Effect of Metschnikowia pulcherrima on Saccharomyces cerevisiae PDH By-Pass in MixedFermentation with Varied Sugar Concentrations of Synthetic Grape Juice and Inoculation Ratios
Abstract
1. Introduction
2. Materials and Method
2.1. Yeast Strain
2.2. Media
2.3. Fermentation Conditions and Sampling
2.4. Pure Cultures
2.5. Co-Cultures
2.6. Sampling
2.7. Enological Parameter Analysis
2.8. Enzyme Activity Analysis
2.9. Quantitative Real-Time PCR
2.10. Statistical Analysis
3. Results
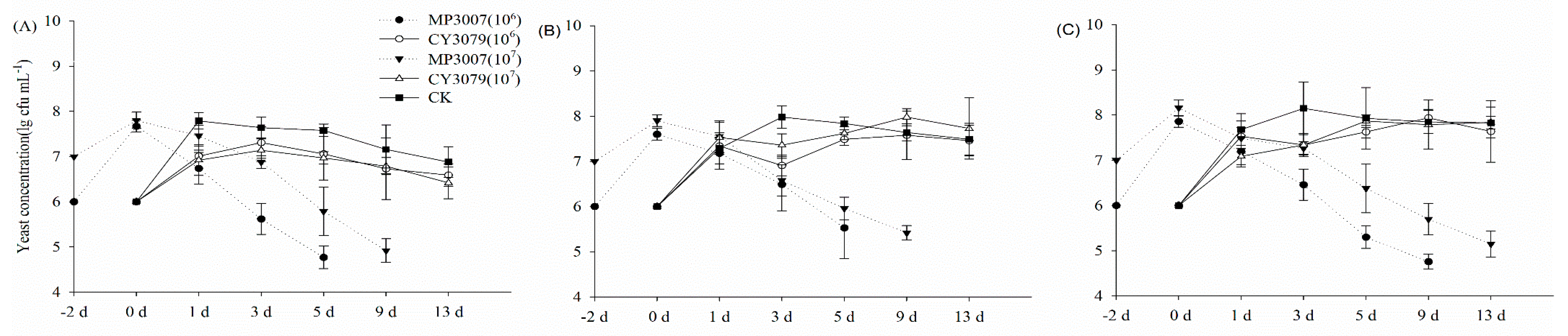
3.1. Fermentation Behavior of Pure and Sequential Cultures
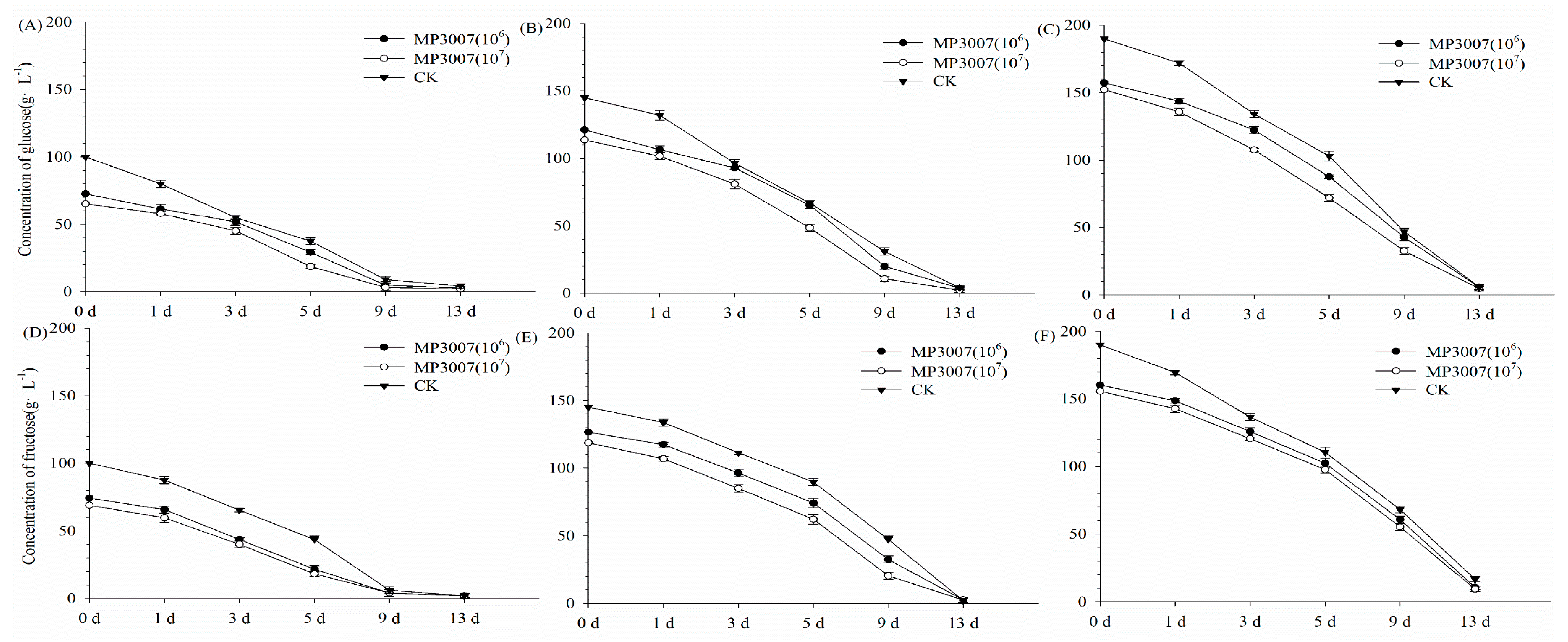
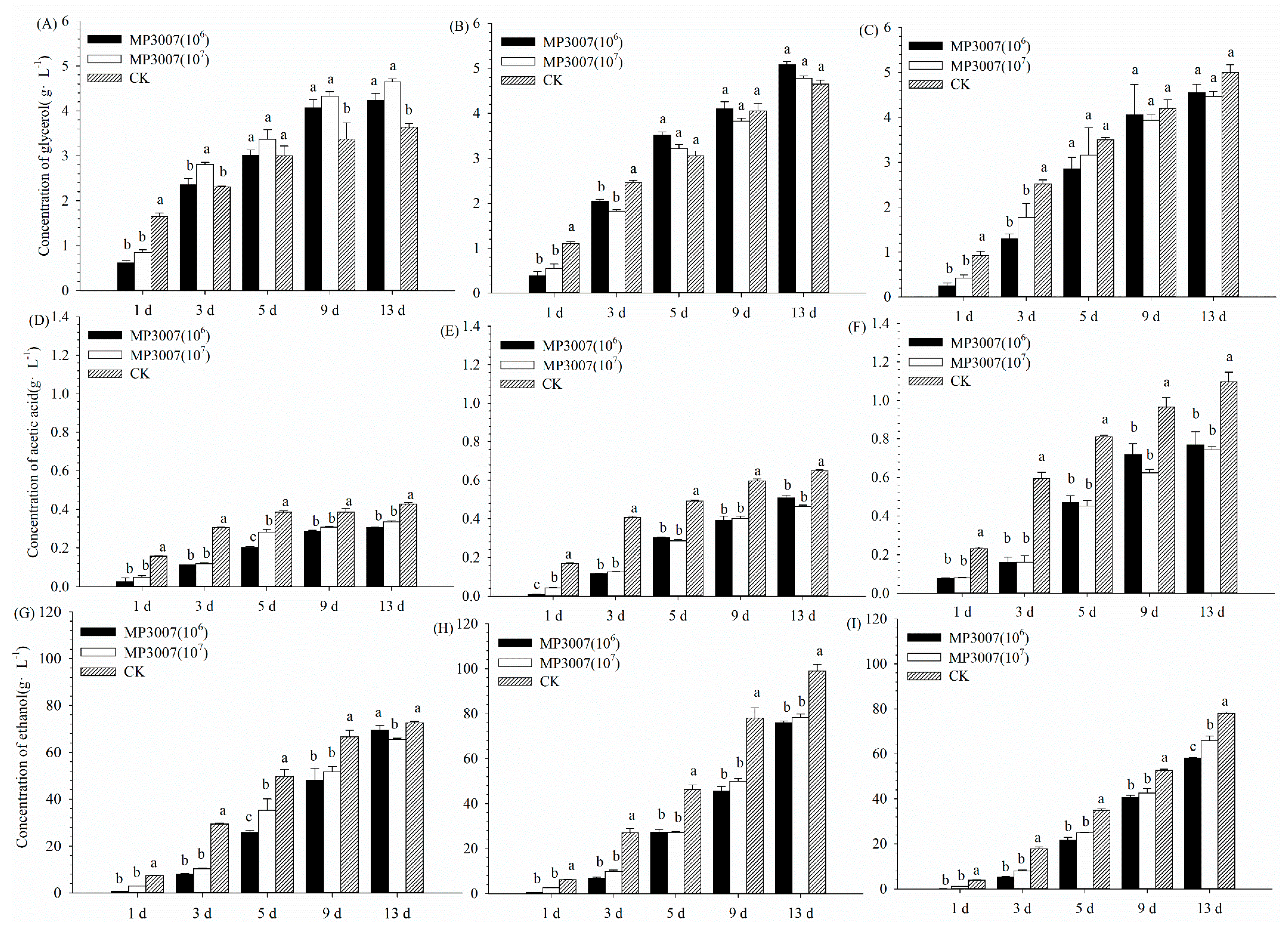
3.2. Metabolite Concentration in the Pure and Mixed Fermentation Processes
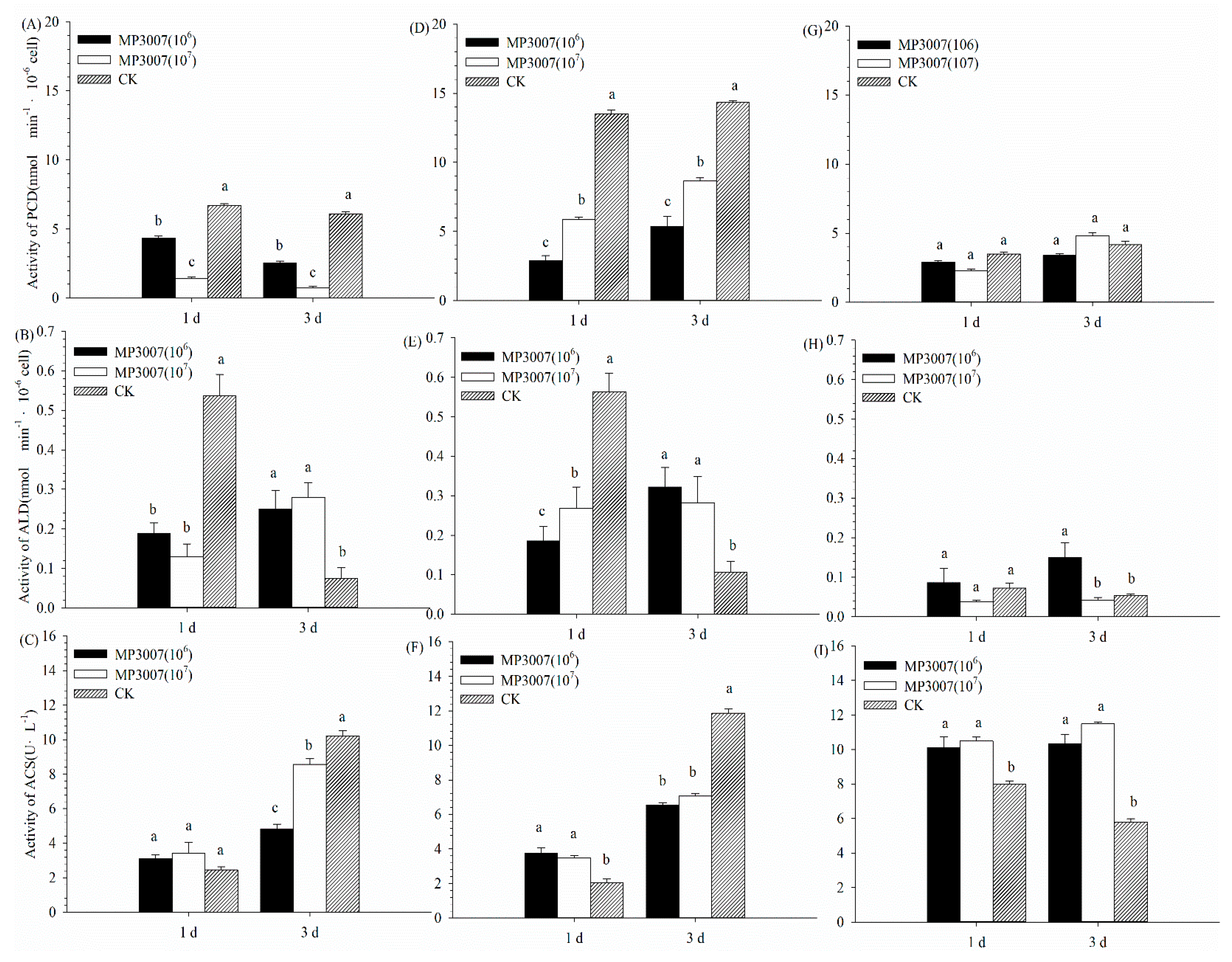
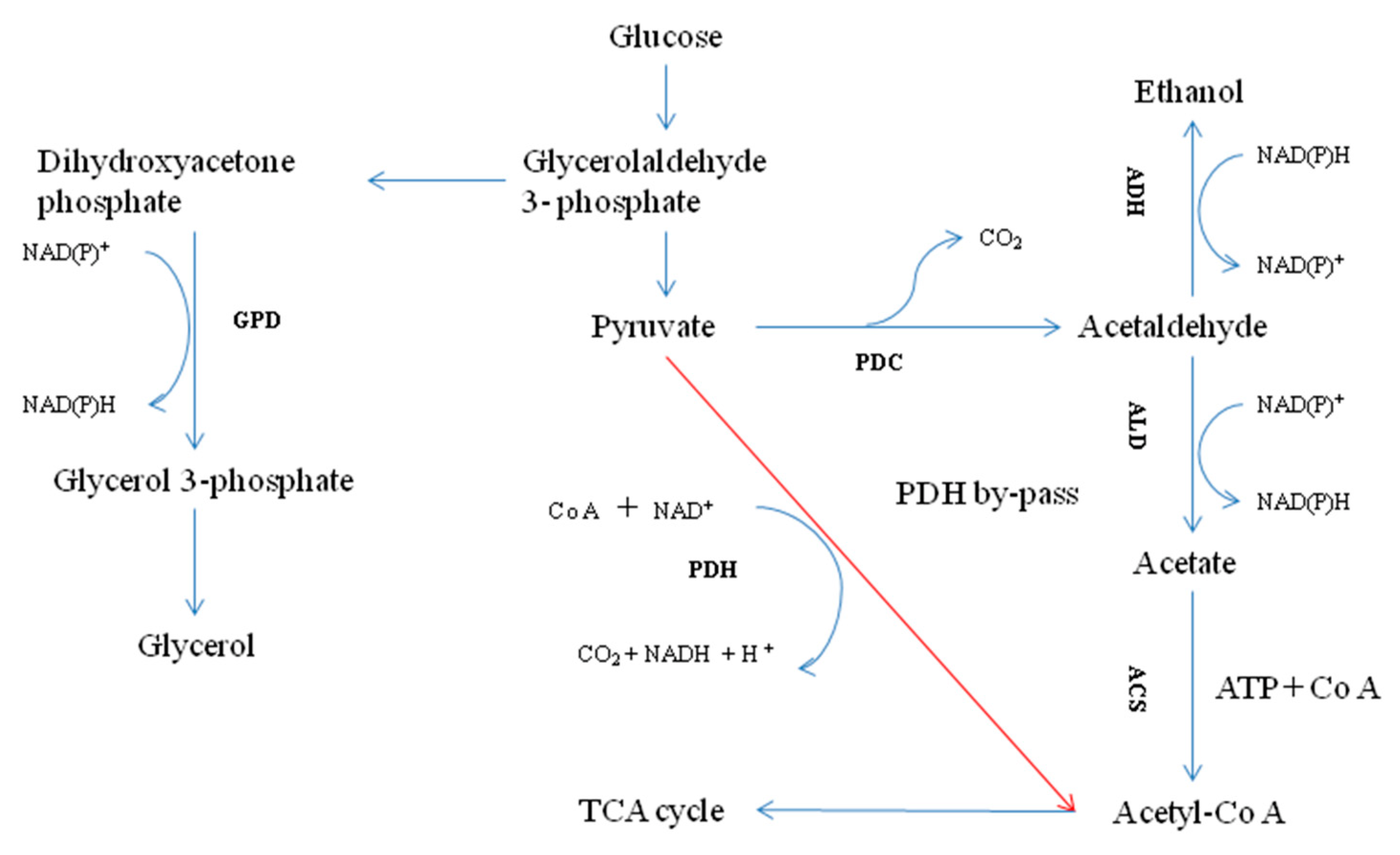
3.3. Enzyme Activities in PDH By-Pass
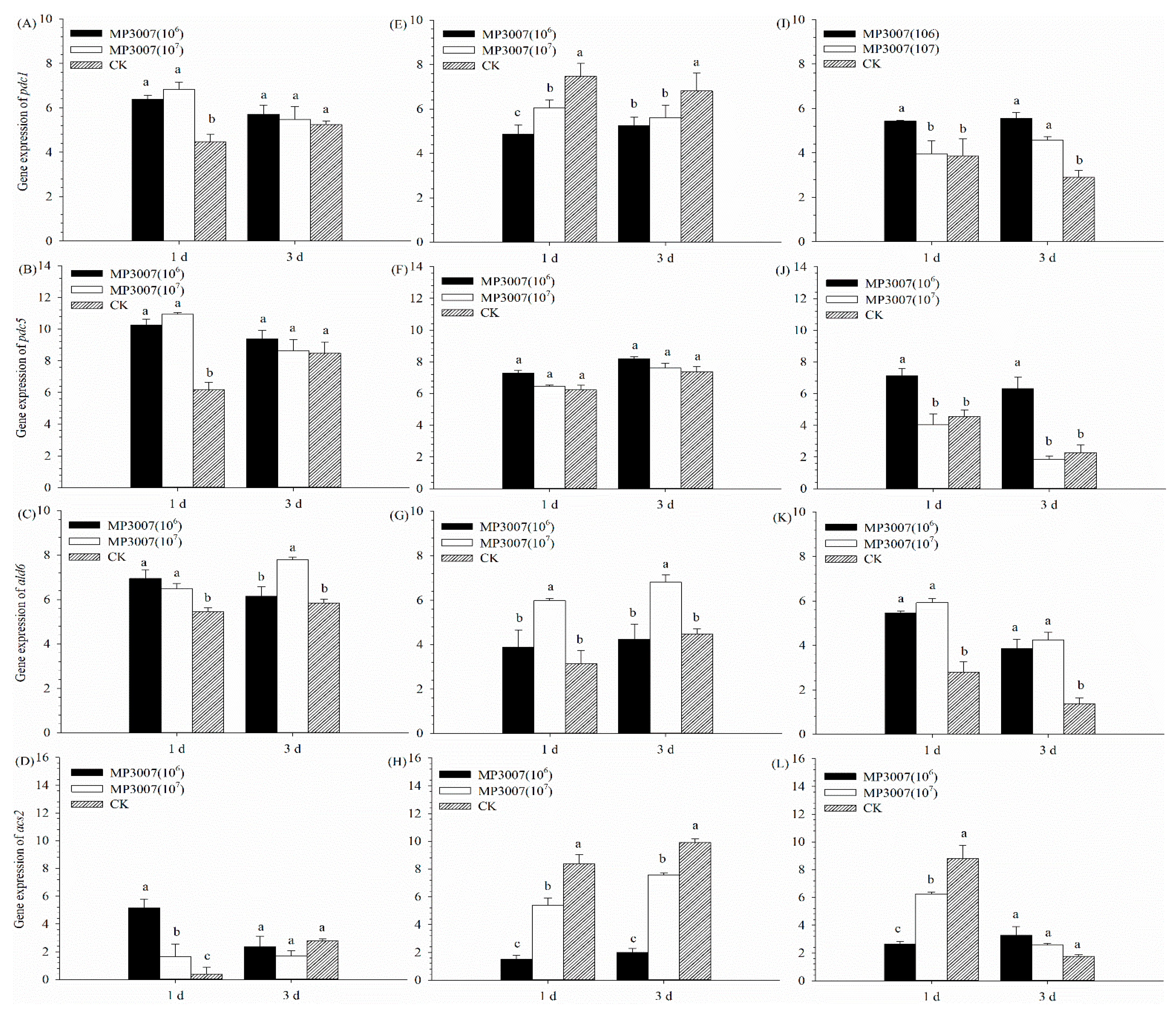
3.4. Gene Expression Analysis
4. Conclusions
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernández-González, M.; Úbeda, J.F.; Briones, A.I. Study of Saccharomyces cerevisiae Wine Strains for Breeding through Fermentation Efficiency and Tetrad Analysis. Curr. Microbiol. 2015, 70, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Cordente, A.G.; Simon, S.; Gemma, B.; Beltran, G.; Jesus, M.T.; Christopher, D.C. Harnessing yeast metabolism of aromatic amino acids for fermented beverage bioflavouring and bioproduction. Appl. Microbiol. Biotechnol. 2019, 103, 4325–4336. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.E.; Margaret, C.; Hennie, J.J.; Vuuren, V. Impact of Yeast Strain on the Production of Acetic Acid, Glycerol, and the Sensory Attributes of Ice wine. Am. J. Enol. Vitic. 2004, 55, 883. [Google Scholar]
- Ribéreau, G.P.; Glories, Y.; Maujean, A. Alcohols and Other Volatile Compounds. In Handbook of Enology; John Wiley and Sons, Ltd.: Hoboken, NJ, USA, 2001; pp. 71–85. [Google Scholar]
