Improvement of Texture, Nutritional Qualities, and Consumers’ Perceptions of Sorghum-Based Sourdough Bread Made with Pediococcus pentosaceus and Weissella confusa Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starter Cultures, Media, and Growth Conditions
2.3. Sourdough and Bread Preparation
2.4. Bread Baking and Qualities
2.5. Proximate Compositions
2.6. Colour Characteristics of the Sourdough Bread Samples
2.7. Textural Properties of the Sourdough Bread Samples
2.8. Scanning Electron Microscopy of the Bread Samples
2.9. Determination of Bioactive Compounds and Radical Scavenging Activities of the Sorghum Sourdough Bread
2.10. Sensory Evaluation
2.11. Shelf-Life Determination of the Bread Samples
2.12. Statistical Analysis
3. Results and Discussion
3.1. External Appearance of the Bread Samples
3.2. Physicochemical Parameters of the Bread Samples
3.3. Colour and Textural Properties
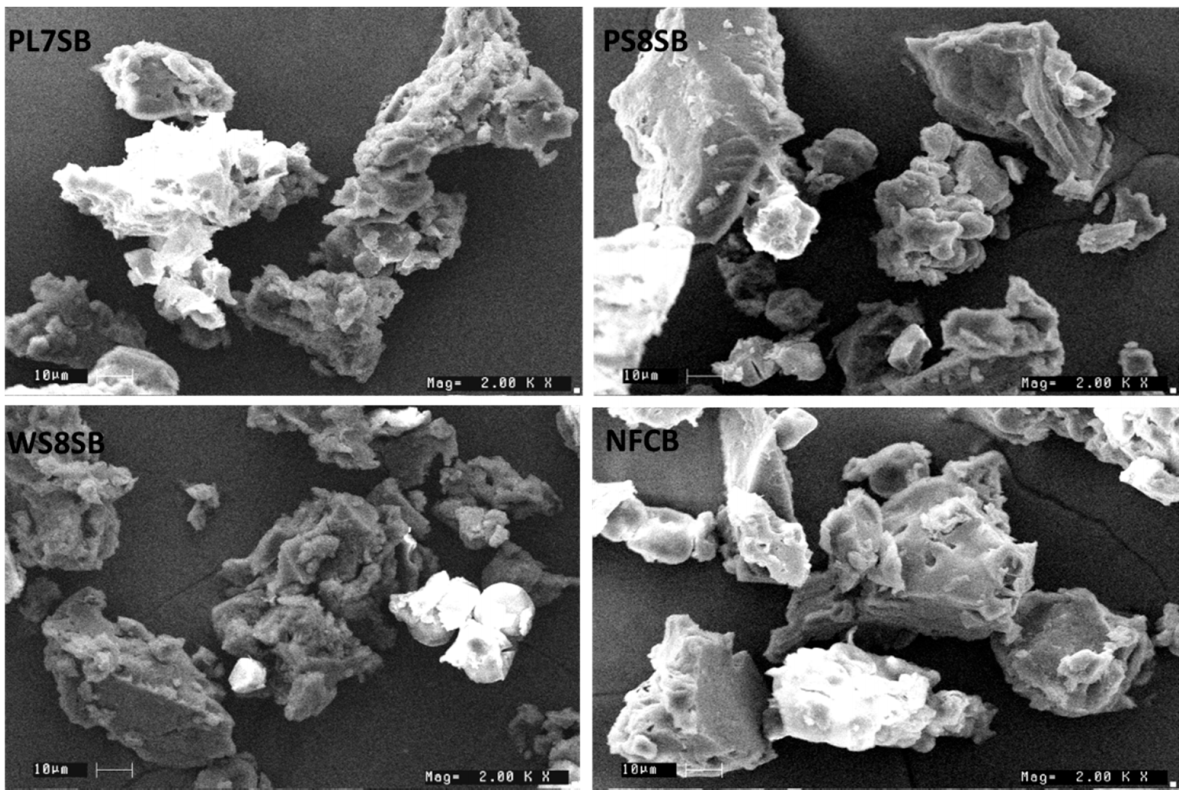
3.4. Microstructure of the Bread Samples
3.5. Bioactive Composition of the Sourdough Breads
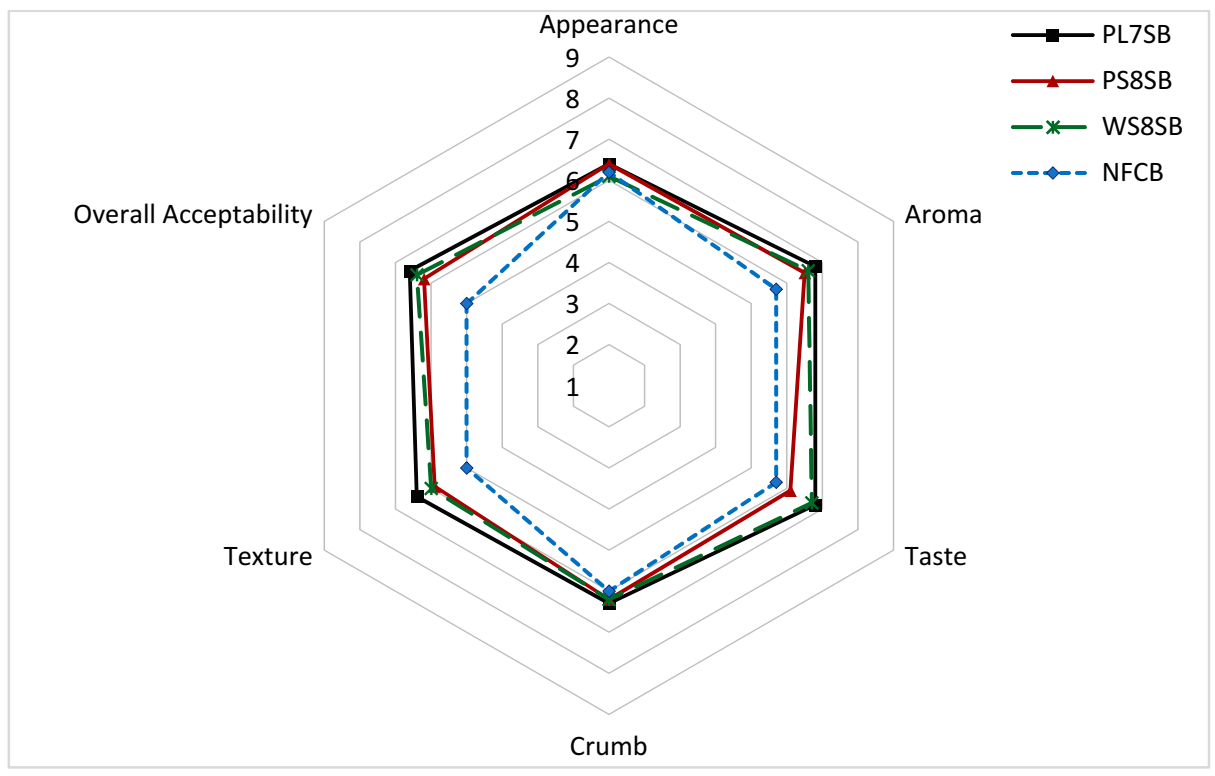
3.6. Sensory Attributes and Shelf–Life of the Sourdough Bread
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demartini, E.; De Marchi, E.; Cavaliere, A.; Mattavelli, S.; Gaviglio, A.; Banterle, A.; Richetin, J.; Perugini, M. Changing attitudes towards healthy food via self-association or nutritional information: What works best? Appetite 2019, 132, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; De Giorgio, R.; Volta, U. Coeliac disease and dermatitis herpetiformis. Lancet 2018, 392, 916–917. [Google Scholar] [CrossRef] [Green Version]
