Abstract
Saccharomyces cerevisiae and Lactobacillus panis are ethanol and lactic acid producers in Maotai-flavor Baijiu fermentation. Understanding their interaction is important to regulate the microbiome composition during fermentation and biosynthesis of ethanol and lactic acid. This study is the first to analyze the interaction between S. cerevisiae and L. panis at different growth phases during co-cultivation. Results showed that the different growth phases of S. cerevisiae modulated L. panis growth. Metabolomics analysis showed that amino acids and nucleoside secreted by S. cerevisiae promote L. panis growth, while ethanol inhibited L. panis growth. Furthermore, S. cerevisiae modulated L. panis cell growth under varying sugar concentrations. Simulated solid-state fermentation demonstrated that regulating the sugar concentration or the ratio of S. cerevisiae to L. panis could inhibit L. panis cell growth and reduce lactic acid accumulation. This study provided an understanding on Maotai-flavor Baijiu microbiome, which might be useful for metabolite regulation.
1. Introduction
The brewing of Chinese liquor (Baijiu) is an open solid-state fermentation process involving various microorganisms [1]. Microorganisms are the core of liquor brewing and play an important role in the yield and quality of the liquor [2,3]. Maotai-flavor liquor (Maotai-flavor Baijiu), a basic flavor of Chinese liquor, uses sorghum as a raw material for pile and cellar fermentation. Maotai-flavor liquor has a complex process involving eight rounds of fermentation [4]. It is famous for its freshness, sweetness, and mellow fragrance, and it is the most popular liquor in China [5]. The unique brewing process of Maotai-flavor liquor creates a complex microbial community. Moreover, the combination and interaction of a wide range of microorganisms creates the unique flavor [6].
In recent years, with the development of high-throughput sequencing technology, there have been many reports on the diversity of microorganisms utilized in Maotai-flavor liquor production [7,8,9]. Saccharomyces cerevisiae, as one of the main fungi in Baijiu fermentation, plays a dominant role in multiple rounds of Maotai-flavor Baijiu and is one of the most important ethanol-producing yeasts. In addition, it has an important contribution to the aroma of Baijiu, and is a producer of many important flavor compounds such as ethyl acetate and 2-phenyl ethanol [10,11,12]. Meanwhile, Lactobacillus is the dominant genus in the entire brewing process [13], and Lactobacillus panis is the most important lactic acid-producing microorganism [14,15]. Whole genome sequencing results showed that L. panis metabolized lactic acid through obligate heterolactic fermentation [16]. Lactic acid is the most important organic acid that influences the flavor of Maotai-flavor liquor [16,17], which will not be lost with distillation in the fermentation process. The continuous accumulation of lactic acid reduces the pH value and has an important impact on the microbial community structure in the brewing microbiome [18]. Preliminary research has mainly focused on the succession of the microbial community structure during the brewing process and the physiological and metabolic characteristics of S. cerevisiae and L. panis [15,19,20]. However, no relevant research has been conducted on the interaction between S. cerevisiae and L. panis.
In this study, we revealed the key factors that affect the growth of L. panis by S. cerevisiae in different growth stages. In addition, the interactions between L. panis and S. cerevisiae are also affected by the content of reducing sugar. On this basis, we realized the regulation of lactic acid production during simulated solid-state Chinese liquor fermentation by regulating the sugar concentration or the ratio of S. cerevisiae to L. panis. Our study might provide guidance for the synthesis and regulation of ethanol and lactic acid and might be beneficial in improving fermented food quality.
2. Materials and Methods
2.1. Strains and Growth Media
S. cerevisiae and L. panis were isolated from the fermentation process of Chinese Moutai-flavor liquor, and the primers NL1 (GCATATCAATAAGCGGAGGAAAAG) and NL4 (GGTCCGTGTTTCAAGACGG) were used to sequence the 26S rRNA gene. The 16S rRNA gene sequencing was performed using the primers 27F (AGTTTGATCMTGGCTCAG) and 1492R (GGTTACCTTGTTACGACTT). S. cerevisiae and L. panis were stored in Yeast Extract Peptone Dextrose Medium (YPD) and modified de Man-Rogosa-Sharpe Medium (MRS) glycerol stock solutions at −80 °C, respectively. S. cerevisiae and L. panis were pre-grown on YPD and MRS agar plates, respectively, and single colonies were enriched in their respective broth medium before inoculation to the fermentation medium. Physiological saline (0.85% NaCl solution) was used to wash the seed solution.
The sorghum “Hong Yingzi,” a unique variety produced in Guizhou, was used as a raw material to prepare a sorghum extract medium for liquid fermentation. Briefly, 500 g of sorghum was mixed with 2 L of ionized water. Then, 20 mL of heat-stabilized α-amylase (40,000 U·mL−) solution was added, and the resulting mixture was boiled using an induction cooker for 2 h, placed in a water bath, and saccharified with glucoamylase at 60 °C for 4 h. Finally, the mixture was filtered through two layers of gauze and centrifuged at 8000× g for 15 min to collect the supernatant (sorghum extract). The sugar content was measured using a Leica refractometer (juan juan, Guangzhou, China). To achieve a final sugar concentration of 10°Bx, the initial dosages of the saccharification and liquefaction enzymes were adjusted. The supernatant was sterilized at 121 °C for 15 min.
A solid-state fermentation medium was prepared to simulate the cellar fermentation system. One kilogram of crushed sorghum was weighed and soaked in deionized water at a ratio of 2:1 for 1 day and sterilized at 121 °C for 20 min. Then, 15 mL of filtered saccharifying enzyme (100,000 U·mL−1) was added. The sugar content in sorghum fermentation medium was regulated by controlling the amount of saccharifying enzyme.