- Casal, M.; Cardoso, H.; Leao, C. Mechanisms regulating the transport of acetic acid in Saccharomyces cerevisiae. Microbiology 1996, 142, 1385–1390. [Google Scholar] [CrossRef]
- Delfini, C.; Costa, A. Effects of the grape must lees and insoluble materials on the alcoholic fermentation rate and the production of acetic acid, pyruvic acid, and acetaldehyde. Am. J. Enol. Vitic. 1993, 44, 86–92. [Google Scholar]
- Barbosa, C.; Falco, V.; Mendes-Faia, A.; Mendes-Ferreira, A. Nitrogen addition influences formation of aroma compounds, volatile acidity and ethanol in nitrogen deficient media fermented by Saccharomyces cerevisiae wine strains. J. Biosci. Bioeng. 2009, 108, 99–104. [Google Scholar] [CrossRef]
- Vilanova, M.; Ugliano, M.; Varela, C.; Siebert, T.; Pretorius, I.S.; Henschke, P.A. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Appl. Microbiol. Biotechnol. 2007, 77, 145–157. [Google Scholar] [CrossRef]
- Graham, H.F. Wine Microbiology and Biotechnology; CRC Press: Boca Raton, FL, USA, 1993; p. 228. [Google Scholar]
- Sahoo, S.; Panda, P.K.; Mishra, S.R. Optimization of some physical and nutritional parameters for the production of hyaluronidase by Streptococcus equi SED 9. Acta Pol. Pharm. 2007, 64, 517. [Google Scholar]
- Llauradó, J.M.; Rozès, N.; Constantí, M.; Mas, A. Study of Some Saccharomyces cerevisiae Strains for Winemaking after Preadaptation at Low Temperatures. J. Agric. Food Chem. 2005, 53, 1003–1011. [Google Scholar] [CrossRef]
- Orli, S.F.N.; Arroyo, L.; Hui, B.K. A comparative study of the wine fermentation performance of Saccharomyces paradoxus under different nitrogen concentrations and glucose/fructose ratios. J. Appl. Microbiol. 2010, 108, 73–80. [Google Scholar] [CrossRef]
