Deep Ocean Water Minerals Promotes the Growth and Cordycepin Production of Cordyceps militaris Fruiting Bodies through Proteomics Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Various Deep Ocean Water Concentrations
2.3. Microorganism and Seed Cultures
2.4. Solid Fermentation of C. militaris in Deep Ocean Water or Ultrapure Water
2.5. Determination of Cordycepin and Adenosine
2.6. Protein Extraction
2.7. 2D Gel Electrophoresis
2.8. Statistical Analysis
3. Results
3.1. Mineral Composition of Deep Ocean Water
3.2. Effect of Deep Ocean Water on the Biomass of C. militaris Fruiting Bodies and the Content of Functional Components
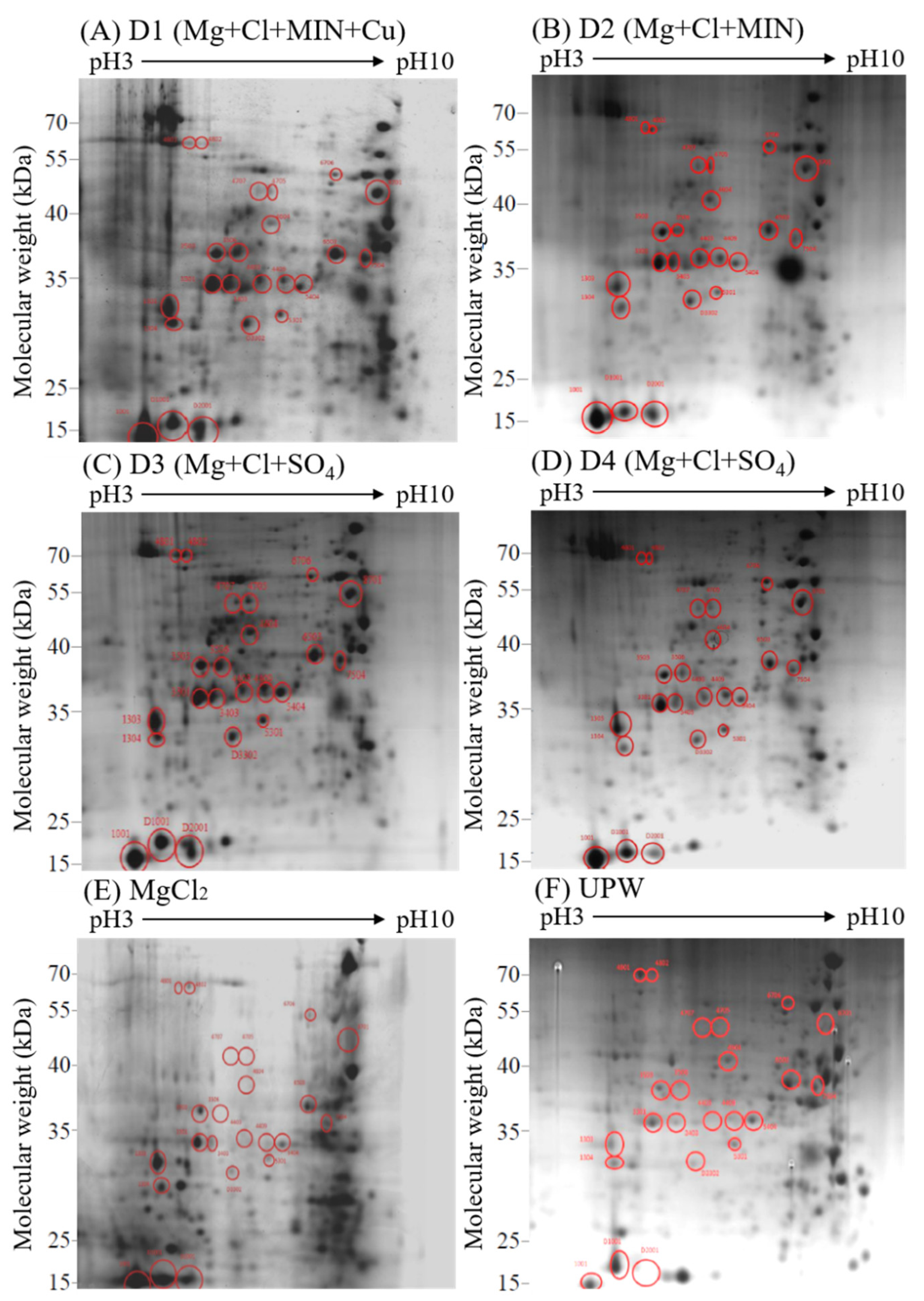
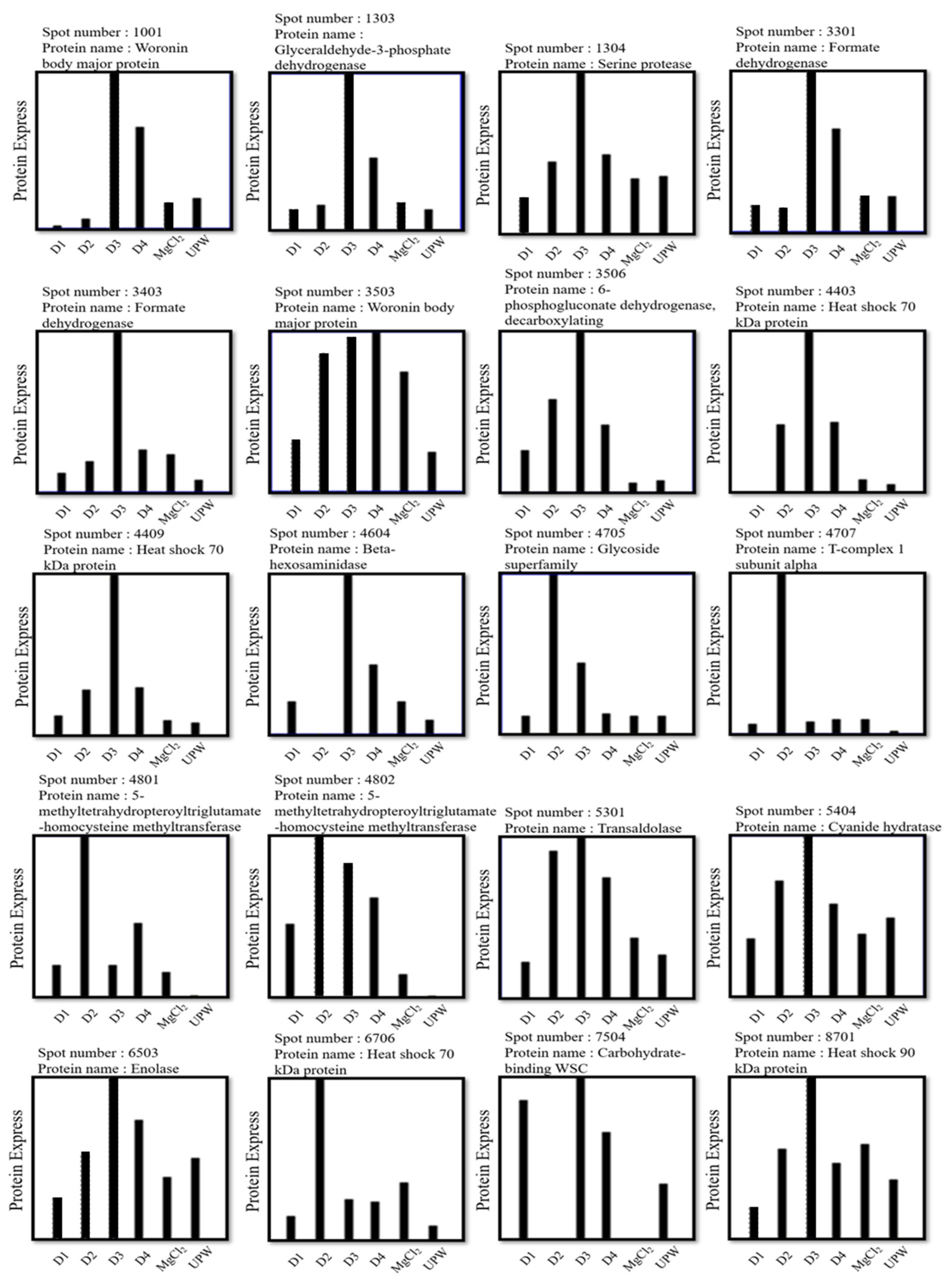
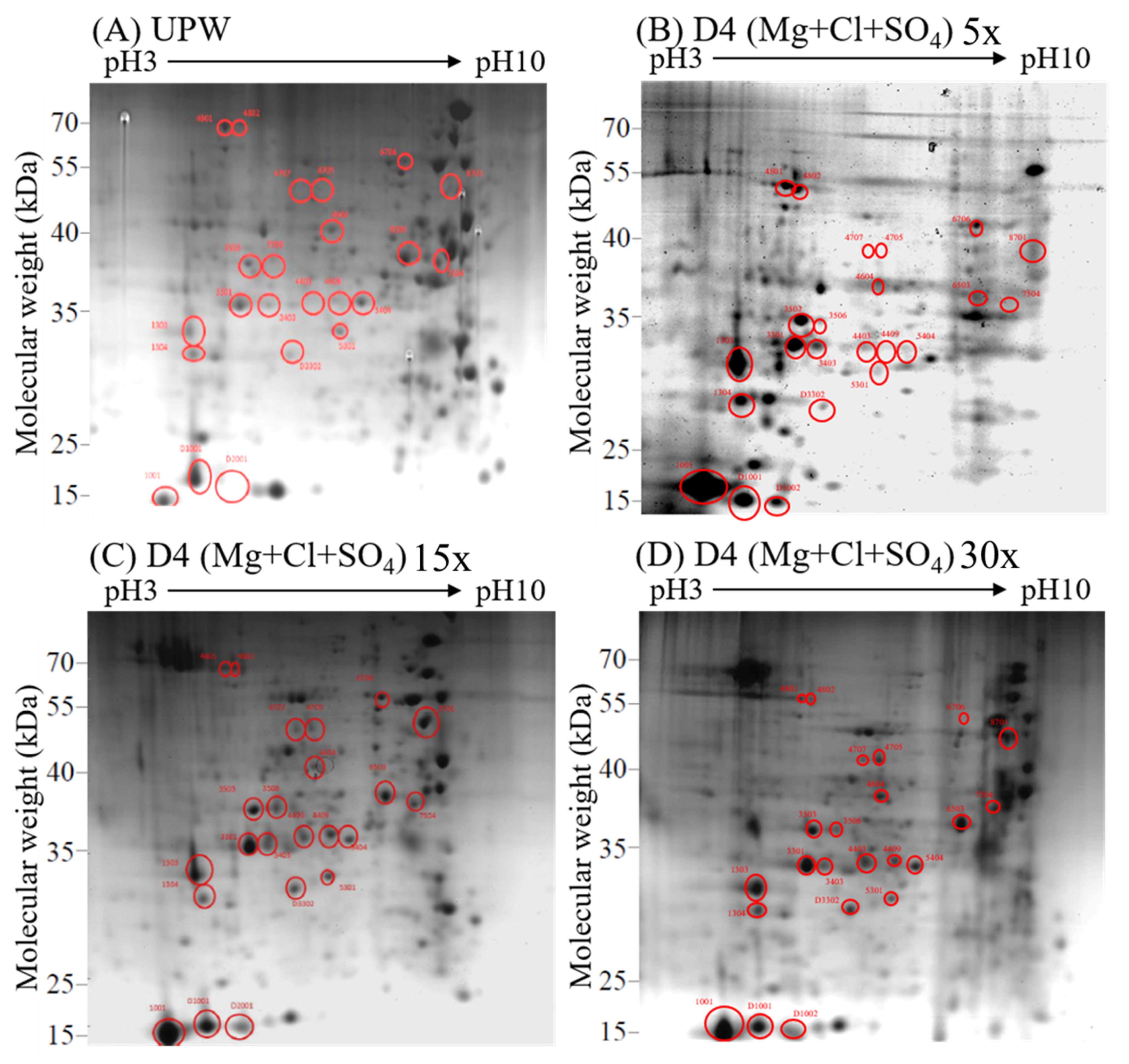
3.3. Effects of Deep Ocean Water with Different Mineral Compositions on the Protein Expression of C. militaris Fruiting Bodies
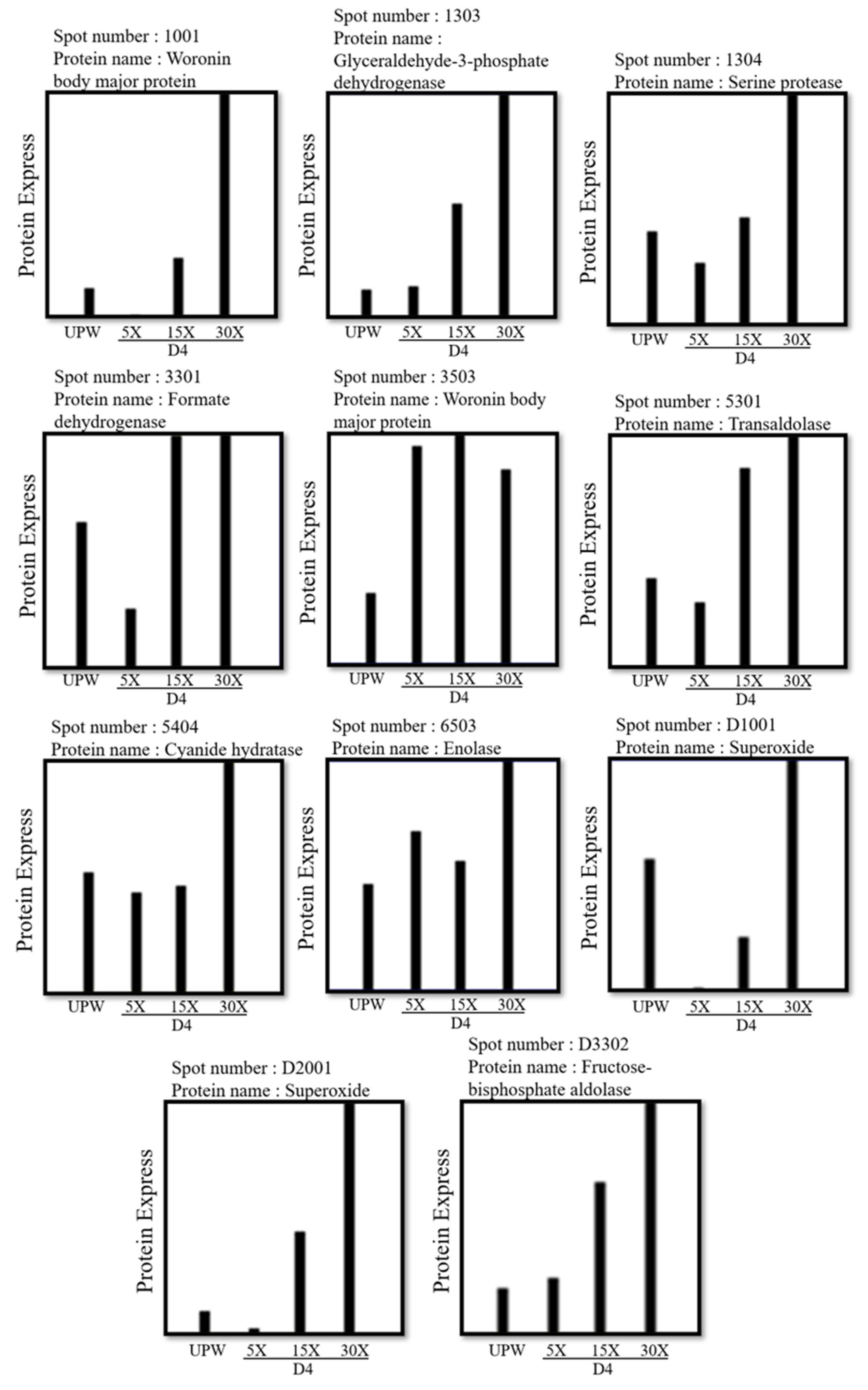
3.4. Effects of Different Concentrations of Deep Ocean Water on the Expression of C. militaris Fruiting Body Proteasome
4. Discussions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, H.S.; Kim, H.A.; Lee, S.H.; Yun, J.W. Anti-obesity and antidiabetic effects of deep sea water on ob/ob mice. Mar. Biotechnol. 2009, 11, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, S.; Yasukawa, T.; Nakagawa, K.; Miyake, M.; Yamasaki, M.; Katahira, K.; Mohri, M.; Shimizu, T.; Hazama, A. Deep-sea water improves cardiovascular hemodynamics in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol. Pharm. Bull. 2008, 31, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Lee, C.L. Comparison of the Improvement Effect of Deep Ocean Water with Different Mineral Composition on the High Fat Diet-Induced Blood Lipid and Nonalcoholic Fatty Liver Disease in a Mouse Model. Nutrients 2021, 13, 1732. [Google Scholar] [CrossRef] [PubMed]
