Abstract
There are two main fermentations associated with the vinification process. Alcoholic fermentation (AF), which is conducted by yeasts and malolactic fermentation (MLF), which takes place as a result of the metabolic activity of lactic acid bacteria (LAB) of the genera Oenococcus, former-Lactobacillus, Pediococcus and Leuconostoc. MLF is defined as the biotransformation of L-malic acid to L-lactic acid and carbon dioxide and in addition to deacidification, contributes significantly to microbial stability and often to the improvement of the sensory profile of wines. Therefore, the abiotic and biotic factors that affect MLF, along with its correlation with quality characteristics, has been in the epicenter of intensive research. In addition, practical issues that accompany MLF have also been considered and adequately assessed. The aim of the present review was to explore and critically discuss MLF from both theoretical and practical perspectives.
1. Introduction
In winemaking, alcoholic fermentation (AF) is traditionally considered as the key determinant. Wine yeasts, through their primary and secondary metabolism, direct the sensory profile of the final product. Malolactic fermentation (MLF), a secondary fermentation that usually follows AF, seems to be equally important, especially in the case of red wines and specific wines, such as Chardonnay [1]. Malolactic fermentation, namely the bioconversion of L-malic acid to L-lactic acid and carbon dioxide, is performed by lactic acid bacteria (LAB) of the genera Oenococcus, former-Lactobacillus, Pediococcus, and Leuconostoc and results in significant physicochemical and organoleptic modifications. Substitution of the dicarboxylic L-malic acid, which is characterized by a harsh taste, with the milder monocarboxylic L-lactic acid, results in deacidification of the wine with concomitant modification of its gustatory and olfactory perception [2]. In addition, removal of L-malic acid, a potential carbon source for some spoilage yeasts [3], enhances the stability of the final product. Finally, a series of modifications are also likely to take place depending on the grape cultivar, the metabolic capacity of the strain(s) driving MLF, and technological parameters [4,5].
The aforementioned reasons triggered intensive research on the nature of MLF, through the elucidation of the genetic and transcriptional organization of MLF, the biotic and abiotic parameters that affect MLF as well as the correlation between growth of malolactic bacteria with wine quality characteristics. At the same time, a number of practical considerations arose, mainly regarding the inoculation protocol and more specifically the temporal relationship between AF and MLF and the compatibility between the microbial strains employed. The recent technological and conceptual advances have improved our knowledge and understanding of the physiology of malolactic bacteria, have provided valuable insights, and have enabled evaluation of their effect beyond the narrow limits of MLF. The aim of the present review was to collect, connect, and comprehensively present all available data regarding the theoretical advances and practical considerations of MLF.
2. Genetic and Transcriptomic Organization of MLF
Malolactic fermentation consists of three steps:
- I.
- L-malate import to the cell,
- II.
- L-malate decarboxylation to L-lactate, and
- III.
- L-lactate release to the growth environment.
In Oenococcus oeni, importing of the L-malate into the cell is driven by a concentration gradient and takes place through a L-malateH- uniport, but only at pH values 3.0–5.6 and L-malate concentrations below 1 mM. At L-malate concentrations above 1 mM, passive diffusion may also take place [6]. Other mechanisms for L-malate transport have also been described, e.g., the malate/lactate antiporter in Lactococcus lactis [7] and the L-malate:proton symport of Lactiplantibacillus plantarum [8]. Once inside, L-malate may be decarboxylated to L-lactate or converted to pyruvate directly or through oxaloacetate, according to the cell needs and the capacity of the strain (Figure 1).
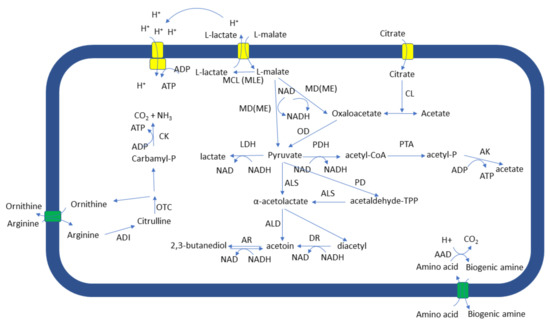
Figure 1.
Pathways of malate, citrate, and arginine metabolism as well as formation of biogenic amines by LAB. AAD: amino acid decarboxylase; ADI: arginine deiminase; AK: acetate kinase, ALD: α-acetolactate decarboxylase, ALS: α-acetolactate synthase, AR: acetoin reductase; CK: carbamate kinase; CL: citrate lyase; DR: diacetyl reductase; LDH: lactate dehydrogenase; MCL (MLE): malate carboxy lyase (malolactic enzyme); MD(ME): malate dehydrogenase (malic enzyme); OD: oxaloacetate decarboxylase; OTC: ornithine transcarboxylase; PD: pyruvate decarboxylase; PDH: pyruvate dehydrogenase; PTA: phosphotransacetylase.
The fate of L-malate is directed by the energetic needs of the cell. Conversion to pyruvate is performed with NAD+ or NADP+ as cofactors. Pyruvate may be further used in catabolic or biosynthetic reactions. Indeed, Qi et al. [9] reported pyruvate accumulation under acid stress and increased flux towards acetyl phosphate, acetoin, and 2,3-butanediol. On the other hand, decarboxylation to lactate consumes a proton; in O. oeni, lactate is released to the growth environment through passive diffusion. This proton translocation across the cell membrane generates or, in the case of wine, sustains the pH difference (ΔpH) as well as the electrical potential (ΔΨ) across the cell membrane. Thus, proton motive force is also sustained, and used by membrane ATPases to generate energy in the form of ATP [10]. Conversion of L-malate to L-lactate is facilitated by acid stress and the flux towards pyruvate seems decreased; however, L-lactate dehydrogenase activity seems to complement the pyruvate pool [9].
The genes that encode for the enzymes that are necessary for MLF are located in the mle operon. This operon consists of three genes. Genes mleA and mleP are 1626 bp and 945 bp long and encode the malolactic enzyme and the malate uniporter, respectively. They are transcribed in one bicistronic message. The gene mleR is transcribed in the opposite direction and encodes a positive regulator protein of the LysR-type, the expression of which, at least in Lc. lactis, is necessary for MLF induction [11,12].
The effect of vinification parameters, namely pH, ethanol, and SO2, on the transcription of the mle operon, has been studied to some extent. In O. oeni, increase of ethanol concentration resulted in upregulation of mleA, mleP, and mleR [13,14]. Lowering of the pH value also resulted in mleP upregulation, but only in the presence of L-malate [15]. Presence of L-malate was also necessary for mleP upregulation during rehydration of the starter before inoculation [16]. As far as the effect of SO2 was concerned, upregulation of mleA and mleP was reported in CCOo medium supplemented with 5 mg/L SO2, but only after 4 h growth at 22 °C and not after 30 min of growth [17]. Similarly, no regulation was observed when the medium was supplemented with 10 mg/L of SO2 [17]. Regarding Lp. plantarum, Miller et al. [18] reported that low pH positively affected the transcription of mleA, while ethanol affected it negatively; on the other hand, Binati et al. [19] reported no differential transcription of the genes associated with MLF during growth in synthetic grape juice medium at 25 °C for 72 h under different conditions of aeration and nitrogen availability.
3. Biotic and Abiotic Parameters Affecting MLF
A series of biotic and abiotic factors may interfere with MLF. These include the pH value, temperature, grapevine cultivar, SO2, organic acids, and the interactions with yeasts. Their individual as well as their combined effect on MLF has been extensively assessed. In the majority of the cases, they affect MLF indirectly, by affecting growth of the LAB that perform it. However, the susceptibility to these factors is a strain-dependent characteristic. Therefore, generalization to species level may be inaccurate or even misleading. In the next paragraphs, the effect that these factors may have on MLF is discussed.
3.1. pH Value
Most wines are characterized by pH values ranging between 3 and 3.8. Therefore, the environment in which LAB will carry out MLF is highly protonated. Given that the cell membrane is highly impermeable to protons, this gradient across the membrane is utilized for energy production [20]. However, organic acids naturally present in grape juice or produced through yeast and LAB catabolic activities, may also be protonated, depending on their pKa value. Undissociated organic acids become more lipophilic and may diffuse through the cell membrane, facilitated by ethanol presence [21]. Once inside the cell, the organic acid is dissociated, triggering acidification of the cytoplasm and accumulation of the anion. Such a condition may be lethal, unless properly addressed by the cell.
In general, at pH values below 3.5, MLF is performed by O. oeni strains while at values above 3.5, other lactobacilli, such as Lp. plantarum or Pediococcus parvulus strains may contribute [22,23,24,25]. Apart from the initial pH value, the evolution of pH value during MLF is equally important and should be taken into consideration for effective MLF. However, this is not adequately studied and the evolution of the pH value during MLF is characterized as rather unpredictable [26].
3.2. Temperature
Temperature affects MLF by controlling microbial growth and the antimicrobial potential of ethanol. Regarding microbial growth, decrease of temperature results in prolongation of lag phase and reduction of specific growth rate and biomass production [27]. On the other hand, increase of temperature enhances ethanol toxicity. As a result, the optimum temperature for MLF seems to range between 15 and 18 °C, depending on the capacity of the microbial strain that performs it [23,28]. However, MLF may also take place at higher temperatures; the impact on the sensorial outcome seems to be dependent upon the temporal relationship between AF and MFL, must composition, and the capacity of each strain [29,30]. More specifically, Sereni et al. [29] reported that the wines produced at 15 and 21 °C presented significant sensorial differences but only when MLF followed AF and not when both fermentations took place concurrently. The wine fermented at the lower temperature was characterized by a greater number of aroma compounds, including ethyl esters, 1-hexanol, and gamma-terpinene. On the contrary, the wine fermented at higher temperature was characterized by diethyl succinate, 2-methylbutanoic acid, isobutyric acid, isopentyl hexanoate, and phenethyl alcohol [29]. Guzzon et al. [30] provided an alternative insight, presenting that even in concurrent AF and MLF, the sensorial outcome may be different under the influence of must composition and microbial strain metabolic capacity.
