Abstract
The present study aimed to characterize melanin pigment extracted from Gluconobacter oxydans FBFS 97. After 14 days of culture at 28 °C in GY (glucose and yeast extract) liquid-state medium, G. oxydans FBFS97 produce the maximum melanin, up to about 12–15 mg/L. The physicochemical characteristics of the extracted melanin showed an ability to dissolve in 1 mol/L NaOH or 1 mol/L KOH, and insolubility in water and most organic solvents, such as chloroform and petroleum ether. The extracted melanin was confirmed to be exact melanin by ultraviolet-visible spectrophotometry, Fourier-transform infrared spectroscopy, thin-layer chromatography, elemental analysis, and scanning electron microscopy. The UV-visible spectrum of G. oxydans FBFS97 exhibited a maximum absorption peak at 230 nm. Extracted melanin demonstrated significant free radical-scavenging activity by DPPH and ABTS methods. The IC50 value of the extracted melanin for scavenging 50% DPPH radicals was 36.94 μg/mL, and the IC50 value of antioxidant activity for ABTS was 4.06 μg/mL. Hence, G. oxydans FBFS97 has the potential to be a new candidate for melanin production.
1. Introduction
The origin of the word “melanin” is the Greek word melas, which means black. Melanins are a group of heterogeneous pigments that are synthesized widely in all living organisms, such as microorganisms, plants, and animals, through oxidative polymerization of different types of indolic and/or phenolic compounds [1,2,3]. Generally, melanin is hydrophobic and a negatively charged dark brown to black pigment, but several other colors have been observed, such as red and yellow [4]. Melanin can be classified according to its monomer units and synthesis process into pheomelanin, allomelanin, eumelanin and pyomelanin. Pheomelanin is produced as a result of oxidation of amino acid l-tyrosine and/or dihydroxyphenylalanine (l-dopa) in the presence of l-cysteine, generating red-yellow-colored molecules. Allomelanin is initially synthesized when one of the following compounds are oxidized: catechols, 4-hydroxyphenylacetic acid, di-hydroxynaphthalene (DHN), γ-glutaminyl-4-hydroxybenzene or tetra-hydroxynaphthalene, caffeic acid, and protocatechualdehyde. Eumelanin is commonly exhibited as a dark brown to black polymer that is formed through the oxidation of the amino acid l-tyrosine and/or l-dopa. Pyomelanin is a type of melanin derived from the oxidation of homogentisic acid (HGA) [5,6,7,8,9].
Melanin plays an important role in microorganisms, since it possesses a large variety of biological functions against chemical stress, such as oxidizing agents and heavy metals, high-temperature damage, and other biochemical stresses, e.g., reactive oxygen species produced by the exposure of solar UV radiation [10,11]. A wide array of biological activities of melanin produced by microorganisms as liver-protecting activity, antitumor, cytotoxic, antiviral, antioxidant, antivenin and anti-inflammatory activities were reported [12,13]. It also shows ability as a metal ion chelator and a vital role against antimicrobial drugs [14]. The role of melanin in humans not only defines skin color but also has an important role against UV radiation, and its deficiency leads to many abnormalities and diseases [15]. AIDS (acquired immunodeficiency syndrome) treatment displays the selective antiviral activity of synthetic soluble melanin against the human immunodeficiency virus [5,12]. Due to its unique chemical composition, melanin shows physicochemical characteristics that allow it to perform as cation exchangers, UV absorbers, amorphous semiconductors, and γ-ray and X-ray absorbers. An additional advantage of melanin is represented in the usage of water-soluble melanin in sunscreens, lenses, paints, solid plastic films, varnishes, and other surface protection formulations to provide greater UV protection. As bacterial strains are easily cultivated and economically durable, they exhibit great potential to produce different types of advanced biocompounds. Many genera of bacteria have been described to synthesize melanin, e.g., Bacillus, Shewanella, Rhizobium, Vibrio, Streptomyces and Aeromonas [11,12,15,16,17].
Lately, the production of melanin pigment by microorganisms has highly attracted attention as an environmentally benign and economical alternative to artificial production [5]. Gluconobacter oxydans is a well-known acetic acid bacterium of biotechnological importance that is exploited to synthesize different types of valuable compounds through a combination of chemical techniques and biocatalysis [18,19].
The purpose of this work was to characterize the melanin extracted from Gluconobacter oxydans FBFS 97 by using ultraviolet-visible absorption, FT-IR spectroscopy, etc. According to our knowledge, this is the first study on the extraction and characterization of melanin pigment from a strain belonging to the acetic acid bacteria group.