- Lung, T.Y.; Liao, L.Y.; Wang, J.J.; Wei, B.L.; Huang, P.Y.; Lee, C.L. Metals of Deep Ocean Water Increase the Anti-Adipogenesis Effect of Monascus-Fermented Product via Modulating the Monascin and Ankaflavin Production. Mar. Drugs 2016, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, S.; Lee, D.Y.; Lee, C. Increasing anti-Abeta-induced neurotoxicity ability of Antrodia camphorata-fermented product with deep ocean water supplementary. J. Sci. Food Agric. 2016, 96, 4690–4701. [Google Scholar] [CrossRef]
- Wang, L.C.; Kuo, I.U.; Tsai, T.Y.; Lee, C.L. Antrodia camphorata-fermented product cultured in deep ocean water has more liver protection against thioacetamide-induced fibrosis. Appl. Microbiol. Biotechnol. 2013, 97, 9955–9967. [Google Scholar] [CrossRef]
- Lee, C.L. The advantages of deep ocean water for the development of functional fermentation food. Appl. Microbiol. Biotechnol. 2015, 99, 2523–2531. [Google Scholar] [CrossRef]
- Wang, L.C.; Lung, T.Y.; Kung, Y.H.; Wang, J.J.; Tsai, T.Y.; Wei, B.L.; Pan, T.M.; Lee, C.L. Enhanced anti-obesity activities of red mold dioscorea when fermented using deep ocean water as the culture water. Mar. Drugs 2013, 11, 3902–3925. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Lee, C.L. Cordyceps cicadae NTTU 868 Mycelium with The Addition of Bioavailable Forms of Magnesium from Deep Ocean Water Prevents the Aβ40 and Streptozotocin-induced Memory Deficit via Suppressing Alzheimer’s Disease Risk Factors and Increasing Magnesium Uptake of Brain. Fermentation 2021, 7, 39. [Google Scholar]
- Hung, Y.P.; Wang, J.J.; Wei, B.L.; Lee, C.L. Effect of the salts of deep ocean water on the production of cordycepin and adenosine of Cordyceps militaris-fermented product. AMB Express 2015, 5, 140. [Google Scholar] [CrossRef]