3.3. Grapevine Cultivar
The grapevine cultivar may affect MLF through the effect that phenolic compounds and organic acids may have on LAB growth. Grape and wine polyphenolic content are largely dependent on grapevine variety and viticultural conditions [31,32,33,34,35,36,37,38,39,40,41,42]. The effect of hydroxybenzoic acids and esters (e.g., 4-hydroxybenzoic acid, ellagic acid, ethylgallate, gallic acid, methylgallate, syringic acid, vanillic acid), hydroxycinnamic acids (e.g., p-coumaric acid, caffeic acid, ferulic acid, sinapic acid), phenolic alcohols (e.g., tryptophol, tyrosol), stilbenes (e.g., ε-viniferin, ampelopsin A, cis-resveratrol, trans-resveratrol, trans-resveratrol-3-O-glucoside, r-viniferin, r2-viniferin), flavan-3-ols (e.g., (+)-catechin, (−)-epicatechin), flavonols (e.g., isorhamnetin, kaempferol, morin, myricetin, quercetin) may have on LAB of enological significance, namely P. pentosaceus, P. parvulus, Lentilactobacillus hilgardii, Lp. plantarum, and O. oeni, has been extensively studied [43,44,45,46,47]. The effect that these compounds may have on the growth of LAB and MLF depends on the nature of the phenolic compound, their concentration, and the capacity of the bacterial strain [43,45,48].
The mode by which phenolic compounds affect the growth of LAB has been in the epicenter of intensive research over the last decade. Changes in the cellular membrane structure and function have been identified as the first key features that determine the fate of LAB and MLF. Indeed, Sirk et al. [49] reported that catechins, and preferably non-galloylated ones, are absorbed into the membrane increasing its fluidity and concomitantly its permeability. The capacity of the strain to reduce membrane fluidity by increasing the saturation degree, by mechanisms such as cyclation of fatty acyl chains or altering the fatty acid composition [50,51], will determine its fate and the degree of adaptation. Another effect that may interfere with energy production and concomitantly bacterial growth is through the inhibition of the arginine deiminase pathway. This pathway may be an important energy source for bacterial growth. On the other hand, intermediate products, namely citrulline and carbamyl phosphate, contain carbamyl groups that react with ethanol and yield ethyl carbamate, a known carcinogen. Alberto et al. [52] demonstrated that gallic and protocatechuic acids may inhibit arginine deiminase pathway depriving energy and reducing the amount of ethyl carbamate precursors. On the other hand, vanillic acid, caffeic acid, rutin, quercetin, and catechin did not seem to have such an effect.
The second key feature that determines the fate of LAB, and concomitantly MLF, is the capacity of the LAB to utilize these compounds and gain energy. The ability of some Lv. brevis strains to decarboxylate hydroxycinnamic acids to their respective vinyl derivatives has been tested [53,54,55,56]. In addition, some strains exhibited the capacity to further reduce these vinyl derivatives to their respective ethyl phenols [55,57]. In the majority of the cases, p-coumaric, ferulic, and caffeic acids were more or less efficiently decarboxylated to vinyl phenol, vinyl guaiacol, and vinyl catechol, respectively. No such activity has been reported for o-coumaric, m-coumaric, cinnamic, and sinapic acids. Similarly, Rodriguez et al. [58] demonstrated the capacity of Lp. plantarum strain CECT 748T to decarboxylate and further reduce p-coumaric, caffeic, and ferulic acids to their respective vinyl and ethyl derivatives. Decarboxylation of gallic and protocatechuic acids as well as reduction of m-coumaric acid were also reported. On the contrary, no such activity was found on phloretic, chlorogenic, ellagic, o-coumaric, cinnamic, sinapic, syringic, salicylic, benzoic, gentisic, veratric, p-hydroxybenzoic, and vanillic acids. Similarly, Lp. plantarum strain 299v degraded caffeic acid to 4-ethylcatechol and ferulic acid to dihydro ferulic acid [59]. The aforementioned capacity may be preceded by tannins degradation through tannase activity. Indeed, several Lp. plantarum strains have been reported to degrade tannins into derivatives that can be further utilized as already described [60,61,62].
On the contrary, metabolism of phenolic compounds by O. oeni has not been adequately studied. The positive effect of some phenolic compounds on some O. oeni strains has been reported [63,64,65] which can probably be assigned to their ability to utilize them.
3.4. Interactions with Yeasts
Yeasts may affect MLF by directly or indirectly interfering with the growth of the bacteria that perform it. This effect may be direct when MLF and AF are simultaneous, and indirect when MLF follows AF. In the first case, it may be attributed to the antagonism for nutrients as well as the toxicity of the metabolites. The yeast metabolites that may affect LAB growth are ethanol, SO2, organic acids, medium chain fatty acids, as well as antimicrobial peptides. The antimicrobial activity of ethanol is well known and has been attributed to membrane damage and protein denaturation; it has been reported to be optimal in water solutions within the range of 60 and 90% [66]. In general, lactobacilli and pediococci seem to be less sensitive to ethanol than O. oeni in synthetic media [22,28]. Recently, genes candidates implicated to ethanol resistance of O. oeni have been studied through heterologous expression to other bacterial species, revealing a complex cell mechanism [67,68]. Regarding ethanol tolerance, the effect of temperature and the wine matrix has also been highlighted [69,70].
The antimicrobial activity of sulfur dioxide is also well known. Molecular sulfur dioxide enters the cell through the cell membrane due to its small size and absence of net charge. Once inside the cell, it yields bisulfite and sulfite, depending on the pH value, and interacts with many cellular components, including proteins, ATP, and cofactors such as NAD+, FAD+, and thiamin, disrupting cell homeostasis [71]. The amount of SO2 needed to prevent bacterial growth depends upon nearly all factors associated with vinification, such as temperature, pH value, ethanol concentration, microbial population, growth stage etc. [71]. Regarding the relevant tolerance of lactobacilli, pediococci, and oenococci, contradictory results are available in the literature [72,73].
Yeasts may also produce peptides with antimicrobial activity [74,75]. Their mode of action includes a variety of effects, including interference with membrane permeability, protein and cell-wall synthesis, enzyme activity, etc. [76]. Several authors have reported the production of such peptides by Saccharomyces cerevisiae and their inhibitory activity against O. oeni and in some cases non-Saccharomyces yeasts [77,78,79,80,81,82].
Organic acids may also affect the growth of the LAB conducting MLF. It has been estimated that organic acids may account for as much as approximately 1% of grape juice solids. L-tartaric acid is the most abundant and may reach 10 g/L. L-malic acid is also present at concentration that may reach 6.5 g/L, whereas citric and ascorbic acids may also be present at concentrations less than 1 g/L. After alcoholic fermentation by S. cerevisiae, L-tartaric and citric acid concentration reduction is very unlikely to occur, but degradation of L-malic acid is possible. However, in the presence of other yeast species, degradation of these organic acids may occur. After AF, production of acetic acid, succinic acid, pyruvic acid, traces of D-lactic acid as well as medium chain fatty acids, is very likely to occur [3]. The effect that these organic acids may have on LAB growth and MLF has been studied to some extent. The stimulatory effect that L-malic acid may have at 2 and 4 g/L, during growth at 20 °C, in a growth medium with pH value 3.5, supplemented with 10% ethanol, has been reported by Fahimi et al. [83]. Regarding MCFA, the effect is type and concentration dependent. Indeed, Capucho and San Ramao [84] reported that decanoic and dodecanoic concentrations below 12.5 and 2.5 mg/L, respectively, had stimulatory effects on O. oeni growth. On the contrary, if the concentration exceeded these limits, an inhibitory effect was noticed.
The effect that yeasts may have on MLF is completely different when MLF follows AF. In that case, there is no antagonism for nutrients. However, LAB have to tolerate the harsh environment that the yeasts’ metabolic activities have created. On the other hand, yeast autolysis, which takes place mostly at the end of alcoholic fermentation, may assist proliferation of LAB. During yeast autolysis, yeast cell walls are becoming available to the microorganisms that can decompose them and utilize their building blocks. Yeast cell walls consist of covalently linked β-1,3 glucan, β-1,6 glucan, chitin, and mannoproteins [85]. Mannoproteins are highly glycosylated polypeptides, the carbohydrate content of which, mannose and glucose in the majority of the cases, may reach 95% of the total mannoprotein weight [86]. Oenococcus oeni has been reported to possess glucosidase and peptidase activities, through which carbohydrates and amino acids are released, providing the necessary carbon, nitrogen, and energy sources [87]. These enzymatic activities are strain-dependent; the importance of mannoprotein utilization for effective MLF has been recently highlighted by Balmaseda et al. [88]. In this study, the O. oeni strain with the lowest mannoprotein utilization capacity exhibited the worst MLF performance among the strains tested. Qualitative and quantitative differences in the mannoproteins provided to O. oeni strains could not be correlated with MLF duration [88,89]. Interestingly, the effect that autolysis has on MLF, may also depend upon the yeast that undergoes autolysis. Indeed, Balmaseda et al. [88] reported that supplementation with lees from different Torulaspora delbrueckii, S. cerevisiae, and Metschnikowia pulcherrima strains, had different effect on MLF performance by O. oeni strains.
4. Correlation between MLF and Wine Quality Characteristics
During malolactic fermentation, a series of bioconversions takes place, which affects the organoleptic characteristics as well as safety of the wine. These depend on the capacity of the strain(s) that drive MLF. In the next paragraphs, the most important activities are summarized.
4.1. Glycosidase Activity
Glycosidase activity may affect the sensorial properties and color stability of the wine. Color stability is largely dependent on monoglucoside anthocyanins. Hydrolysis of these glycoconjugates releases anthocyanidins that may be subsequently spontaneously converted to colorless derivatives [90]. Regarding the sensorial properties, when released from their glycosides, some benzene derivatives, monoterpenes, and C13-norisoprenoids affect aroma, while ethyl esters, carboxylic acids, aliphatic alcohols, and lactones affect taste [91]. Thus, hydrolysis of their glucoconjugates releases them and concomitantly increases the organoleptic complexity of the wine. This hydrolysis is catalyzed by glucosidases specific to the carbohydrate moiety. In the case of monosaccharides, this carbohydrate moiety may be β-D-apiofuranoside, β-L-arabinofuranoside, β-D-glucopyranoside, β-L-rhamnopyranoside, or β-D-xylopyranoside. In addition, β-D-glucopyranosidase activity is required in the case of dissacharides, in order to remove the additional carbohydrate, which is β-D-glucopyranoside [91].