2.2. Fermentation
For liquid fermentation, L. panis and S. cerevisiae were inoculated in 100 mL of sorghum juice extract in an Erlenmeyer flask, with a final cell concentration of 106 CFU·mL−1. The flasks were capped with fermentation plugs to achieve anaerobic and uninterrupted fermentation conditions. Twenty-four flasks were used for different inoculation conditions, and three flasks were randomly selected for observation at 0, 6, 12, 24, 36, 48, 60, and 72 h. Each medium was statically cultured at 30 °C. The sugar content in sorghum fermentation medium was adjusted by controlling the amount of glucoamylase.
For solid-state fermentation, L. panis was added to the sorghum fermentation medium at a final concentration of 106 CFU·mL−1, and the S. cerevisiae inoculum in the co-culture system was adjusted to 104, 105, and 106 CFU·mL−1. The medium was anaerobically cultured at 30 °C for 16 days. All experiments were performed in triplicate. −1
2.3. Determination of Biomass
The biomass of S. cerevisiae and L. panis in a single and co-culture fermentation was measured using plate dilution and counting in red Bengal medium and MRS agar medium (supplemented with 0.05 g·L−1 natamycin), respectively [21].
2.4. Determination of Lactic Acid and Ethanol Content
The contents of lactic acid and ethanol were analyzed by the UV detector (210 nm) and the differential detector of high-performance liquid chromatography (HPLC) (Agilent 1200 series, Santa Clara, CA, USA) using an Aminex HPX-87H column (300 × 7.8 mm, Berkeley, CA, USA). The mobile phase was 5 mmol·L−1 H2SO4, and the flow rate was 0.6 mL·min−1. The column temperature was 40 °C, and the injection volume was 10 μL. The concentrations of lactic acid and ethanol in the fermented samples were determined using the peak area of the standard sample. Six standards of different concentrations were used to construct a standard curve. The cells were removed through centrifugation at 8000× g for 10 min, the supernatant was filtered through a 0.22 μm syringe filter, and HPLC was performed [22]. For solid-state fermentation, 3 g of sample was collected, mixed with 9 mL of deionized water, ultrasonicated for 30 min, and centrifuged at 8000× g, 4 °C, for 10 min. The supernatant was collected and analyzed using the same procedure mentioned above [23].
2.5. Untargeted Metabolome Analysis
The fermentation supernatant was collected at different time points following a previous protocol [24]. Briefly, S. cerevisiae (106 CFU·mL−1) was cultured in sorghum medium for 12 h and centrifuged at 8000× g for 15 min. The supernatant was filtered through a 0.22 µm poly(1,1-difluoroethylene) (PVDF) sterile syringe filter and used to culture L. panis (106 CFU·mL−1) for 48 h. After incubation, the supernatant was collected from three independent set-ups: inoculum without S. cerevisiae, 12 h co-incubation with S. cerevisiae, and 48 h co-incubation with L. panis, and three parallel samples were taken at each time point. The collected samples were filtered through a 0.22 µm PVDF sterile syringe filter, diluted in 0.1% formic acid, and analyzed in an AB SCIEX QTRAP 4500 LC-MS/MS system with the following conditions: column (T3 1.8 µm, 100mm × 2.1 mm), column temperature: 40 °C, mobile phase, A: water (0.1% FA), B: acetonitrile (0.1% FA), and gradient elution at a flow rate of 0.3 mL·min−1. The proportion of mobile phase B was 2% in the first 3 min, and gradually increased to 75% in 7 min. Then, the proportion of mobile phase B increased from 75% to 100% and the flow rate increased from 0.3 to 0.6 mL·min−1 in 0.5 min, and remained for 6 min, then decreased to 2% in 0.5 min, subsequently decreased to 0.3 mL·min−1, and remained for 3 min. A total of 150 compounds were collected simultaneously in positive and negative ESI mode using a Turbo V ion source at 600 °C and multiple reactive ion detection mode fractional scanning (MRM). The ion spray voltage was 5.5 kV, and the equimixed linear ion trap scanning mode (MRM-IDA-EPI) was used to generate the MRM chromatogram for quantitative analysis and two-stage scanning mass spectrometry for qualitative analysis.
2.6. Effects of Co-Culture on Cell Morphology
To observe the morphology of microorganisms under mono- and co-culture conditions, samples were incubated at 30 °C for 48 h, collected, and then analyzed using cold-field scanning electron microscopy [25,26].
2.7. Statistical Analysis
Each treatment was performed in triplicate. All statistical analyses and data plotting were performed using Microsoft Office Excel 2019 (Microsoft, Redmond, WA, USA), OriginPro 2021 (OriginLab, Northampton, MA, USA), and Adobe Illustrator 2020 (Adobe Systems Incorporated, San Jose, CA, USA). A p-value < 0.05 was considered significant using a the two-tailed t-test.
3. Results
3.1. Effect of Co-Culture on the Growth and Metabolic Characteristics of S. cerevisiae and L. panis
Figure 1 shows the fermented biomass and characteristic metabolites of S. cerevisiae and L. panis in monoculture and co-culture systems at 106 CFU·mL−1. There was no significant difference in the biomass of S. cerevisiae in monoculture and co-culture environments (Figure 1A), as well as in the ethanol content between different environments (Figure 1B). These results indicate that L. panis had no significant effect on the growth and metabolism of S. cerevisiae.