2. Materials and Methods
2.1. Pigment Production Conditions
A 1 mL freshly prepared GY (10% glucose and 1% yeast extract) culture suspension of G. oxydans FBFS 97 was inoculated in 200 mL GY in a 500 mL Erlenmeyer flask. After that, this liquid-state media was incubated at 28 °C in a rotary shaker at 200 rpm for 10–14 days [20].
2.2. Melanin Extraction
Melanin pigment was extracted from G. oxydans FBFS 97 bacterial liquid media according to the method of Sajjan et al. [21] with minor changes. Briefly, the 10–14 days grown cells of G. oxydans FBFS 97 bacterial strain were disrupted by using a Vibra-Cell ultrasonic processer in an ice bath. The disrupted broth was acidified with 1 N HCl to pH 2, and allowed to stand for one week at room temperature to precipitate the black pigment, and then was centrifuged for 10 min at 8000× g. The obtained black pigment pellet was boiled in 10 mL of 7 M HCl for 2 h at 100 °C, then collected by centrifugation at 8000× g for 15 min. The resulting pellet was washed three times with 15 mL of 0.1 N HCl, and then with deionized water five times to remove the acid. After that, this pellet was washed with chloroform, ethyl acetate, and ethanol three times to wash away lipids and other residues. Finally, the resultant purified melanin was dried in the air, then ground to a fine powder in a mortar and stored at −20 °C until testing.
2.3. Characterization of the Melanin Produced by G. oxydans FBFS 97
2.3.1. Biochemical and Solubility Tests of Melanin Extracted
The solubility of the extracted melanin pigment was tested with distilled hot/cold water and organic solvents such as ethanol, acetone, methanol, chloroform, hexane, ethyl acetate, DMSO (dimethyl sulfoxide), acetic acid, and petroleum ether. Also, precipitation in 1 mol/L HCl and 1% (w/v) FeCl3 was evaluated and reacted with a strong oxidizing agent, such as 30% H2O2.
2.3.2. Ultraviolet-Visible Spectroscopy (UV-Vis)
The extracted melanin powder was dissolved in a 0.01 mol/L NaOH solution. The ultraviolet-visible (UV-vis) spectrum of the resulting solution was recorded in the wavelength range of 200–800 nm using a UV-1601 UV-vis spectrophotometer (Shimadzu, Kyoto, Japan).
2.3.3. Fourier-Transform Infrared Spectroscopy
A Fourier transform infrared (FT-IR) spectrometer (Thermo Electron Corporation, Waltham, MA, USA) with a range from 500 to 4000 cm−1 was utilized to identify the functional groups and interpretation of the melanin structure formed by G. oxydans FBFS 97. The extracted melanin powder was mixed with KBr in an agate mortar and ground for a few seconds to break up the melanin and KBr lumps, then pressed into a disc under vacuum using a spectra Lab Pelletizer. The spectrum was recorded at room temperature and 500–4000 cm−1 with a resolution of 4 cm−1.
2.3.4. Elemental Composition Analysis
The percentage element compositions of C, H, N, and S in the extracted melanin pigment were determined with about 4 mg of sample weight using a Vario-Micro Cube elemental analyzer.
2.3.5. Thin-Layer Chromatography
Thin-layer chromatography (TLC) was carried out on silica gel TLC plates based on the method of Özdemir and Keleş, 2018 [22]. The extracted powdered melanin was dissolved in 0.1 M NaOH solution. The prepared solvent system was comprised of n-butanol: acetic acid: deionized water (70:20:10). It was performed on silica gel layers. Then, the retention factor (Rƒ) value was calculated based on the following equation:
2.3.6. Scanning Electron Microscopy
The morphology of the extracted melanin powder was examined using scanning electron microscopy (SEM) (SU 1510, Hitachi Corp., Tokyo, Japan). The microcapsule powder was mounted on a specimen holder using double scotch tape and coated with a thin layer of gold under vacuum. Finally, the sputtered coated samples were scanned with an accelerating beam voltage of 5 kV.