Several O. oeni, Lp. plantarum, Levilactobacillus brevis, and Pediococcus spp. strains have been reported to possess the aforementioned enzyme activities [92,93,94,95,96,97,98,99,100,101,102,103,104,105]. Growth stage, ethanol content, residual glucose and fructose concentrations, molecular sulfur dioxide, pH value, and temperature have been reported to affect glucosidase activity, in a strain-dependent manner [92,93,94,102,105].
Novel insights were provided by Spano et al. [99], Olguin et al. [106], and the studies of Li et al. [103] on the transcriptomic response of β-glucosidase genes to oenologically relevant stresses. Spano et al. [99] reported that the presence of K2S2O5, ethanol and low pH value (3.5) repressed the transcription of the Lp. plantarum strain Lp90 β-glucosidase gene. Olguin et al. [106] reported that the transcription of a β-glucosidase gene of four O. oeni strains revealed a strain-dependent response to culture conditions. However, no correlation with measured β-glucosidase activity could be established. On the contrary, Li et al. [103] managed to establish a correlation between the transcription levels of β-glucosidase genes OEOE-0224 and OEOE-1210 with the measured β-glucosidase activity of whole cells of O. oeni strain SD-2a and the transcription levels of β-glucosidase gene OEOE-1569 with the measured β-glucosidase activity of the disrupted lysate of the strain, suggesting a possible causal relationship.
4.2. Esterase Activity
Microbial esterases are involved in both biosynthetic and hydrolytic reactions, affecting quantitatively and qualitatively the ester content and concomitantly the wine aroma [107]. Although most esters are produced by yeasts during alcoholic fermentation [91], modification of their concentration has been reported after MLF [105,108,109,110,111,112,113,114,115], indicating that the LAB that drive MLF may possess such activity. Indeed, strain dependent esterase activity of 23 O. oeni, 16 Lactobacillus spp., and 11 Pediococcus spp. strains against pNP-acetate, pNP-butyrate, and pNP-octanoate was reported by Matthews et al. [116]. The widespread nature of esterase occurrence among LAB and the strain dependent activity was further verified by Perez-Martin et al. [117] and Diez-Ozaeta et al. [118] against pNP-octanoate in the first study and pNP-acetate and pNP-octanoate in the second. Finally, Gammacurta et al. [119] thoroughly studied the effect of yeast strain and cultivar on ester metabolism by LAB and concluded that only ethyl 2-hydroxy-3-methylbutanoate and ethyl 2-hydroxy-4-methylpentanoate were strongly influenced by the LAB strain, irrespective the yeast strain that carried out the alcoholic fermentation and the grapevine cultivar.
Esterase activity has been reported to be affected by pH, ethanol, and temperature either as single stresses or in combination, in a strain-dependent manner [117,118,120].
Cinnamoyl esterases have drawn specific attention since they are involved in the release of hydroxycinnamic acids from their esters. Unbound hydroxycinnamic acids may then serve as substrates for conversion to volatile phenols by Brettanomyces/Dekkera bruxellensis and some wine LAB [121]. Occurrence of cinnamoyl esterases has been reported so far in some O. oeni strains [122,123,124] and their activity has been characterized as strain-dependent [121].
4.3. Citrate Metabolism
The ability of the bacterial strain that performs MLF to degrade citrate is particularly important due to the higher amounts of lactate, acetate, diacetyl, and acetoin that may be produced. Citrate is transported into the cell via citrate or malate permease and converted to acetate and oxaloacetate through the action of citrate lyase (Figure 1). Acetate is excreted from the cell while the oxaloacetate is decarboxylated to pyruvate through oxaloacetate decarboxylase. Pyruvate may be then further utilized through a number of anabolic and catabolic processes, including the ones leading to the production of lactate, acetate, diacetyl, and acetoin. Phenolic compounds, carbohydrate type, and concentration as well as pH value, seem to have a pronounced effect on citrate catabolism. Rozes et al. [125] reported that phenolic compounds reduced glucose and fructose catabolism rate by O. oeni and enhanced citrate catabolism, leading to increased acetic acid production. Glucose seems to suppress citrate permease and citrate lyase and thus citrate metabolism [126,127,128]. On the contrary, fructose may lead to the production of high concentrations of diacetyl and acetoin by both O. oeni and Lp. plantarum [128]. Regarding the effect of pH value, more citrate was metabolized by O. oeni at low pH values (3.0 and 3.5) whereas Lp. plantarum seemed to prefer high pH values (4.0 and 5.0) [128].
4.4. Production of Exopolysaccharides
Lactic acid bacteria may produce exopolysaccharides (EPS), which are extracellular polymers with variable composition, size, and degree of branching. Therefore, their spatial arrangement, charge, and capacity to interact with other molecules, such as proteins, alcohols, esters etc., may vary [129]. The fact that the LAB of wine are all capable of producing various EPS derived from several biosynthetic pathways, while the implicated genes, either organized in clusters or isolated, are well conserved between the bacterial species, indicating their importance for the bacteria cell. Although their exact physiological role has not yet been thoroughly understood, they assist in survival of harsh wine environments and in their persistence during the winemaking process [130].
From an enological perspective, it seems that both structure and EPS localization, capsular or free, should be taken into consideration. For instance, the production of β-1,3-glucans can not only cause wine spoilage and economic loss, but at the same time helps the bacteria to adhere on biotic and abiotic surfaces [130,131,132]. In that case, the solution for the winemaker is the simultaneous enzymatic action of β-glucanase and lysozyme, as recommended by Coulon et al. [133]. On the other hand, possible positive roles in sensorial perception and lyoprotective ability to freeze drying are currently under scrutiny [134].
4.5. Biogenic Amines Production
Biogenic amine production is another strain-dependent characteristic and may take place by both yeasts and LAB. They are produced as a response to harsh environmental conditions, in an attempt to deacidify the cytosol. This is achieved through the coordinated action of a membrane antiport and specific amino acid decarboxylases (Figure 1). Each amino acid decarboxylation consumes one proton, and biogenic amine excretion drives ATP synthesis through proton motive force [135,136]. Such systems have been described for histidine/histamine, tyrosine/tyramine, ornithine/putrescine, and lysine/cadaverine [135,137,138], with the first three being the ones mostly produced during MLF [139]. Interestingly, the capacity of many strains to degrade biogenic amines through monoamino-oxidases or multicopper oxidases has been reported [140,141], providing with new insights into biogenic amine accumulation and additional strategies for their removal. Several factors have been reported to affect biogenic amine accumulation. These include agricultural practices, through their effect on the concentration of the amino acid precursors and technological parameters, including storage conditions, mostly through their effect on the biodiversity of the microecosystem [142].
A very interesting insight was provided by Landete et al. [143]. In that study, the effect of enological factors on the activity of histidine decarboxylase enzyme of Le. hilgardii, P. parvulus and O. oeni as well as the transcription levels of the histidine decarboxylase gene (hdc) of Le. hilgardii were assessed. It was reported that increases of pH value, temperature, lactic acid, tartaric acid, ethanol, and SO2 concentration had no effect on the activity of the enzyme of preadapted cultures of the strains assessed. On the contrary, the activity was reduced upon increase of malic acid, citric acid, and glucose concentration. These results were in concordance with the transcriptomic response of hdc.
4.6. Ethyl Carbamate (Urethane) Production
Ethyl carbamate is produced through the reaction of ethanol with carbamyl compounds. Such compounds may arise from arginine catabolism by yeasts and LAB (Figure 1). In the case of the LAB, arginine is usually catabolized through the arginine deiminase pathway which results in the production of one molecule of ornithine, carbamyl phosphate, and ATP per arginine molecule. Both catabolic products contain carbamoyl groups and thus may react with ethanol. The factors that affect ethyl carbamate production include the concentration of the reactants, as well as storage temperature and time; pH value does not seem to affect it significantly [144,145].
5. Practical Considerations of MLF
Malolactic fermentation (MLF) can be spontaneous or can be induced by wine inoculation with selected LAB starter cultures. The process of spontaneous MLF is manifested by the growth and metabolism of indigenous LAB, with dominant species O. oeni, which are perfectly adapted to the environmental conditions of each medium and space [131]. However, in this case, the bioconversion of malic acid into lactic acid can be slow or incomplete, while undesirable compounds, such as volatile phenols and diacetyl overproduction as well as potentially hazardous compounds, such as biogenic amines can be produced as previously discussed. Certainly, the use of selected LAB starters can help minimize these risks while the moment of inoculation seems to be crucial for the winemaking process.
5.1. Malolactic Starters
In addition to O. oeni, there are several LAB that have been used as MLF starters belonging to the species Lp. plantarum, Le. hilgardii, Lv. brevis, Lacticaseibacillus casei, and Pediococcus spp. [146]. Each has demonstrated different properties with significant strain dependence. Under high pH conditions, Lp. plantarum strains have shown particularly interesting results [147], not only for their ability to induce MLF, but also for their homo-fermentative catabolism of hexoses, which minimizes the risk of acetic acid production [148]. Furthermore, Lp. plantarum has been found to possess a complex enzymatic system, such as β-glucosidases, proteases, esterases, and decarboxylases, which could play a significant role in modifying wine organoleptic properties [24,86,149]. Regarding the genus Pediococcus, it is generally considered a wine spoilage microorganism [150]. However, some findings have indicated that the presence of certain strains of Pediococcus spp. may contribute positively to a wine’s sensory profile [150,151,152]. In recent years, mixed inoculation strategies have also been employed. The use of blended cultures of Lp. plantarum and O. oeni as MLF starters, can facilitate the rapid consumption of malic acid, while contributing significantly to the volatile profile of wine [153].
5.2. Inoculation Protocol
The yeast and bacteria species and strains as well as vinification parameters should be taken into consideration when designing an inoculation protocol. The interplay between these factors affects growth and metabolic activity of the microbial starters employed and concomitantly wine quality (Figure 2). Selection of the yeast strains should not be solely based on their ethanol production capacity, factors that should also be taken into consideration include the release of antimicrobial peptides, medium-chain fatty acids and mannoproteins. Similarly, lactic acid bacteria population and growth phase should also be considered for effective MLF. A bidirectional interaction between these and vinification parameters, such as SO2, oxygen, temperature, pH and cultivar exists and define the inoculation protocol that should be employed.