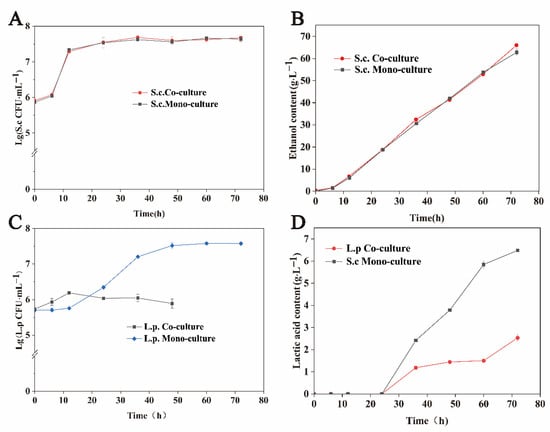
Figure 1.
Effects of monoculture and co-culture on the growth and key metabolite production of S. cerevisiae and L. panis. (A) Cell growth of S. cerevisiae under monoculture (inoculation with 106 CFU·mL−1 of S. cerevisiae) and co-culture (inoculation with both 106 CFU·mL−1 of S. cerevisiae and 106 CFU·mL−1 of L. panis) conditions. (B) Cell growth of L. panis under monoculture (inoculation with 106 CFU·mL−1 of L. panis) and co-culture (inoculation with both 106 CFU·mL−1 of S. cerevisiae and 106 CFU·mL−1 of L. panis) conditions. (C) The contents of ethanol produced by S. cerevisiae under monoculture (inoculation with 106 CFU·mL−1 of S. cerevisiae) and co-culture (inoculation with both 106 CFU·mL−1 of S. cerevisiae and 106 CFU·mL−1 of L. panis) conditions. No L. panis was detected at 60 and 72 h under co-culture conditions. (D) The contents of lactic acid produced by L. panis under monoculture (inoculation with 106 CFU·mL−1 of L. panis) and co-culture (inoculation with both 102 CFU·mL−1 of S. cerevisiae and 106 CFU·mL−1 of L. panis) conditions.
Interestingly, L. panis growth in the co-cultivation was different at different periods (Figure 1C). Therefore, the exponential growth of S. cerevisiae may have a significant effect on the growth of L. panis. The biomass of L. panis in the co-culture system (1.55 × 106 CFU·mL−1) was 2.69-fold higher than that in the monoculture system (5.76 × 105 CFU·mL−1) at 12 h of fermentation. S. cerevisiae significantly inhibited the growth of L. panis after 12 h of fermentation. Compared with the monoculture, the biomass of L. panis was reduced by 97%, and lactic acid was not detected during co-incubation with S. cerevisiae at 48 h. When the inoculation concentration of S. cerevisiae was reduced to 102 CFU·mL−1, the lactic acid content in the fermentation broth was 1.44 g·L−1 after incubation for 48 h, which was 38.0% of the monoculture (Figure 1D). L. panis growth and metabolism were also significantly inhibited. These results suggest that the effect of S. cerevisiae on the growth of L. panis may be affected by different factors in different growth phases.
3.2. Identifying Metabolites Secreted by S. cerevisiae for Promoting L. panis Growth
To determine the metabolites that allow S. cerevisiae to promote the growth of L. panis in the early stage of fermentation, we also examined whether the cell-free filtrate of S. cerevisiae in the early stage of fermentation had an effect on the growth of L. panis (Figure 2A). Clearly, contact between S. cerevisiae and L. panis and signal induction were not required. In addition, we found that the cell-free filtrate still had a certain promoting effect after passing through the 3 kDa filter. To identify the specific metabolites involved, we collected cell-free filtrates at several time points and analyzed with non-targeted metabolomics analysis. The sample preparation for metabolomics analysis was as follows: (1) supernatant without S. cerevisiae, (2) supernatant after S. cerevisiae inoculation for 6 h, (3) supernatant after S. cerevisiae inoculation for 12 h, (4) harvesting supernatant after S. cerevisiae inoculation for 12 h and inoculating L. panis, then collecting supernatant after L. panis inoculation for 24 h, and (5) supernatant after L. panis inoculation for 48 h. We assumed that potential metabolites that promote L. panis growth would accumulate with the growth of S. cerevisiae and then be consumed by L. panis, which should show an increase during S. cerevisiae growth and a decrease after inoculating L. panis (Figure 2B). If the fold change of a certain metabolite is more than 1.5 after inoculating L. panis for 48 h, the metabolites are regarded as potential metabolites for promoting L. panis growth. A total of 101 compounds were detected. Heterogeneity among samples was eliminated through normalization, and the detected metabolites were analyzed using cluster analysis (Supplementary Figure S1). There are a total of seven compounds (L-lysine, 5-S-methyl-5-thioadenosine, D-biotin, Pyruvic acid, L-methionine, Inosine, 2-deoxycytidine 5-monophosphate) with dynamic changes of more than 1.5 times, which are assumed to be substances that promote the growth of L. panis (Figure 2C).
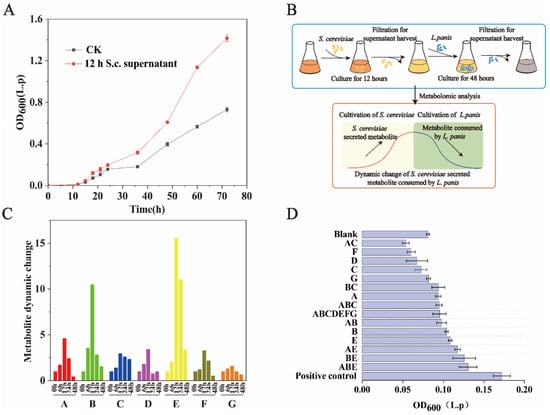
Figure 2.