- Patel, S.; Shibamoto, T. Effect of Different Strains of Saccharomyces cerevisiae on Production of Volatiles in Napa Gamay Wine and Petite Sirah Wine. J. Agric. Food Chem. 2002, 50, 5649–5653. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, Y.; Watanabe, M. Effects of yeast strains and environmental conditions on formation of organic acids in must during fermentation. J. Ferment. Technol. 1981, 59, 27–32. [Google Scholar]
- Torrens, J.; Pilar, U.; Riu, A.M. Different commercial yeast strains affecting the volatile and sensory profile of cava base wine. Int. J. Food Microbiol. 2008, 124, 48–57. [Google Scholar] [CrossRef]
- Sadoudi, S.; Rousseaux, V.; David, H.; Alexandre, R.; Tourdot, M. Metschnikowia pulcherrima Influences the Expression of Genes Involved in PDH By-pass and Glyceropyruvic Fermentation in Saccharomyces cerevisiae. Front. Microbiol. 2017, 8, 1137. [Google Scholar] [CrossRef] [PubMed]
- Verduyn, C.; Postma, E.; Scheffers, W.A.; Van Dijken, J.P. Physiology of Saccharomyces cerevisiae in Anaerobic Glucose-Limited Chemostat Culturesx. J. Gen. Microbiol. 1990, 136, 395–403. [Google Scholar] [CrossRef]
- Remize, F.; Sablayrolles, J.M.; Dequin, S. Re-assessment of the influence of yeast strain and environmental factors on glycerol production in wine. J. Appl. Microbiol. 2000, 88, 371–378. [Google Scholar] [CrossRef]
- Pronk, J.T.; Steensma, H.Y.; Van, D.J.P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 2010, 12, 1607–1633. [Google Scholar] [CrossRef]
- Hohmann, S.; Håkan, C. Autoregulation may control the expression of yeast pyruvate decarboxylase structural genes PDC1 and PDC5. FEBS J. 2010, 188, 615–621. [Google Scholar] [CrossRef]
- Morita, K.; Matsuda, F.; Okamoto, K.; Ishii, J.; Kondo, A.; Shimizu, H. Repression of mitochondrial metabolism for cytosolic pyruvate-derived chemical production in Saccharomyces cerevisiae. Microb. Cell Fact. 2019, 18, 177. [Google Scholar] [CrossRef]