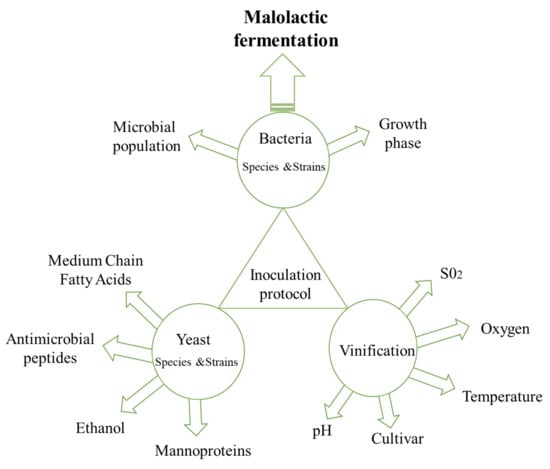
Figure 2.
Biotic and abiotic factors affecting MLF.
Temporal relationship between AF and MLF is a major practical issue. Theoretically, MLF can precede, follow, or take place simultaneously with AF [69,71,146,154,155]. The effect that yeast and LAB metabolic coexistence or succession may have on wine quality has been extensively studied. The option to conduct MLF before AF, although promising [155], is rarely used since oxygen availability may result in increased acetic acid production. This is also the case regarding simultaneous MLF and AF; therefore, LAB are inoculated usually 24 h after yeast inoculation, since oxygen needs to be depleted before LAB become metabolically active. Metabolic coexistence of yeasts and LAB will result in antagonism for the available nutrients and energy sources. This, along with the metabolites produced may result in growth inhibition of either microorganism [156,157]. In Table 1, studies that comparatively assess the outcome of simultaneous and sequential MLF and AF are presented. In only a couple of cases, co-inoculation had a negative effect on the viability of the yeast or the LAB strains employed. It is generally accepted that simultaneous MLF and AF significantly reduces the overall winemaking time, and thus the risk for microbial spoilage, since sulfuring takes place earlier. In addition, ethanol content is not affected by co-inoculation. However, modification of wine composition and sensorial properties may take place. More accurately, increase of volatile acidity, decrease of color intensity and tonality, and enhancement of floral, vegetable, red, and ripe fruit tones, have been recorded. However, this effect seems to be winery, cultivar, vintage, fermentation temperature, and microbial strain dependent. Therefore, proper arrangement of the aforementioned parameters is necessary for successful simultaneous MLF and AF fermentation.

Table 1.
Studies that assess the effect of different inoculation strategy on wine quality.
6. Conclusions
MLF is gaining more and more attention from the wine industry, confirming the fact that it is much more than a deacidification process. The metabolic potential of LAB is complex and continuous efforts are being made to elucidate it. As already discussed, through the activation of various metabolic pathways, LAB significantly influence the organoleptic profile of wine, contributing to the enhancement of aroma and flavor complexity and at the same time impart microbiological stability. However, the same bacteria could produce volatile phenols, which negatively affect wine aromas or biogenic amines, which represent a risk to consumer health. This uncertainty can be controlled with proper malolactic fermentation management. The right inoculation moment as well as the selection of the appropriate strain, are critical points for a successful completion of MLF, leading to wines with desired characteristics. In the future, it will be important to investigate the genomic diversity of LAB, as the basis of their promising metabolic characteristics. New strains, ideal for a region’s unique climate, red versus white wines, or even grape variety, deserve to be thoroughly explored. Different genera as well as mixture of species/strains could be considered for future application as malolactic starters.
Author Contributions
Conceptualization, S.P., Y.K. and M.D.; resources, S.P., V.S., A.T., Y.K. and M.D.; writing—original draft preparation, S.P. and M.D.; writing—review and editing, S.P., V.S., A.T., Y.K. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Semon, M.J.; Edwards, C.G.; Forsyth, D.; Dinn, C. Inducing malolactic fermentation in Chardonnay musts and wines using different strains of Oenococcus oeni. Aust. J. Grape Wine Res. 2001, 7, 52–59. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Troianou, V.; Paramithiotis, S.; Proksenia, N.; Kotseridis, Y. Evaluation of malolactic starters in white and rosé winemaking of Moschofilero wines. Appl. Sci. 2022, 12, 5722. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Mendes-Faia, A. The role of yeasts and lactic acid bacteria on the metabolism of organic acids during winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef] [PubMed]
- Lerm, E.; Engelbrecht, L.; du Toit, M. Malolactic fermentation: The ABC’s of MLF. S. Afr. J. Enol. Vitic. 2010, 31, 186–212. [Google Scholar] [CrossRef]
- Virdis, C.; Sumby, K.; Bartowsky, E.; Jiranek, V. Lactic acid bacteria in wine: Technological advances and evaluation of their functional role. Front. Microbiol. 2021, 11, 612118. [Google Scholar] [CrossRef]
- Salema, M.; Poolman, B.; Lolkema, J.S.; Loureiro Dias, M.; Konings, W.N. Uniport of monoanionic L-malate in membrane vesicles from Leuconostoc oenos. Eur. J. Biochem. 1994, 225, 289–295. [Google Scholar] [CrossRef]
- Poolman, B.; Molenaar, D.; Smid, E.J.; Ubbink, T.; Abee, T.; Renault, P.P.; Konings, W.N. Malolactic fermentation: Electrogenic malate uptake and malate/lactate antiport generate metabolic energy. J. Bacteriol. 1991, 173, 6030–6037. [Google Scholar] [CrossRef]
- Olsen, E.B.; Russell, J.B.; Henick-Kling, T. Electrogenic L-malate transport by Lactobacillus plantarum: A basis for energy derivation from malolactic fermentation. J. Bacteriol. 1991, 173, 6199–6206. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Chen, X.; Wei, G.; Tao, S.; Fan, M. Altered metabolic strategies: Elaborate mechanisms adopted by Oenococcus oeni in response to acid stress. J. Agric. Food Chem. 2021, 69, 2906–2918. [Google Scholar] [CrossRef]
- Versari, A.; Parpinello, G.P.; Cattaneo, M. Leuconostoc oenos and malolactic fermentation in wine: A review. J. Ind. Microbiol. Biotechnol. 1999, 23, 447–455. [Google Scholar] [CrossRef]
- Renault, P.; Gaillardin, C.; Heslot, H. Product of the Lactococcus lactis gene required for malolactic fermentation is homologous to a family of positive regulators. J. Bacteriol. 1989, 171, 3108–3114. [Google Scholar] [CrossRef] [PubMed]
- Labarre, C.; Divies, C.; Guzzo, J. Genetic organization of the mle Locus and identification of a mleR-like gene from Leuconostoc oenos. Appl. Environ. Microbiol. 1996, 62, 4493–4498. [Google Scholar] [CrossRef] [PubMed]
- Olguin, N.; Champomier-Verges, M.; Anglade, P.; Baraige, F.; Cordero-Otero, R.; Bordons, A.; Zagorec, M.; Reguant, C. Transcriptomic and proteomic analysis of Oenococcus oeni PSU-1 response to ethanol shock. Food Microbiol. 2015, 51, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Rantsiou, K.; Majumder, A.; Jacobsen, S.; Pessione, E.; Svensson, B.; Garcia-Moruno, E.; Cocolin, L. Complementing DIGE proteomics and DNA subarray analyses to shed light on Oenococcus oeni adaptation to ethanol in wine-simulated conditions. J. Proteom. 2015, 123, 114–127. [Google Scholar] [CrossRef]
- Augagneur, Y.; Ritt, J.-F.; Linares, D.M.; Remize, F.; Tourdot-Marechal, R.; Garmyn, D.; Guzzo, J. Dual effect of organic acids as a function of external pH in Oenococcus oeni. Arch. Microbiol. 2007, 188, 147–157. [Google Scholar] [CrossRef]
- Costantini, A.; Vaudano, E.; Rantsiou, K.; Cocolin, L.; Garcia-Moruno, E. Quantitative expression analysis of mleP gene and two genes involved in the ABC transport system in Oenococcus oeni during rehydration. Appl. Microbiol. Biotechnol. 2011, 91, 1601–1609. [Google Scholar] [CrossRef]
- Onetto, C.A.; Costello, P.J.; Kolouchova, R.; Jordans, C.; McCarthy, J.; Schmidt, S.A. Analysis of transcriptomic response to SO2 by Oenococcus oeni growing in continuous culture. Microbiol. Spectr. 2021, 9, e01154-21. [Google Scholar] [CrossRef]
- Miller, B.J.; Franz, C.M.A.P.; Cho, G.-S.; du Toit, M. Expression of the malolactic enzyme gene (mle) from Lactobacillus plantarum under winemaking conditions. Curr. Microbiol. 2011, 62, 1682–1688. [Google Scholar] [CrossRef]
- Binati, R.L.; Du Toit, M.; Snoep, J.L.; Salvetti, E.; Torriani, S. Transcriptional and metabolic response of wine-related Lactiplantibacillus plantarum to different conditions of aeration and nitrogen availability. Fermentation 2021, 7, 68. [Google Scholar] [CrossRef]
- Lund, P.A.; De Biase, D.; Liran, O.; Scheler, O.; Mira, N.P.; Cetecioglu, Z.; Noriega Fernández, E.; Bover-Cid, S.; Hall, R.; Sauer, M.; et al. Understanding how microorganisms respond to acid pH is central to their control and successful exploitation. Front. Microbiol. 2020, 11, 556140. [Google Scholar] [CrossRef]
- Patra, M.; Salonen, E.; Terama, E.; Vattulainen, I.; Faller, R.; Lee, B.W.; Holopainen, J.; Karttunen, M. Under the influence of alcohol: The effect of ethanol and methanol on lipid bilayers. Biophys. J. 2006, 90, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.R.; Wibowo, D.J.; Lee, T.H.; Fleet, G.H. Growth and metabolism of lactic acid bacteria during and after malolactic fermentation of wines at different pH. Appl. Environ. Microbiol. 1986, 51, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, H.; Formisyn, P.; Gerbaux, V. Malolatic fermentation of wine: Study of the influence of some physico-chemical factors by experimental design assays. J. Appl. Bacteriol. 1995, 79, 640–650. [Google Scholar] [CrossRef]