- Navarro, V.; Fernández-Gil, M.D.P.; Simón, E.; Bustamante, M.Á. Gluten: General Aspects and International Regulations for Products for Celiac People. In Nutritional and Analytical Approaches of Gluten-Free Diet in Celiac Disease; Springer: Cham, Switzerland, 2017; pp. 15–27. [Google Scholar]
- Sharma, N.; Bhatia, S.; Chunduri, V.; Kaur, S.; Sharma, S.; Kapoor, P.; Kumari, A.; Garg, M. Pathogenesis of Celiac Disease and Other Gluten Related Disorders in Wheat and Strategies for Mitigating Them. Front. Nutr. 2020, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igbinedion, S.O.; Ansari, J.; Vasikaran, A.; Gavins, F.N.; Jordan, P.; Boktor, M.; Alexander, J.S. Non-celiac gluten sensitivity: All wheat attack is not celiac. World J. Gastroenterol. 2017, 23, 7201–7210. [Google Scholar] [CrossRef]
- Encina-Zelada, C.R.; Cadavez, V.; Monteiro, F.; Teixeira, J.A.; Gonzales-Barron, U. Combined effect of xanthan gum and water content on physicochemical and textural properties of gluten-free batter and bread. Food Res. Int. 2018, 111, 544–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USDA-FAS. Grain: World Markets and Trade. 2018. Available online: https://ipad.fas.usda.gov/ (accessed on 5 December 2021).