- Hung, Y.P.; Lee, C.L. Higher Anti-Liver Fibrosis Effect of Cordyceps militaris-Fermented Product Cultured with Deep Ocean Water via Inhibiting Proinflammatory Factors and Fibrosis-Related Factors Expressions. Mar. Drugs 2017, 15, 168. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Lee, S.Y.; Sung, G.H.; Kim, S.H.; Choi, H.K. Metabolic profiles and free radical scavenging activity of Cordyceps bassiana fruiting bodies according to developmental stage. PLoS ONE 2013, 8, e73065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Xu, Y.; Shen, J. Cordycepin inhibits lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-alpha production via activating amp-activated protein kinase (AMPK) signaling. Int. J. Mol. Sci. 2014, 15, 12119–12134. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Yoo, S.K.; Kim, S.W.; Hwang, S.Y.; Sohn, S.H.; Kim, I.W.; Kim, S.K. Cordycepin (3’-deoxyadenosine) attenuates age-related oxidative stress and ameliorates antioxidant capacity in rats. Exp. Gerontol 2012, 47, 979–987. [Google Scholar] [CrossRef]
- Raethong, N.; Laoteng, K.; Vongsangnak, W. Uncovering global metabolic response to cordycepin production in Cordyceps militaris through transcriptome and genome-scale network-driven analysis. Sci. Rep. 2018, 8, 9250. [Google Scholar] [CrossRef]
- Xia, Y.; Luo, F.; Shang, Y.; Chen, P.; Lu, Y.; Wang, C. Fungal Cordycepin Biosynthesis Is Coupled with the Production of the Safeguard Molecule Pentostatin. Cell Chem. Biol. 2017, 24, 1479–1489.e4. [Google Scholar] [CrossRef]
- Feng, K.; Wang, L.Y.; Liao, D.J.; Lu, X.P.; Hu, D.J.; Liang, X.; Zhao, J.; Mo, Z.Y.; Li, S.P. Potential molecular mechanisms for fruiting body formation of Cordyceps illustrated in the case of Cordyceps sinensis. Mycology 2017, 8, 231–258. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, F.; Wang, T.; Lyu, Y.M.; Alteen, M.G.; Cai, Z.P.; Cui, Z.L.; Liu, L.; Voglmeir, J. Characterization of Stackebrandtia nassauensis GH 20 Beta-Hexosaminidase, a Versatile Biocatalyst for Chitobiose Degradation. Int. J. Mol. Sci. 2019, 20, 1243. [Google Scholar] [CrossRef]
