Growth Performance, Nutrient Digestibility, Blood Profiles, and Gut Integrity of Growing Pigs Fed Pickled Fish Residue with Decreased Salt Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Preparation of Pickled Freshwater Fish Residue
2.3. Animals and Treatments
2.4. Growth Performance
2.5. Nutrient Digestibility
2.6. Blood Profiles
2.7. Intestinal Morphology
2.8. Statistical Analysis
3. Results
3.1. Nutrient Composition and Feed Evaluation
3.2. Growth Performance
3.3. Nutrient Digestibility
3.4. Blood Profiles
3.5. Intestinal Morphology
4. Discussion
4.1. Nutrient Composition and Feed Evaluation
4.2. Growth Performance
4.3. Nutrient Digestibility
4.4. Blood Profiles
4.5. Intestinal Morphology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Behera, S.S.; El-Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Rodpai, R.; Sanpool, O.; Thanchomnang, T.; Wangwiwatsin, A.; Sadaow, L.; Phupiewkham, W.; Boonroumkaew, P.; Intapan, P.M.; Mallewong, W. Investigating the microbiota of fermented fish products (Pla-ra) from different communities of northeastern Thailand. PLoS ONE 2020, 16, e0245227. [Google Scholar] [CrossRef]
- Ogata, Y.; Ishigaki, T.; Nakagawa, M.; Yamada, M. Effect of increasing salinity on biogas production in waste landfills with leachate recirculation: A lab-scale model study. Biotechnol. Rep. 2016, 10, 111–116. [Google Scholar] [CrossRef]
- Thai Agricultural Standard TAS 7023-2018 “PLA-RA”. Available online: https://www.acfs.go.th/standard/download/eng/PLA-RA-ENG.pdf (accessed on 5 November 2021).
- Shawk, D.J.; Goodband, R.D.; Tokach, M.D.; Dritz, S.S.; DeRouchey, J.M.; Woodworth, J.C.; Lerner, A.B.; Williams, H.E. Effects of added dietary salt on pig growth performance. Transl. Anim. Sci. 2018, 2, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Richart, E.; Nunes, R.V.; Castilha, L.D.; Silva, Y.L.; Cella, P.S. Nutritional evaluation of Tilapia filleting waste meal for swine in the nursery phase. Rev. Caatinga 2016, 29, 473–480. [Google Scholar] [CrossRef][Green Version]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- European Medicines Agency Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R0006&from=EN (accessed on 21 November 2020).