- Palavecino, P.M.; Ribotta, P.D.; E León, A.; Bustos, M.C. Gluten-free sorghum pasta: Starch digestibility and antioxidant capacity compared with commercial products. J. Sci. Food Agric. 2019, 99, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Galassi, E.; Taddei, F.; Ciccoritti, R.; Nocente, F.; Gazza, L. Biochemical and technological characterization of two C4 gluten-free cereals: Sorghum bicolorand Eragrostis tef. Cereal Chem. J. 2020, 97, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Ogunsakin, A.; Vanajakshi, V.; Anu-Appaiah, K.; Vijayendra, S.; Walde, S.; Banwo, K.; Sanni, A.; Prabhasankar, P. Evaluation of functionally important lactic acid bacteria and yeasts from Nigerian sorghum as starter cultures for gluten-free sourdough preparation. LWT 2017, 82, 326–334. [Google Scholar] [CrossRef]
- Banwo, K.; Asogwa, F.C.; Ogunremi, O.R.; Adesulu-Dahunsi, A.; Sanni, A. Nutritional profile and antioxidant capacities of fermented millet and sorghum gruels using lactic acid bacteria and yeasts. Food Biotechnol. 2021, 35, 199–220. [Google Scholar] [CrossRef]
- Banwo, K.; Fasuyi, T.O.; Olojede, A.O. Potentials of Lactobacillus plantarum and Pichia kudriavzevii in co-fermentation of sourdough from millet. Int. J. Food Sci. Technol. 2021, 56, 857–864. [Google Scholar] [CrossRef]
- Sanni, A.; Onilude, A.; Fatungase, M. Production of sour maize bread using starter-cultures. World J. Microbiol. Biotechnol. 1997, 14, 101–106. [Google Scholar] [CrossRef]
- Hanis-Syazwani, M.; Boarinwa, I.; Lasekan, O.; Muhammad, K. Influence of starter culture on the physicochemical properties of rice bran sourdough and physical quality of sourdough bread. Food Res. 2018, 2, 340–349. [Google Scholar] [CrossRef]
- Rogalski, E.; Vogel, R.F.; Ehrmann, M.A. Monitoring of Lactobacillus sanfranciscensis strains during wheat and rye sourdough fermentations by CRISPR locus length polymorphism PCR. Int. J. Food Microbiol. 2020, 316, 108475. [Google Scholar] [CrossRef]
- Fernández-Peláez, J.; Paesani, C.; Gómez, M. Sourdough Technology as a Tool for the Development of Healthier Grain-Based Products: An Update. Agronomy 2020, 10, 1962. [Google Scholar] [CrossRef]
- Denkova, R.; Ilieva, S.; Denkova, Z.; Georgieva, L.; Yordanova, M.; Nikolova, D.; Evstatieva, Y. Production of wheat bread without preservatives using sourdough starters. Biotechnol. Biotechnol. Equip. 2014, 28, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Suo, B.; Nie, W.; Wang, Y.; Ma, J.; Xing, X.; Huang, Z.; Xu, C.; Li, Z.; Ai, Z. Microbial diversity of fermented dough and volatile compounds in steamed bread prepared with traditional Chinese starters. LWT 2020, 126, 109350. [Google Scholar] [CrossRef]
- Olojede, A.; Sanni, A.; Banwo, K. Rheological, textural and nutritional properties of gluten-free sourdough made with functionally important lactic acid bacteria and yeast from Nigerian sorghum. LWT 2019, 120, 108875. [Google Scholar] [CrossRef]