- Seixas, C.; Cruto, T.; Tavares, A.; Gaertig, J.; Soares, H. CCTalpha and CCTdelta chaperonin subunits are essential and required for cilia assembly and maintenance in Tetrahymena. PLoS ONE 2010, 5, e10704. [Google Scholar] [CrossRef]
- Bertels, L.K.; Fernandez Murillo, L.; Heinisch, J.J. The Pentose Phosphate Pathway in Yeasts-More Than a Poor Cousin of Glycolysis. Biomolecules 2021, 11, 725. [Google Scholar] [CrossRef]
- Beere, H.M.; Wolf, B.B.; Cain, K.; Mosser, D.D.; Mahboubi, A.; Kuwana, T.; Tailor, P.; Morimoto, R.I.; Cohen, G.M.; Green, D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000, 2, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, W.; Pan, Y.; Liu, G. Metabolic engineering of Acremonium chrysogenum for improving cephalosporin C production independent of methionine stimulation. Microb. Cell Factories 2018, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.; Gurr, S.J. Fungi, fungicide discovery and global food security. Fungal Genet. Biol. FGB 2020, 144, 103476. [Google Scholar] [CrossRef] [PubMed]
- Tishkov, V.I.; Popov, V.O. Protein engineering of formate dehydrogenase. Biomol Eng. 2006, 23, 89–110. [Google Scholar] [CrossRef]
- Dumestre, A.; Chone, T.; Portal, J.; Gerard, M.; Berthelin, J. Cyanide Degradation under Alkaline Conditions by a Strain of Fusarium solani Isolated from Contaminated Soils. Appl. Environ. Microbiol. 1997, 63, 2729–2734. [Google Scholar] [CrossRef]
- Leonhardt, Y.; Beck, J.; Ebel, F. Functional characterization of the Woronin body protein WscA of the pathogenic mold Aspergillus fumigatus. Int. J. Med. Microbiol. IJMM 2016, 306, 165–173. [Google Scholar] [CrossRef]