- National Research Council (NRC). Nutrient Requirement of Swine, 11th ed.; National Academic Press: Washington, DC, USA, 2012. [Google Scholar]
- Wang, T.; Adeola, O. Digestibility index marker type, but not inclusion level affects apparent digestibility of energy and nitrogen markey recovery in growing pigs regardless of added oat bran. J. Anim. Sci. 2018, 96, 2817–2825. [Google Scholar] [CrossRef]
- Adeola, O. Digestion and Balance Techniques in Pigs. In Swine Nutrition, 2nd ed.; Lewis, A.J., Southern, L.L., Eds.; CRC Press: Washington, DC, USA, 2001; pp. 903–916. [Google Scholar]
- Wang, C.; Huang, Z.; Yu, K.; Ding, R.; Ye, K.; Dai, C.; Xu, X.; Zhou, G.; Li, C. High-salt diets has a certain impact on protein digestion and gut microbiota: A sequencing and proteome combined study. Front. Microbiol. 2017, 8, 1838. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; El-Hack, M.E.A.; Noreldin, A.E.; Batiha, G.E.; Beshbishy, A.M.; Ohran, H.; Khafaga, A.F.; Othman, S.I.; Allam, A.A.; Swelum, A.A. High salt diet affects the reproductive health in animals: An overview. Animals 2020, 10, 590. [Google Scholar] [CrossRef]
- Fereidoun, H.; Bahram, A.; Sadraddin, K.; Ahmad, R.; Pouria, H. Determination of tolerable and toxic salt concentrations in drinking water in rat, pig, and rabbit. Toxicol. Environ. Chem. 2018, 90, 115–1123. [Google Scholar] [CrossRef]
- Neves, C.M.S.S.; Held, C.; Mohammad, S.; Schleinitz, M.; Coutinhoa, J.A.P.; Freire, M.G. Effect of salts on the solubility of ionic liquid in water: Experimental and electrolyte Perturbed-Chain Statistical Associating Fluid Theory. Phys. Chem. Chem. Phys. 2015, 17, 32044–32052. [Google Scholar] [CrossRef]
- Mielcarek, K.; Puścion-Jakubik, A.; Gromkowska-Kępka, K.J.; Soroczyńska, J.; Naliwajko, S.K.; Markiewicz-Żukowska, R.; Moskwa, J.; Nowakowski, P.; Borawska, M.H.; Socha, K. Proximal composition and nutritive value of raw, smoked and pickled freshwater fish. Foods 2020, 9, 1879. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Huang, C.; Wu, X.; Li, T.; Huang, R.; Kang, P.; Hu, Q.; Chu, W.; Kong, X. Nutrient digestibility response to graded dietary levels of sodium chloride in weanling pigs. J. Sci. Food Agric. 2008, 88, 940–944. [Google Scholar] [CrossRef]