2.3.7. Antioxidant Activity of the Extracted Melanin
The antioxidant activity of the extracted melanin was determined using a DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical-scavenging assay following the previously detailed method of Anissi et al. [23] with slight modifications. Briefly, the 0.1 mmol/L of DPPH solution was prepared and left in the refrigerator for two hours. A DPPH 5 mL solution was mixed with 0.1 mL of sample. Then, the reaction mixture was shaken vigorously and incubated in the darkness at room temperature. The absorbance of the resulting solution was measured at 515 nm. The decrease absorbance of the DPPH solution indicated to the DPPH radical-scavenging activity increasing. Finally, the percentage radical-scavenging activity was calculated using the following equation:
Scavenging (%) = (A control − (A sample − A blank))/A control × 100
The tests were performed in triplicate and the extract concentration providing 50% of antioxidant activity (IC50) was obtained through plotting the antioxidant activity against the extracted melanin concentration.
The 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cation-scavenging activity of the extracted melanin from G. oxydans FBFS 97 strain was determined according to the procedure of Re et al. [24]. Briefly, the 2.45 mM of potassium persulfate and 7 mM of ABTS solution were mixed and placed for 16 h at room temperature under dark conditions. ABTS solution was diluted with absolute ethanol until the absorbance was 0.70 ± 0.02 at 734 nm. A series of 0.2 mL of samples were incubated with 3 mL of ABTS solution in the darkness for 60 min at room temperature, and the absorbance was measured at 734 nm (A1). The ABTS solution was replaced with an equal volume of absolute ethanol and then the absorbance was recorded (A2). The same amount of distilled water was utilized instead of the melanin solution, and the absorbance was measured (A0). The ABTS radical-scavenging rate of the extracted melanin was calculated using the following equation:
ABTS scavenging activity (%) = (1 − (A1 − A2)/A0) × 100
3. Results and Discussion
3.1. The Characterization of Extracted Melanin Produced by G. oxydans FBFS97
3.1.1. Preliminary Properties of the Extracted Melanin
The maximum yield of melanin was obtained within 14 days of the incubation time, which was estimated at 12–15 mg/L (Figure 1). The solubility tests of the extracted melanin from G. oxydans FBFS 97 are shown in Table 1. The extracted melanin was black, as shown in Figure 2, and insoluble in water either hot or cold, ethanol, acetone, chloroform, hexane, ethyl acetate, acetic acid, petroleum ether, and DMSO. It was also sparingly soluble in methanol. However, the extracted melanin revealed solubility in 1 mol/L NaOH and 1 mol/L KOH. It showed precipitation in 1 mol/L HCl and displayed a positive reaction for polyphenols with 1% (w/v) FeCl3 by producing a flocculent brown precipitate. Also, the extracted melanin of G. oxydans FBFS 97 was positive for decolorization by 30% hydrogen peroxide. All these characteristics resemble those displayed in the typical melanin pigment. The obtained results were almost similar to those of the melanin extracted from Spissiomyces endophytica SDBR-CMU319 and synthetic dopa melanin [25] (Figure 3).
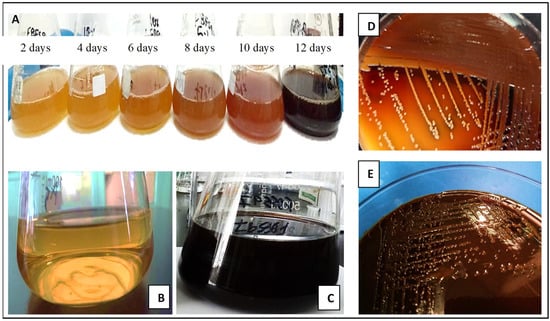
Figure 1.
Melanin production by G. oxydans FBFS 97 in glucose yeast extract broth at different elapsed times (A), GY broth was used as the negative control (B), culture broth after 14 days of incubation (C), melanin production on GYC agar plates after 10 days (D) and after 14 days (E) of incubation.

Table 1.
Physical and chemical properties of the extracted melanin produced by G. oxydans FBFS 97.

Figure 2.
Melanin pigment of G. oxydans FBFS 97 during the extraction process (A), purified melanin powder (B,C).

Figure 3.
The solubility tests of extracted melanin from G. oxydans FBFS 97. A brown precipitate formed during the FeCl3 test (A), solubility test in 1 mol/L NaOH (B), solubility test in 1 mol/L KOH (C), solubility in MeOH (D), H2O2 decolorization test (E), solubility in DMSO (F).