- American Association of Cereal Chemistry (AACC). International Method 10-05.01. Guidelines for Measurement of Volume by Rapeseed Displacement. Approved Methods of Analysis of the American Association of Cereal Chemistry, 11th ed.; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Kiskini, A.; Kapsokefalou, M.; Yanniotis, S.; Mandala, I. Effect of Iron Fortification on Physical and Sensory Quality of Gluten-Free Bread. Food Bioprocess Technol. 2011, 5, 385–390. [Google Scholar] [CrossRef]
- Asp, N.G.; Johansson, C.G.; Hallmer, H.; Siljestroem, M. Rapid enzymic assay of insoluble and soluble dietary fiber. J. Agric. Food Chem. 1983, 31, 476–482. [Google Scholar] [CrossRef]
- Matos, M.E.; Rosell, C.M. Relationship between instrumental parameters and sensory characteristics in gluten-free breads. Eur. Food Res. Technol. 2012, 235, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Radha, C.; Prakash, V. Structural and Functional Properties of Heat-processed Soybean Flour: Effect of Proteolytic Modification. Food Sci. Technol. Int. 2009, 15, 453–463. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; El-Beltagi, H.S.; El-Salam, S.M.A.; Omran, A.A. Protein Solubility, Digestibility and Fractionation after Germination of Sorghum Varieties. PLoS ONE 2012, 7, e31154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olojede, A.; Sanni, A.; Banwo, K. Effect of legume addition on the physiochemical and sensorial attributes of sorghum-based sourdough bread. LWT 2019, 118, 108769. [Google Scholar] [CrossRef]
- Axel, C.; Röcker, B.; Brosnan, B.; Zannini, E.; Furey, A.; Coffey, A.; Arendt, E.K. Application of Lactobacillus amylovorus DSM19280 in gluten-free sourdough bread to improve the microbial shelf life. Food Microbiol. 2015, 47, 36–44. [Google Scholar] [CrossRef]
- Santos, F.G.; Aguiar, E.V.; Rosell, C.M.; Capriles, V.D. Potential of chickpea and psyllium in gluten-free breadmaking: Assessing bread’s quality, sensory acceptability, and glycemic and satiety indexes. Food Hydrocoll. 2021, 113, 106487. [Google Scholar] [CrossRef]
- Rinaldi, M.; Paciulli, M.; Caligiani, A.; Scazzina, F.; Chiavaro, E. Sourdough fermentation and chestnut flour in gluten-free bread: A shelf-life evaluation. Food Chem. 2017, 224, 144–152. [Google Scholar] [CrossRef]
- Verni, M.; Rizzello, C.G.; Coda, R. Fermentation Biotechnology Applied to Cereal Industry By-Products: Nutritional and Functional Insights. Front. Nutr. 2019, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Edema, M.O.; Sanni, A.I. Functional properties of selected starter cultures for sour maize bread. Food Microbiol. 2008, 25, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.E.; Rosell, C.M. Understanding gluten-free dough for reaching breads with physical quality and nutritional balance. J. Sci. Food Agric. 2015, 95, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Mihhalevski, A.; Nisamedtinov, I.; Hälvin, K.; Ošeka, A.; Paalme, T. Stability of B-complex vitamins and dietary fiber during rye sourdough bread production. J. Cereal Sci. 2013, 57, 30–38. [Google Scholar] [CrossRef]
- Gänzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Sabanis, D.; Lebesi, D.; Tzia, C. Effect of dietary fibre enrichment on selected properties of gluten-free bread. LWT 2009, 42, 1380–1389. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.-S.; Zannini, E.; Czerny, M.; Arendt, E.K. Influence of dextran-producing Weissella cibaria on baking properties and sensory profile of gluten-free and wheat breads. Int. J. Food Microbiol. 2014, 172, 83–91. [Google Scholar] [CrossRef]
- Maidana, S.D.; Finch, S.; Garro, M.; Savoy, G.; Gänzle, M.; Vignolo, G. Development of gluten-free breads started with chia and flaxseed sourdoughs fermented by selected lactic acid bacteria. LWT 2020, 125, 109189. [Google Scholar] [CrossRef]
- Verheyen, C.; Albrecht, A.; Elgeti, D.; Jekle, M.; Becker, T. Impact of gas formation kinetics on dough development and bread quality. Food Res. Int. 2015, 76, 860–866. [Google Scholar] [CrossRef]
- Litwinek, D.; Gambuś, H.; Zięć, G.; Sabat, R.; Wywrocka-Gurgul, A.; Berski, W. The comparison of quality and chemical composition of breads baked with residual and commercial oat flours and wheat flour. J. Microbiol. Bio-Technol. Food Sci. 2021, 2, 1734–1743. [Google Scholar]
- Movahed, S.; Rooshenas, G.; Ahmadichenarbon, H. Evaluation of the effect of liquid sourdough method on dough yield, bread yield and organoleptic properties Iranian Lavash bread. World Appl. Sci. J. 2011, 15, 1054–1058. [Google Scholar]
- Miller, R.A.; Hoseney, R.C. Role of salt in baking. Cereal Foods World 2008, 53, 4. [Google Scholar] [CrossRef]
- Schober, T.J. Manufacture of gluten-free specialty breads and confectionery products. In Gluten-Free Food Science and Technology; Gallagher, E., Ed.; Wiley-Blackwell: Oxford, UK, 2009; pp. 130–180. [Google Scholar]
- Phattanakulkaewmorie, N.; Paseephol, T.; Moongngarm, A. Chemical compositions and physico-chemical properties of malted sorghum flour and characteristics of gluten free bread. World Acad. Sci. Eng. Technol. 2011, 81, 454–460. [Google Scholar]
- Sandri, L.T.B.; Santos, F.G.; Fratelli, C.; Capriles, V.D. Development of gluten-free bread formulations containing whole chia flour with acceptable sensory properties. Food Sci. Nutr. 2017, 5, 1021–1028. [Google Scholar] [CrossRef] [Green Version]
- Hayta, M.; Ertop, M.H. Physicochemical, textural and microbiological properties of optimised wheat bread formulations as affected by differently fermented sourdough. Qual. Assur. Saf. Crop. Foods 2019, 11, 283–293. [Google Scholar] [CrossRef]
- Cantatore, V.; Filannino, P.; Gambacorta, G.; De Pasquale, I.; Pan, S.; Gobbetti, M.; Di Cagno, R. Lactic Acid Fermentation to Re-cycle Apple By-Products for Wheat Bread Fortification. Front. Microbiol. 2019, 10, 2574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komlenić, D.K.; Ugarčić-Hardi, Ž.; Jukić, M.; Planinić, M.; Bucić-Kojić, A.; Strelec, I. Wheat dough rheology and bread quality effected by Lactobacillus brevis preferment, dry sourdough and lactic acid addition. Int. J. Food Sci. Technol. 2010, 45, 1417–1425. [Google Scholar] [CrossRef]