- Wang, Z.S.; Gu, Y.X.; Yuan, Q.S. Effect of nutrition factors on the synthesis of superoxide dismutase, catalase, and membrane lipid peroxide levels in Cordyceps militaris mycelium. Curr. Microbiol. 2006, 52, 74–79. [Google Scholar] [CrossRef]
- Raethong, N.; Wang, H.; Nielsen, J.; Vongsangnak, W. Optimizing cultivation of Cordyceps militaris for fast growth and cordycepin overproduction using rational design of synthetic media. Comput. Struct. Biotechnol. J. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Gu, C.; Cooper, D.M. Ca(2+), Sr(2+), and Ba(2+) identify distinct regulatory sites on adenylyl cyclase (AC) types VI and VIII and consolidate the apposition of capacitative cation entry channels and Ca(2+)-sensitive ACs. J. Biol. Chem. 2000, 275, 6980–6986. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Huang, Z. Structural analysis and antioxidant activities of polysaccharides from cultured Cordyceps militaris. Int. J. Biol. Macromol. 2013, 58, 18–22. [Google Scholar] [CrossRef]
- Yagura, T.; Nagata, I.; Kuma, K.; Uchino, H. Increased cyclic nucleotide phosphodiesterase (PDE) and calmodulin activities in soluble fraction of Graves’ thyroid: Analysis of increase in Ca2+ dependence of PDE activities. J. Clin. Endocrinol. Metab. 1985, 60, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, X.; Wang, S.; Zhu, S.; Rong, R.; Xu, X. Complex effect of zinc oxide nanoparticles on cadmium chloride-induced hepatotoxicity in mice: Protective role of metallothionein. Metallomics 2017, 9, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.D.; Klipp, E.; Cowen, L.E.; Brown, A.J. Fungal Hsp90: A biological transistor that tunes cellular outputs to thermal inputs. Nat. Rev..Microbiol. 2012, 10, 693–704. [Google Scholar] [CrossRef] [PubMed]
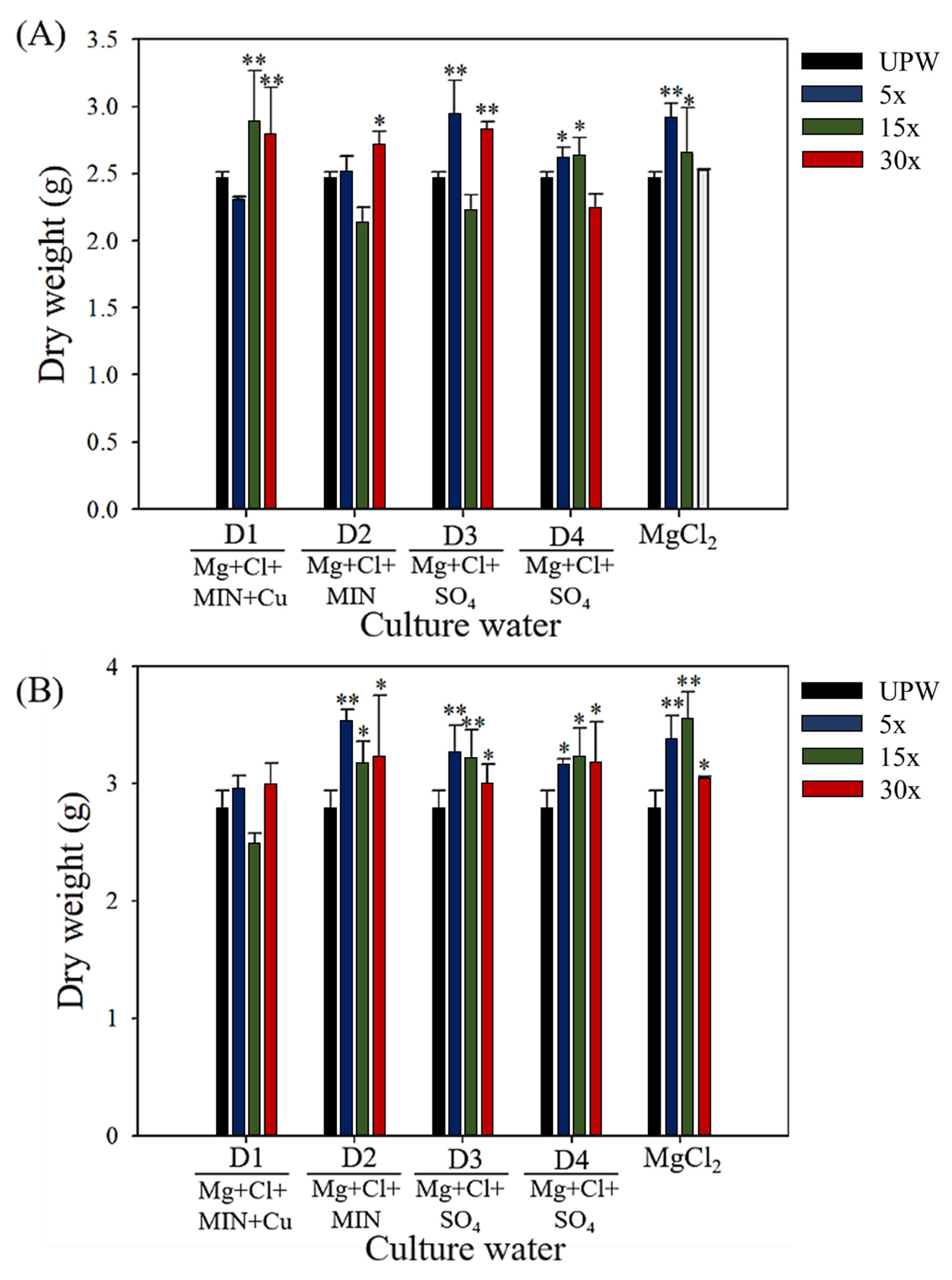

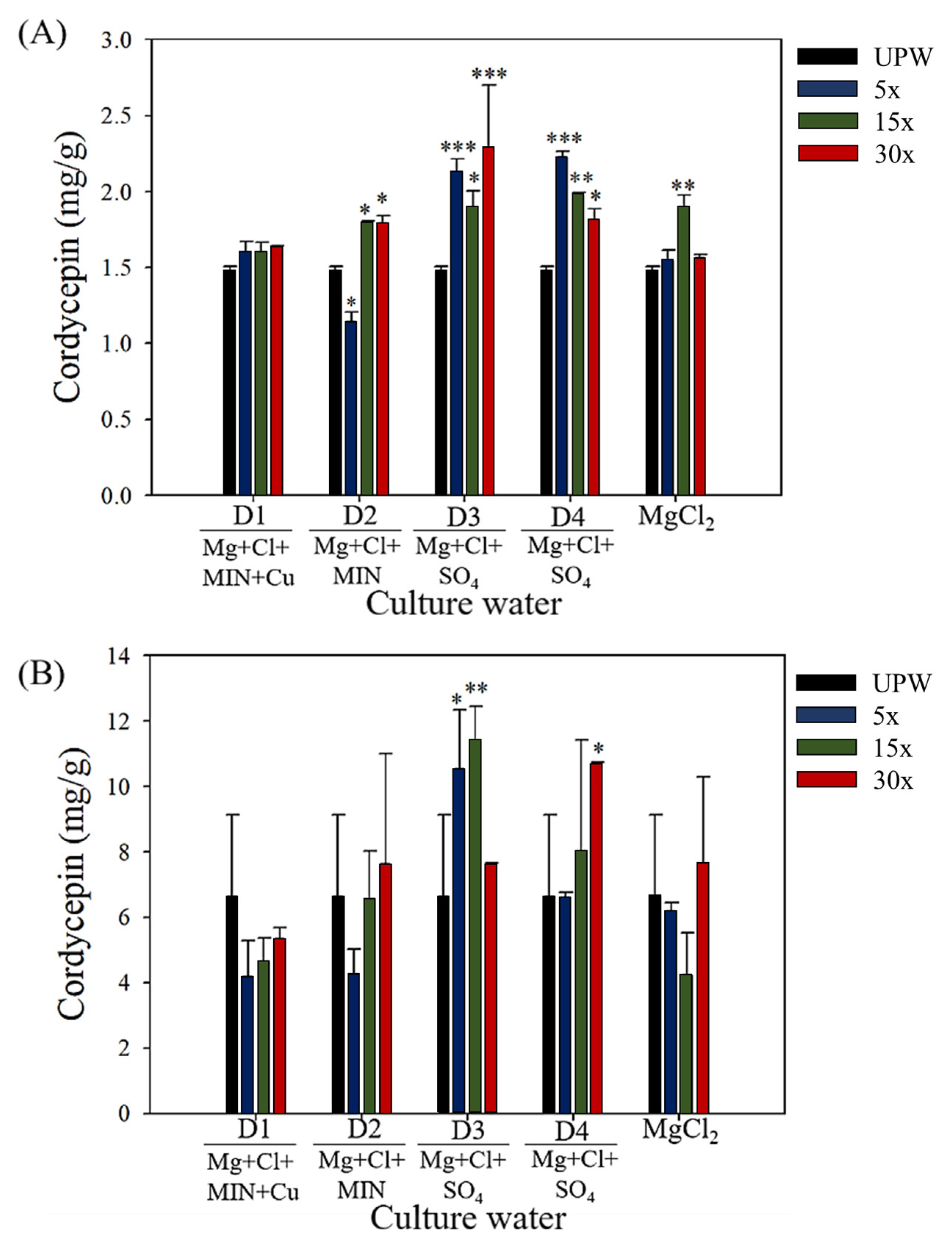