Identification of the key metabolites secreted by exponentially growing S. cerevisiae that promote L. panis growth. (A) Effects of fermentation supernatant of S. cerevisiae (12 h) on cell growth of L. panis. CK: Fermentation supernatant from cultivating S. cerevisiae at 0 h. (B) Sample preparation for metabolomics analysis to identify potential key metabolites secreted by exponentially growing S. cerevisiae. (C) Dynamic changes of seven potential key metabolites (compared to 0 h). 6 h, 12 h: fermentation supernatant of S. cerevisiae incubated for 6 and 12 h, respectively; 24 h, 48 h: fermentation supernatant from cultivating L. panis for 24 and 48 h, respectively. A: L-lysine, B: 5-S-methyl-5-thioadenosine, C: D-biotin, D: Pyruvic acid, E: L-methionine, F: Inosine, G: 2-deoxycytidine 5-monophosphate. (D) Effects of adding identified metabolites to sorghum fermentation medium on the growth of L. panis. A: L-lysine, B: 5-S-Methyl-5-thioadenosine, C: D-biotin, D: Pyruvic acid, E: L-methionine, F: Inosine, G: 2-deoxycytidine 5-monophosphate.
Next, the seven target compounds of corresponding concentrations were added through exogenous combination. Compared with the blank, the growth of L. panis was significantly improved (p < 0.05), especially when the combination of 5-S-Methyl-5-thioadenosine, lysine, and methionine were added (Figure 2D), which increased the biomass of L. panis by 60.4%, indicating that S. cerevisiae can promote the growth of L. panis by secreting amino acids and adenosine compounds during the fermentation process. The influence of macromolecular substances and the database limitation might be the reason why the combination of exogenous addition cannot fully achieve the effect of the positive control.
3.3. S. cerevisiae Inhibits the Growth of L. panis by Metabolite Inhibition
Space, nutrition, and inhibition of metabolic products restrict the coexistence of different microorganisms [27,28,29]. Figure 1C shows that S. cerevisiae has strong inhibitory effects on the growth of L. panis during the middle and late growth stages. To determine the mechanism underlying the interaction between S. cerevisiae and L. panis, we collected the fermentation broth of S. cerevisiae grown for 24, 48, and 72 h, and tested the cell-free filtrate of these samples for the influence of the growth of L. panis. The experimental results are shown in Figure 3A. Notably, S. cerevisiae did not need direct contact to inhibit the growth of L. panis, and spatial competition should not be the main inhibiting factor. Different ethanol concentrations were added to the sorghum fermentation medium according to the detected concentration of ethanol in the fermentation broth. L. panis was gradually inhibited with the increasing ethanol concentration (Figure 3B).
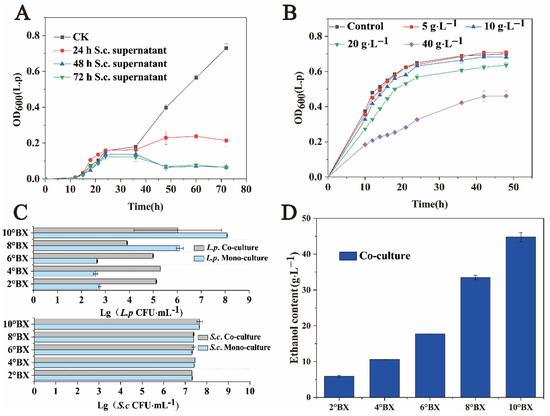
Figure 3.
Cell growth and key metabolite production of L. panis and S. cerevisiae under different conditions. (A) Effects of fermentation supernatant of S. cerevisiae with cell growth of L. panis. (B) Effects of ethanol on cell growth of L. panis. (C) Colony numbers of S. cerevisiae (106 CFU·mL−1) and L. panis (106 CFU·mL−1) after 48 h of co-culture and monoculture at varying sugar concentrations. 2°Bx: Approximately 15 g·L−1 reducing sugar, 4°Bx: Approximately 32 g·L−1 reducing sugar, 6°Bx: Approximately 46 g·L−1 reducing sugar, 8°Bx: Approximately 64 g·L−1 reducing sugar, 10°Bx: Approximately 80 g·L−1 reducing sugar. (D) Ethanol concentration after 48 h of co-culture at different sugar concentrations.
In addition, the content of reducing sugar is a dynamic process in liquor fermentation [30]. Therefore, we studied the effect of sorghum juice extracts with different reducing sugar contents on microbial growth. As shown in Figure 3C, the biomass of S. cerevisiae in monoculture and co-culture in medium with a reducing sugar gradient was not significantly different at 48 h of fermentation. We also observed that, in the medium with a low reducing sugar concentration, L. panis could survive only when co-cultured with S. cerevisiae. The biomass of L. panis in co-culture with a low reducing sugar content (2–6°Bx) was much higher than that in monoculture. Although L. panis in the monoculture system at the later stage of fermentation was almost undetectable compared to the co-culture system, it still maintained a certain survival. This may be related to the low nutrient environment. Meanwhile, the low ethanol production of S. cerevisiae (Figure 3B) was not enough to inhibit L. panis, and as previously observed, small molecules such as amino acids secreted by S. cerevisiae maintain a certain growth requirement of L. panis. In the co-culture condition with low sugar, L. panis can thrive at a certain number. These results also showed that nutritional competition was not the main factor that restricted the growth of L. panis by S. cerevisiae. At 48 h, the ethanol contents of the two gradients with higher sugar content (8°Bx and 10°Bx) were 33.49 ± 0.66 g·L−1 and 44.76 ± 1.32 g·L−1 (Figure 3D). As mentioned above, a higher ethanol concentration significantly inhibited the growth of L. panis. Taken together, these results suggest that ethanol, the characteristic metabolite of S. cerevisiae, is the main inhibitory factor against L. panis growth, rather than competition for nutrition and survival.