- Datta, S.; Annapure, U.; Timson, D. Different specificities of two aldehyde dehydrogenases from Saccharomyces cerevisiae var. boulardii. Biosci. Rep. 2017, 37, BSR20160529. [Google Scholar] [CrossRef]
- Tessier, W.D.; Meaden, P.G.; Dickinson, F.M.; Melvin, M. Identification and disruption of the gene encoding the k+-activated acetaldehyde dehydrogenase of Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2010, 1, 29–34. [Google Scholar]
- Kozak, B.U.; van Rossum, H.M.; Niemeijer, M.S.; Marlous, V.D.; Kirsten, B.; Liang, W. Replacement of the initial steps of ethanol metabolism in Saccharomyces cerevisiae by ATP-independent acetylating acetaldehyde dehydrogenase. FEMS Yeast Res. 2016, 16, fow006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saigal, D.; Cunningham, S.J.; Farrés, J.; Weiner, H. Molecular cloning of the mitochondrial aldehyde dehydrogenase gene of Saccharomyces cerevisiae by genetic complementation. J. Bacteriol. 1991, 173, 3199–3208. [Google Scholar] [CrossRef]
- Wang, X.; Mann, C.J.; Bai, Y.; Ni, L.; Weiner, H. Molecular cloning, characterization, and potential roles of cytosolic and mitochondrial aldehyde dehydrogenases in ethanol metabolism in Saccharomyces cerevisiae. J. Bacteriol. 1998, 180, 822–830. [Google Scholar] [CrossRef]
- Vanden, B.M.; Jong, G.P.; Kortland, C.; Dijken, J.; Pronk, J.; Steensma, H. The two acetyl-coenzyme A synthetases of Saccharomyces cerevisiae differ with respect to kinetic properties and transcriptional regulation. J. Biol. Chem. 1996, 271, 28953–28959. [Google Scholar] [CrossRef] [PubMed]
- Vanden, B.M.; Steensma, H. ACS2, a Saccharomyces cerevisiae gene encoding acetyl-coenzyme A synthetase, essential for growth on glucose. Eur. J. Biochem. 1995, 231, 704–713. [Google Scholar]
