MIOX: Inositol oxygenase; UDH: Uronate dehydrogenase. The figure of own authorship is based on KEGG pathways (www.genome.jp—accessed on 13 September 2022) and in the literature cited in the text.
4.1.2. 2,3-Butanediol Production from Glucose in K. phaffii
2,3-butanediol (2,3-BD) (C4H10O2) is another bulk platform biochemical that microorganisms can produce from lignocellulose-derived sugars. It can be converted into methyl ethyl ketone, gamma-butyrolactone, and 1,3-butadiene with potential applications in the fuel, polymer, food, and pharmaceutical industries [78]. Heterologous production of 2,3-BD via GRAS microorganisms, such as E. coli and S. cerevisiae, has been reported, with the highest production of 100 g/L of 2,3-BD in S. cerevisiae from glucose and galactose. K. phaffii strains were engineered for the production of 2,3-BD isomer (2R, 3R)-2,3-BD with the expression of codon-optimized sequences of acetolactate synthetase (AlsS) and acetolactate decarboxylase (AlsD) retrieved from B. subtilis under pGAP promoter, and also a 2,3-butanediol dehydrogenase (BDH1) from S. cerevisiae was overexpressed in K. phaffii, thus, the heterologous BDH1 did not increased the 2,3-BD production. [41]. The highest 2,3-BD production from glucose was achieved in fed-batch optimized cultivation, reaching 74 g/L with yield and productivity of 0.3 and 0.81 g/L/h, respectively, with 99% enantiopurity (Table 2). This result was close to the 100 g/L production of 2,3-BD previously achieved in S. cerevisiae by [79] with 0.35 yield and productivity of 0.33 g/L/h with 98% enantiopurity.
4.1.3. Inositol Production from Glucose in K. phaffii
Myo-inositol (inositol) (C6H12O6) is a carbocyclic sugar present in microorganisms, plants, and animals. Dietary inositol has been found to benefit treatments of neurological and endocrine diseases and is also regarded as an essential nutrient for aquatic animals [43,80]. Recently, K. phaffii was exploited for inositol production through enhancing its native inositol biosynthesis pathway [43]. The production of 0.71 g/L was obtained by the overexpression of endogenous inositol-3-phosphate synthase (PpIPS) and the knock out of inositol transporters PpITR1 and PpITR2, and B-subunit of 6-phosphofructokinase gene pfk2. The introduction of heterologous inositol-3-phosphate synthase (IPS), inositol monophosphatase (IMP) (Figure 3), and the further substitution of promoters zwf (encoding glucose-6-phosphate dehydrogenase), pgi (encoding glucose-6-phosphate isomerase), and pfk1 (encoding α-subunit of 6-phosphofructokinase) for glycerol-induced pGUT, could improve inositol production up to 30.71 g/L in high-cell-density fermentation, where glycerol was first used as sole carbon source then glucose was added into the medium for inositol production when wet cell weight reached about 0.2 g/mL (Table 2).
4.1.4. Glucaric Acid Production from Glucose in K. phaffii
Glucaric acid (C6H10O8) is a natural valuable organic acid that can be found in fruits, vegetables, and mammals. Glucaric acid have a range of utilizations in the chemical, pharmaceutical, and food industry and has also been identified as a “top value-added chemical from biomass” for its potential use in the formation of biodegradable detergents and polymers, and metal complexation agents [81]. The co-expression of heterologous mouse MIOX and urinate dehydrogenase (Udh) from P. putida in K. phaffii led to the accumulation of 107 mg/L of glucaric acid from glucose. In contrast, the co-expression of native MIOX and Udh produced no glucaric acid from the same carbon source (Figure 3). The optimization of cultivation with the addition of myo-inositol increased glucaric acid concentration from 785.4 mg/L to 1697.6 mg/L in flasks, and production reached 6.61 g/L in fed-batch culture, the highest among other hosts such as E. coli and Granulobacter sp. (Table 2) [42].
4.2. Metabolic Engineering of K. phaffii to Produce Renewables Chemicals from Xylose
Xylose is another abundant substrate for biotechnological processes. In yeast species that naturally utilize xylose, the metabolism of the pentose starts with its conversion to xylitol, mediated by a NAD(P)H-dependent xylose reductase, followed by the conversion to xylulose, mediated by an NAD+-dependent xylitol dehydrogenase. Xylulose is therefore phosphorylated by a xylulokinase and enters the pentose phosphate pathway (PPP) as xylulose-5P (Figure 4). An isomerase pathway found mainly in bacteria allows direct conversion of xylose to xylulose via xylose isomerase. In addition, Archaea can assimilate xylose by a non-phosphorylate pathway (NP) converting it into xylonolactone by a xylose dehydrogenase reaction. Xylonolactone will be further converted into xylonate rather spontaneously or with the aid of a xylonolactonase. Xylonate is then dehydrated into 2-keto-3-D-deoxypentanoate to enter the central carbon metabolism [82].
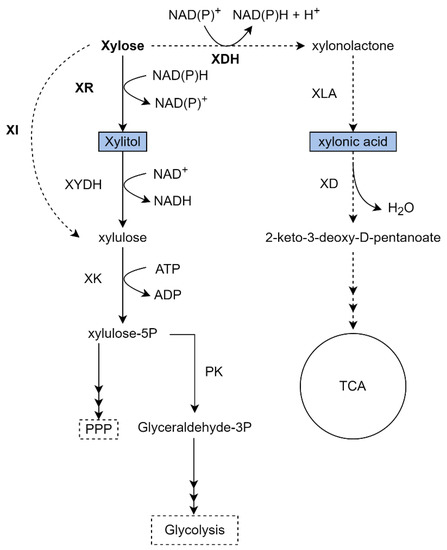
Figure 4.
Metabolic pathways to xylose assimilation and production of a variety of chemicals by K. phaffii. Black arrows represent the native xylose metabolism, dotted arrows represent an overexpressed/heterologous pathway. Bold enzyme names represent an overexpressed/heterologous enzyme, and the blue boxes represents the product formation. Some reactions are summarized for better comprehension. XR: Xylose reductase; XI: Xylose isomerase; XYDH: Xylitol dehydrogenase; XK: Xylulo kinase; PK: Phosphoketolase; XDH: Xylose dehydrogenase; XLA: Xylono lactonase; XD: Xylonate dehydratase. The figure of own authorship is based on KEGG pathways (www.genome.jp—accessed on 13 September 2022) and in the literature cited in the text.
Despite Komagataella, species are generally described as non-xylose utilizing organisms; an examination of K. phaffii genome revealed genes encoding for putative XR and a putative XDH homologous to other yeasts, such as S. cerevisiae (56% identity) and Kluyveromyces marxianus (57% identity) [83]. Recent findings also show that several Komagataella species, including K. phaffii, can grow on xylose as the sole carbon source, suggesting a hidden pathway of xylose utilization [21]. The introduction of a XI from Orpinomyces sp. in K. phaffii, in association with adaptive evolution, effectively enhanced the flux of xylose towards PPP (Figure 4), enhancing the consumption rate of xylose from 0.193 g/g to 0.378 g/g after 50 generations [83]. Recent advances in using xylose as a substrate for chemical production in K. phaffii refer to xylitol [84] and xylonic acid [45] production (Table 2).
4.2.1. Xylitol Production from Xylose in K. phaffii
Xylitol (C5H12O5) is a natural occurring pentahydroxy sugar alcohol found in a variety of fruits, vegetables, and mushrooms, with high application potential in personal care, pharmaceuticals, and the food industry, as a sweetener that is similar to sucralose but does not require insulin to be metabolized [4,84]; current production is around 200,000 tons [74]. The global market of xylitol exceeded USD 880 million and is expected to expand to over USD 1 billion by 2026 [84]. The biological production of xylitol in yeasts occurs in a single step where xylose is reduced to xylitol by a XR (Figure 4). Recombinant K. phaffii strains for xylitol production from xylose and hemicellulose hydrolysate were obtained through (over)expression of XR (XYL1) from P. stipitis solely or in combination with glucose dehydrogenase (gdh) from Bacillus subtilis, so that the oxidation of glucose could be used to re-generate NAD(P)H required by the XR reaction [44]. The highest conversion rate by the recombinant strain was 320 mM of xylitol from 400 mM of xylose, with a productivity value of 2.44 g/L/h in a defined medium with both modifications. 300 mM of xylitol from 420 mM of xylose, with a productivity value of 0.46 g/L/h, was obtained in hemicellulose hydrolysate (Table 2).
4.2.2. Xylonic Acid Production from Xylose in K. phaffii
Xylonic acid (C5H10O6) is an organic compound obtained from the microbial oxidation of xylose by naturally or engineered species. It has been reported for serval industrial applications such as a cement additive, plasticizer, cleaner agent, polyamide and polyester co-polymerization, and as a precursor of other value-added chemicals such as ethylene glycol, glycolic acid, and 1,2,4-butanetriol (reviewed in [5]). Several archaea and some bacteria produce xylonic acid by the conversion of xylose to xylonolactone by a xylose dehydrogenase (XDH), which is then converted to xylonic acid in a spontaneous hydration reaction catalyzed by a xylonolactonase (XLA) (Figure 4) [5,82]. Yeasts, on the contrary, are not naturally capable of producing xylonic acid. Therefore, various yeast strains have conquered xylonic acid by the introduction of a heterologous xylose dehydrogenase pathway [5].
The recent construction of K. phaffii strains with putative heterologous XDHs was described in [45]. To this end, 11 putative protein sequences of XDH from bacteria and filamentous fungi were identified and overexpressed in K. phaffii. Six strains were able to produce xylonic acid. Strains with bacterial XDHs were able to produce tenfold more xylonic acid than the strains with fungal XDHs. The best strain produced up to 37 g/L of xylonic acid in defined medium and up to 11 g/L in sugarcane bagasse hydrolysate, with the highest yield of 0.96 g/g and 0.43 g/g, respectively (Table 2).
5. Renewables from Methanol and CO2
Methanol is produced in large volumes worldwide, with a production capacity achieving more than 100 million metric tons per year. This C1 nonfood feedstock can be derived from fossil raw materials or renewable resources such as biomass, glycerol, methane, and carbon dioxide, rendering it a flexible and attractive alternative to sugar-based substrates [85]. In addition, methanol is more reduced than most carbohydrates, which can enhance the yields of metabolites produced by microorganisms when using this carbon source as a substrate or co-substrate [86].
Several studies have focused on engineering relevant industrial microorganisms, such as E. coli and S. cerevisiae, for methanol metabolism [87]. However, implementing the methanol assimilation pathway in non-native methylotrophs is quite challenging. It involves a complete change in the microorganism’s lifestyle, requiring, at least, the capacity to build essential cellular metabolites from a single carbon molecule and tolerance to the highly toxic intermediate compounds produced. Despite the achievements, until now, growth performances of the engineered cells on methanol are far from those observed for natural methylotrophs [87].
Several native methylotrophic microorganisms, including K. phaffii, Ogataea (Hansenula) polymorpha, and Bacillus methanolicus, are naturally capable of utilizing C1 compounds as a source of energy and carbon through different metabolic routes [85,88,89]. Among them, K. phaffii presents some prominent properties that make this yeast an interesting host for biotechnological processes. For example, we can cite resistance to high concentrations of methanol and other stressful industrial conditions, growth to high cell densities, and ability to express recombinant protein efficiently [7,85]. In addition, several tools are available for synthetic and metabolic engineering for this yeast and well-established fermentation processes [90,91]. Therefore, K. phaffii has been widely used for the recombinant protein production of several biopharmaceuticals and industrial enzymes, as demonstrated in this review (as discussed in the next section), and, more recently, has also been employed for the production of chemicals and other valued added compounds, although mainly using sugars as carbon sources as previously presented here [14,85].
In K. phaffii, methanol metabolism initiates with its oxidation to formaldehyde. Then, the formaldehyde can follow either an assimilatory or dissimilatory pathway. In the assimilatory pathway, localized in peroxisomes, formaldehyde is condensed to phosphosugars and enters the central metabolism, yielding biomass. In the dissimilatory pathway, localized both in peroxisome and in the cytosol, the formaldehyde is oxidized to CO2, yielding NADH (Figure 5) [92].
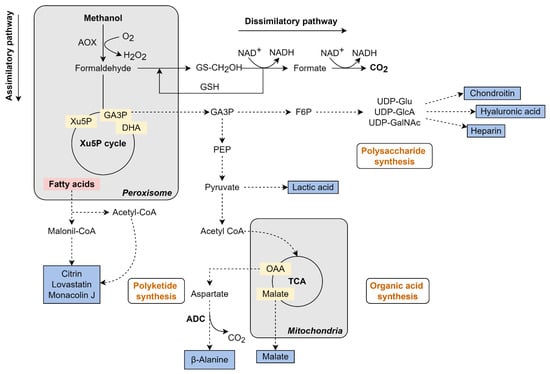
Figure 5.