- Duangsai, P.; Gawborisut, S. Effects of drying temperature on quality parameters of Thai fermented fish dip (Jaew Bong). Int. J. Food Stud. 2020, 9, 251–263. [Google Scholar] [CrossRef]
- Klocek, C.; Nowicki, J.; Brudzisz, B.; Pabiańczyk, M. Color preferences in pigs. Sci. Ann. Polish Soc. Anim. Prod. 2016, 12, 123–129. [Google Scholar]
- Noh, H.S.; Ingale, S.L.; Lee, S.H.; Kim, K.H.; Kwon, I.K.; Kim, Y.H.; Jo, C.B. Effects of citrus pulp, fish by-product and Bacillus subtilis fermentation biomass on growth performance, nutrient digestibility, and fecal microflora of weanling pigs. J. Anim. Sci. Technol. 2014, 56, 10. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.S.; Jensen, S.K.; Jakobsen, K. Development of digestive enzymes in pigs with emphasis on lipolytic activity in the stomach and pancreas. J. Anim. Sci. 1997, 75, 437–445. [Google Scholar] [CrossRef]
- Teodorowicz, M.; Hendriks, W.H.; Wichers, H.J.; Savelkoul, H.F.J. Immunomodulation by processed animal feed: The role of Millard reaction products and advanced glycation end-products (AGEs). Front. Immunol. 2018, 9, 2088. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, I.H.; Kim, D.H.; Joo, Y.H.; Kim, S.C. Influence of fermented fish meal supplementation on growth performance, blood metabolites, and fecal microflora of weaning pigs. R. Braz. Zootec. 2017, 46, 433–437. [Google Scholar] [CrossRef]
- Mmanda, F.P.; Lindberg, J.E.; Haldén, A.N.; Mtolera, M.S.P.; Kitula, R.; Lundh, T. Digestibility of local feed ingredients in Talipia Oreochromis niloticus jeveniles, determined on faeces collected by siphoning or stripping. Fishes 2020, 5, 32. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Kim, I.H. Supplemental effects of fish oil and powdered/coated docosahexaenoic acid on the growth performance, nutrient digestibility, blood profile and fecal coliform and lactic acid bacteria counts in weaner pigs. Anim. Feed Sci. Technol. 2021, 275, 114885. [Google Scholar] [CrossRef]
- Choct, M. Managing gut health through nutrition. Br. Poult. Sci. 2009, 50, 9–15. [Google Scholar] [CrossRef]