3.4. Effects of Co-Culture on Microbial Morphology
Ethanol stress can lead to increased cell membrane permeability and damage cell membrane integrity in Lactobacillus [31]; thus, the effects of ethanol stress on the metabolic activity and membrane structure of Lactobacillus should be inhibited. In this study, we performed a cold-field scanning electron microscope analysis to observe the morphology of microorganisms cultured for 48 h in different environments (Figure 4). Compared with the results of a single culture, the morphology of S. cerevisiae cells under co-culture did not change significantly. Contrarily, the morphology of L. panis changed significantly. The bacterial cells were clearly atrophied, damaged, and destroyed, and the cell count was reduced, which in turn affected its ability to metabolize lactic acid (Figure 1D). These results further indicated that ethanol, the characteristic metabolite of S. cerevisiae, was the main factor that inhibited the growth and metabolism of L. panis. S. cerevisiae exerts stress and damage to L. panis by secreting ethanol, thereby reducing its survival rate.
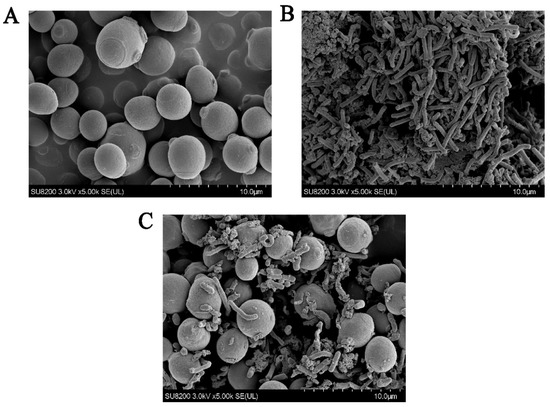
Figure 4.
Cell morphology of S. cerevisiae and L. panis under different conditions observed using scanning electron microscopy in cold field. (A) Monoculture of S. cerevisiae and (B) L. panis. (C) Co-culture of S. cerevisiae and L. panis.
3.5. Controlling Lactic Acid Production Using Solid-State Simulated Fermentation
Simulated solid-state fermentation can realistically demonstrate the liquor brewing process. The initial concentration of yeast in the in situ system was 105–106 CFU·g−1. By adjusting the initial inoculum of S. cerevisiae, the influence of different ratios of S. cerevisiae on the growth and metabolism of L. panis was studied in a solid-state simulated fermentation environment. The initial inoculation amounts of S. cerevisiae were 0, 104, 105, and 106 CFU·g−1, and the initial inoculation amount of L. panis was constant at 106 CFU·g−1. Figure 5A shows the effect of different inoculation ratios of S. cerevisiae to L. panis on cell growth of L. panis. In a solid-state fermentation environment, with an increase in fermentation time, low inoculum of S. cerevisiae still showed a strong effect on the growth of L. panis. With an increase in the volume, the inhibition effect was more evident. Similarly, lactic acid production in different fermentation environments showed similar trends. In a single culture, the maximum lactic acid content in the environment can reach 3.30 ± 0.13 g·L−1. Co-culture with 104 CFU·g−1 S. cerevisiae after 16 days revealed a lactic acid content of 0.43 ± 0.01 g·L−1, which was 87.0% lower than that in monoculture. Furthermore, increasing inoculum of S. cerevisiae decreased lactic acid production. When S. cerevisiae and L. panis were co-cultured, almost no lactic acid was detected after 16 days of anaerobic fermentation (Figure 5B).
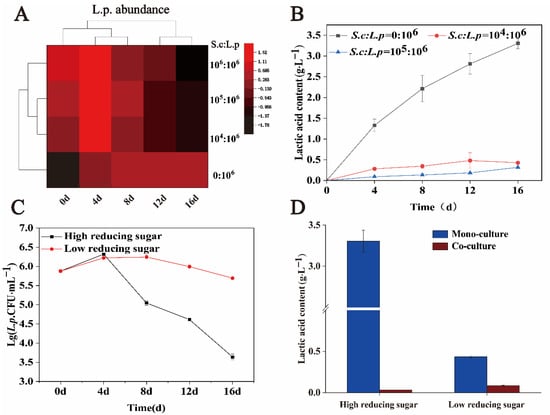
Figure 5.
Effects of sugar concentration and the ratio of S. cerevisiae to L. panis in the simulated solid-state fermentation system on cell growth and lactic acid production of L. panis. (A) Influence of different inoculation ratios of S. cerevisiae to L. panis on cell growth of L. panis. (B) Influence of different inoculation ratios of S. cerevisiae to L. panis on lactic acid production of L. panis. (C) Cell growth of L. panis under monoculture conditions with different reducing sugar concentrations. (D) Lactic acid produced by L. panis under monoculture and co-culture conditions at different reducing sugar concentrations after 16 days of fermentation.
Next, we studied the effects of high and low reducing sugar content on the growth and lactic acid metabolism of L. panis in 106 CFU·g−1 S. cerevisiae solid-state fermentation. During the whole fermentation process, the biomass of viable bacteria in the group with high reducing sugar content was significantly lower than that in the group with low reducing sugar content. After 16 days of fermentation, the biomass of L. panis with high reducing sugar content was (4.4 ± 0.79) × 103 CFU·g−1, while that of L. panis with low reducing sugar content was (4.97 ± 0.50) × 105 CFU·g−1, which was 113 times higher than that of the high reducing sugar content (Figure 5C). Simultaneously, we compared the effects of S. cerevisiae on lactic acid yield under different reducing sugar contents. The lactic acid content of monocultured L. panis was significantly higher than that of co-cultured in high or low reducing sugar content environments. In addition, when co-cultured with S. cerevisiae, the lactic acid content detected in the high reducing sugar environment was lower than that in the low reducing sugar environment (Figure 5D). These results suggest that increasing the amount of S. cerevisiae or regulating the content of reducing sugars in the environment can control the abundance of L. panis and the production of lactic acid during brewing.