Metabolic pathways that led to methanol assimilation and production of a variety of chemicals by K. phaffii. Black arrows represent the native methanol metabolism, dotted arrows represent an overexpressed/heterologous pathway. Bold enzyme names represent an overexpressed/heterologous enzyme, and the blue boxes represents the product formation. Some reactions are summarized for better comprehension, also, not all cofactors are represented in the figure. AOX: Alcohol oxidase; Xu5P: Xylulose-5-phosphate; DHA: Dihydroxyacetone; GAP: Glyceraldehyde-3-phosphate; XuMP: Xylulose-monophosphate cycle; PEP: Phosphoenol pyruvate; F6P: Fructose-6-phosphate; OAA: Oxalacetate; ADC: L-aspartate decarboxylase. The figure of own authorship is based on KEGG pathways (www.genome.jp—accessed on 13 September 2022) and in the literature cited in the text.
The first enzymatic reaction occurs in the peroxisomes, where methanol is oxidized to formaldehyde with the release of hydrogen peroxide (H2O2) by the action of the key-enzyme alcohol oxidase (AOX). As H2O2 is toxic to the cell, the compartmentalization of the process in the peroxisomes aid in protecting the cell until the catalase converts it into H2O and O2 as well as could alleviate the toxicity caused by formaldehyde accumulation (Figure 5) [85]. K. phaffii has two isoforms of AOX, encoded by the genes AOX1 and AOX2, which share 97% of identity at the protein level. Among them, AOX1 represents the major AOX protein expressed during growth on methanol, whose content could reach 20% to 30% of the total protein [85].
In the assimilatory pathway, formaldehyde is then combined with xylulose-5-phosphate (X5P) by the enzyme dihydroxyacetone synthase (Das1 and Das2 in K. phaffii), generating dihydroxyacetone (DHA) and glyceraldehyde-3-phosphate (GAP). A total of 1 mol of GAP are produced from 3 mol of methanol, which is used to generate biomass and energy. X5P is recycled by sugar phosphates interconversions catalyzed by PPP isoenzymes in the peroxisomes [89,92]. This compartmentalized and cyclic C1 assimilation process is called Xylulose-Monophosphate cycle (XuMP) (Figure 5) [89].
Part of the formaldehyde generated in the methanol oxidation reaction follows the dissimilatory pathway. In this case, the formaldehyde flows into the cytosol and reacts with glutathione through the formaldehyde dehydrogenase, S-formylglutathione hydrolase, and formate dehydrogenase enzymes, yielding CO2 and NADH (Figure 5) [89]. Although the C1 carbon is lost in the process, it is an essential route for energy generation (in the form of the reducing power NADH) and formaldehyde detoxification, thereby affecting the ability of cell growth on methanol [85].
5.1. K. phaffii Engineering for CO2 Assimilation
Exploring the methanol-assimilation pathway, a K. phaffii strain able to grow using CO2 as a sole carbon source, was recently reported [92]. In the synthetic autotroph strain, the peroxisomal pathway for methanol assimilation was rewired to a CO2-fixation pathway that resembles the Calvin-Benson-Bassham cycle (CBB). For this, eight heterologous genes were expressed and combined with the XuMP: six CBB enzymes (RuBisCO form II encoded by cbbM, PGK1, TDH3, TPI1, TKL1, PRK) and two chaperones (groEL, groES) to assist RuBisCO folding. In addition, the native genes DAS1, DAS2, and AOX1, were deleted to separate the CO2-fixation machinery from energy generation. Thus, methanol was used to provide energy via the dissimilatory pathway [92]. Moreover, adaptive laboratory evolution (ALE) was employed to increase the growth rates of the strain on CO2, resulting in a gain of more than 2-fold (from 0.008 h−1 to 0.018 h−1) [92].
The Improved phenotypes of these evolved CO2-assimilating K. phaffii yeasts were further investigated [93]. Reverse genetic engineering was used to evaluate the mutations found in the genes encoding for the heterologous phosphoribulokinase (PRK) and the native nicotinic acid mononucleotide adenylyltransferase (NMA1). The results showed that lower activities of the mutated enzymes, which affect the availability of ATP and other consequences, are beneficial to the autotrophic phenotype. Furthermore, through a second evolution round, it was identified that facilitating peroxisomal import can be another engineering strategy to improve the autotrophic growth of K. phaffii [93].
These studies represent a remarkable advance in microbial utilization of one carbon (C1) feedstocks, since synthetic autotrophs can be used as an industrial platform for the production of several value-added chemicals and enzymes. Besides, this approach may have a significant role in the circular bioeconomy, promoting more sustainable biotechnological bioprocesses and also contributing to mitigating the atmospheric greenhouse gas CO2 [92,93]. However, despite the huge potential of the implementation of the CO2-assimilating pathway, many challenges should be addressed before it can be successfully explored in industrial production bioprocesses.
5.2. Metabolic Engineering of K. phaffii for Production of Renewables from Methanol
C1 substrates, such as CO2, methane, and methanol, have been considered next-generation feedstocks since they are relatively inexpensive due to their natural abundance, low production cost, or availability as industrial by-products [94]. In particular, methanol has gained attention as it can be produced directly from methane and CO2 using renewable resources [87]. Considering that, the native capacity of K. phaffii to metabolize methanol has been the focus of several studies to develop biotechnological fermentation processes. Although methanol has been widely employed in several bioprocesses related to recombinant protein expression by K. phaffii, only more recently, methanol has been explored as a substrate or co-substrate for the synthesis of various chemical compounds. We can cite, for example, the production of β -alanine, organic acids, alkenes, fatty acids, and pharmaceutical compounds, such as methyl salicylic acid and lovastatin (Table 2) [14,46,47,48,49,50,85].
5.2.1. β-Alanine Production from Methanol in K. phaffii
β-alanine is a natural β-amino acid that can be used to synthesize several nitrogen-containing compounds for the food and pharmaceutical industry. Biological production of β -alanine is obtained via L-aspartate decarboxylation. Considering that aspartate is one of the main amino acid pools in K phaffii, different bacterial L-aspartate decarboxylase (ADC) genes were expressed in the yeast to evaluate β-alanine production (Figure 5) [46]. Further improvement on β-alanine synthesis was achieved by increasing the ADC copy number and the supply of the C4 precursor aspartate. The engineered strain produced 1.2 g/L of β -alanine in shake-flask culture and reached a titer of 5.6 g/L in a fermenter using a two-stage strategy with high initial biomass (Table 2). This study was the first attempt to produce amino acids in K. phaffii using methanol as substrate, indicating the great potential of the yeast for fermentative production of amino acids [46].
5.2.2. Organic Acids Production from Methanol in K. phaffii
Malic acid, like other C4 dicarboxylic acids, and lactic acid, are on the top of the most relevant bio-based chemicals that can be produced through microbial processes from renewable resources [74,81]. Both compounds have high commercial value and a broad range of applications in the industrial sector, with a global market size estimated at USD 182.6 million in 2018 for malic acid (https://www.grandviewresearch.com/industry-analysis/malic-acid-market—accessed on 17 August 2022) and USD 2.9 billion in 2021 for lactic acid (https://www.grandviewresearch.com/industry-analysis/lactic-acid-and-poly-lactic-acid-market—accessed on 17 August 2022).
Considering that, K. phaffii has been engineered to produce organic acids using methanol [47,48]. The expression of multiple copies of the D-lactate dehydrogenase gene from Leuconostoc mesenteroides via gene integration into the rDNA locus of K. phaffii and post-transformational vector amplification, led to a D-lactic acid production of 3.48 g/L by the recombinant cells after 96 h of cultivation [47]. In another study, the conversion of methanol into malic acid was reported [48]. Guo et al. [48] evaluated three different malic acid accumulation modules in K. phaffii. Among them, the reductive TCA pathway derived from Rhizopus oryzae showed the best results for malic acid production. Additional metabolic engineering was also tested to optimize the strain, which included: (i) overexpression of the SpMAE transporter, (ii) deletion of genes related to by-product formation, and (iii) modifications on the methanol metabolism pathway. The resulting strain could produce 2.79 g/L of malic acid from methanol [48]. Although a malic acid titer of 42.28 g/L has been already obtained by a recombinant K. phaffii strain during the cultivation on glucose and using methanol as inducer [95], it was the first report of malic acid production using methanol as the only carbon source (Table 2) (Figure 5).
5.2.3. Biopolymers Production from Methanol in K. phaffii
Biopolymers have also been produced in K. phaffii. Hyaluronic acid is a glycosaminoglycan polysaccharide with remarkable biological activity. Because of that, it is widely used in the medical and pharmaceutical sectors, with a global market valued at USD 9.1 billion in 2019 (reviewed in [96]). The commercial production of hyaluronic acid is mainly from animal tissue extraction and, more recently, by fermentation using the pathogenic bacteria Streptococcus sp. [96]. However, attempts have been developed to produce this polysaccharide using other non-pathogenic host strains [53]. Considering that, K. phaffii was engineered to express the hyaluronan synthase (xhasA2) and UDP-glucose dehydrogenase (xhasB) from Xenopus laevis, in combination with the overexpression of native genes involved with the hyaluronic acid biosynthesis (hasC, hasD, hasE). The engineered yeasts produced an amount of 0.8–1.7 g/L of hyaluronic acid with a molecular weight from 1.2 to 2.5 MDa (Table 2), using glucose as the main substrate and methanol as co-substrate and inducer (Figure 5) [53].
Exploring the natural occurrence of the biosynthesis pathway of sulfation in the yeast, the production of chondroitin sulfate, another clinically relevant biopolymer, has been proposed and implemented in K. phaffii (Figure 5). Chondroitin is a glycosaminoglycan composed of sulfated disaccharides repetitions of glucuronic acid (GlcA) and N-acetylgalactosamine (GalNAc) linked by β (1 → 3) and β (1 → 4) bonds [51]. Initially, an engineered strain was constructed by introducing the chondroitin biosynthesis pathway harboring the genes kfoC and kfoA from Escherichia coli, which led to the synthesis of the intermediate metabolites UDP-GlcA and UDP-GalNAc, and tuaD from Bacillus subtilis, which encoded a chondroitin polymerase responsible for the polysaccharide formation. The resulting strain produced 5.5 mg/L of chondroitin [51]. For further improvement, combined strategies were employed, such as codon optimization, expression of the chondroitin-4-O-sulfotransferase (C4ST), and increased supply of the 3′-phosphoadenosine-5′-phosphosulfate (PAPS, a universal donor for sulfonated molecules). As a result, the yeast was capable of synthesizing 2.1 g/L of chondroitin sulfate from methanol [51].
Recently, this previously engineered strain [51] was used as a host to express sulfotransferases and an epimerase for in vitro biosynthesis of heparin [52]. Taking into consideration the successful expression of all required enzymes, the authors proposed the construction of a yeast platform for complete biosynthesis of heparin from methanol. The recombinant K. phaffii, co-expressing heparosan synthesis enzymes (tuaD, KfiC, KfiA), bifunctional enzyme NDST, sulfotransferases (2-OST, 6-OST and 3-OST), and epimerase C5 epi, showed production of 2.08 g/L of the bioengineered heparin, with a molecular weight of 349 kDa, in fed-batch cultures [52]. Heparin is a linear and unbranched glycosaminoglycan widely used as an anticoagulant drug. The global market was valued at USD 7.3 billion in 2021 and is growing with the increasing worldwide demand (https://www.grandviewresearch.com/industry-analysis/heparin-market—accessed on 17 August 2022).
5.2.4. Polyketides Production from Methanol in K. phaffii
The potential of K. phaffii as a microbial chassis for polyketide biosynthesis has also been evaluated (Figure 5) [49,50,97]. Polyketides are a class of secondary metabolites produced by several organisms, showing structural diversity and bioactivity, making some interesting pharmaceutical drugs [14,50]. The synthesis of the polyketide 6-methyl salicylic acid (6MSA) was described by Gao et al. [50]. In K. phaffii, through overexpression of the 6-methyl salicylic acid synthase gene (atX) from Aspergillus terreus and phosphopantetheinyl transferase gene (npgA) from Aspergillus nidulans, a production of 2.2 g/L 6MSA was achieved during cultivation in bioreactor [50]. Later, the same research group reported the synthesis of the polyketide citrinin by K. phaffii [97]. For this, the citrinin biosynthetic pathway, which genes are derived from Monascus purpureus (pksCT, mpl1, mpl2, and mpl4) and Monascus ruber (mpl6, mpl7), was introduced into the yeast together with the expression of the transferase npgA-encoding gene. The engineered strain was able to accumulate 0.6 mg/L of the polyketide using methanol as a carbon source and inducer [97].
Lovastatin, a common pharmaceutical drug used in hypercholesterolemia treatment, and its precursor monacolin J, are other examples of polyketides produced in K. phaffii [49]. The commercial product is obtained mainly from fermentation using an Aspergillus terreus strain. The metabolic engineering of other microbial hosts, such as K. phaffii, can be an alternative to reduce the long fermentation period required by filamentous fungi and limit multiple byproduct formation [49]. For production in K. phaffii, the complex monacolin J and lovastatin biosynthetic pathways were assembled and implemented in the yeast, resulting in 3.3 and 21 mg/L of lovastatin and monacolin J, respectively. Using a buffered medium, an improvement in product concentration of approximately three times was observed during shake flask cultivation.