- Du Toit, M.; Engelbrecht, L.; Lerm, E.; Krieger-Weber, S. Lactobacillus: The next generation of malolactic fermentation starter cultures—An overview. Food Bioprocess Technol. 2011, 4, 876–906. [Google Scholar] [CrossRef]
- Cinquanta, L.; De Stefano, G.; Formato, D.; Niro, S.; Panfili, G. Effect of pH on malolactic fermentation in southern Italian wines. Eur. Food Res. Technol. 2018, 244, 1261–1268. [Google Scholar] [CrossRef]
- Lerena, M.C.; Rojo, M.C.; Sari, S.; Mercado, L.A.; Krieger-Weber, S.; Combina, M. Malolactic fermentation induced by Lactobacillus plantarum in Malbec wines from Argentina. S. Afr. J. Enol. Vitic. 2016, 37, 115–123. [Google Scholar] [CrossRef]
- Pimentel, M.S.; Silva, M.H.; Cortes, I.; Mendes Faia, A. Growth and metabolism of sugar and acids of Leuconostoc oenos under different conditions of temperature and pH. J. Appl. Bacteriol. 1994, 76, 42–48. [Google Scholar] [CrossRef]
- Guerzoni, M.E.; Sinigaglia, M.; Gardini, F.; Ferruzzi, M.; Torriani, S. Effects of pH, temperature, ethanol, and malate concentration on Lactobacillus plantarum and Leuconostoc oenos: Modelling of the malolactic activity. Am. J. Enol. Vitic. 1995, 46, 368–374. [Google Scholar]
- Sereni, A.; Phan, Q.; Osborne, J.; Tomasino, E. Impact of the timing and temperature of malolactic fermentation on the aroma composition and mouthfeel properties of Chardonnay wine. Foods 2020, 9, 802. [Google Scholar] [CrossRef]
- Guzzon, R.; Roman, T.; Larcher, R. Impact of different temperature profiles on simultaneous yeast and bacteria fermentation. Ann. Microbiol. 2020, 70, 44. [Google Scholar] [CrossRef]
- Ojeda, H.; Andary, C.; Creaba, E.; Carbonneau, A.; Deloire, A. Influence of pre- and postveraison water deficit on synthesis and concentration of skin phenolic compounds during berry growth of Vitis vinifera var. Shiraz. Am. J. Enol. Vitic. 2002, 53, 261–267. [Google Scholar]
- Rodriguez Montealegre, R.; Romero Peces, R.; Chacon Vozmediano, J.L.; Martinez Gascuena, J.; Garcia Romero, E. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compost. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Guidoni, S.; Oggero, G.; Cravero, S.; Rabino, M.; Cravero, M.C.; Balsari., P. Manual and mechanical leaf removal in the bunch zone (Vitis vinifera L., cv Barbera): Effects on berry composition, health, yield and wine quality, in a warm temperate area. J. Int. Sci. Vigne Vin 2008, 42, 49–58. [Google Scholar] [CrossRef]
- Gamero, E.; Moreno, D.; Talaverano, I.; Prieto, M.H.; Guerra, M.T.; Valdés, M.E. Effects of irrigation and cluster thinning on Tempranillo grape and wine composition. S. Afr. J. Enol. Vitic. 2014, 35, 196–204. [Google Scholar] [CrossRef]
- Ramos, M.C.; Jones, G.V.; Yuste, J. Spatial and temporal variability of cv. Tempranillo phenology and grape quality within the Ribera del Duero DO (Spain) and relationships with climate. Int. J. Biometeorol. 2015, 59, 1849–1860. [Google Scholar] [CrossRef]
- Barroso, J.M.; Pombeiro, L.; Rato, A.E. Impacts of crop level, soil and irrigation management in grape berries of cv ‘Trincadeira’ (Vitis vinifera L.). J. Wine Res. 2016, 28, 1–12.
- Blank, M.; Hofmann, M.; Stoll, M. Seasonal differences in Vitis vinifera L. cv. Pinot noir fruit and wine quality in relation to climate. OENO One 2019, 2, 189–203. [Google Scholar]
- Echeverría, G.; Ferrer, M.; Mirás-Avalos, J.M. Effects of soil type on vineyard performance and berry composition in the Río de la Plata coast (Uruguay). OENO One 2017, 51, 251–261. [Google Scholar] [CrossRef]
- Izcara, S.; Morante-Zarcero, S.; de Andrés, M.T.; Arroyo, T.; Sierra, I. A comparative study of phenolic composition and antioxidant activity in commercial and experimental seedless table grapes cultivated in a Mediterranean climate. J. Food Meas. Charact. 2021, 15, 1916–1930. [Google Scholar] [CrossRef]
- Karaman, H.T.; Küskü, D.Y.; Söylemezoğlu, G. Phenolic compounds and antioxidant capacities in grape berry skin, seed and stems of six wine grape varieties grown in Turkey. Acta Sci. Pol. Hortorum Cultus 2021, 20, 15–25. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; Liu, H.; Tang, M.; Yao, Y.; Zhai, H.; Gao, Z.; Du, Y. Effects of flower cluster tip removal on phenolics and antioxidant activity of grape berries and wines. Am. J. Enol. Vitic. 2021, 72, 298–306. [Google Scholar] [CrossRef]
- Luzio, A.; Bernardo, S.; Correia, C.; Moutinho-Pereira, J.; Dinis, L.-T. Phytochemical screening and antioxidant activity on berry, skin, pulp and seed from seven red Mediterranean grapevine varieties (Vitis vinifera L.) treated with kaolin foliar sunscreen. Sci. Hortic. 2021, 281, 109962. [Google Scholar] [CrossRef]
- García-Ruiz, A.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Bartolomé, B. Comparative study of the inhibitory effects of wine polyphenols on the growth of enological lactic acid bacteria. Int. J. Food Microbiol. 2011, 145, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Zimdars, S.; Caspers-Weiffenbach, R.; Wegmann-Herr, P.; Weber, F. Stilbenes can impair malolactic fermentation with strains of Oenococcus oeni and Lactobacillus plantarum. Am. J. Enol. Vitic. 2021, 72, 56–63. [Google Scholar] [CrossRef]
- Collombel, I.; Campos, F.M.; Hogg, T. Changes in the composition of the lactic acid bacteria behavior and the diversity of Oenococcus oeni isolated from red wines supplemented with selected grape phenolic compounds. Fermentation 2019, 5, 1. [Google Scholar] [CrossRef]
- Figueiredo, A.R.; Campos, F.; Freitas, V.; Hogg, T.; Couto, J.A. Effect of phenolic aldehydes and flavonoids on growth and inactivation of Oenococcus oeni and Lactobacillus hilgardii. Food Microbiol. 2008, 25, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Sabel, A.; Bredefeld, S.; Schlander, M.; Claus, H. Wine phenolic compounds: Antimicrobial properties against yeasts, lactic acid and acetic acid bacteria. Beverages 2017, 3, 29. [Google Scholar] [CrossRef]
- Devi, A.; Anu-Appaiah, K.A. Diverse physiological and metabolic adaptations by Lactobacillus plantarum and Oenococcus oeni in response to the phenolic stress during wine fermentation. Food Chem. 2018, 268, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Sirk, T.W.; Brown, E.F.; Friedman, M.; Sum, A.K. Molecular binding of catechins to biomembranes: Relationship to biological activity. J. Agric. Food Chem. 2009, 57, 6720–6728. [Google Scholar] [CrossRef]
- Teixeira, H.; Goncalves, M.G.; Rozes, N.; Ramos, A.; San Romao, M.V. Lactobacillic acid accumulation in the plasma membrane of Oenococcus oeni: A response to ethanol stress? Microb. Ecol. 2002, 43, 146–153. [Google Scholar] [CrossRef]
- Grandvalet, C.; Assad-Garcia, J.S.; Chu-Ky, S.; Tollot, M.; Guzzo, J.; Gresti, J. Changes in membrane lipid composition in ethanol- and acid-adapted Oenococcus oeni cells: Characterization of the cfa gene by heterologous complementation. Microbiology 2008, 154, 2611–2619. [Google Scholar] [CrossRef]
- Alberto, M.R.; Manca de Nadra, M.C.; Arena, M.E. Influence of phenolic compounds on the growth and arginine deiminase system in a wine lactic acid bacterium. Braz. J. Microbiol. 2012, 43, 167–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.N. The influence of Brettanomyces/Dekkera sp. yeasts and lactic acid bacteria on the ethylphenol content of red wines. Am. J. Enol. Vitic. 1995, 46, 463–468. [Google Scholar]
- Cavin, J.F.; Andioc, V.; Etievant, P.X.; Divies, C. Ability of wine lactic acid bacteria to metabolize phenol carboxylic acids. Am. J. Enol. Vitic. 1993, 44, 76–80. [Google Scholar]
- De las Rivas, B.; Rodríguez, H.; Curiel, J.A.; Landete, J.M.; Muñoz, R. Molecular screening of wine lactic acid bacteria degrading hydroxycinnamic acids. J. Agric. Food Chem. 2009, 57, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Curiel, J.A.; Rodríguez, H.; Landete, J.M.; de las Rivas, B.; Muñoz, R. Ability of Lactobacillus brevis strains to degrade food phenolic acids. Food Chem. 2010, 120, 225–229. [Google Scholar] [CrossRef]
- Couto, J.A.; Campos, F.M.; Figueiredo, A.R.; How, T.A. Ability of lactic acid bacteria to produce volatile phenols. Am. J. Enol. Vitic. 2006, 57, 166–171. [Google Scholar]
- Rodriguez, H.; Landete, J.M.; de las Rivas, B.; Munoz, R. Metabolism of food phenolic acids by Lactobacillus plantarum CECT 748T. Food Chem. 2008, 107, 1393–1398. [Google Scholar] [CrossRef]
- Rogozinska, M.; Korsak, D.; Mroczek, J.; Biesaga, M. Catabolism of hydroxycinnamic acids in contact with probiotic Lactobacillus. J. Appl. Microbiol. 2021, 131, 1464–1473. [Google Scholar] [CrossRef]