4. Discussion
Varieties of microorganisms participate in the metabolism of the brewing of liquor and several metabolites affect the succession of the microbial community. Therefore, the microbial community during fermentation is a dynamic process. The interactions between microorganisms may not be static [32]. Lactic acid is one of the main organic acids produced by solid-state fermentation of liquor. A small amount of lactic acid can react with ethanol produced by ethanol-metabolizing microorganisms such as S. cerevisiae to produce ethyl lactate, which has an important contribution to the smell and taste of liquor. However, as a non-volatile organic acid, lactic acid gradually accumulates along the process, observing a pH decrease in the subsequent rounds, which will affect the growth of many functional microorganisms and cause the quality of liquor to decline [18,33]. Here, we have developed a co-cultivation system of two important microorganisms, S. cerevisiae and L. panis, during the brewing process of Maotai-flavor liquor, to explore their interactions at different periods. In our study, we found that early on, S. cerevisiae could promote the growth of L. panis, while the growth and metabolism of L. panis in the middle and late stages were significantly inhibited by S. cerevisiae (Figure 1C). Previous studies have reported that the growth of S. cerevisiae is stressed by high concentrations of lactic acid [17]. During the whole process, the growth and metabolism of S. cerevisiae did not change significantly, which may be due to the low accumulation of lactic acid in the early rounds.
In different traditional fermented foods, S. cerevisiae and Lactobacillus often show different interactions [34]. A previous study demonstrated that S. cerevisiae may increase the viability of Lactobacillus rhamnosus strains through direct cell contact and copolymerization mediated by cell surface and cell wall components or by metabolites [35]. In our study, we proved that the early promotion effect does not require interspecies contact and signal induction. Moreover, using metabolomics, we found that S. cerevisiae could stimulate the growth of L. panis by secreting amino acids and nucleoside compounds (Figure 2), which is consistent with the previous study, that found that S. cerevisiae created survival conditions for lactic acid bacteria through nitrogen source overflow in Kefir [36]. Furthermore, we ruled out the influence of nutrition and living space on the growth of L. panis, and through ethanol concentration experiments and electron microscope observations, we determined that ethanol is a representative metabolite that inhibits the growth of L. panis. High concentrations of ethanol damage the cell morphology and membrane structure of L. panis (Figure 3 and Figure 4).
Simulating solid-state fermentation is beneficial to better reproduce the interaction between S. cerevisiae and L. panis. By simulating solid-state fermentation experiments, we observed similar results to those of liquid-state fermentation. Recent experiments have shown that sugar content has an important effect on the growth and metabolism of microorganisms [35]. Based on these results, we studied the effects of different inoculation amounts of S. cerevisiae and the sugar content of solid fermentation media on the growth and metabolism of L. panis and production of lactic acid. Increasing the sugar content or intake of S. cerevisiae can reduce the accumulation of lactic acid in the environment, providing a new option to regulate the content of lactic acid in Maotai-flavor liquor and the quality of liquor (Figure 5). In the future, we will use transcriptomics to comprehensively examine the molecular mechanism by which S. cerevisiae affects the expression of genes of L. panis at the molecular level to reveal their interaction mechanism more accurately and comprehensively. Different temperatures have a significant impact on lactic acid production. Different temperatures can affect cell growth by affecting the catalytic activity of intracellular enzymes, and the transcription levels of genes in the lactic acid synthesis pathway can change significantly. The transcription level of lactate dehydrogenase (numbered 32 and 33) of the Pediococcus acidilactici MT25 strain at 37 °C was the highest, which is consistent with its lactic acid production during liquid fermentation. However, when the transcription level of lactate dehydrogenase in the Lactobacillus amylovorus MT30 strain was greatly reduced at 45 °C, lactic acid production during liquid fermentation was significantly increased. These data indicate that lactic acid production by lactic acid bacteria under different conditions is the result of systemic global regulation of endogenous metabolic pathways. It may be necessary to comprehensively analyze its lactic acid production capacity under specific conditions from more levels, such as the key enzymes under specific conditions, and factors such as catalytic activity, cell growth, and carbon source consumption rate.
5. Conclusions
In conclusion, the interaction between S. cerevisiae and L. panis during different growth stages was analyzed and the mechanism was explored. Metabolomics analysis showed that the amino acids and nucleosides secreted by S. cerevisiae promote the growth of L. panis, while ethanol inhibits the growth of L. panis. In addition, we found that S. cerevisiae regulates the cell growth of L. panis under different reducing sugar concentrations. Simulated solid-state fermentation experiments show that adjusting the concentration of reducing sugars or the ratio of S. cerevisiae to L. panis can inhibit the growth of L. panis and reduce the accumulation of lactic acid. This study is helpful to understand the microbial community metabolism of Chinese Moutai-flavor liquor and provides a basis for regulating metabolism.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation8010033/s1, Figure S1: Cluster analysis heat map of metabolites detected by metabolomics.
Author Contributions
Y.L. and B.W. performed the experiments; Y.L., J.L., G.D. and J.C. conceived the project and supervised the research; Y.L., B.W., X.Z., F.Y. and L.W. designed the experiments, analyzed the data, and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Key Research and Development Program of China (2018YFA0900300), National Natural Science Foundation of China (32172349).