Furthermore, the pathway was split into two modules and implemented in two separated strains to reduce the metabolic burden and avoid the accumulation of intermediate metabolites. During bioreactor cultivation, the co-culture system enabled the production of 250 mg/L of lovastatin and 593 mg/L monacolin J using methanol as substrate. These results demonstrated that microbial consortium could be an efficient strategy for metabolite production. However, it depends on cell internalization of the intermediate compounds [14]. This study was the first report of biosynthesis of lovastatin in a heterologous host; additionally, the compound production was comparable to that obtained with the native A. terreus strain [49].
As can be seen, several studies demonstrate the potential of K. phaffii as a microbial chassis to produce value-added chemicals using methanol as a carbon source. However, methanol fermentation efficiency is still lower than sugar-based processes. To overcome the current proof-of-concept stage, some problems should be addressed, including the cellular toxicity of methanol and the intermediate formaldehyde, as well as the carbon loss (via the dissimilatory pathway) that reduce product yields [85,88]. Therefore, further improvements are required for the industrial application of K. phaffii in methanol bioconversions.
6. Komagataella phaffii Application in Protein Production
Komagataella phaffii has been one of the most used species for the cost-effective production of recombinant protein, capable of expressing a range of enzymes with industrial and pharmaceutical relevance (Table 4). This fact is due to the ability of this microorganism to grow to very high cell densities (biomass over 100 g/L using methanol) and produce amounts of recombinant protein ranging from milligrams to grams for both research (laboratory) and industry purposes [19,98]. Anteriorly, Pichia pastoris (include K. phaffii and K. pastoris strains) first caught attention by being used as a single-cell protein (SCP) by Phillips Petroleum and commercialized for animal supplementation [72,99]. Later, with more advanced genetic manipulation of K. phaffii and the isolation of different promoters, especially the alcohol oxidase genes (AOX), glyceraldehyde-3-phosphate dehydrogenase (GAP), and the 3-phosphoglyceratekinase gene (PGK1), the development of expression vectors, new techniques, and protocols for genetic transformation, culminated in the creation of the current successful heterologous expression system [99,100,101].

Table 4.
Examples of heterologous proteins expressed in K. phaffii and production yields.
Among the main advantages of recombinant expression in K. phaffii are rapid growth in cheaper culture media, scaling up of protein production, easy maintenance, and genetic manipulation. In addition, these organisms are capable of performing most post-translational modifications such as glycosylation (N or O), formation of disulfide bridges, and adequate folding of the protein of interest, allowing the production of a cost-competitive enzyme with minimal processing in the following steps [6,72,99]. Moreover, the choice of a promoter (constitutive or induced), selection marks (dominant or auxotrophic), and signal peptides for extracellular secretion (when desired), are essential for the successful production of the protein of interest. Additionally, integrated vectors into the genome potentially increase the genetic stability of the engineered strains even in continuous and large-scale fermentation processes [99].
Several studies have reported the production of recombinant proteins in K. phaffii, registering more than 500 biopharmaceutical proteins, such as insulin, anti-microbial peptides, human serum albumin (HSA), and epidermal growth factor [98]. These proteins represent a growing market in the therapeutics biotechnology business. Since K. phaffii has the status GRAS, a range of approved biopharmaceuticals such as vaccines is being extensively studied for production in this yeast. For instance, the production of protein candidates for subunit vaccines has been expressed in K. phaffii to be used against malaria [102], dengue [103], chikungunya [104], tuberculosis [105,125], and SARS [126,127]. Further, the expression of SARS-CoV-2 Spike RBD (receptor binding domain) in K. phaffii was scaled-up reaching yields above 45 mg/L of 90% pure protein. This work provided an alternative for the production of neutralizing antibodies and the possible development of SARS-CoV-2 vaccines to combat the COVID-19 pandemic [106].
Additionally, other important therapeutic proteins were produced at high levels in K. phaffii, such as biologically active human proinsulin [107] and human epidermal growth [108]. The interleukin-1beta involved in different physiological responses was successfully expressed in K. phaffii, and its activity was detected to inhibit the growth of B16 melanoma cells [109]. Currently, different active antimicrobials against pathogens (bacteria and fungi) like Hispidalin [110], the Hybrid cecropinA-thanatin [111], PAF102 peptide (under GAP regulation) [112], and fowlicidins [113] have been produced in K. phaffii. Moreover, K. phaffii achieved one of the highest concentrations (8.86 g/L) of a human serum albumin, an important protein with different therapeutic functions [114]. All these cited proteins were expressed under the regulation of the AOX promoter unless otherwise specified.
The global market for recombinant proteins is expected to reach USD 1.7 billion by 2026 [128]. In the food, feed, detergent, and clothes industries, many relevant enzymes for biotechnology applications have been produced in K. phaffii, such as lignocellulolytic enzymes (responsible for the degradation of main components of the plant cell wall, such as cellulose, hemicellulose, pectin, lignin, and others) [116,129]. A wide range of these enzymes is naturally produced by fungal strains like Trichoderma spp., Penicillium spp., Aspergillus spp., Humicola spp., Thermomyces, and Myceliophthora spp. [130]. To produce purified and high yields of the targeted proteins from these fungi, using heterologous expression in K. phaffii is highly favorable. Additionally, an important fact is that K. phaffii does not secrete endogenous lignocellulolytic enzymes in significant amounts. Thus, recombinant strains of this yeast can provide heterologous enzyme preparations without the need for many purification steps, reducing the time and cost of the process [99,116].
Cellulases such as endoglucanases [115], cellobiohydrolases [116], and β-glucosidases [131], have been expressed in K. phaffii allowing purification, biochemical characterization, and biotechnological applications. These proteins can be used in paper and pulp, textile, and detergent industries, and applied in biorefineries for the bioconversion of lignocellulosic biomass [132]. In this context, to aid in the degradation of cellulose chains, the oxidative enzymes called lytic polysaccharide monooxygenases (LPMOs) and classified as auxiliary activity (AA) proteins have been produced in K. phaffii [118,133], as well as expansins (AA proteins), which act in synergy with cellulases to enhance sugar release from the substrate [119].
Likewise, hemicellulases like xylanases and β-xilosidases have been extensively expressed in K. phaffii. These enzymes can be applied in the textile, paper, and pulp industries, and hemicellulose deconstruction [120,134,135]. Other enzymes that can be utilized with xylanases and β-xilosidases to increase hemicellulose degradation have also been expressed in this yeast, including feruloyl esterase, acetyl xylan esterase, and α-L-arabinofuranosidases [121,122,123]. The recombinant mannanases and pectinases produced in K. phaffii are very relevant for the food industry and have been used in preparation for prebiotics and juice clarification, respectively [117,136]. Furthermore, successfully recombinant lipases produced in K. phaffii expression system are being studied as biocatalysts for the conversion of biomass fatty acids in biodiesel [124,137].
Several proteins of industrial and medical importance have been produced in K. phaffii and approved by regulatory institutions so far, noting the efficient expression system established in this yeast. Meaningful progress has been made with improved protein expression levels with different approaches. However, protein production still needs to be optimized and maximized for large-scale processes utilized in industry.
7. Strategies to Develop and Optimize Fermentative Processes
The production of proteins and chemicals in K. phaffii can be a powerful tool to address global needs. However, despite the increasing development of this microbial platform, their fermentation processes have still not achieved the same maturity as conventional biochemical processes [138,139,140]. There is a disparity between a promising laboratory scale and its industrial application; although many studies have demonstrated proof-of-concept pathways for new molecules, a much smaller amount of these works have focused on bioprocess design, optimization strategies, mathematical modeling, or scaling-up processes [139,141,142,143]. Integrating strain engineering and fermentation strategies is crucial for improving bioprocess efficiency. The technology transfer of bioprocesses in host systems is usually a result of iterative process optimizations and biological re-design cycles [144]. In this sense, a deep understanding of cell factories, process development, optimization, and scaling-up must be part of the same improvement. As strain engineering has been discussed in the previous sections, this one will focus on fermentation characteristics and strategies to produce a maximum amount of product in the minimum process time with quality specifications.
Despite the existence of conditions widely used in the fermentative processes in K. phaffii, optimal conditions differ according to the target product, the combination of genetic construction, or whether the production occurs under the control of inducible or constitutive promoters [98,140]. The most successful process development in K. phaffii suggests that the optimized process moves away from standard protocols and adapts process parameters to specific strain/product/bioreactor [140,145]. The main factors are discussed in detail in the following and summarized on Table 5.

Table 5.
Strategies for process development, simulation, and optimization with K. phaffii.
7.1. Fermentation Parameters
The efficiency of fermentation processes is strongly influenced by operational parameters such as temperature, pH, dissolved oxygen (DO), medium composition, and osmolality. For K. phaffii cultivation, several authors use a similar range of optimal set-point and control strategies for these parameters, mainly based on standard protocols (Table 5) [98,138,142,154].
The required growth temperature for K. phaffii is 28–30 °C [98,155]. A temperature greater than 32 °C could cause cell death and reduce protein expression. For heterologous protein production, in some cases, reducing the cultivation temperature may improve protein production by increasing yeast viability (despite decreasing growth rate), decreasing folding stress, and reducing proteolytic activity against the target protein [98,155]. Besides that, decreasing the cultivation temperature increases the oxygen solubility and consequently improves the oxygen transfer rate [155]. Studies focused on the temperature effect indicated that the improvement in efficiency depends on the target product and should be evaluated in each case [155,159].
The pH value for K. phaffii cultivation range from 5 to 6.5. pH, values above 8 reduce cell viability [155,160]. The optimum value is strongly dependent on the recombinant protein or metabolite produced. pH value of 5.5 contributes to minimizing the harmful effect of proteases [140,159].
The set point for DO concentration in K. phaffii fermentation is usually kept at 20–30% saturated [155]. The higher the cell density in fermentation, the more onerous it is to maintain this concentration [151,155]. The limited oxygen transfer capacity is an essential factor in the K. phaffii process development. Due to the low solubility of oxygen in cultivation media, in K. phaffii cultivation at high cell densities, the maximum oxygen transfer capacity of the equipment can determine the maximum amount of biomass that can be reached [151]. Moreover, studies related to recombinant protein production observed that oxygen-limiting conditions results in higher protein production [138,151].
The large-scale production of K. phaffii also depends on low-cost culture media. New medium formulations have been evaluated for specific studies; no generic composition works in all cases [145,155]. Media optimization can be an effective alternative to increase growth rate and product yield; however, this option is not widely explored for K. phaffii [145,160]. The most cited medium for K. phaffii fermentation is the basal salt (BSM) medium. Still, some studies report a potential increase of product titers at similar cell densities or improved per-cell productivity by adding nutrients in the basal medium [160].
7.2. Operation Mode
Regarding operation mode, the fed-batch culture, with gradual feeding of the substrate, is the most popular method used for the recombinant production process in K. phaffii. This strategy achieves high cell densities and high product titers and prevents substrate inhibition or catabolite repression [9,161]. In fed-batch culture, exponential feeding profiles have been considered the most effective procedure to achieve a pseudo-stationary state and, consequently, constant and controlled specific growth, consumption, and production rates [140,154]. Common feeding strategies such as pulse, constant rates, ramp, or step-based additions, have been considered obsolete since they do not provide the physiological needs of cells and do not allow optimal performance [138,155,157]. Without changing growth parameters, the combination of feeding profiles and carbon-starving is recommended for recombinant protein overexpression in fed-batch cultures [138,150]. Although advantageous in some aspects, this strategy reduces productivity values and is not always better than exponential feeding [154].
The use of high-cell-density fermentation (HCDF) in fed-batch mode has been the most adopted strategy in recent studies to achieve the high-yield of enzymes or chemicals production [140,142]. However, there are still difficulties in establishing a robust HCDF process, mainly when methanol is used as a carbon source and inducer [161]. Since high methanol concentrations are toxic, it must be continuously added and maintained at a proper level in fermentation. Then, cell growth, substrate consumption, and product formation will depend on the methanol feeding strategy [161]. Although HCDF contributes to increased productivity, it can also imply accumulation of protease, especially harmful in heterologous protein production. Some strategies can be adopted to overcome the problem, such as the pH of the fermentation medium controlled in a range of 3.0–7.0, the addition of amino acid-rich supplements, or using a protease-deficient K. phaffii host strain [142].