- Nishitani, Y.; Sasaki, E.; Fujisawa, T.; Osawa, R. Genotypic analyses of lactobacilli with a range of tannase activities isolated from human feces and fermented foods. Syst. Appl. Microbiol. 2004, 27, 109–117. [Google Scholar] [CrossRef]
- Vaquero, I.; Marcobal, A.; Muñoz, R. Tannase activity by lactic acid bacteria isolated from grape must and wine. Int. J. Food Microbiol. 2004, 96, 199–204. [Google Scholar] [CrossRef]
- Jiménez, N.; Esteban-Torres, M.; Mancheño, J.M.; de las Rivas, B.; Muñoz, R. Tannin degradation by a novel tannase enzyme present in some Lactobacillus plantarum strains. Appl. Environ. Microbiol. 2014, 80, 2991–2997. [Google Scholar] [CrossRef] [PubMed]
- Reguant, C.; Bordons, A.; Arola, L.; Rozes, N. Influence of phenolic compounds on the physiology of Oenococcus oeni from wine. J. Appl. Microbiol. 2000, 88, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Chasseriaud, L.; Krieger-Weber, S.; Deleris-Bou, M.; Sieczkowski, N.; Jourdes, M.; Teissedre, P.L.; Claisse, O.; Lonvaud-Funel, A. Hypotheses on the effects of enological tannins and total red wine phenolic compounds on Oenococcus oeni. Food Microbiol. 2015, 52, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Breniaux, M.; Dutilh, L.; Petrel, M.; Gontier, E.; Campbell-Sills, H.; Deleris-Bou, M.; Krieger, S.; Teissedre, P.L.; Jourdes, M.; Reguant, C.; et al. Adaptation of two groups of Oenococcus oeni strains to red and white wines: The role of acidity and phenolic compounds. J. Appl. Microbiol. 2018, 125, 1117–1127. [Google Scholar] [CrossRef]
- McDonnell, G.; Denver Russell, A. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Yuan, L.; Zhao, H.; Liu, L.; Peng, S.; Li, H.; Wang, H. Heterologous expression of the puuE from Oenococcus oeni SD-2a in Lactobacillus plantarum WCFS1 improves ethanol tolerance. J. Basic Microbiol. 2019, 59, 1134–1142. [Google Scholar] [CrossRef]
- Liu, S.; Skory, C.; Liang, X.; Mills, D.; Qureshi, N. Increased ethanol tolerance associated with the pntAB locus of Oenococcus oeni and Lactobacillus buchneri. J. Ind. Microbiol. Biotechnol. 2019, 46, 1547–1556. [Google Scholar] [CrossRef]
- Henick-Kling, T. Malolactic Fermentation. In Wine Microbiology and Biotechnology, 1st ed.; Fleet, G.H., Ed.; Harwood Academic Publishers: Chur, Switzerland, 1993; pp. 289–326. [Google Scholar]
- Gockowiak, H.; Henschke, P.A. Interaction of pH, ethanol concentration and wine matrix on induction of malolactic fermentation with commercial ‘direct inoculation’ starter cultures. Aust. J. Grape Wine Res. 2003, 9, 200–209. [Google Scholar] [CrossRef]
- Fugelsang, K.C.; Edwards, C.G. Wine Microbiology, Practical Applications and Procedures, 2nd ed.; Springer Science+Business Media LLC: New York, NY, USA, 2007. [Google Scholar]
- Hood, A. Inhibition of growth of wine lactic-acid bacteria by acetaldehyde bound sulphur dioxide. Aust. Grape. Grapegrow. Winemak. 1983, 232, 34–43. [Google Scholar]
- Davis, C.R.; Wibowo, D.; Fleet, G.H.; Lee, T.H. Properties of wine lactic acid bacteria: Their potential enological significance. Am. J. Enol. Vitic. 1988, 39, 137–142. [Google Scholar]
- Mannazzu, I.; Domizio, P.; Carboni, G.; Zara, S.; Comitini, F.; Budroni, M.; Ciani, M. Yeast killer toxins: From ecological significance to application. Crit. Rev. Biotechnol. 2019, 39, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Shavandi, A.; Mirdamadi, S.; Soleymanzadeh, N.; Motahari, P.; Mirdamadi, N.; Moser, M.; Subra, G.; Alimoradi, H.; Goriely, S. Bioactive peptides from yeast: A comparative review on production methods, bioactivity, structure-function relationship, and stability. Trends Food Sci. Technol. 2021, 118, 297–315. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Dick, K.J.; Molan, P.C.; Eschembruch, R. The isolation from Saccharomyces cerevisiae of two antibacterial cationic proteins that inhibit malolactic bacteria. Vitis 1992, 31, 105–116. [Google Scholar]
- Comitini, F.; Ferretti, R.; Clementi, F.; Mannazzu, I.; Ciani, M. Interactions between Saccharomyces cerevisiae and malolactic bacteria: Preliminary characterization of a yeast proteinaceous compound(s) active against Oenococcus oeni. J. Appl. Microbiol. 2005, 99, 105–111. [Google Scholar] [CrossRef]
- Osborne, J.P.; Edwards, C.G. Inhibition of malolactic fermentation by a peptide produced by Saccharomyces cerevisiae during alcoholic fermentation. Int. J. Food Microbiol. 2007, 118, 27–34. [Google Scholar] [CrossRef]
- Albergaria, H.; Francisco, D.; Gori, K.; Arneborg, N.; Gírio, F. Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl. Microb. Cell Physiol. 2010, 86, 965–972. [Google Scholar] [CrossRef]
- Mendoza, L.M.; Manca, M.C.; Farías, M.E. Antagonistic interaction between yeasts and lactic acid bacteria of oenological relevance partial characterization of inhibitory compounds produced by yeasts. Food. Res. Int. 2010, 43, 1990–1998. [Google Scholar] [CrossRef]
- Branco, P.; Francisco, D.; Chambon, C.; Hébraud, M.; Arneborg, N.; Almeida, M.G.; Caldeira, J.; Albergaria, H. Identification of novel GAPDH-derived antimicrobial peptides secreted by Saccharomyces cerevisiae and involved in wine microbial interactions. Appl. Microbiol. Biotechnol. 2014, 98, 843–853. [Google Scholar] [CrossRef]
- Fahimi, N.; Brandam, C.; Taillandier, P. A mathematical model of the link between growth and L-malic acid consumption for five strains of Oenococcus oeni. World J. Microbiol. Biotechnol. 2014, 30, 3163–3172. [Google Scholar] [CrossRef]
- Capucho, I.; San Ramao, M.V. Effect of ethanol and fatty acids on malolactic activity of Leoconostoc oenos. Appl. Microbiol. Biotech. 1994, 42, 723–726. [Google Scholar]
- Lipke, P.N.; Ovalle, R. Cell wall architecture in yeast: New structure and new challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar] [CrossRef] [PubMed]
- Vejarano, R. Non-Saccharomyces in winemaking: Source of mannoproteins, nitrogen, enzymes, and antimicrobial compounds. Fermentation 2020, 6, 76. [Google Scholar] [CrossRef]
- Alexandre, H.; Costello, P.J.; Remize, F.; Guzzo, J.; Guilloux-Benatier, M. Saccharomyces cerevisiae–Oenococcus oeni interactions in wine: Current knowledge and perspectives. Int. J. Food Microbiol. 2004, 93, 141–154. [Google Scholar] [CrossRef]
- Balmaseda, A.; Rozes, N.; Bordons, A.; Reguant, C. Simulated lees of different yeast species modify the performance of malolactic fermentation by Oenococcus oeni in wine-like medium. Food Microbiol. 2021, 99, 103839. [Google Scholar] [CrossRef]
- Ferrando, N.; Araque, I.; Ortís, A.; Thornes, G.; Bautista-Gallego, J.; Bordons, A.; Reguant, C. Evaluating the effect of using non-Saccharomyces on Oenococcus oeni and wine malolactic fermentation. Food Res. Int. 2020, 138, 109779. [Google Scholar] [CrossRef]
- Huang, H.T. Decolorization of anthocyanins by fungal enzymes. J. Agric. Food Chem. 1995, 3, 141–146. [Google Scholar] [CrossRef]
- Matthews, A.; Grimaldi, A.; Walker, M.; Bartowsky, E.; Grbin, P.; Jiranek, V. Lactic acid bacteria as a potential source of enzymes for use in vinification. Appl. Environ. Microbiol. 2004, 70, 5715–5731. [Google Scholar] [CrossRef]
- Grimaldi, A.; McLean, H.; Jiranek, V. Identification and partial characterization of glycosidic activities of commercial strains of the lactic acid bacterium, Oenococcus oeni. Am. J. Enol. Vitic. 2000, 51, 362–369. [Google Scholar]
- Grimaldi, A.; Bartowsky, E.; Jiranek, V. A survey of glycosidase activities of commercial wine strains of Oenococcus oeni. Int. J. Food Microbiol. 2005, 105, 233–244. [Google Scholar] [CrossRef]
- Grimaldi, A.; Bartowsky, E.; Jiranek, V. Screening of Lactobacillus spp. and Pediococcus spp. for glycosidase activities that are important in oenology. J. Appl. Microbiol. 2005, 99, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Boido, E.; Lloret, A.; Medina, K.; Carrau, F.; Dellacassa, E. Effect of glycosidase activity of Oenococcus oeni on the glycosylated flavor precursors of tannat wine during malolactic fermentation. J. Agric. Food Chem. 2002, 50, 2344–2349. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.K.; Zoecklein, B.W.; Whiton, R.S. Quantification of glycosidase activity in selected strains of Brettanomyces bruxellensis and Oenococcus oeni. Am. J. Enol. Vitic. 2002, 53, 303–307. [Google Scholar]
- Ugliano, M.; Genovese, A.; Moio, L. Hydrolysis of wine aroma precursors during malolactic fermentation with four commercial starter cultures of Oenococcus oeni. J. Agric. Food Chem. 2003, 51, 5073–5078. [Google Scholar] [CrossRef] [PubMed]
- D’Incecco, N.; Bartowsky, E.; Kassara, S.; Lante, A.; Spettoli, P.; Henschke, P. Release of glycosidically bound flavour compounds of Chardonnay by Oenococcus oeni during malolactic fermentation. Food Microbiol. 2004, 21, 257–265. [Google Scholar] [CrossRef]
- Spano, G.; Rinaldi, A.; Ugliano, M.; Moio, L.; Beneduce, L.; Massa, S. A b-glucosidase gene isolated from wine Lactobacillus plantarum is regulated by abiotic stresses. J. Appl. Microbiol. 2005, 98, 855–861. [Google Scholar] [CrossRef]