- Becker, S.L.; Gould, S.A.; Petry, A.L.; Kellesvig, L.M.; Patience, J.F. Adverse effects on growth performance and bone development in nursery pigs fed diets marginally deficient in phosphorus with increasing calcium to available phosphorus ratios. J. Anim. Sci. 2020, 98, 1–8. [Google Scholar] [CrossRef]
- Oster, M.; Just, F.; Büsing, K.; Wolf, P.; Polley, C.; Vollmar, B.; Muráni, E.; Ponsuksili, S.; Wimmers, K. Toward improved phosphorus efficiency in monogastrics-interplay of serum, minerals, bone, and immune system after divergent dietary phosphorus supply in swine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, 917–925. [Google Scholar] [CrossRef]
- Ebeledike, E.U.; Nwokedi, G.I.U.; Ndu, O.O.; Okoye, F.B.C.; Ochiogu, I.S. Calcium and phosphorus contents of body parts of some domestic animals used as meat source in Nigeria. Asian Pac. J. Trop. Med. 2010, 3, 395–398. [Google Scholar] [CrossRef]
- Hasan, K.M.; Tamanna, N.; Haque, A. Biochemical and histopathological profiling of Wistar rat treated with Brassica napus as a supplementary feed. Food Sci. Hum. 2018, 7, 77–82. [Google Scholar] [CrossRef]
- Unger, V.; Grosse-Siestrup, C.; Fehrenberg, C.; Fischer, A.; Meissler, M.; Groneberg, D.A. Reference values and physiological characterization of a specific isolated pig kidney perfusion model. J. Occup. Med. Toxicol. 2007, 2, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barszcz, M.; Taciak, M.; Tuśnio, A.; Ćobanová, K.; Grešáková, L. The effect of organic and inorganic zinc source, used in combination with potato fiber, on growth, nutrient digestibility and biochemical blood profile in growing pigs. Livest. Sci. 2019, 227, 37–43. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented food the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Kelly, D.; King, T.P. Luminal Bacteria: Regulation of Gut Function and Immunity. In Gut Environment of Pigs, 1st ed.; Piva, A., Knudsen, K.E.B., Lindberg, J.E., Eds.; Nottingham University Press: Nottingham, UK, 2001; p. 113. [Google Scholar]
- Yang, Z.; Liao, S.F. Physiological effects of dietary amino acids on gut health and functions of swine. Front. Vet. Sci. 2019, 6, 169. [Google Scholar] [CrossRef]
- Lindberg, J.E. Fiber effects in nutrition and gut health in pigs. J. Anim. Sci. Biotechnol. 2014, 5, 15. [Google Scholar] [CrossRef]