Although the fed-batch operation is the most used method, continuous mode production has become a trend. In continuous culture, fresh medium is continuously added to the bioreactor, and culture broth and cell are removed at the same flow rate. Then, the specific growth rate (µ) is kept close to µmax. This fermentation mode presents advantages compared to fed-batch, such as higher productivity, lower spending on utilities, and the simplification of the process by operation under steady-state, which provides highly robust and reliable data [138,155,162]. Continuous culture is considered the best alternative for obtaining accurate physiological data for reliable strain characterization and process [155]. However, using the continuous operation mode in the industry also presents some challenges, including a higher risk of genetic instability and contamination, demand for a higher level of automation, and other product-specific problems [138,155]. As far as we know, for K. phaffii, successful continuous fermentations have been reported on the laboratory scale [15,154,155,159], but there are no continuous biomanufacturing processes.
In the process development of continuous fermentation, the dilution rate (D), the same value of µ, defined by mass balance, is the most critical parameter to be considered [163]. The most reported type of cell growth kinetics is described by models based on the Monod equation [140,155]. However, equation parameters or a pattern of YP/X (product to biomass yield) behavior as a function of µ is challenging to be compared since different strains using different substrates and metabolic routes are evaluated.
Regardless of the strategy used for process optimization, some factors must be considered as design criteria. Depending on strain engineering and culture conditions, an optimum specific growth rate (µopt) can be determined for better performance. The maximization of conventional parameters must be considered, for example, final titer and specific product formation rate (qP), usually for high added-value products, and YP/S (product to substrate yield), typically for low-cost products [138,157,162].
Usually, a high initial cell concentration and a low specific growth rate are recommended for higher product titers, since growth-associated biomass is a by-product and has to be controlled not to exceed the limit concentration [138,140,162]. Based on this, the relationship between qP and µ becomes crucial information to compare efficiency in different conditions. This relationship is considered by some authors the key factor in bioprocess development [138,140,155]. An empirical understanding of the relationship between growth and product formation is usually necessary, since lacking a theoretical base [140]. This observation of product formation kinetics would define conditions to control the fed-batch process.
The recombinant protein production is usually growth-associated, and the optimum qP is reached at high µ [138,154]. When applicable, positive growth associated with production (specific product formation rate increases with the specific growth rate) is the better approach to evolving strains while maintaining high titers and yield [138,140,162]. This strategy was used to couple succinate overproduction with growth through the deletion of genes in E. coli [141] and to improve an extracellular human granulocyte-macrophage colony-stimulating factor expression in K. phaffii [140]. Genome-scale models have allowed to couple growth and production for most metabolites [141]. When it is impossible to biochemically couple target metabolism with growth dependence, or the growth coupling is limited to some cultivation conditions, engineered strain, with increased tolerance to stresses and inhibitors, is mandatory [141]. In this case, to preserve strain performance, the selection of strains with improved growth and loss of production must be avoided over the generations [138]. One possible alternative to ensure stable strain performance, and decoupling growth and production, is to activate product pathways only after cell density reaches the stationary phase [141].
The maximum specific growth rate (µmax) is another crucial parameter for bioprocess development since it defines the maximum substrate consumption and, consequently, the upper limit of substrate assimilation in the case of the fed-batch mode [140,162]. Usually, strains engineered to produce heterologous proteins present µmax lower than values observed for a non-engineered strain [140]. One more critical factor is the relationship between yield and productivity. One of these variables cannot be individually optimized to the detriment of the other [138,157]. Mathematical tools to define a global optimum solution based on goal criteria can be helpful, especially when production kinetics are nonlinear.
One frequent issue in process development that must be considered is the chemical inhibition by media, metabolites, or products, mainly when employing complex and crude carbon sources. In these cases, a selected strain is unlikely to provide all the desired properties for an optimum process naturally. Adaptive laboratory evolution (ALE) can be an alternative to overcome these physiological limitations and offer new targets for the next design step [164,165]. ALE has been shown to be useful in many studies to improve the strains of K. phaffii for different purposes [155,165]. Although ALE is a powerful method to accelerate the development of efficient recombinant hosts, this evolution can drive the population towards a low-production high-fitness phenotype [164,165]. The combination of growth-coupled production with ALE has been responsible for high-production strains that preserve phenotype [141]. Combining multi-omic studies may also help in this selection stage [165].
As may be seen, some parameters of development are common to bioprocess in K. phaffii. However, the challenges, in general, are specific to the product and must be considered particularly in each case [9,138].
7.3. Mathematical Models
Despite the advances, the process development and optimization for biological systems are usually empirical. Mathematical models can reduce experimental work and costs, proposing favorable and non-intuitive operating conditions, and designing optimizing control strategies. These models may be considered and integrated to direct the process to the best performance at macroscopic or microscopic levels.
At a microscopic level, two types of mathematical models are available for comprehending cellular metabolism: the kinetic model and the genome-scale metabolic model [158]. The kinetic model can be a limited approach for understanding cellular metabolism because enzyme kinetics parameters of all reactions are unavailable [158]. Then, the use of genome-scale modeling (GEM), which depends on genome sequence data, is very helpful in identifying engineering targets and developing efficient bioprocess strategies. Several GEMs are available for K. phaffii, with constant refinement and evolution [90,153,158].
For a macroscopic approach, the established techniques of design of experiments (DoE), artificial neural networks (ANN), and genetic algorithms (GA), have proven to be robust for fermentation process optimization [166]. Usually, these techniques are associated with fermentation media development or early process optimization [166]. The combination of GEM and DoE could determine the effect of genetic and culture conditions in K. phaffii to produce thaumatin, a sweetener [158].
The dynamic macroscopic models of bioprocesses, based on mass and energy balances, are essential to understanding how cells behave to changes in environmental conditions and to optimizing bioreactor fermentation; however, a limited number of these models are available for K. phaffii, usually with simple formulations [153,167]. Recently, an extensive macroscopic bioreactor model was constructed for K. phaffii [167], which describes substrates and other medium components, biomass, total protein, and off-gas components in different operating conditions.
Model predictive control (MPC) has gained prominence for bioprocess optimization [152]. This practice considers dynamic and static interactions between input, output, and disturbance variables, and uses a predictive process model to synchronize the control estimate with the optimum set points calculations [152]. Simulation in K. phaffii fermentation process, a highly nonlinear system, employing the MPC method, has been shown to be accurate in describing experimental data [167]. Still, it remains underused for this kind of bioprocess.
Despite the advances, observing all limitations in integrating cells, metabolites, and processes, with precision in predicting all responses, remain a challenge [164,167]. Gradually, multipurpose models are beginning to be used as an efficient tool in systematic metabolic engineering and bioprocess optimization.
Table 5 summarizes strategies discussed above applied to the development, simulation, and optimization of processes with K. phaffii. Although some techniques are still incipient for this host system, there is a clear need to apply efforts in this area to establish industrial fermentation processes with K. phaffii.
8. Challenges for Industrial Processes with K. phaffii
Currently, K. phaffii is responsible for more than 300 licensed industrial processes and 70 different types of commercial products, such as enzymes and biopharmaceuticals [138,145]. The www.pichia.com accessed on 13 September 2022 website provides a list of products on the market or in the industry development stage using this yeast. Compared to its use as a protein expression system, the K. phaffii as a cell factory for the production of chemicals has been less exploited, despite increasing use in recent years.
Until then, the underestimated use of K. phaffii as a cell factory or expression system in industrial-scale processes could be mainly related to challenges such as economic competitiveness, low titers and yields, and scaling-up adversities. Furthermore, despite the numerous advantages of producing chemicals using bio-based methods, there are few cases in which bioprocessing competes with the fossil-based chemical industry in terms of time and cost [164].
As highlighted earlier, an efficient bioprocess in K. phaffii and other microorganisms requires an optimized and stable host strain with improved metabolic rates; and an effective and reproducible fermentation process, operating with a high titer, yield, and productivity. However, to produce marketed chemicals or proteins, it is also mandatory a successful scaling-up of a bioprocess, designed to be scalable, without loss of efficiency or significant increase in cost; a technically and cost-effective downstream on a large-scale process; a rigorously and constantly monitored and controlled process. All these factors are intrinsically interconnected and must be considered together to establish the industrial fermentation process [15,141].
Despite the recent advances in K. phaffii industrial utilization, the scaling-up process also has some drawbacks due to strain limitations. Unlike S. cerevisiae, K. phaffii strains are mainly prototype, which means that they have few genetic markers, they change their mating type by aggregating, and there are just a few strains used in industry and research, bringing limitations to its genetic pool [15]. Another critical metabolic limitation is glucose uptake. The glucose uptake is reduced and does not exceed the yeast’s respiratory capacities. Compared to S. cerevisiae, the maximum glucose uptake is almost tenfold lower in K. phaffii, which may reduce process efficiency [14]. Presumably, the strict glucose uptake and high PPP flux can lead to the production of complex undesired secondary metabolites as fermentative byproducts, such as terpenoids and carotenoids [15]. Thus, the recent advances in enhancing cellular function, development of tools, promoters, and markers, and designing new expression platforms, allowed the construction of various strains capable of producing renewable chemicals (Table 2). However, the limitations in carbon assimilation (consumption capacity and rates) and byproduct formation are still the main drawbacks in the industrial use of recombinant K. phaffii due to low production titers, yields, and/or productivity.
Scale-up is considered the most critical bottleneck for contemporary biotechnology to establish in a large-scale industry [143,144]. Usually, process engineering does not receive the same interest or develop at the same speed as biological systems’ genetic and metabolic engineering. The financial investment to scale up a bioprocess is typically more significant than the cost to develop a lab-scale production. This can cost USD 100 million to USD 1 billion and take 3–10 years to scale the transition [144]. Thus, several lab-scale bioprocesses cannot overcome the Death Valley of process development.
Industrial-scale bioreactors are substantially different from laboratory cultures in several aspects. Then, if a process optimization on a small scale is not well designed, it cannot address the real problem of the industrial scale. Previous scale-up criteria for fermentation processes were based on keeping constant process engineering parameters, such as power input/volume ratio (P/V), impeller tip speed, or volumetric mass transfer coefficient (kLa), and thus have not resulted in universally valid standards for successful scale-up [138,144]. In this context, the most successful technology transfers to the full-scale fermentation process resulting from scale-down models, where strains are evaluated under critical aspects of the large-scale fermentation environment [139,141,144,168]. This systematic method of bioprocess and strain engineering for scale-up requires a large-scale view of production conditions on a small scale to define combinations of a host, path, and process, that will meet the requirements of industrial production. The successful use of this approach considers successive tiers to experimental evaluation, but it starts using the same raw materials and feeding algorithms as used on an industrial scale. Even on a laboratory scale, industrial-scale and process control aspects must be considered to minimize scale-up risk. The selection of parameters for a well-established evaluation is particular to the process, strain, and product type.
Even when systems biology and process development were planned with increasing bioreactor volume, other factors not previously observed can become an obstacle. Examples of these differences in scale are: the hydrostatic pressure gradient of the reactor, which can influence enzyme activities, metabolic flux, or the concentration of dissolved gases in the medium; or poor mixing and dead zones created with increasing bioreactor volume, which results in the gradients of parameters such as pH, temperature, dissolved oxygen and the concentration of nutrients; mass transfer limitations; yeast metabolism generates a large amount of heat and therefore need efficient cooling. These stress conditions can trigger genetic and physiological responses in the host strain and lead to difficulties in monitoring and controlling large-scale cultivation [138,141,151,164]. In this sense, a rigorous study for transitioning the bioprocess to the industrial scale is a mandatory stage. Using multi-omic characterization and the integration of fluid dynamics and cell metabolism, or building mathematic multiscale models, can be helpful, as discussed previously [164].
Regarding the downstream, the fermentation process strongly influences this stage by requiring the separation of high cell density and the purification of desired product out of a complex matrix. The downstream must be considered part of the bioprocess, and its development and optimization must use the same logic applied to the process development [139,141,144,166]. In the same way, the technical and economic feasibility of downstream processing will also depend on the product and should be evaluated in each case [138,143,154].
A better understanding of the interaction between strain and bioprocess and the ability to use new and sophisticated tools has brought us closer to the market availability of several new renewable molecules produced by K. phaffii.
9. Conclusions
The Komagataella species are versatile yeasts capable of producing a range of bio compounds of interest and heterologous proteins. With the increasing number of strains and new molecular tools and methodologies, these yeasts are becoming even more competitive hosts when compared to others. For instance, K. phaffii has the advantage of being eukaryotic (when compared to bacteria) and presents undeveloped potential regarding fermentative cultivation (compared with S. cerevisiae). Besides this fact, K. phaffii is one of the major microbial platforms for the production of recombinant proteins, that are constantly employed in different industries, such as pharmaceuticals, food, feed, detergents, and others. More recent results demonstrate K. phaffii feasibility to produce complex chemicals via metabolic engineering. As can be seen, several studies highlight the potential of K. phaffii for being a microbial chassis for the production of value-added compounds using glycerol, glucose, xylose, and methanol as carbon source. Despite the great advancements in the field, further improvements are required for the industrial application of K. phaffii in methanol bioconversions.