3.1.2. Ultraviolet-Visible Spectroscopy (UV-Vis)
The maximum value of the ultraviolet-visible absorption spectrum of melanin extracted from G. oxydans FBFS 97 strain was observed at 230 nm in the ultraviolet region, but it decreased progressively as the wavelength increased toward the visible region, as represented in Figure 4. The spectra of the G. oxydans FBFS 97 extracted melanin did not show other absorption peaks between 230 and 800 nm regions. This result is similar to the previous study by Deepthi and Rosamma (2014), which revealed that the maximum absorbance of the melanin pigment produced by Streptomyces bikiniensis M8 was observed at 230 nm with decreased absorbance towards the visible region as a result of the presence of the very complex conjugated structure, which is the characteristic property of melanin [26]. All melanins exhibit higher absorption in the UV region because this is characteristic of the absorption profile of aromatic compounds, such as melanin [27].
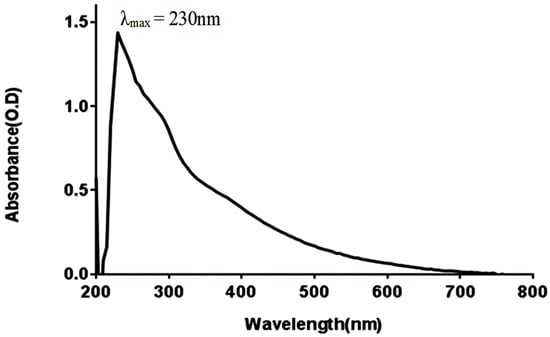
Figure 4.
UV-visible absorbance spectrum of melanin produced by G. oxydans FBFS 97.
3.1.3. Fourier-Transform Infrared Spectroscopy
The FT-IR spectrum was used to confirm that the extracted melanin from G. oxydans FBFS 97 was melanin. The appearance of broad absorbance at 3278.18 cm−1, which can be attributed to the polymeric groups OH or NH stretching vibrations, whereas an absorption peak at 2928.07 cm−1 that exhibited a weak absorption band was assigned to the stretching vibration of the aliphatic C-H group [28,29]. The peak that appeared in the region 1500–1650 cm−1 was assigned aromatic ring C=C stretching. The spectrum absorbance at 1529 cm−1 appeared due to the presence of NH bending vibration. The absorption band at 1073.27 cm−1 was assigned to CO groups of ester, acid, and phenol. The peaks that appeared in the region 1400–1500 cm−1 were attributed to aliphatic C-H groups in the melanin pigment, at 1451.68 cm−1 (CH2-CH3) bending characteristic of melanin pigment. A wagging vibration peak of NH at 697.93 cm−1 weak bands below 700 cm−1 was attributed to alkene C-H substitution in the melanin pigment [21] (Figure 5).
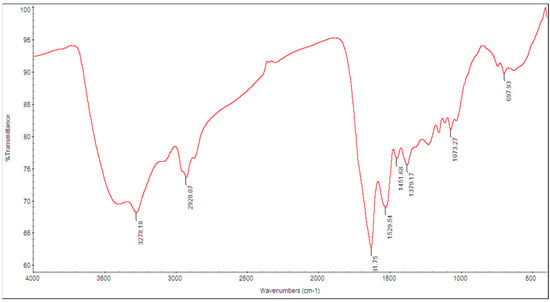
Figure 5.
FT-IR spectrum of the melanin produced by G. oxydans FBFS 97.
3.1.4. Elemental Composition Analysis
The extracted melanin obtained from G. oxydans FBFS 97 was composed of C, H, N, O, and S with a content percentage of 53.81, 5.13, 5.95, 34.38, and 0.73 respectively Table 2. In addition, the empirical formula of C: N:H of the extracted melanin was (11:12:1). The obtained results obviously showed that the content of elements was different from those of other melanins previously reported, which suggests that the content of elements in different melanins varying might be due to the change in media compositions [30]. Notably, the extracted melanin displayed a low content of nitrogen compared with its high content of carbon, which is the characteristic property of dopa melanin [25]. In general, melanins synthesized from the DOPA-pathway are divided into eumelanin and pheomelanin. Eumelanin contained 5.1−9% nitrogen and 0−1% sulfur, while pheomelanin contained 8−11% nitrogen and 9−12% sulfur [25]. Thus, the extracted melanin in our study could be classified as a dopa-melanin. These results also indicated that the extracted melanin from G. oxydans FBFS 97 contains more carboxylate and aliphatic groups.

Table 2.
Elemental composition analysis of the melanin produced by G. oxydans FBFS 97.