- Xia, L.; Yang, Y.; Wang, J.; Jing, Y.; Yang, Q. Impact of TGEV infection on the pig small intestine. Virol. J. 2018, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Buchon, N.; Broderick, N.A.; Poidevin, M.; Pradervand, S.; Lemaitre, B. Drosophila intestinal response to bacterial infection: Activation of host defense and stem cell proliferation. Cell Host Microbe. 2009, 5, 200–211. [Google Scholar] [CrossRef] [PubMed]
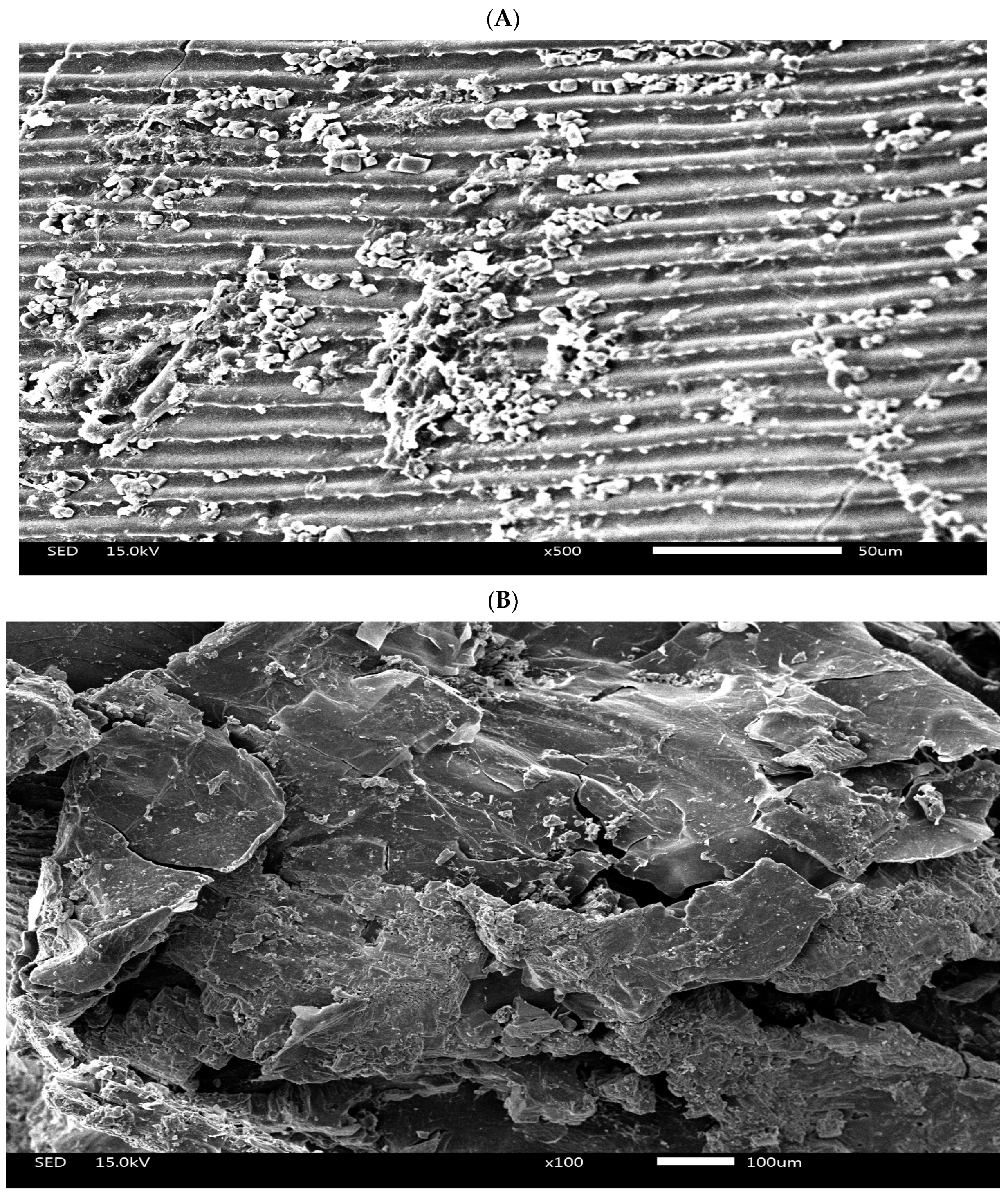
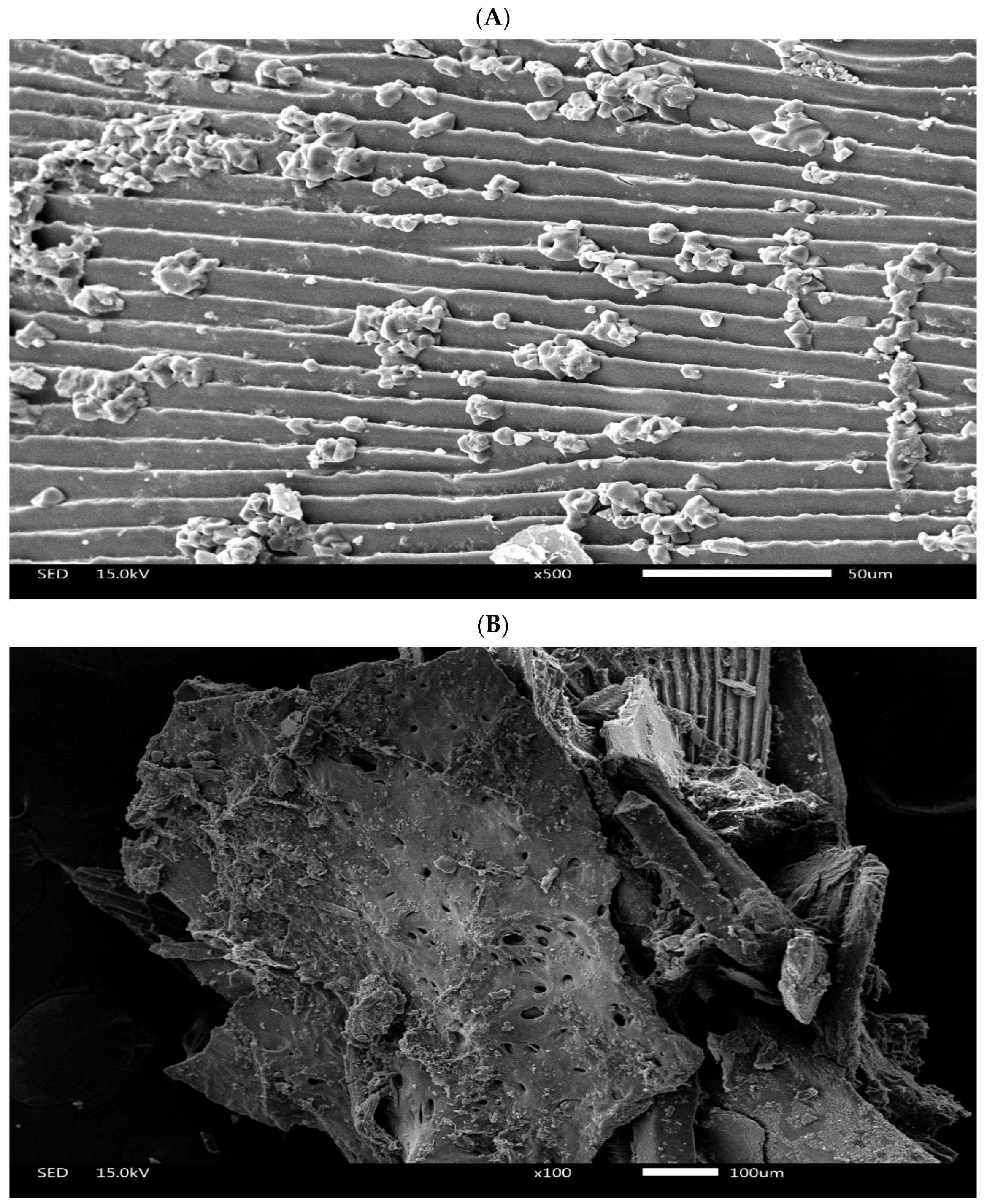
| Ingredient | CON | PFR5 | PFR10 | PFR15 |
|---|---|---|---|---|
| Corn | 39.22 | 37.85 | 35.17 | 32.17 |
| Soybean meal (45.6%) | 27.92 | 25.68 | 23.58 | 21.58 |
| Cassava meal | 15 | 15 | 15 | 15 |
| Rice bran | 10 | 10 | 10 | 10 |
| Rice bran oil | 5 | 5 | 5 | 5 |
| Pickle fish residue | 0 | 5 | 10 | 15 |
| L-Lysine (78%) | 0.47 | 0.47 | 0.47 | 0.47 |
| DL-Methionine (99%) | 0.31 | 0.31 | 0.31 | 0.31 |
| L-Threonine (99%) | 0.22 | 0.22 | 0.22 | 0.22 |
| Salt | 0.22 | 0.22 | - | - |
| Limestone | 1.39 | - | - | - |
| Vitamin-mineral premix 2 | 0.25 | 0.25 | 0.25 | 0.25 |
| Total | 100 | 100 | 100 | 100 |
| Calculated compositions (%) | ||||
| Metabolizable energy (kcal/kg) | 3400 | 3400 | 3400 | 3400 |
| Crude protein | 18.50 | 18.50 | 18.50 | 18.50 |
| Ether extract | 3.36 | 3.35 | 3.29 | 3.22 |
| Lysine | 1.12 | 1.12 | 1.12 | 1.12 |
| Methionine + Cysteine | 0.72 | 0.69 | 0.66 | 0.64 |
| Threonine | 0.88 | 0.84 | 0.81 | 0.75 |
| Tryptophan | 0.19 | 0.19 | 0.19 | 0.19 |
| Calcium | 0.66 | 0.86 | 1.58 | 2.31 |
| Total phosphorus | 0.53 | 0.86 | 1.18 | 1.51 |
| NaCl | 0.43 | 0.43 | 0.43 | 0.65 |
| Fiber | 4.83 | 5.08 | 5.30 | 5.52 |
| Analyzed compositions (%) | ||||
| Crude protein | 18.57 | 18.52 | 18.49 | 18.54 |
| Ether extract | 3.41 | 3.35 | 3.32 | 3.29 |
| Crude fiber | 5.13 | 5.22 | 5.24 | 5.37 |
| NaCl | 0.41 | 0.44 | 0.43 | 0.62 |
| Item | Before Treating | After Treating |
|---|---|---|
| Moisture | 1.98 | 1.47 |
| Ash | 61.12 | 62.25 |
| Crude protein | 20.52 | 22.80 |
| Ether extract | 1.45 | 1.58 |
| Crude fiber | 0.86 | 0.87 |
| Nitrogen free extract 1 | 14.07 | 11.03 |
| Calcium | 12.95 | 14.68 |
| Phosphorus | 7.00 | 6.92 |
| Gross energy (kcal/kg) | 2261.84 | 2685.60 |
| NaCl | 26.99 | 4.32 |
| Color score 2 | ||