| Elements | D1 (1×) | D2 (1×) | D3 (1×) | D4 (1×) |
|---|---|---|---|---|
| Sodium | 1.22 | 1.13 | 0.37 | 0.42 |
| Potassium | 1.41 | 1.32 | 0.17 | 0.22 |
| Calcium | 6.11 | 5.80 | <0.01 | <0.01 |
| Magnesium | 20.65 | 20.65 | 20.65 | 20.65 |
| Selenium | <0.01 | <0.01 | <0.01 | <0.01 |
| Molybdenum | <0.01 | <0.01 | <0.01 | <0.01 |
| Strontium | 0.12 | 0.12 | <0.01 | <0.01 |
| Iron | <0.01 | <0.01 | <0.01 | <0.01 |
| Zinc | <0.01 | <0.01 | <0.01 | <0.01 |
| Copper | 0.30 | <0.01 | <0.01 | <0.01 |
| Chloride | 66.79 | 62.38 | 61.54 | 46.29 |
| Barium | 4.40 | 5.83 | 1.71 | 1.18 |
| Sulfate | 1.51 | 1.77 | 3.78 | 3.71 |
| Phosphate | <0.01 | <0.01 | <0.01 | <0.01 |
| Silicate | <0.01 | <0.01 | <0.01 | <0.01 |
| Nitrite | <0.01 | <0.01 | <0.01 | <0.01 |
| Nitrate | 0.01 | 0.01 | <0.01 | <0.01 |
| Spot (SPP) | Source | Protein Name | Mass | pI | Peptide Match | Sequence | Score |
|---|---|---|---|---|---|---|---|
| 1001 | Cordyceps militaris | Woronin body major protein | 58,476 | 7.00 | 361(293) | 13 | 10620 |
| 1303 | Cordyceps militaris | Glyceraldehyde-3-phosphate dehydrogenase | 36,053 | 20.67 | 418(280) | 19(19) | 7794 |
| 1304 | Cordyceps militaris | Serine protease | 56,785 | 5.06 | 341(239) | 13(13) | 8478 |
| 3301 | Cordyceps militaris | Formate dehydrogenase | 52,097 | 28.23 | 376(314) | 23(22) | 10811 |
| 3403 | Cordyceps militaris | Formate dehydrogenase | 52,097 | 10.64 | 403(326) | 20(19) | 10124 |
| 3503 | Cordyceps militaris | Woronin body major protein | 58,476 | 14.42 | 277(246) | 25(24) | 9528 |
| 3506 | Cordyceps militaris | 6-phosphogluconate dehydrogenase, decarboxylating | 57,840 | 3.22 | 148(104) | 16(16) | 3499 |
| 4403 | Cordyceps militaris | Heat shock 70 kDa protein | 71,507 | 5.59 | 209(194) | 20(20) | 8436 |
| 4409 | Cordyceps militaris | Heat shock 70 kDa protein | 71,507 | 6.54 | 242(231) | 20(20) | 26577 |