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information Files. The datasets generated and analyzed during the current study are also available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, X.S.; Du, H.; Zhang, Y.; Xu, Y. Environmental Microbiota Drives Microbial Succession and Metabolic Profiles during Chinese Liquor Fermentation. Appl. Environ. Microbiol. 2018, 84, e02369-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.M.; Meng, Y.J.; Wang, Y.L.; Zhou, Q.W.; Li, A.J.; Liu, G.Y.; Li, J.X.; Xing, X.H. Prokaryotic communities in multidimensional bottom-pit-mud from old and young pits used for the production of Chinese Strong-Flavor Baijiu. Food Chem. 2020, 312, 126084. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Q.; Wang, P.; Lin, J.C.; Huang, L.; Xu, Y. Synergistic Effect in Core Microbiota Associated with Sulfur Metabolism in Spontaneous Chinese Liquor Fermentation. Appl. Environ. Microbiol. 2017, 83, e01475-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, G.Y.; Zhu, Y.; Xu, Y. Mystery behind Chinese liquor fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.; Chen, L. Diversity of yeast species during fermentative process contributing to Chinese Maotai-flavour liquor making. Lett. Appl. Microbiol. 2012, 55, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wu, Q.; Wang, L.; Wang, D.Q.; Chen, L.Q.; Xu, Y. Improving flavor metabolism of Saccharomyces cerevisiae by mixed culture with Bacillus licheniformis for Chinese Maotai-flavor liquor making. J. Ind. Microbiol. Biotechnol. 2015, 42, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Cai, W.C.; Wang, W.P.; Shu, N.; Zhang, Z.D.; Hou, Q.C.; Shan, C.H.; Guo, Z. Analysis of microbial diversity and functional differences in different types of high-temperature Daqu. Food Sci. Nutr. 2021, 9, 1003–1016. [Google Scholar] [CrossRef]
- Zuo, Q.C.; Huang, Y.G.; MinGuo. Evaluation of bacterial diversity during fermentation process: A comparison between handmade and machine-made high-temperature Daqu of Maotai-flavor liquor. Ann. Microbiol. 2020, 70, 1–10. [Google Scholar] [CrossRef]
- Song, Z.W.; Du, H.; Zhang, Y.; Xu, Y. Unraveling Core Functional Microbiota in Traditional Solid-State Fermentation by High-Throughput Amplicons and Metatranscriptomics Sequencing. Front. Microbiol. 2017, 8, 1294. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Xi-Bin, L.; Yao-Ling, W.; Xiang-Lian, Z.; Qiao-Ling, Z.; Liang-Qiang, C.; Jie, Y.; Ru-Ye, L.; Fan, Y.; He-Yu, W.; et al. Diversity of yeasts from fermented grain in Maotai liquor fermentation. Mycosystema 2019, 38, 620–630. [Google Scholar] [CrossRef]
- Plata, C.; Millan, C.; Mauricio, J.C.; Ortega, J.M. Formation of ethyl acetate and isoamyl acetate by various species of wine yeasts. Food Microbiol. 2003, 20, 217–224. [Google Scholar] [CrossRef]
- Carmona, M.; Zamarro, M.A.T.; BláZquez, B.; Durante-RodríGuez, G.; JuáRez, J.F.; Valderrama, J.A.S.; Barragán, M.A.J.L.; García, J.L.; Díaz, E. Anaerobic Catabolism of Aromatic Compounds: A Genetic and Genomic View. Microbiol. Mol. Biol. Rev. 2009, 73, 71–133. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.L.; Hao, F.; Lv, X.B.; Chen, B.; Yang, Y.B.; Zeng, X.L.; Yang, F.; Wang, H.Y.; Wang, L. Diversity of lactic acid bacteria in Moutai-flavor liquor fermentation process. Food Biotechnol. 2020, 34, 212–227. [Google Scholar] [CrossRef]
- Lv, X.; Wu, Y.L.; Hao, F.; Zeng, X.L. Analysis of Microbial Diversity in the 1st and 2nd Fermentation Cycle of Jiangxiang Baijiu based on High Throughput Sequencing Technology. Brew. Technol. 2019, 3, 52–58. [Google Scholar] [CrossRef]
- Yang, F.; Chen, L.Q.; Liu, Y.F.; Li, J.H.; Wang, L.; Chen, J. Identification of microorganisms producing lactic acid during solid-state fermentation of Maotai flavour liquor. J. Inst. Brew. 2019, 125, 171–177. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, Q.L.; Liu, Y.F.; Li, J.H.; Wang, L.; Chen, J. Lactic acid biosynthesis pathways and important genes of Lactobacillus panis L7 isolated from the Chinese microbiome. Food Biosci. 2020, 36, 100627. [Google Scholar] [CrossRef]
- Deng, N.; Du, H.; Xu, Y. Cooperative Response of Pichia kudriavzevii and Saccharomyces cerevisiae to Lactic Acid Stress in Baijiu Fermentation. J. Agric. Food Chem. 2020, 68, 4903–4911. [Google Scholar] [CrossRef]