Author Contributions
Conceptualization, C.V.G.C.C. and J.R.M.d.A.; writing—original draft preparation, C.V.G.C.C., D.T., L.M.M.F., L.T.D.B., L.A.S., M.N.d.M.F. and T.F.P. writing—review and editing, C.V.G.C.C. and J.R.M.d.A.; visualization, C.V.G.C.C.; funding acquisition, J.R.M.d.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Foundation for Research Support of the Federal District (FAP_DF) grant number 00193-00000762/2021-92.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leong, H.Y.; Chang, C.K.; Khoo, K.S.; Chew, K.W.; Chia, S.R.; Lim, J.W.; Chang, J.S.; Show, P.L. Waste Biorefinery towards a Sustainable Circular Bioeconomy: A Solution to Global Issues. Biotechnol. Biofuels 2021, 14, 1–15. [Google Scholar] [CrossRef]
- Muscat, A.; de Olde, E.M.; Ripoll-Bosch, R.; Van Zanten, H.H.E.; Metze, T.A.P.; Termeer, C.J.A.M.; van Ittersum, M.K.; de Boer, I.J.M. Principles, Drivers and Opportunities of a Circular Bioeconomy. Nat. Food 2021, 2, 561–566. [Google Scholar] [CrossRef]
- Kardung, M.; Cingiz, K.; Costenoble, O.; Delahaye, R.; Heijman, W.; Lovrić, M.; van Leeuwen, M.; M’barek, R.; van Meijl, H.; Piotrowski, S.; et al. Development of the Circular Bioeconomy: Drivers and Indicators. Sustainability 2021, 13, 413. [Google Scholar] [CrossRef]
- Carneiro, C.V.G.; Silva, F.C.D.P.E.; Almeida, J.R. Xylitol Production: Identification and Comparison of New Producing Yeasts. Microorganisms 2019, 7, 484. [Google Scholar] [CrossRef]
- Trichez, D.; Carneiro, C.V.G.C.; Braga, M.; Almeida, J.R.M. Recent Progress in the Microbial Production of Xylonic Acid. World J. Microbiol. Biotechnol. 2022, 38, 127. [Google Scholar] [CrossRef] [PubMed]
- Ata, Ö.; Ergün, B.G.; Fickers, P.; Heistinger, L.; Mattanovich, D.; Rebnegger, C.; Gasser, B. What Makes Komagataella Phaffii Non-Conventional? FEMS Yeast Res. 2021, 21, foab059. [Google Scholar] [CrossRef]
- Bernauer, L.; Radkohl, A.; Lehmayer, L.G.K.; Emmerstorfer-Augustin, A. Komagataella Phaffii as Emerging Model Organism in Fundamental Research. Front. Microbiol. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Chiang, C.-J.; Ho, Y.-J.; Hu, M.-C.; Chao, Y.-P. Rewiring of Glycerol Metabolism in Escherichia Coli for Effective Production of Recombinant Proteins. Biotechnol. Biofuels 2020, 13, 205. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z. Engineering Strategies for Enhanced Production of Protein and Bio-Products in Pichia Pastoris. Biotechnol. Adv. 2018, 36, 182–195. [Google Scholar] [CrossRef]
- Näätsaari, L.; Mistlberger, B.; Ruth, C.; Hajek, T.; Hartner, F.S.; Glieder, A. Deletion of the Pichia Pastoris KU70 Homologue Facilitates Platform Strain Generation for Gene Expression and Synthetic Biology. PLoS ONE 2012, 7, e39720. [Google Scholar] [CrossRef]
- Ito, Y.; Watanabe, T.; Aikawa, S.; Nishi, T.; Nishiyama, T.; Nakamura, Y.; Hasunuma, T.; Okubo, Y.; Ishii, J.; Kondo, A. Deletion of DNA Ligase IV Homolog Confers Higher Gene Targeting Efficiency on Homologous Recombination in Komagataella Phaffii. FEMS Yeast Res. 2018, 18, foy074. [Google Scholar] [CrossRef]
- Weninger, A.; Hatzl, A.-M.; Schmid, C.; Vogl, T.; Glieder, A. Combinatorial Optimization of CRISPR/Cas9 Expression Enables Precision Genome Engineering in the Methylotrophic Yeast Pichia Pastoris. J. Biotechnol. 2016, 235, 139–149. [Google Scholar] [CrossRef]
- Fischer, J.E.; Glieder, A. Current Advances in Engineering Tools for Pichia Pastoris. Curr. Opin. Biotechnol. 2019, 59, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Peña, D.A.; Gasser, B.; Zanghellini, J.; Steiger, M.G.; Mattanovich, D. Metabolic Engineering of Pichia Pastoris. Metab. Eng. 2018, 50, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Gasser, B.; Mattanovich, D. A Yeast for All Seasons—Is Pichia Pastoris a Suitable Chassis Organism for Future Bioproduction? FEMS Microbiol. Lett. 2018, 365, fny181. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Matsuda, M.; Maeda, K.; Mikata, K. The Phylogenetic Relationships of Methanol-Assimilating Yeasts Based on the Partial Sequences of 18S and 26S Ribosomal RNAs: The Proposal of Komagataella Gen. Nov. (Saccharomycetaceae). Biosci. Biotechnol. Biochem. 1995, 59, 439–444. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Robnett, C.J. Identification and Phylogeny of Ascomycetous Yeasts from Analysis of Nuclear Large Subunit (26S) Ribosomal DNA Partial Sequences. Antonie Van Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef]
- Guilliermond, A. Zygosaccharomyces Pastori, Nouvelle Espèce de Levures à Copulation Hétérogamique. Bull. Société Mycol. Fr. 1920, 36, 203–211. [Google Scholar]
- Heistinger, L.; Gasser, B.; Mattanovich, D. Microbe Profile: Komagataella Phaffii: A Methanol Devouring Biotech Yeast Formerly Known as Pichia Pastoris. Microbiology 2020, 166, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T. The Yeasts—A Taxonomy Study; Elsevier Science: Amsterdam, The Netherlands, 2011; Volume 5, ISBN 978-0-08-093127-2. [Google Scholar]
- Heistinger, L.; Dohm, J.C.; Paes, B.G.; Koizar, D.; Troyer, C.; Ata, Ö.; Steininger-Mairinger, T.; Mattanovich, D. Genotypic and Phenotypic Diversity among Komagataella Species Reveals a Hidden Pathway for Xylose Utilization. Microb. Cell Factories 2022, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P. Description of Komagataella Phaffii Sp. Nov. and the Transfer of Pichia Pseudopastoris to the Methylotrophic Yeast Genus Komagataella. Int. J. Syst. Evol. Microbiol. 2005, 55, 973–976. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, K.; Lin, Y.-C.; Tiels, P.; Van Hecke, A.; Glinka, S.; Weber-Lehmann, J.; Rouzé, P.; Van de Peer, Y.; Callewaert, N. Genome Sequence of the Recombinant Protein Production Host Pichia Pastoris. Nat. Biotechnol. 2009, 27, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Love, K.R.; Shah, K.A.; Whittaker, C.A.; Wu, J.; Bartlett, M.C.; Ma, D.; Leeson, R.L.; Priest, M.; Borowsky, J.; Young, S.K.; et al. Comparative Genomics and Transcriptomics of Pichia Pastoris. BMC Genom. 2016, 17, 550. [Google Scholar] [CrossRef] [PubMed]
- Mattanovich, D.; Graf, A.; Stadlmann, J.; Dragosits, M.; Redl, A.; Maurer, M.; Kleinheinz, M.; Sauer, M.; Altmann, F.; Gasser, B. Genome, Secretome and Glucose Transport Highlight Unique Features of the Protein Production Host Pichia Pastoris. Microb. Cell Factories 2009, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.M.; Fávaro, L.C.L.; Quirino, B.F. Biodiesel Biorefinery: Opportunities and Challenges for Microbial Production of Fuels and Chemicals from Glycerol Waste. Biotechnol. Biofuels 2012, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A Promising and Abundant Carbon Source for Industrial Microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef]
- Tomàs-Gamisans, M.; Ødum, A.S.R.; Workman, M.; Ferrer, P.; Albiol, J. Glycerol Metabolism of Pichia Pastoris (Komagataella Spp.) Characterised by 13C-Based Metabolic Flux Analysis. New Biotechnol. 2019, 50, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Prielhofer, R.; Cartwright, S.P.; Graf, A.B.; Valli, M.; Bill, R.M.; Mattanovich, D.; Gasser, B. Pichia Pastoris Regulates Its Gene-Specific Response to Different Carbon Sources at the Transcriptional, Rather than the Translational, Level. BMC Genom. 2015, 16, 167. [Google Scholar] [CrossRef] [PubMed]
- Çelik, E.; Ozbay, N.; Oktar, N.; Çalık, P. Use of Biodiesel Byproduct Crude Glycerol as the Carbon Source for Fermentation Processes by Recombinant Pichia Pastoris. Ind. Eng. Chem. Res. 2008, 47, 2985–2990. [Google Scholar] [CrossRef]
- Kumokita, R.; Bamba, T.; Inokuma, K.; Yoshida, T.; Ito, Y.; Kondo, A.; Hasunuma, T. Construction of an l-Tyrosine Chassis in Pichia Pastoris Enhances Aromatic Secondary Metabolite Production from Glycerol. ACS Synth. Biol. 2022, 11, 2098–2107. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, D. Toward Glycerol Biorefinery: Metabolic Engineering for the Production of Biofuels and Chemicals from Glycerol. Biotechnol. Biofuels 2016, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Miao, J.; Luo, W.; Li, G.; Du, Y.; Yu, X. Crude Glycerol from Biodiesel as a Carbon Source for Production of a Recombinant Highly Thermostable β-Mannanase by Pichia Pastoris. Biotechnol. Lett. 2018, 40, 135–141. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent Advances in Lactic Acid Production by Microbial Fermentation Processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Hashemi, S.M.B. Lactic Acid Production—Producing Microorganisms and Substrates Sources-State of Art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef] [PubMed]
- de Lima, P.B.A.; Mulder, K.C.L.; Melo, N.T.M.; Carvalho, L.S.; Menino, G.S.; Mulinari, E.; de Castro, V.H.; dos Reis, T.F.; Goldman, G.H.; Magalhães, B.S.; et al. Novel Homologous Lactate Transporter Improves L-Lactic Acid Production from Glycerol in Recombinant Strains of Pichia Pastoris. Microb. Cell Factories 2016, 15, 158. [Google Scholar] [CrossRef]
- Melo, N.; Mulder, K.; Nicola, A.; Carvalho, L.; Menino, G.; Mulinari, E.; Parachin, N. Effect of Pyruvate Decarboxylase Knockout on Product Distribution Using Pichia Pastoris (Komagataella Phaffii) Engineered for Lactic Acid Production. Bioengineering 2018, 5, 17. [Google Scholar] [CrossRef]
- Tamires Moreira Melo, N.; Pontes, G.C.; Procópio, D.P.; de Gois e Cunha, G.C.; Eliodório, K.P.; Costa Paes, H.; Basso, T.O.; Parachin, N.S. Evaluation of Product Distribution in Chemostat and Batch Fermentation in Lactic Acid-Producing Komagataella Phaffii Strains Utilizing Glycerol as Substrate. Microorganisms 2020, 8, 781. [Google Scholar] [CrossRef]
- Fina, A.; Brêda, G.C.; Pérez-Trujillo, M.; Freire, D.M.G.; Almeida, R.V.; Albiol, J.; Ferrer, P. Benchmarking Recombinant Pichia Pastoris for 3-hydroxypropionic Acid Production from Glycerol. Microb. Biotechnol. 2021, 14, 1671–1682. [Google Scholar] [CrossRef]