- Viana, F.; Gil, J.V.; Genovés, S.; Vallés, S.; Manzanares, P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef]
- Shekhawat, K.; Bauer, F.F.; Setati, M.E. Impact of oxygenation on the performance of three non-Saccharomyces yeasts in co-fermentation with Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 2479–2491. [Google Scholar] [CrossRef]
- Hu, K.; Jin, G.-J.; Xu, Y.-H.; Tao, Y.-S. Wine aroma response to different participation of selected Hanseniaspora uvarum in mixed fermentation with Saccharomyces cerevisiae. Food Res. Int. 2018, 108, 119–127. [Google Scholar] [CrossRef]
- Varela, C.; Sengler, F.; Solomon, M.; Curtin, C. Volatile flavour profile of reduced alcohol wines fermented with the non-conventional yeast species Metschnikowia pulcherrima and Saccharomyces uvarum. Food Chem. 2016, 209, 57–64. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Cabellos, J.M.; Arroyo, T. Sequential Non-Saccharomyces and Saccharomyces cerevisiae Fermentations to Reduce the Alcohol Content in Wine. Fermentation 2020, 6, 60. [Google Scholar] [CrossRef]
- Ivit, N.N.; Longo, R.; Kemp, B. The Effect of Non-Saccharomyces and Saccharomyces Non-Cerevisiae Yeasts on Ethanol and Glycerol Levels in Wine. Fermentation 2020, 6, 77. [Google Scholar] [CrossRef]
- Escribano, V.R.; González, A.L.; Portu, J.; Garijo, P.; López, A.I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Wine aroma evolution throughout alcoholic fermentation sequentially inoculated withnon-Saccharomyces/Saccharomyces yeasts. Food Res. Int. 2018, 112, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Vaudano, E.; Noti, O.; Costantini, A.; Garcia-Moruno, E. Identifification of reference genes suitable for normalization of RT-qPCR expression data in Saccharomyces cerevisiae during alcoholic ermentation. Biotechnol. Lett. 