3.1.5. Thin-Layer Chromatography
Thin-layer chromatography (TLC) was performed to detect the purity of extracted melanin from G. oxydans FBFS 97 (Figure 6). The obtained results of TLC analysis revealed a violet-colored spot and the value of Rƒ calculated was 0.68, which was ascribed to the melanin pigment. This result is similar to that of Kiran et al., 2017 [31]. A study by Madhusudhan et al., 2014, carried out to detect the synthesis potential of both insoluble and soluble melanins by Streptomyces lusitanus DMZ-3 strain showed that the results of TLC using the different proportions of organic solvents (such as chloroform, hexane, butanol, acetic acid, and methanol) revealed that the separated bands were equal to the Rƒ value of standard melanin at 0.68 [12].
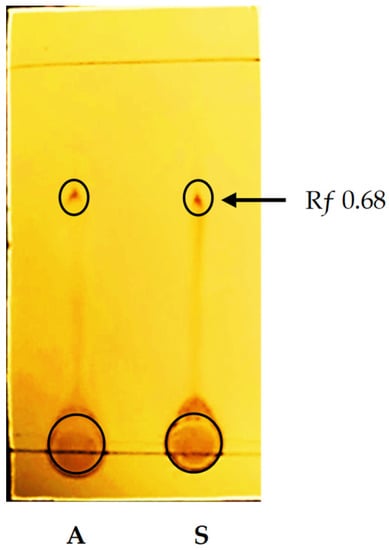
Figure 6.
TLC image for the melanin from G. oxydans FBFS 97. Extracted melanin (A), and the reference standard (S).
3.1.6. Scanning Electron Microscopy
This is a powerful method for the morphological characterization and particle size distribution of different types of melanin [9]. The obtained results of the present study displayed that the melanin synthesized by G. oxydans FBFS 97 was appeared in the aggregates of small spherical by using scanning electron microscopy (SEM) imaging (Figure 7). These results were similar to previous studies of melanin synthesized by several microorganisms such as the extracted melanin from Streptomyces glaucescens NEAE-H, which appeared in small spheres using SEM imaging [5], as well as similar to the natural (Sepia officinalis) melanin, which has a well-known defined spherical structure. The extracted melanin from FBFS 97 was structurally similar to the other microbial melanins, such as the melanin produced by Mycosphaerella fijiensis fungi, which consists of a spherical granular body arrangement in clusters with different sizes and aggregations [32]. Moreover, the morphological characterization of the purified melanin pigment synthesized by E. coli (BL21) using the electron microscopic image analysis showed that the obtained melanin is formed by aggregation of numerous spherical granules with unusual size distributions [33].
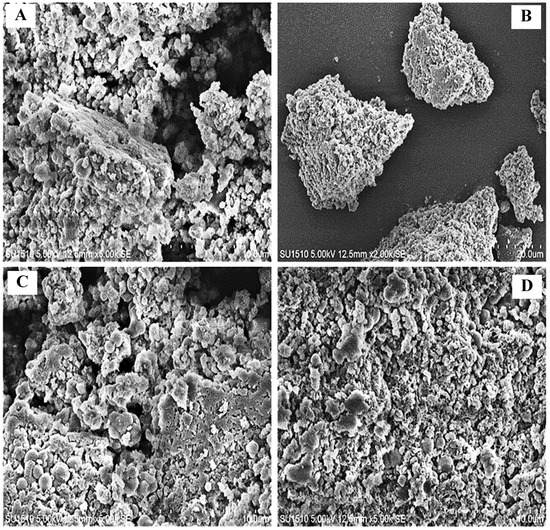
Figure 7.
SEM images of the extracted melanin produced by G. oxydans FBFS 97. The aggregates of small spheres of the extracted melanin by G. oxydans FBFS 97 (A–D).
3.2. Antioxidant Activities of the Extracted Melanin Produced by G. oxydans FBFS 97
3.2.1. DPPH Radical-Scavenging Activity
The properties of antioxidant activity, especially radical activities, are significant because of the deleterious role of free radicals in biological systems. The excessive accumulation of free radicals accelerated lipids oxidation and caused severe damages on the adjacent biological macromolecules. DPPH is considered the simplest and the most accurate method to estimate the radical scavenging capacity of antioxidants in all methods [34]. The antioxidant compounds in the sample react with DPPH to transform it to 1,1-dipheyl-2-(2,4,6-trinitropheyl) hydrazine due to the hydrogen donating ability. The scavenging potential of the antioxidant components is evaluated depending on their ability for discoloration. The DPPH radical scavenging activity of the melanin extracted from G. oxydans FBFS 97 increased with increasing concentrations and exhibited a dose–response relationship. At a concentration of 1.56 µg/mL, the DPPH-scavenging rate of extracted melanin was 1.2 ± 0.082%, which increased with increasing concentration to 2.86 ± 0.20% at 3.13 µg/mL, and reached 7.58 ± 0.53% at 6.25 µg/mL (Figure 8). The half-maximal inhibitory concentration (IC50) of the extracted melanin from G. oxydans FBFS 97, which effects scavenging of 50% DPPH free radicals, was calculated as 36.94 µg/mL (Table 3).