| L* | 52.85 | 51.64 |
| a* | 7.53 | 7.59 |
| b* | 13.34 | 16.46 |
| Item | Dietary Supplement Level | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | PFR5 | PFR10 | PFR15 | Treatment | Linear | Quadratic | ||
| Number of pigs | 24 | 24 | 24 | 24 | ||||
| Initial BW (kg) | 23.48 | 23.73 | 23.67 | 23.73 | 0.243 | 0.861 | - | - |
| Final BW (kg) | 48.49 | 49.89 | 50.82 | 49.67 | 1.619 | 0.141 | 0.150 | 0.072 |
| ADG (g) | 595.48 | 622.98 | 646.55 | 617.54 | 14.951 | 0.162 | 0.199 | 0.078 |
| ADFI (g) | 1471 | 1447 | 1433 | 1459 | 26.191 | 0.769 | 0.688 | 0.354 |
| G:F ratio | 0.405 b | 0.431 a,b | 0.452 a | 0.423 a,b | 0.010 | 0.027 | 0.101 | 0.013 |
| Item | Dietary Supplement Level | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | PFR5 | PFR10 | PFR15 | Treatment | Linear | Quadratic | ||
| Number of pigs | 3 | 3 | 3 | 3 | ||||
| Apparent total tract digestibility (%) | ||||||||
| Dry matter | 92.59 | 93.03 | 93.53 | 93.15 | 0.416 | 0.495 | 0.272 | 0.353 |
| Crude protein | 89.57 c | 92.18 a,b | 93.97 a | 91.89 b | 0.623 | 0.006 | 0.012 | 0.005 |
| Ash | 46.74 | 53.45 | 55.61 | 51.96 | 4.007 | 0.484 | 0.347 | 0.228 |
| Ether extract | 77.81 b | 83.25 a | 84.69 a | 82.32 a | 1.274 | 0.021 | 0.028 | 0.014 |
| Item | Dietary Supplement Level | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | PFR5 | PFR10 | PFR15 | Treatment | Linear | Quadratic | ||
| Number of pigs | 5 | 5 | 5 | 5 | ||||
| 3 weeks | ||||||||
| Total Ca (mmol/L) | 2.54 | 2.63 | 2.61 | 2.71 | 5.581 | 0.930 | 0.562 | 0.982 |
| Phosphorus (mmol/L) | 3.08 | 2.97 | 3.18 | 2.92 | 3.416 | 0.926 | 0.844 | 0.803 |
| Creatinine (µmol/L) | 119.97 | 127.83 | 122.83 | 116.17 | 0.086 | 0.909 | 0.758 | 0.543 |
| ALP (U/L) | 420.33 | 453.88 | 392.57 | 456.17 | 0.022 | 0.736 | 0.826 | 0.749 |
| AST (U/L) | 68.34 b | 70.67 b | 58.43 b | 96.18 a | 0.132 | 0.019 | 0.053 | 0.034 |
| 6 weeks | ||||||||
| Total Ca (mmol/L) | 2.62 | 2.54 | 2.69 | 2.74 | 7.022 | 0.785 | 0.454 | 0.663 |
| Phosphorus (mmol/L) | 2.87 | 3.04 | 3.19 | 3.13 | 3.222 | 0.894 | 0.512 | 0.722 |
| Creatinine (µmol/L) | 131.83 | 121.67 | 130.50 | 124.33 | 0.136 | 0.727 | 0.683 | 0.789 |
| ALP (U/L) | 444.77 | 427.83 | 469.39 | 432.83 | 0.019 | 0.942 | 0.981 | 0.853 |