- Siripong, W.; Wolf, P.; Kusumoputri, T.P.; Downes, J.J.; Kocharin, K.; Tanapongpipat, S.; Runguphan, W. Metabolic Engineering of Pichia Pastoris for Production of Isobutanol and Isobutyl Acetate. Biotechnol. Biofuels 2018, 11, 1. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z. Production of (2R, 3R)-2,3-Butanediol Using Engineered Pichia Pastoris: Strain Construction, Characterization and Fermentation. Biotechnol. Biofuels 2018, 11, 35. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, X.; Wang, C.; Du, G.; Chen, J.; Kang, Z. Production of Glucaric Acid from Myo-Inositol in Engineered Pichia Pastoris. Enzym. Microb. Technol. 2016, 91, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.; Luo, H.; Wang, Y.; Wang, Y.; Tu, T.; Qin, X.; Su, X.; Huang, H.; Yao, B.; et al. Metabolic Engineering of Pichia Pastoris for Myo-Inositol Production by Dynamic Regulation of Central Metabolism. Microb. Cell Factories 2022, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Louie, T.M.; Louie, K.; DenHartog, S.; Gopishetty, S.; Subramanian, M.; Arnold, M.; Das, S. Production of Bio-xylitol from Xylose by an Engineered P.Pastoris. Microb. Cell Factories 2021, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Ramos, T.G.S.; Justen, F.; Carneiro, C.V.G.C.; Honorato, V.M.; Franco, P.F.; Vieira, F.S.; Trichez, D.; Rodrigues, C.M.; Almeida, J.R.M. Xylonic Acid Production by Recombinant Komagataella Phaffii Strains Engineered with Newly Identified Xylose Dehydrogenases. Bioresour. Technol. Rep. 2021, 16, 100825. [Google Scholar] [CrossRef]
- Miao, L.; Li, Y.; Zhu, T. Metabolic Engineering of Methylotrophic Pichia Pastoris for the Production of β-Alanine. Bioresour. Bioprocess. 2021, 8, 89. [Google Scholar] [CrossRef]
- Yamada, R.; Ogura, K.; Kimoto, Y.; Ogino, H. Toward the Construction of a Technology Platform for Chemicals Production from Methanol: D-Lactic Acid Production from Methanol by an Engineered Yeast Pichia Pastoris. World J. Microbiol. Biotechnol. 2019, 35, 37. [Google Scholar] [CrossRef]
- Guo, F.; Dai, Z.; Peng, W.; Zhang, S.; Zhou, J.; Ma, J.; Dong, W.; Xin, F.; Zhang, W.; Jiang, M. Metabolic Engineering of Pichia Pastoris for Malic Acid Production from Methanol. Biotechnol. Bioeng. 2021, 118, 357–371. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, X.; Xu, Q.; Bai, C.; Kong, C.; Liu, Q.; Yu, J.; Peng, Q.; Zhou, X.; Zhang, Y.; et al. Engineered Monoculture and Co-Culture of Methylotrophic Yeast for de Novo Production of Monacolin J and Lovastatin from Methanol. Metab. Eng. 2018, 45, 189–199. [Google Scholar] [CrossRef]
- Gao, L.; Cai, M.; Shen, W.; Xiao, S.; Zhou, X.; Zhang, Y. Engineered Fungal Polyketide Biosynthesis in Pichia Pastoris: A Potential Excellent Host for Polyketide Production. Microb. Cell Factories 2013, 12, 77. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, W.; Wang, Y.; Sheng, J.; Xu, R.; Li, J.; Du, G.; Kang, Z. Biosynthesis of Non-Animal Chondroitin Sulfate from Methanol Using Genetically Engineered Pichia Pastoris. Green Chem. 2021, 23, 4365–4374. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhou, Z.; Wang, P.; Xi, X.; Hu, S.; Xu, R.; Du, G.; Li, J.; Chen, J.; et al. Synthesis of Bioengineered Heparin by Recombinant Yeast Pichia Pastoris. Green Chem. 2022, 24, 3180–3192. [Google Scholar] [CrossRef]
- Jeong, E.; Shim, W.Y.; Kim, J.H. Metabolic Engineering of Pichia Pastoris for Production of Hyaluronic Acid with High Molecular Weight. J. Biotechnol. 2014, 185, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Ashok, S.; Park, S. Recent Advances in Biological Production of 3-Hydroxypropionic Acid. Biotechnol. Adv. 2013, 31, 945–961. [Google Scholar] [CrossRef] [PubMed]
- de Fouchécour, F.; Sánchez-Castañeda, A.-K.; Saulou-Bérion, C.; Spinnler, H.É. Process Engineering for Microbial Production of 3-Hydroxypropionic Acid. Biotechnol. Adv. 2018, 36, 1207–1222. [Google Scholar] [CrossRef]
- Tashiro, Y.; Rodriguez, G.M.; Atsumi, S. 2-Keto Acids Based Biosynthesis Pathways for Renewable Fuels and Chemicals. J. Ind. Microbiol. Biotechnol. 2015, 42, 361–373. [Google Scholar] [CrossRef]
- Weber, C.; Farwick, A.; Benisch, F.; Brat, D.; Dietz, H.; Subtil, T.; Boles, E. Trends and Challenges in the Microbial Production of Lignocellulosic Bioalcohol Fuels. Appl. Microbiol. Biotechnol. 2010, 87, 1303–1315. [Google Scholar] [CrossRef]
- Wess, J.; Brinek, M.; Boles, E. Improving Isobutanol Production with the Yeast Saccharomyces Cerevisiae by Successively Blocking Competing Metabolic Pathways as Well as Ethanol and Glycerol Formation. Biotechnol. Biofuels 2019, 12, 173. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Luo, H.; Wang, Y.; Wang, Y.; Tu, T.; Qin, X.; Huang, H.; Bai, Y.; Yao, B.; et al. Metabolic Engineering of Komagataella Phaffii for Synergetic Utilization of Glucose and Glycerol. Yeast 2022, 39, 412–421. [Google Scholar] [CrossRef]
- Wang, B.; He, M.; Wang, X.; Tang, H.; Zhu, X. A Multi-Model Predictive Control Method for the Pichia Pastoris Fermentation Process Based on Relative Error Weighting Algorithm. Alex. Eng. J. 2022, 61, 9649–9660. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol Production from Renewable Sources: Current Perspectives and Technological Progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Chen, H. Chemical Composition and Structure of Natural Lignocellulose. In Biotechnology of Lignocellulose; Springer: Dordrecht, The Netherlands, 2014; pp. 25–71. ISBN 978-94-007-6897-0. [Google Scholar]
- Chandel, A.K.; Garlapati, V.K.; Jeevan Kumar, S.P.; Hans, M.; Singh, A.K.; Kumar, S. The Role of Renewable Chemicals and Biofuels in Building a Bioeconomy. Biofuels Bioprod. Bioref. 2020, 14, 830–844. [Google Scholar] [CrossRef]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Liu, C.-G.; Xiao, Y.; Xia, X.-X.; Zhao, X.-Q.; Peng, L.; Srinophakun, P.; Bai, F.-W. Cellulosic Ethanol Production: Progress, Challenges and Strategies for Solutions. Biotechnol. Adv. 2019, 37, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, D.; Zhao, X. Conversion of Lignocellulose to Biofuels and Chemicals via Sugar Platform: An Updated Review on Chemistry and Mechanisms of Acid Hydrolysis of Lignocellulose. Renew. Sustain. Energy Rev. 2021, 146, 111169. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A Review on the Pretreatment of Lignocellulose for High-Value Chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Almeida, J.R.; Modig, T.; Petersson, A.; Hähn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Increased Tolerance and Conversion of Inhibitors in Lignocellulosic Hydrolysates BySaccharomyces Cerevisiae. J. Chem. Technol. Biotechnol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Sjulander, N.; Kikas, T. Origin, Impact and Control of Lignocellulosic Inhibitors in Bioethanol Production—A Review. Energies 2020, 13, 4751. [Google Scholar] [CrossRef]
- Paes, B.G.; Steindorff, A.S.; Formighieri, E.F.; Pereira, I.S.; Almeida, J.R.M. Physiological Characterization and Transcriptome Analysis of Pichia Pastoris Reveals Its Response to Lignocellulose-Derived Inhibitors. AMB Expr. 2021, 11, 2. [Google Scholar] [CrossRef]
- Bustos, C.; Quezada, J.; Veas, R.; Altamirano, C.; Braun-Galleani, S.; Fickers, P.; Berrios, J. Advances in Cell Engineering of the Komagataella Phaffii Platform for Recombinant Protein Production. Metabolites 2022, 12, 346. [Google Scholar] [CrossRef]
- Lamsen, E.N.; Atsumi, S. Recent Progress in Synthetic Biology for Microbial Production of C3–C10 Alcohols. Front. Microbiol. 2012, 3, 196. [Google Scholar] [CrossRef] [PubMed]
- Jong, E.d.; Stichnothe, H.; Bell, G.; Jørgensen, H. Task 42-Bio-Based Chemicals: A 2020 Update; IEA Bioenergy: Vienna, Austria, 2020. [Google Scholar]
- Zhao, E.M.; Zhang, Y.; Mehl, J.; Park, H.; Lalwani, M.A.; Toettcher, J.E.; Avalos, J.L. Optogenetic Regulation of Engineered Cellular Metabolism for Microbial Chemical Production. Nature 2018, 555, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Motta-Mena, L.B.; Reade, A.; Mallory, M.J.; Glantz, S.; Weiner, O.D.; Lynch, K.W.; Gardner, K.H. An Optogenetic Gene Expression System with Rapid Activation and Deactivation Kinetics. Nat. Chem. Biol. 2014, 10, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, N.M.; Binod, P.; Sindhu, R.; Awasthi, M.K.; Pandey, A. Microbial Engineering for the Production of Isobutanol: Current Status and Future Directions. Bioengineered 2021, 12, 12308–12321. [Google Scholar] [CrossRef] [PubMed]
- Hazeena, S.H.; Sindhu, R.; Pandey, A.; Binod, P. Lignocellulosic Bio-Refinery Approach for Microbial 2,3-Butanediol Production. Bioresour. Technol. 2020, 302, 122873. [Google Scholar] [CrossRef]
- Lian, J.; Chao, R.; Zhao, H. Metabolic Engineering of a Saccharomyces Cerevisiae Strain Capable of Simultaneously Utilizing Glucose and Galactose to Produce Enantiopure (2R,3R)-Butanediol. Metab. Eng. 2014, 23, 92–99. [Google Scholar] [CrossRef]
- Li, Y.; Han, P.; Wang, J.; Shi, T.; You, C. Production of Myo-inositol: Recent Advance and Prospective. Biotechnol. Appl. Biochem. 2022, 69, 1101–1111. [Google Scholar] [CrossRef]
- Braga, M.; Ferreira, P.M.; Almeida, J.R.M. Screening Method to Prioritize Relevant Bio-based Acids and Their Biochemical Processes Using Recent Patent Information. Biofuels Bioprod. Bioref. 2021, 15, 231–249. [Google Scholar] [CrossRef]
- Francois, J.M.; Alkim, C.; Morin, N. Engineering microbial pathways for production of bio-based chemicals from lignocellulosic sugars: Current status and perspectives. Biotechnol. Biofuels 2020, 13, 118. [Google Scholar] [CrossRef]
- Li, P.; Sun, H.; Chen, Z.; Li, Y.; Zhu, T. Construction of Efficient Xylose Utilizing Pichia Pastoris for Industrial Enzyme Production. Microb. Cell Factories 2015, 14, 22. [Google Scholar] [CrossRef]
- Araújo, D.; Costa, T.; Freitas, F. Biovalorization of Lignocellulosic Materials for Xylitol Production by the Yeast Komagataella Pastoris. Appl. Sci. 2021, 11, 5516. [Google Scholar] [CrossRef]
- Guo, Y.; Liao, Y.; Wang, J.; Ma, C.; Qin, J.; Feng, J.; Li, Y.; Wang, X.; Chen, K. Methylotrophy of Pichia Pastoris: Current Advances, Applications, and Future Perspectives for Methanol-Based Biomanufacturing. ACS Sustain. Chem. Eng. 2022, 10, 1741–1752. [Google Scholar] [CrossRef]
- Whitaker, W.B.; Sandoval, N.R.; Bennett, R.K.; Fast, A.G.; Papoutsakis, E.T. Synthetic Methylotrophy: Engineering the Production of Biofuels and Chemicals Based on the Biology of Aerobic Methanol Utilization. Curr. Opin. Biotechnol. 2015, 33, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Peiro, C.; Vicente, C.M.; Jallet, D.; Heux, S. From a Hetero- to a Methylotrophic Lifestyle: Flash Back on the Engineering Strategies to Create Synthetic Methanol-User Strains. Front. Bioeng. Biotechnol. 2022, 10, 907861. [Google Scholar] [CrossRef]
- Cotton, C.A.; Claassens, N.J.; Benito-Vaquerizo, S.; Bar-Even, A. Renewable Methanol and Formate as Microbial Feedstocks. Curr. Opin. Biotechnol. 2020, 62, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Rußmayer, H.; Buchetics, M.; Gruber, C.; Valli, M.; Grillitsch, K.; Modarres, G.; Guerrasio, R.; Klavins, K.; Neubauer, S.; Drexler, H.; et al. Systems-Level Organization of Yeast Methylotrophic Lifestyle. BMC Biol. 2015, 13, 80. [Google Scholar] [CrossRef]