- Michlmayr, H.; Schümann, C.; Barreira Braz Da Silva, N.M.; Kulbe, K.D.; Del Hierro, A.M. Isolation and basic characterization of a b-glucosidase from a strain of Lactobacillus brevis isolated from a malolactic starter culture. J. Appl. Microbiol. 2010, 108, 550–559. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Hollmann, A.; Brizuela, N.; La Hens, D.V.; Tymczyszyn, E.; Semorile, L. Growth and consumption of l-malic acid in wine-like medium by acclimated and non-acclimated cultures of Patagonian Oenococcus oeni strains. Folia Microbiol. 2016, 61, 365–373. [Google Scholar] [CrossRef]
- Fia, G.; Millarini, V.; Granchi, L.; Bucalossi, G.; Guerrini, S.; Zanoni, B.; Rosi, I. Beta-glucosidase and esterase activity from Oenococcus oeni: Screening and evaluation during malolactic fermentation in harsh conditions. Food Sci. Technol. 2018, 89, 262–268. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Fan, L.; Wang, F.; Liu, X.; Zhang, H.; Zhou, J. Assessment of β-D glucosidase activity and bgl gene expression of Oenococcus oeni SD-2a. PLoS ONE 2020, 15, e0240484. [Google Scholar] [CrossRef]
- López-Seijas, J.; García-Fraga, B.; da Silva, A.F.; Zas-García, X.; Lois, L.C.; Gago-Martínez, A. Evaluation of malolactic bacteria associated with wines from Albariño variety as potential starters: Screening for quality and safety. Foods 2020, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, N.S.; Arnez-Arancibia, M.; Semorile, L.; Pozo-Bayón, M.Á.; Bravo-Ferrada, B.M.; Tymczyszyn, E.E. β-Glucosidase activity of Lactiplantibacillus plantarum UNQLp 11 in different malolactic fermentations conditions: Effect of pH and ethanol content. Fermentation 2021, 7, 22. [Google Scholar] [CrossRef]
- Olguin., N.; Alegret, J.O.; Bordons, A.; Reguant, C. β-Glucosidase activity and bgl gene expression of Oenococcus oeni strains in model media and cabernet sauvignon wine. Am. J. Enol. Vitic. 2011, 62, 99–105. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Laurent, M.H.; Henick-Kling, T.; Acree, T.E. Changes in the aroma and odor of Chardonnay wines due to malolactic fermentation. Wein-Wiss 1994, 49, 3–10. [Google Scholar]
- De Revel, G.; Martin, N.; Pripis-Nicolau, L.; Lonvaud-Funel, A.; Bertrand, A. Contribution to the knowledge of malolactic fermentation influence on wine aroma. J. Agric. Food Chem. 1999, 47, 4003–4008. [Google Scholar] [CrossRef]
- Maicas, S.; Gil, J.V.; Pardo, I.; Ferrer, S. Improvement of volatile composition of wines by controlled addition of malolactic bacteria. Food Res. Int. 1999, 32, 491–496. [Google Scholar] [CrossRef]
- Delaquis, P.; Cliff, M.; King, M.; Girard, B.; Hall, J.; Reynolds, A. Effect of two commercial malolactic cultures on the chemical and sensory properties of Chancellor wines vinified with different yeasts and fermentation temperatures. Am. J. Enol. Vitic. 2000, 51, 42–48. [Google Scholar]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Gambaro, A.; Boido, E.; Zlotejablko, A.; Medina, K.; Lloret, A.; Dellacassa, E.; Carrau, F. Effect of malolactic fermentation on the aroma properties of Tannat wine. Aust. J. Grape Wine Res. 2001, 7, 27–32. [Google Scholar] [CrossRef]
- Vilanova, M.; Martinez, C. First study of determination of aromatic compounds of red wine from Vitis vinifera cv. Castanãl grown in Galica (NW Spain). Eur. Food Res. Technol. 2007, 224, 431–436. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Lavilla, M.; Amarita, F. Wine aroma profile modification by Oenococcus oeni strains from Rioja Alavesa region: Selection of potential malolactic starters. Int. J. Food Microbiol. 2021, 356, 109324. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.; Grbin, P.R.; Jiranek, V. A survey of lactic acid bacteria for enzymes of interest to oenology. Aust. J. Grape Wine Res. 2006, 12, 235–244. [Google Scholar] [CrossRef]
- Pérez-Martín, F.; Seseña, S.; Izquierdo, P.M.; Palop, M.L. Esterase activity of lactic acid bacteria isolated from malolactic fermentation of red wines. Int. J. Food Microbiol. 2013, 163, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Diez-Ozaeta, I.; Lavilla, M.; Amarita, F. Technological characterisation of potential malolactic starters from Rioja Alavesa winemaking region. LWT-Food Sci. Technol. 2020, 134, 109916. [Google Scholar] [CrossRef]
- Gammacurta, M.; Lytra, G.; Marchal, A.; Marchand, S.; Barbe, J.C.; Moine, V.; de Revel, G. Influence of lactic acid bacteria strains on ester concentrations in red wines: Specific impact on branched hydroxylated compounds. Food Chem. 2018, 239, 252–259. [Google Scholar] [CrossRef]
- Matthews, A.; Grbin, P.; Jiranek, V. Biochemical characterisation of the esterase activities of wine lactic acid bacteria. Appl. Microbiol. Biotechnol. 2007, 77, 329–337. [Google Scholar] [CrossRef]
- Collombel, I.; Melkonian, C.; Molenaar, D.; Campos, F.M.; Hogg, T. New insights into cinnamoyl esterase activity of Oenococcus oeni. Front. Microbiol. 2019, 10, 2597. [Google Scholar] [CrossRef]
- Chescheir, S.; Philbin, D.; Osborne, J.P. Impact of Oenococcus oeni on wine hydroxycinnamic acids and volatile phenol production by Brettanomyces bruxellensis. Am. J. Enol. Viticult. 2015, 66, 357–362. [Google Scholar] [CrossRef]
- Madsen, M.G.; Edwards, N.K.; Petersen, M.A.; Mokwena, L.; Swiegers, J.H.; Arneborg, N. Influence of Oenococcus oeni and Brettanomyces bruxellensis on hydroxycinnamic acids and volatile phenols of aged wine. Am. J. Enol. Viticult. 2016, 68, 23–29. [Google Scholar] [CrossRef]
- Zepeda-Mendoza, M.L.; Edwards, N.K.; Madsen, M.G.; Abel-Kistrup, M.; Puetz, L.; Sicheritz-Ponten, T.; Swiegers, J.H. Influence of Oenococcus oeni and Brettanomyces bruxellensis aged wine microbial taxonomic and functional potential profiles. Am. J. Enol. Viticult. 2018, 69, 321–333. [Google Scholar] [CrossRef]
- Rozes, N.; Arola, L.; Bordons, A. Effect of phenolic compounds on the co-metabolism of citric acid and sugars by Oenococcus oeni from wine. Lett. Appl. Microbiol. 2003, 36, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Medina de Figueroa, R.B.; Alvarez, F.; Pesce de Ruiz Holgado, A.; Oliver, G.; Sesma, F. Citrate utilization by homo- and heterofermentative lactobacilli. Microbiol. Res. 2000, 154, 313–320. [Google Scholar] [CrossRef]
- Palles, T.; Beresford, T.; Condon, S.; Cogan, T. Citrate metabolism in Lactobacillus casei and Lactobacillus plantarum. J. Appl. Microbiol. 1998, 85, 147–154. [Google Scholar] [CrossRef]
- Pretorius, N.; Engelbrecht, L.; Du Toit, M. Influence of sugars and pH on the citrate metabolism of different lactic acid bacteria strains in a synthetic wine matrix. J. Appl. Microbiol. 2019, 127, 1490–1500. [Google Scholar] [CrossRef]
- Welman, A.D.; Maddox, I.S. Exopolysaccharides from lactic acid bacteria: Perspectives and challenges. Trends Biotechnol. 2003, 21, 269–274. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Dols-Lafargue, M. Exopolysaccharides producing lactic acid bacteria in wine and other fermented beverages: For better or for worse? Foods 2021, 10, 2204. [Google Scholar] [CrossRef]
- Lonvaud-Funel, A. Lactic acid bacteria and the quality improvement and depreciation of wine. Antonie Van Leeuwenhoek 1999, 76, 317–331. [Google Scholar] [CrossRef]
- Walling, E.; Gindreau, E.; Lonvaud Funel, A. La biosynthese d’exopolysaccharides par des souches de Pediococcus damnosus isolees du vin Mise au point d’outils moleculaires de detectio. Lait 2001, 289, 289–300. [Google Scholar] [CrossRef][Green Version]
- Coulon, J.; Houlès, A.; Dimopoulou, M.; Maupeu, J.; Dols-Lafargue, M. Lysozyme resistance of the ropy strain Pediococcus parvulus IOEB 8801 is correlated with beta-glucan accumulation around the cell. Int. J. Food Microbiol. 2012, 159, 25–29. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Raffenne, J.; Claisse, O.; Miot-Sertier, C.; Iturmendi, N.; Moine, V.; Coulon, J.; Dols-Lafargue, M. Oenococcus oeni exopolysaccharide biosynthesis, a tool to improve malolactic starter performance. Front. Microbiol. 2018, 9, 1276. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, D.; Bosscher, J.S.; Ten Brink, B.; Driessen, A.J.M.; Konings, W.N. Generation of a proton motive force by histidine decarboxylation and electrogenic histidine/histamine antiport in Lactobacillus buchneri. J. Bacteriol. 1993, 175, 2864–2870. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion on risk-based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393–2486. [Google Scholar] [CrossRef]
- Soksawatmaekhin, W.; Kuraishi, A.; Sakata, K.; Kashiwagi, K.; Igarashi, K. Excretion and uptake of cadaverine by CadB and its physiological functions in Escherichia coli. Mol. Microbiol. 2004, 51, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Wolken, W.A.; Lucas, P.M.; Lonvaud-Funel, A.; Lolkema, J.S. The mechanism of the tyrosine transporter TyrP supports a proton motive tyrosine decarboxylation pathway in Lactobacillus brevis. J. Bacteriol. 2006, 188, 2198–2206. [Google Scholar] [CrossRef]