2011, 33, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Cankorur-Cetinkaya, A.; Dereli, E.; Eraslan, S.; Karabekmez, E.; Dikicioglu, D.; Kirdar, B. A Novel Strategy for Selection and Validation of Reference Genes in Dynamic Multidimensional Experimental Design in Yeast. PLoS ONE 2012, 7, e38351. [Google Scholar]
- Pan, X.; Wu, J.; Zhang, W.; Liu, J.; Yang, X.; Liao, X.; Hu, X.; Lao, F. Effects of sugar matrices on the release of key aroma compounds in fresh and high hydrostatic pressure processed Tainong mango juices. Food Chem. 2020, 338, 128117. [Google Scholar] [CrossRef]
- Kántor, A.; Hutková, J.; Petrová, J. Antimicrobial activity of pulcherrimin pigment produced by Metschnikowia pulcherrima against various yeast species. J. Microbiol. Biotechnol. Food Sci. 2017, 5, 282–285. [Google Scholar] [CrossRef]
- Oro, L.; Ciani, M.; Comitini, F. Antimicrobial activity of Metschnikowia pulcherrima on wine yeasts. J. Appl. Microbiol. 2014, 116, 1209–1217. [Google Scholar] [CrossRef]
- Mestre, M.V.; Maturano, Y.P.; Mercado, L. Evaluation of different co-inoculation time of non-Saccharomyces/Saccharomyces yeasts in order to obtain reduced ethanol wines. BIO Web Conf. 2016, 7, 02025. [Google Scholar] [CrossRef]
- Zhang, B.; Ivanova, P.V.; Duan, C. Distinctive chemical and aromatic composition of red wines produced by Saccharomyces cerevisiae co-fermentation with indigenous and commercial non-Saccharomyces strains. Food Biosci. 2021, 41, 100925. [Google Scholar] [CrossRef]
- Hu, L.; Liu, R.; Wang, X.; Zhang, X. The Sensory Quality Improvement of Citrus Wine through Co-Fermentations with Selected Non-Saccharomyces Yeast Strains and Saccharomyces cerevisiae. Microorganisms 2020, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Q.-Q.; Xu, Y.-H.; Li, A.-H.; Tao, Y.