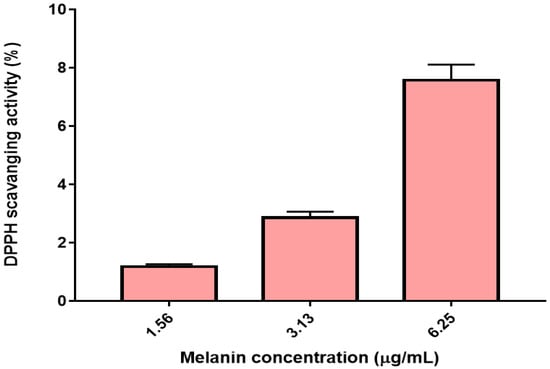
Figure 8.
The scavenging ability of the melanin produced by G. oxydans FBFS 97 on DPPH radicals.

Table 3.
The IC50 value was defined as the concentration of G. oxydans FBFS97 melanin.
3.2.2. ABTS Radical-Scavenging Activity
The scavenging ability of the extracted melanin from FBFS 97 on ABTS radical was determined and is shown in Figure 9. The scavenging effects of G. oxydans FBFS 97 extracted melanin at a concentration of 0.20 µg/mL was 4.16 ± 0.29% which increased in a concentration-dependent manner to 6.71 ± 0.47% at a concentration of 0.39 µg/mL, and 11.14 ± 0.78% at a concentration of 0.78 µg/mL (Figure 8). Then, the IC50 value of antioxidant activity for ABTS of the melanin from G. oxydans FBFS 97 was determined as 4.06 µg/mL (Table 3). Another study by El-Naggar and El-Ewasy 2017, revealed that the purified melanin pigment of Streptomyces glaucescens strain NEAE-H showed good antioxidant activity since 100 μg/mL melanin exhibited a percentage inhibition of 57.2% radical scavenging activity, which was comparable to that of standard antioxidant ascorbic acid showing activity of 89.6% [5]. Melanin pigment interacts with free radicals and other reactive species readily due to the presence of unpaired electrons in its molecules and acts as an antioxidant, suggesting its use as a raw cosmetic material to minimize toxin-induced tissue destruction. Melanin interacts with free radicals via the simple one-electron transfer processes [35].
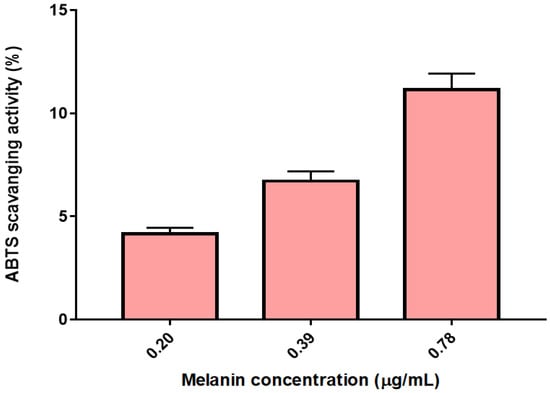
Figure 9.
The scavenging ability of the extracted melanin produced by G. oxydans FBFS 97 on ABTS.
4. Conclusions
Melanin pigment was successfully extracted and characterized from G. oxydans FBFS 97. To our knowledge, this study represents the first study on the extraction and characterization of melanin pigment from a strain belonging to the acetic acid bacteria group. The extracted melanin showed the physicochemical characteristics of typical melanin. Furthermore, the extracted melanin possessed antioxidant activities, indicating that it may be exploited in biotechnological applications. Thus, G. oxydans FBFS 97 can be considered a promising candidate for bacterial melanin production.