- De, S.; Mattanovich, D.; Ferrer, P.; Gasser, B. Established Tools and Emerging Trends for the Production of Recombinant Proteins and Metabolites in Pichia Pastoris. Essays Biochem. 2021, 65, 293–307. [Google Scholar] [CrossRef]
- Zahrl, R.J.; Peña, D.A.; Mattanovich, D.; Gasser, B. Systems Biotechnology for Protein Production in Pichia Pastoris. FEMS Yeast Res. 2017, 17, fox068. [Google Scholar] [CrossRef]
- Gassler, T.; Sauer, M.; Gasser, B.; Egermeier, M.; Troyer, C.; Causon, T.; Hann, S.; Mattanovich, D.; Steiger, M.G. The Industrial Yeast Pichia Pastoris Is Converted from a Heterotroph into an Autotroph Capable of Growth on CO2. Nat. Biotechnol. 2020, 38, 210–216. [Google Scholar] [CrossRef]
- Gassler, T.; Baumschabl, M.; Sallaberger, J.; Egermeier, M.; Mattanovich, D. Adaptive Laboratory Evolution and Reverse Engineering Enhances Autotrophic Growth in Pichia Pastoris. Metab. Eng. 2022, 69, 112–121. [Google Scholar] [CrossRef]
- Zhang, C.; Ottenheim, C.; Weingarten, M.; Ji, L. Microbial Utilization of Next-Generation Feedstocks for the Biomanufacturing of Value-Added Chemicals and Food Ingredients. Front. Bioeng. Biotechnol. 2022, 10, 874612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ge, C.; Deng, L.; Tan, T.; Wang, F. C4-Dicarboxylic Acid Production by Overexpressing the Reductive TCA Pathway. FEMS Microbiol. Lett. 2015, 362, fnv052. [Google Scholar] [CrossRef] [PubMed]
- Ucm, R.; Aem, M.; Lhb, Z.; Kumar, V.; Taherzadeh, M.J.; Garlapati, V.K.; Chandel, A.K. Comprehensive Review on Biotechnological Production of Hyaluronic Acid: Status, Innovation, Market and Applications. Bioengineered 2022, 13, 9645–9661. [Google Scholar] [CrossRef]
- Xue, Y.; Kong, C.; Shen, W.; Bai, C.; Ren, Y.; Zhou, X.; Zhang, Y.; Cai, M. Methylotrophic Yeast Pichia Pastoris as a Chassis Organism for Polyketide Synthesis via the Full Citrinin Biosynthetic Pathway. J. Biotechnol. 2017, 242, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, M.; Rezaee, S.; Farsiani, H. Pichia Pastoris: A Highly Successful Expression System for Optimal Synthesis of Heterologous Proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef]
- Ahmad, M.; Hirz, M.; Pichler, H.; Schwab, H. Protein Expression in Pichia Pastoris: Recent Achievements and Perspectives for Heterologous Protein Production. Appl. Microbiol. Biotechnol. 2014, 98, 5301–5317. [Google Scholar] [CrossRef]
- Cereghino, J.L.; Cregg, J.M. Heterologous Protein Expression in the Methylotrophic Yeast Pichia Pastoris. FEMS Microbiol. Rev. 2000, 24, 45–66. [Google Scholar] [CrossRef]
- de Almeida, J.R.M.; de Moraes, L.M.P.; Torres, F.A.G. Molecular Characterization of the 3-Phosphoglycerate Kinase Gene (PGK1) from the Methylotrophic Yeast Pichia Pastoris. Yeast 2005, 22, 725–737. [Google Scholar] [CrossRef]
- Spiegel, H.; Schinkel, H.; Kastilan, R.; Dahm, P.; Boes, A.; Scheuermayer, M.; Chudobová, I.; Maskus, D.; Fendel, R.; Schillberg, S.; et al. Optimization of a Multi-Stage, Multi-Subunit Malaria Vaccine Candidate for the Production in Pichia Pastoris by the Identification and Removal of Protease Cleavage Sites: Optimization of a Malaria Vaccine Candidate. Biotechnol. Bioeng. 2015, 112, 659–667. [Google Scholar] [CrossRef]
- Tripathi, L.; Mani, S.; Raut, R.; Poddar, A.; Tyagi, P.; Arora, U.; de Silva, A.; Swaminathan, S.; Khanna, N. Pichia Pastoris-Expressed Dengue 3 Envelope-Based Virus-like Particles Elicit Predominantly Domain III-Focused High Titer Neutralizing Antibodies. Front. Microbiol. 2015, 6, 1005. [Google Scholar] [CrossRef]
- Saraswat, S.; Athmaram, T.N.; Parida, M.; Agarwal, A.; Saha, A.; Dash, P.K. Expression and Characterization of Yeast Derived Chikungunya Virus Like Particles (CHIK-VLPs) and Its Evaluation as a Potential Vaccine Candidate. PLoS Negl. Trop. Dis. 2016, 10, e0004782. [Google Scholar] [CrossRef] [PubMed]
- Farsiani, H.; Mosavat, A.; Soleimanpour, S.; Sadeghian, H.; Akbari Eydgahi, M.R.; Ghazvini, K.; Sankian, M.; Aryan, E.; Jamehdar, S.A.; Rezaee, S.A. Fc-Based Delivery System Enhances Immunogenicity of a Tuberculosis Subunit Vaccine Candidate Consisting of the ESAT-6:CFP-10 Complex. Mol. BioSyst. 2016, 12, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- Argentinian AntiCovid Consortium. Structural and Functional Comparison of SARS-CoV-2-Spike Receptor Binding Domain Produced in Pichia Pastoris and Mammalian Cells. Sci. Rep. 2020, 10, 21779. [Google Scholar] [CrossRef] [PubMed]
- Baeshen, M.N.; Bouback, T.A.F.; Alzubaidi, M.A.; Bora, R.S.; Alotaibi, M.A.T.; Alabbas, O.T.O.; Alshahrani, S.M.; Aljohani, A.A.M.; Munshi, R.A.A.; Al-Hejin, A.; et al. Expression and Purification of C-Peptide Containing Insulin Using Pichia Pastoris Expression System. BioMed Res. Int. 2016, 2016, 3423685. [Google Scholar] [CrossRef] [PubMed]
- Eissazadeh, S.; Moeini, H.; Dezfouli, M.G.; Heidary, S.; Nelofer, R.; Abdullah, M.P. Production of Recombinant Human Epidermal Growth Factor in Pichia Pastoris. Braz. J. Microbiol. 2017, 48, 286–293. [Google Scholar] [CrossRef]
- Li, P.; Yang, G.; Geng, X.; Shi, J.; Li, B.; Wang, Z.; Zhang, Q.; Yang, Y.; Xu, C. High-Level Secretory Expression and Purification of Recombinant Human Interleukin 1 Beta in Pichia Pastoris. Protein Pept. Lett. 2016, 23, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.-M.; Li, W.-J.; Shi, L.-Y.; Lv, Y.-J.; Sun, X.-Q.; Hu, J.-C.; Fan, Z.-C. Expression, Purification and Characterization of a Recombinant Antimicrobial Peptide Hispidalin in Pichia Pastoris. Protein Expr. Purif. 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, M.; Chen, X.; Yang, G.; Yang, T.; Yu, L.; Hui, L.; Wang, X. Expression and Antibacterial Activity of Hybrid Antimicrobial Peptide CecropinA-Thanatin in Pichia Pastoris. Front. Lab. Med. 2018, 2, 23–29. [Google Scholar] [CrossRef]
- Popa, C.; Shi, X.; Ruiz, T.; Ferrer, P.; Coca, M. Biotechnological Production of the Cell Penetrating Antifungal PAF102 Peptide in Pichia Pastoris. Front. Microbiol. 2019, 10, 1472. [Google Scholar] [CrossRef]
- Xing, L.-W.; Tian, S.-X.; Gao, W.; Yang, N.; Qu, P.; Liu, D.; Jiao, J.; Wang, J.; Feng, X.-J. Recombinant Expression and Biological Characterization of the Antimicrobial Peptide Fowlicidin-2 in Pichia Pastoris. Exp. Ther. Med. 2016, 12, 2324–2330. [Google Scholar] [CrossRef]
- Zhu, W.; Gong, G.; Pan, J.; Han, S.; Zhang, W.; Hu, Y.; Xie, L. High Level Expression and Purification of Recombinant Human Serum Albumin in Pichia Pastoris. Protein Expr. Purif. 2018, 147, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Várnai, A.; Tang, C.; Bengtsson, O.; Atterton, A.; Mathiesen, G.; Eijsink, V.G. Expression of Endoglucanases in Pichia Pastoris under Control of the GAP Promoter. Microb. Cell Factories 2014, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Mellitzer, A.; Weis, R.; Glieder, A.; Flicker, K. Expression of Lignocellulolytic Enzymes in Pichia Pastoris. Microb. Cell Factories 2012, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ning, C.; Yuan, M.; Fu, X.; Yang, S.; Wei, X.; Xiao, M.; Mou, H.; Zhu, C. High-Efficiency Expression of a Superior β-Mannanase Engineered by Cooperative Substitution Method in Pichia Pastoris and Its Application in Preparation of Prebiotic Mannooligosaccharides. Bioresour. Technol. 2020, 311, 123482. [Google Scholar] [CrossRef]
- Rodrigues, K.B.; Macêdo, J.K.A.; Teixeira, T.; Barros, J.S.; Araújo, A.C.B.; Santos, F.P.; Quirino, B.F.; Brasil, B.S.A.F.; Salum, T.F.C.; Abdelnur, P.V.; et al. Recombinant Expression of Thermobifida Fusca E7 LPMO in Pichia Pastoris and Escherichia Coli and Their Functional Characterization. Carbohydr. Res. 2017, 448, 175–181. [Google Scholar] [CrossRef]
- Matsuyama, K.; Sunagawa, N.; Igarashi, K. Mutation of Cysteine Residues Increases Heterologous Expression of Peach Expansin in the Methylotrophic Yeast Pichia Pastoris. Plant Biotechnol. 2020, 37, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Miao, T.; Basit, A.; Liu, J.; Zheng, F.; Rahim, K.; Lou, H.; Jiang, W. Improved Production of Xylanase in Pichia Pastoris and Its Application in Xylose Production From Xylan. Front. Bioeng. Biotechnol. 2021, 9, 690702. [Google Scholar] [CrossRef]
- Juturu, V.; Aust, C.; Wu, J.C. Heterologous Expression and Biochemical Characterization of Acetyl Xylan Esterase from Coprinopsis Cinerea. World J. Microbiol. Biotechnol. 2013, 29, 597–605. [Google Scholar] [CrossRef]
- Zhang, S.-B.; Wang, L.; Liu, Y.; Zhai, H.-C.; Cai, J.-P.; Hu, Y.-S. Expression of Feruloyl Esterase A from Aspergillus Terreus and Its Application in Biomass Degradation. Protein Expr. Purif. 2015, 115, 153–157. [Google Scholar] [CrossRef]
- Zheng, F.; Liu, J.; Basit, A.; Miao, T.; Jiang, W. Insight to Improve α-L-Arabinofuranosidase Productivity in Pichia Pastoris and Its Application on Corn Stover Degradation. Front. Microbiol. 2018, 9, 3016. [Google Scholar] [CrossRef]
- Adina, S.R.; Suwanto, A.; Meryandini, A.; Puspitasari, E. Expression of Novel Acidic Lipase from Micrococcus Luteus in Pichia Pastoris and Its Application in Transesterification. J. Genet. Eng. Biotechnol. 2021, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Chen, X.; Zhu, K.; Xu, H. Immunogenicity of Heparin-Binding Hemagglutinin Expressed by Pichia Pastoris GS115 Strain. Iran. J. Basic Med. Sci. 2018, 21, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Du, L.; Chag, S.M.; Ma, C.; Tricoche, N.; Tao, X.; Seid, C.A.; Hudspeth, E.M.; Lustigman, S.; Tseng, C.-T.K.; et al. Yeast-Expressed Recombinant Protein of the Receptor-Binding Domain in SARS-CoV Spike Protein with Deglycosylated Forms as a SARS Vaccine Candidate. Hum. Vaccines Immunother. 2014, 10, 648–658. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chag, S.M.; Poongavanam, M.V.; Biter, A.B.; Ewere, E.A.; Rezende, W.; Seid, C.A.; Hudspeth, E.M.; Pollet, J.; McAtee, C.P.; et al. Optimization of the Production Process and Characterization of the Yeast-Expressed SARS-CoV Recombinant Receptor-Binding Domain (RBD219-N1), a SARS Vaccine Candidate. J. Pharm. Sci. 2017, 106, 1961–1970. [Google Scholar] [CrossRef]
- Markets Recombinant Proteins Market by Product, Application, End User-Global Forecasts (2022–2026). Available online: https://www.marketsandmarkets.com/Market-Reports/recombinant-proteins-market-70095015.html (accessed on 8 August 2022).