- Landete, J.M.; Ferrer, S.; Pardo, I. Biogenic amine production by lactic acid bacteria, acetic bacteria and yeast isolated from wine. Food Control 2007, 18, 1569–1574. [Google Scholar] [CrossRef]
- Callejon, S.; Sendra, R.; Ferrer, S.; Pardo, I. Identification of a novel enzymatic activity from lactic acid bacteria able to degrade biogenic amines in wine. Appl. Microbiol. Biotechnol. 2014, 98, 185–198. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Ladero, V.; Fernandez, M.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic amines degradation by Lactobacillus plantarum: Toward a potential application in wine. Front. Microbiol. 2012, 3, 122. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Ray, R.C. Biogenic Amines in Wine. In Winemaking: Basics and Applied Aspects; Joshi, V.K., Ray, R.C., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2021; pp. 452–467. [Google Scholar]
- Landete, J.M.; Pardo, I.; Ferrer, S. Regulation of hdc expression and HDC activity by enological factors in lactic acid bacteria. J. Appl. Microbiol. 2008, 105, 1544–1551. [Google Scholar] [CrossRef]
- Xue, J.; Fu, F.; Liang, M.; Zhao, C.; Wang, D.; Wu, Y. Ethyl carbamate production kinetics during wine storage. S. Afr. J. Enol. Vitic. Stellenbosch. 2015, 36, 277–284. [Google Scholar] [CrossRef][Green Version]
- Leca, J.M.; Pereira, V.; Miranda, A.; Vilchez, J.L.; Marques, J.C. New insights into ethyl carbamate occurrence in fortified wines. LWT-Food Sci. Technol. 2021, 150, 111566. [Google Scholar] [CrossRef]
- Sumby, K.; Bartle, L.; Grbin, P.; Jiranek, V. Measures to improve wine malolactic fermentation. Appl. Microbiol. Biotechnol. 2019, 103, 2033–2051. [Google Scholar] [CrossRef] [PubMed]
- Bou, M.; Krieger, S. Alcohol-Tolerant Malolactic Strains for the Maturation of Wines with Average or High pH. U.S. Patent 7,625,745, 14 February 2012. [Google Scholar]
- Krieger-Weber, S.; Heras, J.M.; Suarez, C. Lactobacillus plantarum, a new biological tool to control malolactic fermentation: A review and an outlook. Beverages 2020, 6, 23. [Google Scholar] [CrossRef]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT-Food Sci. Technol. 2016, 73, 557–566. [Google Scholar] [CrossRef]
- Wade, M.E.; Strickland, M.T.; Osborne, J.P.; Edwards, C.G. Role of Pediococcus in winemaking. Aust. J. Grape Wine Res. 2018, 25, 7–24. [Google Scholar] [CrossRef]
- Juega, M.; Costantini, A.; Bonello, F.; Cravero, M.C.; Martinez-Rodriguez, A.J.; Carrascosa, A.V.; Garcia-Moruno, E. Effect of malolactic fermentation by Pediococcus damnosus on the composition and sensory profile of Albariño and Caiño white wines. J. Appl. Microbiol. 2014, 116, 586–595. [Google Scholar] [CrossRef]
- Strickland, M.T.; Schopp, L.M.; Edwards, C.G.; Osborne, J.P. Impact of Pediococcus spp. on Pinot noir wine quality and growth of Brettanomyces. Am. J. Enol. Vitic. 2016, 67, 188–198. [Google Scholar] [CrossRef]
- Brizuela, N.S.; Franco-Luesma, E.; Bravo-Ferrada, B.M.; Pérez-Jiménez, M.; Semorile, L.; Tymczyszyn, E.E.; Pozo-Bayon, M.A. Influence of Patagonian Lactiplantibacillus plantarum and Oenococcus oeni strains on sensory perception of Pinot Noir wine after malolactic fermentation. Aust. J. Grape Wine Res. 2021, 27, 118–127. [Google Scholar] [CrossRef]
- Bartle, L.; Sumby, K.; Sundstrom, J.; Jiranek, V. The microbial challenge of winemaking: Yeast-bacteria compatibility. FEMS Yeast Res. 2019, 19, foz040. [Google Scholar] [CrossRef]
- Lucio, O.; Pardo, I.; Heras, J.M.; Krieger-Weber, S.; Ferrer, S. Use of starter cultures of Lactobacillus to induce malolactic fermentation in wine. Aust. J. Grape Wine Res. 2017, 23, 15–21. [Google Scholar] [CrossRef]
- Comitini, F.; Ciani, M. The inhibitory activity of wine yeast starters on malolactic bacteria. Ann. Microbiol. 2007, 57, 61–66. [Google Scholar] [CrossRef]
- Bayrock, D.P.; Ingledew, W.M. Inhibition of yeast by lactic acid bacteria in continuous culture: Nutrient depletion and/or acid toxicity? J. Ind. Microbiol. Biotechnol. 2004, 31, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo Canas, P.M.; García Romero, E.; Perez-Martín, F.; Prieto, S.; Palop Herreros, M.L. Sequential inoculation versus co-inoculation in Cabernet Franc wine fermentation. Food Sci. Technol. Int. 2014, 21, 1–10. [Google Scholar]
- Versari, A.; Patrizi, C.; Parpinello, G.P.; Mattioli, A.U.; Pasini, L.; Meglioli, M.; Longhini, G. Effect of co-inoculation with yeast and bacteria on chemical and sensory characteristics of commercial Cabernet Franc red wine from Switzerland. J. Chem. Technol. Biotechnol. 2015, 91, 876–882. [Google Scholar] [CrossRef]
- Jussier, D.; Morneau, A.D.; de Orduna, R.M. Effect of simultaneous inoculation with yeast and bacteria on fermentation kinetics and key wine parameters of cool-climate Chardonnay. Appl. Environ. Microbiol. 2006, 72, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Testa, B.; Succi, M.; Tremonte, P.; Sorrentino, E.; Di Renzo, M.; Strollo, D.; Coppola, R. Inoculum strategies and performances of malolactic starter Lactobacillus plantarum M10: Impact on chemical and sensorial characteristics of Fiano Wine. Microorganisms 2020, 8, 516. [Google Scholar] [CrossRef]
- Guzzon, R.; Moser, S.; Davide, S.; Villegas, T.R.; Malacarne, M.; Larcher, R.; Nardi, T.; Vagnoli, P.; Krieger-Weber, S. Exploitation of simultaneous alcoholic and malolactic fermentation of Incrocio Manzoni, a traditional Italian white wine. S. Afr. J. Enol. Vitic. 2016, 37, 124–131. [Google Scholar] [CrossRef][Green Version]
- Mendoza, L.; Merín, M.; Morata, V.; Farías, M. Characterization of wines produced by mixed culture of autochthonous yeasts and Oenococcus oeni from the northwest region of Argentina. J. Ind. Microbiol. Biotechnol. 2011, 38, 1777–1785. [Google Scholar] [CrossRef]
- Massera, A.; Soria, A.; Catania, C.; Krieger, S.; Combina, M. Simultaneous inoculation of Malbec (Vitis vinifera) musts with yeast and bacteria: Effects on fermentation performance, sensory and sanitary attributes of wines. Food Technol. Biotechnol. 2009, 47, 192–201. [Google Scholar]
- Hu, K.; Zhao, H.; Kang, X.; Ge, X.; Zheng, M.; Hu, Z.; Tao, Y. Fruity aroma modifications in Merlot wines during simultaneous alcoholic and malolactic fermentations through mixed culture of S. cerevisiae, P. fermentans, and L. brevis. LWT-Food Sci. Technol. 2022, 154, 112711. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.C.; de Revel, G. Co-inoculation with yeast and LAB under winery conditions: Modification of the aromatic profile of Merlot wines. S. Afr. J. Enol. Vitic. 2013, 34, 223–232. [Google Scholar] [CrossRef]
- Tristezza, M.; di Feo, L.; Tufariello, M.; Grieco, F.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. Simultaneous inoculation of yeasts and lactic acid bacteria: Effects on fermentation dynamics and chemical composition of Negroamaro wine. LWT-Food Sci. Technol. 2016, 66, 406–412. [Google Scholar] [CrossRef]
- Knoll, C.; Fritsch, S.; Schnell, S.; Grossmann, M.; Krieger-Weber, S.; du Toit, M.; Rauhut, D. Impact of different malolactic fermentation inoculation scenarios on Riesling wine aroma. World J. Microbiol. Biotechnol. 2012, 28, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Anu-Appaiah, K.A. Mixed malolactic co-culture (Lactobacillus plantarum and Oenococcus oeni) with compatible Saccharomyces influences the polyphenolic, volatile and sensory profile of Shiraz wine. LWT-Food Sci. Technol. 2021, 135, 110246. [Google Scholar] [CrossRef]
- Abrahamse, C.E.; Bartowsky, E.J. Timing of malolactic fermentation inoculation in Shiraz grape must and wine: Influence on chemical composition. World J. Microbiol. Biotechnol. 2012, 28, 255–265. [Google Scholar] [CrossRef]
- Munoz, V.; Beccaria, B.; Abreo, E. Simultaneous and successive inoculations of yeasts and lactic acid fermentation of an unsulfited Tannat grape must. Braz. J. Microbiol. 2014, 45, 59–66. [Google Scholar] [CrossRef]
- Izquierdo-Cañas, P.M.; Ríos-Carrasco, M.; García-Romero, E.; Mena-Morales, A.; Heras-Manso, J.M.; Cordero-Bueso, G. Co-existence of inoculated yeast and lactic acid bacteria and their impact on the aroma profile and sensory traits of Tempranillo red wine. Fermentation 2020, 6, 17. [Google Scholar] [CrossRef]
- Izquierdo Cañas, P.M.; Pérez-Martín, F.; Romero, E.G.; Prieto, S.S.; Herreros, M.L.P. Influence of inoculation time of an autochthonous selected malolactic bacterium on volatile and sensory profile of Tempranillo and Merlot wines. Int. J. Food Microbiol. 2012, 156, 245–254. [Google Scholar] [CrossRef]
- Plavsa, T.; Jagatic Korenika, A.-M.; Lukic, I.; Bubola, M.; Jeromel, A. Influence of different malolactic fermentation techniques on changes in chemical properties and volatile compounds of cv. Teran red wine (Vitis vinifera L.). J. Cent. Eur. Agric. 2021, 22, 582–595. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).