Author Contributions
A.E.N. performed the experiments; A.E.N. wrote the manuscript; A.E.N., F.C., and N.S.A.-B. revised the manuscript; and F.C. supervised. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors would like to thank Fouad Hayel Saeed for his support and encouragement over the past few years.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Płonka, P.; Grabacka, M. Melanin synthesis in microorganisms: Biotechnological and medical aspects. Acta Biochim. Polonica. 2006, 53, 3. [Google Scholar] [CrossRef]
- Tarangini, K.; Mishra, S. Production, characterization and analysis of melanin from isolated marine Pseudomonas sp. using vegetable waste. Res. J. Eng. Sci. 2013, 2278, 9472. [Google Scholar]
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014. [Google Scholar] [CrossRef]
- Toledo, A.V.; Franco, M.E.E.; Lopez, S.M.Y.; Troncozo, M.I.; Saparrat, M.C.N.; Balatti, P.A. Melanins in fungi: Types, localization and putative biological roles. Physiol. Mol. Plant Pathol. 2017, 99, 2–6. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; El-Ewasy, S.M. Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glaucescens NEAE-H. Sci. Rep. 2017, 7, 1–19. [Google Scholar] [CrossRef]
- Lindgren, J.; Moyer, A.; Schweitzer, M.H.; Sjövall, P.; Uvdal, P.; Nilsson, D.E.; Heimdal, J.; Engdahl, A.; Gren, J.A.; Schultz, B.P. Interpreting melanin-based coloration through deep time: A critical review. Proc. R. Soc. B. Biol. Sci. 2015, 282, 20150614. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Cai, X.; Wang, P.; Guo, Y.; Liu, X.; Li, B.; Wang, X. Biofilm formation and heat stress induce pyomelanin production in deep-sea Pseudoalteromonas sp. SM9913. Front. Microbiol. 2017, 8, 1822. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. New insight into melanin for food packaging and biotechnology applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 4629–4655. [Google Scholar] [CrossRef] [PubMed]
- Pralea, I.-E.; Moldovan, R.-C.; Petrache, A.-M.; Ilieș, M.; Hegheș, S.-C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [PubMed]
- Allam, N.G.; Abd El-Zaher, E.H. Protective role of Aspergillus fumigatus melanin against ultraviolet (UV) irradiation and Bjerkandera adusta melanin as a candidate vaccine against systemic candidiasis. Afr. J. Biotechnol. 2012, 11, 6566–6577. [Google Scholar]
- Wang, L.-F.; Rhim, J.-W. Isolation and characterization of melanin from black garlic and sepia ink. LWT 2019, 99, 17–23. [Google Scholar] [CrossRef]
- Madhusudhan, D.N.; Mazhari, B.B.Z.; Dastager, S.G.; Agsar, D. Production and cytotoxicity of extracellular insoluble and droplets of soluble melanin by Streptomyces lusitanus DMZ-3. BioMed. Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Pombeiro-Sponchiado, S.R.; Sousa, G.S.; Andrade, J.C.; Lisboa, H.F.; Gonçalves, R.C. Production of melanin pigment by fungi and its biotechnological applications. Melanin 2017, 1, 47–75. [Google Scholar]
- Abbas, M.; D’Amico, F.; Morresi, L.; Pinto, N.; Ficcadenti, M.; Natali, R.; Ottaviano, L.; Passacantando, M.; Cuccioloni, M.; Angeletti, M.; et al. Structural, electrical, electronic and optical properties of melanin films. Eur. Phys. J. E 2009, 28, 285–291. [Google Scholar] [CrossRef]
- Lambert, M.W.; Maddukuri, S.; Karanfilian, K.M.; Elias, M.L.; Lambert, W.C. The physiology of melanin deposition in health and disease. Clin. Dermatol. 2019, 37, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiao, Y.; Chai, B.; Qiu, C.; Chen, X. Identification and molecular characterization of the homogentisate pathway responsible for pyomelanin production, the major melanin constituents in Aeromonas media WS. PLoS ONE 2015, 10, e0120923. [Google Scholar] [CrossRef]
- Ye, M.; Guo, G.-Y.; Lu, Y.; Song, S.; Wang, H.-Y.; Yang, L. Purification, structure and anti-radiation activity of melanin from Lachnum YM404. Int. J. Biol. Macromol. 2014, 63, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zeng, W.; Du, G.; Chen, J.; Zhou, J. Identification of NAD-dependent xylitol dehydrogenase from Gluconobacter oxydans WSH-003. ACS Omega 2019, 4, 15074–15080. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.A.R.; Oliveira, S.S.d.S.; Lima, S.F.; do Nascimento, R.P.; Baptista, A.R.d.S.; Fiaux, S.B. The industrial versatility of Gluconobacter oxydans: Current applications and future perspectives. World J. Microbiol. Biotechnol. 2022, 38, 134. [Google Scholar] [CrossRef]