| AST (U/L) | 79.25 a,b | 81.82 a,b | 69.39 b | 98.92 a | 0.158 | 0.034 | 0.122 | 0.051 |
| Item | Dietary Supplement Level | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | PFR5 | PFR10 | PFR15 | Treatment | Linear | Quadratic | ||
| Number of pigs | 5 | 5 | 5 | 5 | 5 | |||
| Villus height (µm) | ||||||||
| Duodenum | 263.79 c | 309.03 b | 330.59 a,b | 357.93 a | 14.275 | 0.004 | 0.001 | 0.542 |
| Jejunum | 326.37 | 341.94 | 349.53 | 321.68 | 15.503 | 0.565 | 0.927 | 0.187 |
| Crypt depth (µm) | ||||||||
| Duodenum | 215.39 | 200.17 | 193.01 | 219.23 | 12..930 | 0.460 | 0.941 | 0.135 |
| Jejunum | 232.02 a | 212.58 b | 204.08 b | 197.71 b | 6.255 | 0.012 | 0.002 | 0.317 |
| Villus height:crypt depth | ||||||||
| Duodenum | 1.23 | 1.57 | 1.72 | 1.69 | 0.122 | 0.053 | 0.015 | 0.166 |
| Jejunum | 1.41 | 1.63 | 1.72 | 1.62 | 0.073 | 0.059 | 0.044 | 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boontiam, W.; Kitipongpysan, S.; Wachirapakorn, C.; Hong, J.; Gawborisut, S.; Saeeaw, A. Growth Performance, Nutrient Digestibility, Blood Profiles, and Gut Integrity of Growing Pigs Fed Pickled Fish Residue with Decreased Salt Content. Fermentation 2022, 8, 3. https://doi.org/10.3390/fermentation8010003
Boontiam W, Kitipongpysan S, Wachirapakorn C, Hong J, Gawborisut S, Saeeaw A. Growth Performance, Nutrient Digestibility, Blood Profiles, and Gut Integrity of Growing Pigs Fed Pickled Fish Residue with Decreased Salt Content. Fermentation. 2022; 8(1):3. https://doi.org/10.3390/fermentation8010003
Chicago/Turabian StyleBoontiam, Waewaree, Sumetee Kitipongpysan, Chalong Wachirapakorn, Jinsu Hong, Somsamorn Gawborisut, and Amporn Saeeaw. 2022. "Growth Performance, Nutrient Digestibility, Blood Profiles, and Gut Integrity of Growing Pigs Fed Pickled Fish Residue with Decreased Salt Content" Fermentation 8, no. 1: 3. https://doi.org/10.3390/fermentation8010003
APA StyleBoontiam, W., Kitipongpysan, S., Wachirapakorn, C., Hong, J., Gawborisut, S., & Saeeaw, A. (2022). Growth Performance, Nutrient Digestibility, Blood Profiles, and Gut Integrity of Growing Pigs Fed Pickled Fish Residue with Decreased Salt Content. Fermentation, 8(1), 3. https://doi.org/10.3390/fermentation8010003