- Azizi, S.; Azizi, M.H.; Moogouei, R.; Rajaei, P. The effect of Quinoa flour and enzymes on the quality of gluten-free bread. Food Sci. Nutr. 2020, 8, 2373–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Chiş, M.S.; Păucean, A.; Man, S.M.; Mureşan, V.; Socaci, S.A.; Pop, A.; Stan, L.; Rusu, B.; Muste, S. Textural and Sensory Features Changes of Gluten Free Muffins Based on Rice Sourdough Fermented with Lactobacillus spicheri DSM 15429. Foods 2020, 9, 363. [Google Scholar] [CrossRef] [Green Version]
- Novotni, D.; Čukelj, N.; Smerdel, B.; Ćurić, D. Quality attributes and firming kinetics of partially baked frozen wholewheat bread with sourdough. Int. J. Food Sci. Technol. 2013, 48, 2133–2142. [Google Scholar] [CrossRef]
- Bartkiene, E.; Mozuriene, E.; Lele, V.; Zokaityte, E.; Gruzauskas, R.; Jakobsone, I.; Juodeikiene, G.; Ruibys, R.; Bartkevics, V. Changes of bioactive compounds in barley industry by-products during submerged and solid state fermentation with antimicrobial Pediococcus acidilactici strain LUHS29. Food Sci. Nutr. 2019, 8, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Antognoni, F.; Mandrioli, R.; Potente, G.; Saa, D.L.T.; Gianotti, A. Changes in carotenoids, phenolic acids and antioxidant capacity in bread wheat doughs fermented with different lactic acid bacteria strains. Food Chem. 2019, 292, 211–216. [Google Scholar] [CrossRef]
- Banwo, K.; Osagbemi, O.; Ajao, O.; Sanni, A. Sourdough Bread from the Blend of Cassava, Sweet Potato, and Soybean Flours Using Lactobacillus Plantarum and Pichia Kudriavzevii. Acta Aliment. 2020, 49, 441–450. [Google Scholar] [CrossRef]
- Calvert, M.D.; Madden, A.A.; Nichols, L.M.; Haddad, N.M.; Lahne, J.; Dunn, R.R.; McKenney, E.A. A review of sourdough starters: Ecology, practices, and sensory quality with applications for baking and recommendations for future research. PeerJ 2021, 9, e11389. [Google Scholar] [CrossRef]
- Fekri, A.; Torbati, M.; Khosrowshahi, A.Y.; Shamloo, H.B.; Azadmard-Damirchi, S. Functional effects of phytate-degrading, probiotic lactic acid bacteria and yeast strains isolated from Iranian traditional sourdough on the technological and nutritional properties of whole wheat bread. Food Chem. 2020, 306, 125620. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, N.; Albanell, E.; Miñarro, B.; Capellas, M. Chestnut flour sourdough for gluten-free bread making. Eur. Food Res. Technol. 2016, 242, 1795–1820. [Google Scholar] [CrossRef]
- Nionelli, L.; Rizzello, C.G. Sourdough-Based Biotechnologies for the Production of Gluten-Free Foods. Foods 2016, 5, 65. [Google Scholar] [CrossRef] [PubMed]



| Organism | Strain | Accession Number | Source | Reference |
|---|---|---|---|---|
| Pediococcus pentosaceus | LD7 | KX017195 | Sorghum sourdough, Nigeria | [10] |
| P. pentosaceus | SA8 | KX017196 | Sorghum sourdough, Nigeria | [10] |
| Weissella confusa | SD8 | KX017197 | Sorghum sourdough, Nigeria | [10] |
| Ingredients (%) | PL7SB | PS8SB | WS8SB | NFCB |
|---|---|---|---|---|
| Sorghum flour | 60 | 60 | 60 | 70 |
| Tap water | 85 | 85 | 85 | 95 |
| Corn starch | 30 | 30 | 30 | 30 |
| Compressed Baker’s yeast | 2 | 2 | 2 | 2 |
| HPMC | 2 | 2 | 2 | 2 |