- Haon, M.; Grisel, S.; Navarro, D.; Gruet, A.; Berrin, J.-G.; Bignon, C. Recombinant Protein Production Facility for Fungal Biomass-Degrading Enzymes Using the Yeast Pichia Pastoris. Front. Microbiol. 2015, 6, 1002. [Google Scholar] [CrossRef]
- Steindorff, A.S.; Serra, L.A.; Formighieri, E.F.; de Faria, F.P.; Poças-Fonseca, M.J.; de Almeida, J.R.M. Insights into the Lignocellulose-Degrading Enzyme System of Humicola Grisea Var. Thermoidea Based on Genome and Transcriptome Analysis. Microbiol. Spectr. 2021, 9, e01088–e01121. [Google Scholar] [CrossRef]
- Liu, W.; Xiang, H.; Zhang, T.; Pang, X.; Su, J.; Liu, H.; Ma, B.; Yu, L. Development of a New High-Cell Density Fermentation Strategy for Enhanced Production of a Fungus β-Glucosidase in Pichia Pastoris. Front. Microbiol 2020, 11, 1988. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, U.; Sohail, M.; Ghanemi, A. Cellulases: From Bioactivity to a Variety of Industrial Applications. Biomimetics 2021, 6, 44. [Google Scholar] [CrossRef]
- Gaber, Y.; Rashad, B.; Hussein, R.; Abdelgawad, M.; Ali, N.S.; Dishisha, T.; Várnai, A. Heterologous Expression of Lytic Polysaccharide Monooxygenases (LPMOs). Biotechnol. Adv. 2020, 43, 107583. [Google Scholar] [CrossRef]
- He, J.; Yu, B.; Zhang, K.; Ding, X.; Chen, D. Expression of Endo-1, 4-Beta-Xylanase from Trichoderma Reesei in Pichia Pastorisand Functional Characterization of the Produced Enzyme. BMC Biotechnol. 2009, 9, 56. [Google Scholar] [CrossRef]
- Michelin, M.; Polizeli, M.d.L.T.M.; Ruzene, D.S.; Silva, D.P.; Vicente, A.A.; Jorge, J.A.; Terenzi, H.F.; Teixeira, J.A. Xylanase and β-Xylosidase Production by Aspergillus Ochraceus: New Perspectives for the Application of Wheat Straw Autohydrolysis Liquor. Appl. Biochem. Biotechnol. 2012, 166, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Pagnonceli, J.; Rasbold, L.M.; Rocha, G.B.; Silva, J.L.C.; Kadowaki, M.K.; Simão, R.C.G.; Maller, A. Biotechnological Potential of an Exo-polygalacturonase of the New Strain Penicillium Janthinellum VI2R3M: Biochemical Characterization and Clarification of Fruit Juices. J. Appl. Microbiol. 2019, 127, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Sena, R.O.; Carneiro, C.; Moura, M.V.H.; Brêda, G.C.; Pinto, M.C.C.; Fé, L.X.S.G.M.; Fernandez-Lafuente, R.; Manoel, E.A.; Almeida, R.V.; Freire, D.M.G.; et al. Application of Rhizomucor Miehei Lipase-Displaying Pichia Pastoris Whole Cell for Biodiesel Production Using Agro-Industrial Residuals as Substrate. Int. J. Biol. Macromol. 2021, 189, 734–743. [Google Scholar] [CrossRef] [PubMed]
- García-Ortega, X.; Cámara, E.; Ferrer, P.; Albiol, J.; Montesinos-Seguí, J.L.; Valero, F. Rational Development of Bioprocess Engineering Strategies for Recombinant Protein Production in Pichia Pastoris (Komagataella Phaffii) Using the Methanol-Free GAP Promoter. Where Do We Stand? New Biotechnol. 2019, 53, 24–34. [Google Scholar] [CrossRef]
- Hill, P.; Benjamin, K.; Bhattacharjee, B.; Garcia, F.; Leng, J.; Liu, C.-L.; Murarka, A.; Pitera, D.; Rodriguez Porcel, E.M.; da Silva, I.; et al. Clean Manufacturing Powered by Biology: How Amyris Has Deployed Technology and Aims to Do It Better. J. Ind. Microbiol. Biotechnol. 2020, 47, 965–975. [Google Scholar] [CrossRef]
- Looser, V.; Bruhlmann, B.; Bumbak, F.; Stenger, C.; Costa, M.; Camattari, A.; Fotiadis, D.; Kovar, K. Cultivation Strategies to Enhance Productivity of Pichia Pastoris: A Review. Biotechnol. Adv. 2015, 33, 1177–1193. [Google Scholar] [CrossRef]
- Wehrs, M.; Tanjore, D.; Eng, T.; Lievense, J.; Pray, T.R.; Mukhopadhyay, A. Engineering Robust Production Microbes for Large-Scale Cultivation. Trends Microbiol. 2019, 27, 524–537. [Google Scholar] [CrossRef]
- Duman-Özdamar, Z.E.; Binay, B. Production of Industrial Enzymes via Pichia Pastoris as a Cell Factory in Bioreactor: Current Status and Future Aspects. Protein J. 2021, 40, 367–376. [Google Scholar] [CrossRef]
- Brooks, S.M.; Alper, H.S. Applications, Challenges, and Needs for Employing Synthetic Biology beyond the Lab. Nat. Commun. 2021, 12, 1390. [Google Scholar] [CrossRef]
- Crater, J.S.; Lievense, J.C. Scale-up of Industrial Microbial Processes. FEMS Microbiol. Lett. 2018, 365, fny138. [Google Scholar] [CrossRef]
- Safder, I.; Khan, S.; Islam, I.-u.; Ali, M.K.; Bibi, Z.; Waqas, M. Pichia Pastoris Expression System: A Potential Candidate to Express Protein in Industrial and Biopharmaceutical Domains. Biomed. Lett. 2018, 4, 1–14. [Google Scholar]
- Emenike, V.N.; Schenkendorf, R.; Krewer, U. Model-Based Optimization of Biopharmaceutical Manufacturing in Pichia Pastoris Based on Dynamic Flux Balance Analysis. Comput. Chem. Eng. 2018, 118, 1–13. [Google Scholar] [CrossRef]
- Azadi, S.; Mahboubi, A.; Naghdi, N.; Solaimanian, R.; Mortazavi, S.A. Evaluation of Sorbitol-Methanol Co-Feeding Strategy on Production of Recombinant Human Growth Hormone in Pichia Pastoris. Iran J. Pharm. Res. 2017, 16, 1555–1564. [Google Scholar] [PubMed]
- D’anjou, M.C.; Daugulis, A.J. A Rational Approach to Improving Productivity in RecombinantPichia Pastoris Fermentation. Biotechnol. Bioeng. 2001, 72, 1–11. [Google Scholar] [CrossRef]
- Moser, J.W.; Prielhofer, R.; Gerner, S.M.; Graf, A.B.; Wilson, I.B.H.; Mattanovich, D.; Dragosits, M. Implications of Evolutionary Engineering for Growth and Recombinant Protein Production in Methanol-Based Growth Media in the Yeast Pichia Pastoris. Microb. Cell Factories 2017, 16, 49. [Google Scholar] [CrossRef]
- Garcia-Ortega, X.; Adelantado, N.; Ferrer, P.; Montesinos, J.L.; Valero, F. A Step Forward to Improve Recombinant Protein Production in Pichia Pastoris : From Specific Growth Rate Effect on Protein Secretion to Carbon-Starving Conditions as Advanced Strategy. Process Biochem. 2016, 51, 681–691. [Google Scholar] [CrossRef]
- Garcia-Ortega, X.; Valero, F.; Montesinos-Seguí, J.L. Physiological State as Transferable Operating Criterion to Improve Recombinant Protein Production in Pichia Pastoris through Oxygen Limitation: Physiological State Control to Improve Protein Specific Productivity in Pichia Pastoris with Oxygen Limitation. J. Chem. Technol. Biotechnol 2017, 92, 2573–2582. [Google Scholar] [CrossRef]
- Rathore, A.S.; Mishra, S.; Nikita, S.; Priyanka, P. Bioprocess Control: Current Progress and Future Perspectives. Life 2021, 11, 557. [Google Scholar] [CrossRef]
- Saitua, F.; Torres, P.; Pérez-Correa, J.R.; Agosin, E. Dynamic Genome-Scale Metabolic Modeling of the Yeast Pichia Pastoris. BMC Syst. Biol. 2017, 11, 27. [Google Scholar] [CrossRef]
- Garrigós-Martínez, J.; Weninger, A.; Montesinos-Seguí, J.L.; Schmid, C.; Valero, F.; Rinnofner, C.; Glieder, A.; Garcia-Ortega, X. Scalable Production and Application of Pichia Pastoris Whole Cell Catalysts Expressing Human Cytochrome P450 2C9. Microb. Cell Factories 2021, 20, 90. [Google Scholar] [CrossRef]
- Nieto-Taype, M.A.; Garcia-Ortega, X.; Albiol, J.; Montesinos-Seguí, J.L.; Valero, F. Continuous Cultivation as a Tool Toward the Rational Bioprocess Development With Pichia Pastoris Cell Factory. Front. Bioeng. Biotechnol. 2020, 8, 632. [Google Scholar] [CrossRef] [PubMed]
- Gasset, A.; Garcia-Ortega, X.; Garrigós-Martínez, J.; Valero, F.; Montesinos-Seguí, J.L. Innovative Bioprocess Strategies Combining Physiological Control and Strain Engineering of Pichia Pastoris to Improve Recombinant Protein Production. Front. Bioeng. Biotechnol. 2022, 10, 818434. [Google Scholar] [CrossRef] [PubMed]
- Ponte, X.; Barrigón, J.M.; Maurer, M.; Mattanovich, D.; Valero, F.; Montesinos-Seguí, J.L. Towards Optimal Substrate Feeding for Heterologous Protein Production in Pichia Pastoris (Komagataella Spp) Fed-Batch Processes under PAOX1 Control: A Modeling Aided Approach: Optimal Feeding for Pichia Pastoris. J. Chem. Technol. Biotechnol. 2018, 93, 3208–3218. [Google Scholar] [CrossRef]
- Patra, P.; Das, M.; Kundu, P.; Ghosh, A. Recent Advances in Systems and Synthetic Biology Approaches for Developing Novel Cell-Factories in Non-Conventional Yeasts. Biotechnol. Adv. 2021, 47, 107695. [Google Scholar] [CrossRef]
- Berrios, J.; Flores, M.-O.; Díaz-Barrera, A.; Altamirano, C.; Martínez, I.; Cabrera, Z. A Comparative Study of Glycerol and Sorbitol as Co-Substrates in Methanol-Induced Cultures of Pichia Pastoris: Temperature Effect and Scale-up Simulation. J. Ind. Microbiol. Biotechnol. 2017, 44, 407–411. [Google Scholar] [CrossRef]
- Matthews, C.B.; Kuo, A.; Love, K.R.; Love, J.C. Development of a General Defined Medium for Pichia Pastoris. Biotechnol. Bioeng. 2018, 115, 103–113. [Google Scholar] [CrossRef]
- Liu, W.-C.; Inwood, S.; Gong, T.; Sharma, A.; Yu, L.-Y.; Zhu, P. Fed-Batch High-Cell-Density Fermentation Strategies for Pichia Pastoris Growth and Production. Crit. Rev. Biotechnol. 2019, 39, 258–271. [Google Scholar] [CrossRef]
- Nieto-Taype, M.A.; Garrigós-Martínez, J.; Sánchez-Farrando, M.; Valero, F.; Garcia-Ortega, X.; Montesinos-Seguí, J.L. Rationale-based Selection of Optimal Operating Strategies and Gene Dosage Impact on Recombinant Protein Production in Komagataella Phaffii (Pichia Pastoris). Microb. Biotechnol. 2020, 13, 315–327. [Google Scholar] [CrossRef]
- de Macedo Robert, J.; Garcia-Ortega, X.; Montesinos-Seguí, J.L.; Guimaraes Freire, D.M.; Valero, F. Continuous Operation, a Realistic Alternative to Fed-Batch Fermentation for the Production of Recombinant Lipase B from Candida Antarctica under the Constitutive Promoter PGK in Pichia Pastoris. Biochem. Eng. J. 2019, 147, 39–47. [Google Scholar] [CrossRef]
- Campbell, K.; Xia, J.; Nielsen, J. The Impact of Systems Biology on Bioprocessing. Trends Biotechnol. 2017, 35, 1156–1168. [Google Scholar] [CrossRef]
- Guirimand, G.; Kulagina, N.; Papon, N.; Hasunuma, T.; Courdavault, V. Innovative Tools and Strategies for Optimizing Yeast Cell Factories. Trends Biotechnol. 2021, 39, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Abt, V.; Barz, T.; Cruz-Bournazou, M.N.; Herwig, C.; Kroll, P.; Möller, J.; Pörtner, R.; Schenkendorf, R. Model-Based Tools for Optimal Experiments in Bioprocess Engineering. Curr. Opin. Chem. Eng. 2018, 22, 244–252. [Google Scholar] [CrossRef]
- Hong, M.S.; Velez-Suberbie, M.L.; Maloney, A.J.; Biedermann, A.; Love, K.R.; Love, J.C.; Mukhopadhyay, T.K.; Braatz, R.D. Macroscopic Modeling of Bioreactors for Recombinant Protein Producing Pichia Pastoris in Defined Medium. Biotechnol. Bioeng. 2021, 118, 1199–1212. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, H.; Luna, M.F.; Stosch, M.; Cruz Bournazou, M.N.; Polotti, G.; Morbidelli, M.; Butté, A.; Sokolov, M. Bioprocessing in the Digital Age: The Role of Process Models. Biotechnol. J. 2020, 15, 1900172. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).