- Wang, S.L.; Wu, Q.; Nie, Y.; Wu, J.F.; Xu, Y. Construction of Synthetic Microbiota for Reproducible Flavor Compound Metabolism in Chinese Light-Aroma-Type Liquor Produced by Solid-State Fermentation. Appl. Environ. Microbiol. 2019, 85, e03090-18. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.J.; Tian, Z.G.; Meng, W.N.; Li, Z.J. Microbial Diversity and Physicochemical Characteristics of the Maotai-Flavored Liquor Fermentation Process. J. Nanosci. Nanotechnol. 2020, 20, 4097–4109. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.J.; Tian, Z.Q.; Meng, W.N.; Li, C.Y.; Li, Z.J. Changes in Microbial Diversity, Physicochemical Characteristics, and Flavor Substances During Maotai-Flavored Liquor Fermentation and Their Correlations. J. Biobased Mater. Bioenergy 2019, 13, 290–307. [Google Scholar] [CrossRef]
- Pang, X.N.; Huang, X.N.; Chen, J.Y.; Yu, H.X.; Wang, X.Y.; Han, B.Z. Exploring the diversity and role of microbiota during material pretreatment of light -flavor Baijiu. Food Microbiol. 2020, 91, 103514. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Kwon, E.Y.; Bae, S.J.; Cho, B.R.; Kim, S.Y.; Hahn, J.S. Improvement of D-Lactic Acid Production in Saccharomyces cerevisiae Under Acidic Conditions by Evolutionary and Rational Metabolic Engineering. Biotechnol. J. 2017, 12, 1700015. [Google Scholar] [CrossRef]
- Kuanyshev, N.; Ami, D.; Signori, L.; Porro, D.; Morrissey, J.P.; Branduardi, P. Assessing physio-macromolecular effects of lactic acid on Zygosaccharomyces bailii cells during microaerobic fermentation. Fems Yeast Res. 2016, 16, fow058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, G.S.; Teng, C.; Xu, D.; Fu, Z.L.; Liu, P.X.; Wu, Q.H.; Yang, R.; Li, X.T. Improving Ethyl Acetate Production in Baijiu Manufacture by Wickerhamomyces anomalus and Saccharomyces cerevisiae Mixed Culture Fermentations. BioMed Res. Int. 2019, 2019, 1470543. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.X.; Du, H.; Xu, Y. Volatile Organic Compound-Mediated Antifungal Activity of Pichia spp. and Its Effect on the Metabolic Profiles of Fermentation Communities. Appl. Environ. Microbiol. 2021, 87, e02992-20. [Google Scholar] [CrossRef]
- Tian, R.Z.; Liu, Y.F.; Cao, Y.T.; Zhang, Z.J.; Li, J.H.; Liu, L.; Du, G.C.; Chen, J. Titrating bacterial growth and chemical biosynthesis for efficient N-acetylglucosamine and N-acetylneuraminic acid bioproduction. Nat. Commun. 2020, 11, 5078. [Google Scholar] [CrossRef]
- van Tatenhove-Pel, R.J.; Rijavec, T.; Lapanje, A.; van Swam, I.; Zwering, E.; Hernandez-Valdes, J.A.; Kuipers, O.P.; Picioreanu, C.; Teusink, B.; Bachmann, H. Microbial competition reduces metabolic interaction distances to the low mu m-range. ISME J. 2021, 15, 688–701. [Google Scholar] [CrossRef]
- Leroy, S.; Lebert, I.; Andant, C.; Talon, R. Interaction in dual species biofilms between Staphylococcus xylosus and Staphylococcus aureus. Int. J. Food Microbiol. 2020, 326, 108653. [Google Scholar] [CrossRef]
- Shangjie, Y.; Yao, J.; Rongqing, Z.; Chongde, W. Interaction between microorganisms in traditional fermented food and its application. Bio-Ind. Technol. 2019, 4, 48–54. [Google Scholar] [CrossRef]
- Zhang, H.X.; Wang, L.; Tan, Y.W.; Wang, H.Y.; Yang, F.; Chen, L.Q.; Hao, F.; Lv, X.B.; Du, H.; Xu, Y. Effect of Pichia on shaping the fermentation microbial community of sauce-flavor Baijiu. Int. J. Food Microbiol. 2021, 336, 108898. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, B.; Jiang, C.; Lu, S.; Yang, Y.; Li, K.; Fan, Z.; Jiang, Y. Effect of Ethanol Stress on the Metabolic Activity of Lactobacillus plantarum D5-5. J. Food Sci. Biotechnol. 2016, 35, 12. [Google Scholar]
- Zhao, C.J.; Schieber, A.; Ganzle, M.G. Formation of taste-active amino acids, amino acid derivatives and peptides in food fermentations—A review. Food Res. Int. 2016, 89, 39–47. [Google Scholar] [CrossRef]
- Clement, H.; Prost, C.; Chiron, H.; Ducasse, M.B.; Della Valle, G.; Courcoux, P.; Onno, B. The effect of organic wheat flour by-products on sourdough performances assessed by a multi-criteria approach. Food Res. Int. 2018, 106, 974–981. [Google Scholar] [CrossRef]
- Garcia, C.; Rendueles, M.; Diaz, M. Liquid-phase food fermentations with microbial consortia involving lactic acid bacteria: A review. Food Res. Int. 2019, 119, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.B.; Lu, Z.R.; Soteyome, T.; Ye, Y.R.; Huang, T.Y.; Liu, J.Y.; Harro, J.M.; Kjellerup, B.V.; Peters, B.M. Polymicrobial interaction between Lactobacillus and Saccharomyces cerevisiae: Coexistence-relevant mechanisms. Crit. Rev. Microbiol. 2021, 47, 386–396. [Google Scholar] [CrossRef]
- Ponomarova, O.; Gabrielli, N.; Sevin, D.C.; Mulleder, M.; Zimgibl, K.; Bulyha, K.; Andrejev, S.; Kafkia, E.; Typas, A.; Sauer, U.; et al. Yeast Creates a Niche for Symbiotic Lactic Acid Bacteria through Nitrogen Overflow. Cell Syst. 2017, 5, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).