| 4604 | Cordyceps militaris | Beta-hexosaminidase | 6877 | 1.01 | 65(59) | 13(13) | 1833 |
| 4705 | Cordyceps militaris | Glycoside superfamily | 69,104 | 2.04 | 131(115) | 12(12) | 3183 |
| 4707 | Cordyceps militaris | T-complex 1 subunit alpha | 31,407 | 2.88 | 86(78) | 19(19) | 2694 |
| 4801 | Cordyceps militaris | 5-methyltetrahydropteroyltriglutamate -homocysteine methyltransferase | 85,587 | 0.12 | 11(10) | 3(3) | 337 |
| 4802 | Cordyceps militaris | 5-methyltetrahydropteroyltriglutamate -homocysteine methyltransferase | 85,587 | 2.46 | 116(105) | 19(19) | 4006 |
| 5301 | Cordyceps militaris | Transaldolase | 35,163 | 5.63 | 155(119) | 14(14) | 3530 |
| Spot | Protein Name | Function |
|---|---|---|
| Carbohydrate metabolism | ||
| 1303 | Glyceraldehyde-3-phosphate dehydrogenase | Participate in the sixth step of glycolysis. It catalyzes the reaction of G3P into 1,3-BPG [17]. |
| 3506 | 6-phosphogluconate dehydrogenase, decarboxylating | Involved in pentose phosphorylation [17]. |
| 4604 | Beta-hexosaminidase | Hydrolysis of N-terminal acetyl group of hexosamine [18]. |
| 4705 | Glycoside superfamily | Hydrolyzed glycosidic bond [19]. |
| 5301 | Transaldolase | Involved in pentose phosphorylation [20]. |
| 6503 | Enolase | Participate in the ninth step of glycolysis. It catalyzes the reaction of 2-PG into PEP [17]. |
| D3302 | Fructose-bisphosphate aldolase | Participate in glycogenogenesis [17]. |
| Protein metabolism | ||
| 1304 | Serine protease | Folding and breaking down proteins [17]. |
| 4403 4409 6706 | Heat shock 70 kDa protein Heat shock 70 kDa protein Heat shock 70 kDa protein | Participate in protein folding, synthesis and assembly [21]. |
| 4707 | T-complex 1 subunit alpha | Folding proteins [19]. |
| 4801 4802 | 5-methyltetrahydropteroyltriglutamate-homocysteine methyltransferase | Transfer methyl on protein [22]. |