-S. Increased glycosidase activities improved the production of wine varietal odorants in mixed fermentation of P. fermentans and high antagonistic S. cerevisiae. Food Chem. 2020, 332, 127426. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.P.; Farina, L.; Carrau, F. A novel extracellular β-glucosidase from Issatchenkia terricola: Isolation, immobilization and application for aroma enhancement of white Muscat wine. Process Biochem. 2011, 46, 385–389. [Google Scholar] [CrossRef]
- Canonico, L.; Ciani, E.; Galli, E.; Comitini, F.; Ciani, M. Evolution of Aromatic Profile of Torulaspora delbrueckii Mixed Fermentation at Microbrewery Plant. Fermentation 2020, 6, 7. [Google Scholar] [CrossRef]
- Barone, E.; Ponticello, G.; Giaramida, P.; Squadrito, M.; Fasciana, T.; Gandolfo, V.; Ardizzone, F.; Monteleone, M.; Corona, O.; Francesca, N.; et al. Use of Kluyveromyces marxianus to Increase Free Monoterpenes and Aliphatic Esters in White Wines. Fermentation 2021, 7, 79. [Google Scholar] [CrossRef]
- Alexandre, H.; Nguyen, V.L.T.; Feuillat, M.; Charpentier, C. Contribution à l’étude des bourbes: Influence sur la fermentescibilitédes moûts. Blood Cells 1994, 146, 11–20. [Google Scholar]
- Noti, O.; Vaudano, E.; Pessione, E. Short-term response of different Saccharomyces cerevisiae strains to hyperosmotic stress caused by inoculation in grape must: RT-qPCR study and metabolite analysis. Food Microbiol. 2015, 52, 49–58. [Google Scholar] [CrossRef]
- Englezos, V.; Cravero, F.; Torchio, F.; Rantsiou, K.; Ortiz-Julien, A.; Lambri, M.; Gerbi, V.; Rolle, L.; Cocolin, L. Oxygen availability and strain combination modulate yeast growth dynamics in mixed culture fermentations of grape must with Starmerella bacillaris and Saccharomyces cerevisiae. Food Microbiol. 2018, 69, 179–188. [Google Scholar] [CrossRef]
| Genes | NCBI Gene ID | 5′-3′ | Primer Size |