- Noman, A.E.; Al-Barha, N.S.; Sharaf, A.-A.M.; Al-Maqtari, Q.A.; Mohedein, A.; Mohammed, H.H.H.; Chen, F. A novel strain of acetic acid bacteria Gluconobacter oxydans FBFS97 involved in riboflavin production. Sci. Rep. 2020, 10, 13527. [Google Scholar] [CrossRef] [PubMed]
- Sajjan, S.; Kulkarni, G.; Yaligara, V.; Lee, K.; Karegoudar, T.B. Purification and physiochemical characterization of melanin pigment from Klebsiella sp. GSK. J. Microbiol. 2010, 20, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Keles, Y.; Özdemir, Ö. Extraction, purification, antioxidant properties and stability conditions of phytomelanin pigment on the sunflower seeds. Int. J. Second. Metab. 2018, 5, 140–148. [Google Scholar] [CrossRef]
- Anissi, J.; El Hassouni, M.; Ouardaoui, A.; Sendide, K. A comparative study of the antioxidant scavenging activity of green tea, black tea and coffee extracts: A kinetic approach. Food Chem. 2014, 150, 438–447. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Watanabe, B.; Matsui, K.; Lumyong, S. Characterization of melanin and optimal conditions for pigment production by an endophytic fungus, Spissiomyces endophytica SDBR-CMU319. PLoS ONE 2019, 14, e0222187. [Google Scholar] [CrossRef]
- Deepthi, A.; Rosamma, P. Actinomycete Isolates from Arabian Sea and Bay of Bengal: Biochemical, Molecular and Functional Characterization. Ph.D. Thesis, Cochin University of Science And Technology, Kochi, India, 2014. [Google Scholar]
- Liu, Q.; Xiao, J.; Liu, B.; Zhuang, Y.; Sun, L. Study on the preparation and chemical structure characterization of melanin from Boletus griseus. Int. J. Mol. Sci. 2018, 19, 3736. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Kumar, C.; Sahu, N.; Narender Reddy, G.; Prasad, R.B.N.; Nagesh, N.; Kamal, A. Production of melanin pigment from Pseudomonas stutzeri isolated from red seaweed H ypnea musciformis. Lett. Appl. Microbiol. 2013, 57, 295–302. [Google Scholar] [CrossRef]
- Seelam, S.D.; Agsar, D.; Shetty, P.R.; Vemireddy, S.; Reddy, K.M.; Umesh, M.; Rajitha, C. Characterization and photoprotective potentiality of lime dwelling Pseudomonas mediated melanin as sunscreen agent against UV-B radiations. J. Photochem. Photobiol. B Biol. 2021, 216, 112126. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, Y.; Tang, B. Physicochemical properties and biological activities of melanin extracted from sunflower testae. Food Sci. Technol. Res. 2018, 24, 1029–1038. [Google Scholar] [CrossRef]
- Kiran, G.S.; Jackson, S.A.; Priyadharsini, S.; Dobson, A.D.; Selvin, J. Synthesis of Nm-PHB (nanomelanin-polyhydroxy butyrate) nanocomposite film and its protective effect against biofilm-forming multi drug resistant Staphylococcus aureus. Sci. Res. 2017, 7, 9167. [Google Scholar] [CrossRef]
- Beltran-Garcia, M.J.; Prado, F.M.; Oliveira, M.S.; Ortiz-Mendoza, D.; Scalfo, A.C.; Pessoa, A., Jr.; Medeiros, M.H.; White, J.F.; Di Mascio, P. Singlet molecular oxygen generation by light-activated DHN-melanin of the fungal pathogen Mycosphaerella fijiensis in black Sigatoka disease of bananas. PLoS ONE 2014, 9, e91616. [Google Scholar] [CrossRef]
- Amin, S.; Rastogi, R.P.; Sonani, R.R.; Ray, A.; Sharma, R.; Madamwar, D. Bioproduction and characterization of extracellular melanin-like pigment from industrially polluted metagenomic library equipped Escherichia coli. Sci. Total Environ. 2018, 635, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Peksel, A.; Arisan-Atac, I.; Yanardag, R. Evaluation of antioxidant and antiacetylcholinesterase activities of the extracts of Pistacia atlantica Desf. Leaves. J. Food Biochem. 2010, 34, 451–476. [Google Scholar] [CrossRef]
- Różanowska, M.; Sarna, T.; Land, E.J.; Truscott, T.G. Free radical scavenging properties of melanin: Interaction of eu-and pheo-melanin models with reducing and oxidising radicals. Free Radic. Biol. Med. 1999, 26, 518–525. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).