| 8701 | Heat shock 90 kDa protein | Folding proteins [21]. |
| Other functional proteins | ||
| 1001 3503 | Woronin body major protein Woronin body major protein | Block the diaphragm hole to prevent cytoplasm from flowing out [23]. |
| 3301 3403 | Formate dehydrogenase Formate dehydrogenase | Catalyzes one-carbon compounds and reduces NAD+ to NADH [24]. |
| 5405 | Cyanide hydratase | Involved in the metabolism of cyanoamino acids [25]. |
| 7504 | Carbohydrate-binding WSC | May be the area where carbohydrates are recognized [26]. |
| D1001 | Superoxide dismutase | Antioxidative enzyme, protection [27]. |
| D1002 | Superoxide dismutase | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Huang, H.-L.; Chen, Y.-H.; Lee, C.-L. Deep Ocean Water Minerals Promotes the Growth and Cordycepin Production of Cordyceps militaris Fruiting Bodies through Proteomics Regulation. Fermentation 2022, 8, 481. https://doi.org/10.3390/fermentation8100481
Lin C-H, Huang H-L, Chen Y-H, Lee C-L. Deep Ocean Water Minerals Promotes the Growth and Cordycepin Production of Cordyceps militaris Fruiting Bodies through Proteomics Regulation. Fermentation. 2022; 8(10):481. https://doi.org/10.3390/fermentation8100481
Chicago/Turabian StyleLin, Chang-Hong, Hsin-Lun Huang, Yen-Hsun Chen, and Chun-Lin Lee. 2022. "Deep Ocean Water Minerals Promotes the Growth and Cordycepin Production of Cordyceps militaris Fruiting Bodies through Proteomics Regulation" Fermentation 8, no. 10: 481. https://doi.org/10.3390/fermentation8100481
APA StyleLin, C.-H., Huang, H.-L., Chen, Y.-H., & Lee, C.-L. (2022). Deep Ocean Water Minerals Promotes the Growth and Cordycepin Production of Cordyceps militaris Fruiting Bodies through Proteomics Regulation. Fermentation, 8(10), 481. https://doi.org/10.3390/fermentation8100481