|---|---|---|---|
| pdc1 | 850733 | CTTACGCCGCTGATGGTTA | 19 |
| (YLR044C) | GGCAATACCGTTCAAAGCAG | 20 | |
| pdc5 | 850825 | GGCTGATGCTTGTGCTTCTA | 20 |
| (YLR134W) | GGGTGTTGTTCGTCAATAGC | 20 | |
| ald6 | 856044 | TCTCTTCTGCCACCACTGAA | 20 |
| (YPL061W) | CCTCTTTCTCTTGGGTCTTGG | 21 | |
| acs2 | 850846 | ATTGGTCCTTTCGCCTCAC | 19 |
| (YLR153C) | GCTGTTCGGCTTCGTTAGA | 19 | |
| pgk1 | 850370 | GGTAACACCGTCATCATTGG | 20 |
| (YCR012W) | AAGCACCACCACCAGTAGAGA | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Tang, X.; Han, X.; He, X.; Han, N.; Ding, Y.; Sun, Y. Effect of Metschnikowia pulcherrima on Saccharomyces cerevisiae PDH By-Pass in MixedFermentation with Varied Sugar Concentrations of Synthetic Grape Juice and Inoculation Ratios. Fermentation 2022, 8, 480. https://doi.org/10.3390/fermentation8100480
Lin X, Tang X, Han X, He X, Han N, Ding Y, Sun Y. Effect of Metschnikowia pulcherrima on Saccharomyces cerevisiae PDH By-Pass in MixedFermentation with Varied Sugar Concentrations of Synthetic Grape Juice and Inoculation Ratios. Fermentation. 2022; 8(10):480. https://doi.org/10.3390/fermentation8100480
Chicago/Turabian StyleLin, Xueqing, Xiaohong Tang, Xiaomei Han, Xi He, Ning Han, Yan Ding, and Yuxia Sun. 2022. "Effect of Metschnikowia pulcherrima on Saccharomyces cerevisiae PDH By-Pass in MixedFermentation with Varied Sugar Concentrations of Synthetic Grape Juice and Inoculation Ratios" Fermentation 8, no. 10: 480. https://doi.org/10.3390/fermentation8100480
APA StyleLin, X., Tang, X., Han, X., He, X., Han, N., Ding, Y., & Sun, Y. (2022). Effect of Metschnikowia pulcherrima on Saccharomyces cerevisiae PDH By-Pass in MixedFermentation with Varied Sugar Concentrations of Synthetic Grape Juice and Inoculation Ratios. Fermentation, 8(10), 480. https://doi.org/10.3390/fermentation8100480