| Salt | 2 | 2 | 2 | 2 |
| Sugar | 4 | 4 | 4 | 4 |
| Baking fat | 1 | 1 | 1 | 1 |
| Sourdough | 20 | 20 | 20 | 0 |
| Physical and Chemical Parameters | Sample | |||
|---|---|---|---|---|
| PL7SB | PS8SB | WS8SB | NFCB | |
| Moisture [%] | 44.74 ± 1.07 a | 42.46 ± 0.18 a | 40.36 ± 8.52 a | 39.41 ± 0.42 a |
| Crude Protein [%] | 5.31 ± 0.17 a | 4.91 ± 0. 52 a | 5.05 ± 0.00 a | 4.55 ± 0.35 a |
| Ash [%] | 2.44 ± 0.03 c | 2.37 ± 0.01 b | 2.43 ± 0.01 c | 2.12 ± 0.00 a |
| Total Lipid [%] | 3.74 ± 0.49 a | 3.23 ± 0.20 a | 4.14 ± 0.24 a | 3.91 ± 0.57 a |
| Soluble Dietary Fibre [%] | 0.46 ± 0.63 a | 1.73 ± 1.36 a | 0.00 ± 0.00 a | 2.70 ± 0.47 a |
| Insoluble Dietary Fibre [%] | 15.51 ± 0.28 a | 13.57 ± 2.69 a | 14.43 ± 1.10 a | 10.55 ± 0.71 a |
| Total Dietary Fibre [%] | 14.43 ± 1.10 a | 15.95 ± 0.90 a | 15.30 ± 4.04 a | 13.25 ± 0.24 a |
| Total Carbohydrate | 31.08 ± 3.41 a | 32.72 ± 12.32 a | 29.35 ± 0.67 a | 36.77 ± 1.10 a |
| Weight (g) | 131.45 ± 2.05 a | 133.00 ± 0.42 a | 132.70 ± 0.57 a | 133.55 ± 0.21 a |
| Specific Volume (g/cm3) | 2.42 ± 0.11 a,b | 2.16 ± 0.09 a | 2.50 ± 0.07 b | 2.46 ± 0.21 a,b |
| Baking Yield | 87.63 ± 1.37 a | 88.67 ± 0.28 a | 88.47 ± 0.38 a | 89.03 ± 0.14 a |
| Shelf Life (days) | 4 | 5 | 5 | 5 |
| Samples | Crumb Colour | Crust Colour | ||||
|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | |
| PL7SB | 35.22 ± 2.79 a | 6.81 ± 0.55 a | 13.04 ± 0.48 a,b | 44.50 ± 6.91 a | 8.72 ± 0.56 a | 16.41 ± 1.67 a |
| PS8SB | 34.16 ± 1.42 a | 6.54 ± 0.48 a | 12.35 ± 0.45 a | 44.03 ± 2.01 a | 8.54 ± 0.30 b | 16.95 ± 1.15 a |
| WS8SB | 34.14 ± 0.75 a | 6.13 ± 0.49 a | 12.37 ± 0.26 a | 37.72 ± 1.71 a | 7.73 ± 0.55 b | 15.39 ± 1.69 a |
| NFCB | 35.98 ± 1.37 a | 5.89 ± 0.52 a | 13.41 ± 0.28 b | 40.76 ± 1.96 a | 7.47 ± 0.64 a,b | 15.04 ± 0.57 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olojede, A.O.; Sanni, A.I.; Banwo, K.; Michael, T. Improvement of Texture, Nutritional Qualities, and Consumers’ Perceptions of Sorghum-Based Sourdough Bread Made with Pediococcus pentosaceus and Weissella confusa Strains. Fermentation 2022, 8, 32. https://doi.org/10.3390/fermentation8010032
Olojede AO, Sanni AI, Banwo K, Michael T. Improvement of Texture, Nutritional Qualities, and Consumers’ Perceptions of Sorghum-Based Sourdough Bread Made with Pediococcus pentosaceus and Weissella confusa Strains. Fermentation. 2022; 8(1):32. https://doi.org/10.3390/fermentation8010032
Chicago/Turabian StyleOlojede, Ayoyinka O., Abiodun I. Sanni, Kolawole Banwo, and Towobola Michael. 2022. "Improvement of Texture, Nutritional Qualities, and Consumers’ Perceptions of Sorghum-Based Sourdough Bread Made with Pediococcus pentosaceus and Weissella confusa Strains" Fermentation 8, no. 1: 32. https://doi.org/10.3390/fermentation8010032
APA StyleOlojede, A. O., Sanni, A. I., Banwo, K., & Michael, T. (2022). Improvement of Texture, Nutritional Qualities, and Consumers’ Perceptions of Sorghum-Based Sourdough Bread Made with Pediococcus pentosaceus and Weissella confusa Strains. Fermentation, 8(1), 32. https://doi.org/10.3390/fermentation8010032







