Effects of Selenium Supplementation on Rumen Microbiota, Rumen Fermentation, and Apparent Nutrient Digestibility of Ruminant Animals: A Review
Abstract
1. Introduction
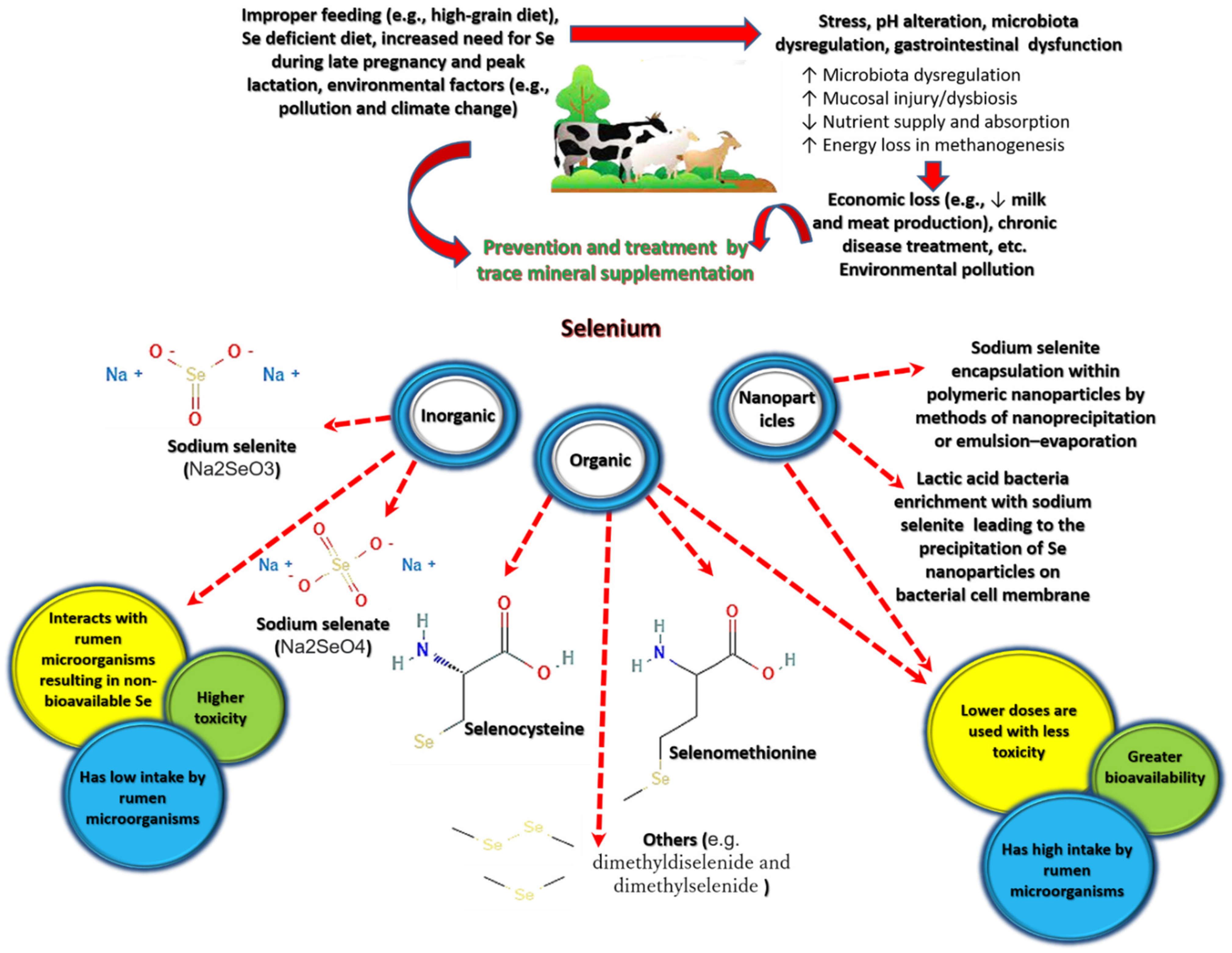
1.1. Selenium in Nature and Its Forms
1.2. Selenium Biokinetics and Bioactive Properties
1.3. Selenium Deficiency in Livestock
1.4. Selenium Use in Livestock
2. Results
| Species | Se Source | VFA | A:P | pH | NH3-N | Digestibility | Enzymes | Microbiota | PD | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Lactating dairy cows | SeY | + | - | - | - | + | ND | ND | ND | [24] |
| Lactating dairy cows | RPSS | + | - | - | - | + | + | +- | ND | [77] |
| Lactating dairy cows | HMSeBA and SS | + | - | - | - | + | ND | ND | ND | [69] |
| Lactating dairy cows | SS and CSS | + | - | - | - | + | + | +- | ND | [26] |
| Lactating dairy cows | SeY | ND | ND | ND | ND | 0 | ND | ND | ND | [99] |
| Beef cattle | SS | 0 | ND | 0 | ND | ND | ND | ND | ND | [89] |
| Beef calves and dairy heifers | SS and SeY | ND | ND | ND | ND | 0 | ND | ND | ND | [97] |
| Dairy calves | SS | + | - | - | 0 | + | + | +- | + | [80] |
| Dairy calves | SeY | ND | ND | ND | ND | 0 | ND | ND | ND | [100] |
| Dairy bulls | SS | + | - | - | - | + | + | +- | + | [78] |
| Dairy bulls | SS and CSS | + | - | - | 0 | + | ND | ND | ND | [79] |
| Steers | SeY | + | - | 0 | - | ND | ND | ND | + | [86] |
| Steers | SeY | 0 | 0 | 0 | 0 | 0 | ND | 0 | 0 | [87] |
| Buffalo heifers | SS | ND | ND | ND | ND | 0 | ND | ND | ND | [96] |
| Male buffalo calves | SS | ND | ND | ND | ND | + | ND | ND | ND | [93] |
| Male buffalo calves | Se # | ND | ND | ND | ND | 0 | ND | ND | ND | [102] |
| Pregnant and lactating ewes | SS | ND | ND | ND | ND | + | ND | ND | ND | [94] |
| Sheep | SeY and SeNps | + | - | - | - | + | ND | ND | + | [73] |
| Sheep | SS and SeY | + | 0 | 0 | - | + | ND | ND | ND | [83] |
| Sheep | SeY | ND | ND | ND | ND | + | ND | ND | ND | [92] |
| Sheep | SS and SeY | ND | ND | ND | ND | ND | + | ND | ND | [105] |
| Lambs | SS, SeY, and SeNps | ND | ND | ND | ND | + | ND | ND | ND | [74] |
| Lambs | SS and SeY | ND | ND | ND | ND | ND | ND | + | ND | [104] |
| Lambs | SS | + | ND | - | ND | ND | ND | + | ND | [85] |
| Male lambs | SSA and SeY | + | 0 | ND | ND | ND | ND | ND | ND | [84] |
| Male lambs | SeY | ND | ND | ND | ND | ND | + | ND | ND | [106] |
| Male lambs | SS | 0 | 0 | 0 | 0 | + | ND | 0 | ND | [88] |
| Male lambs | SS and Jevsel-101 * | ND | ND | ND | ND | 0 | ND | ND | ND | [101] |
| Male lambs | SS and SeY | ND | ND | ND | ND | + | ND | ND | ND | [90] |
| Male lambs | SS | ND | ND | ND | ND | 0 | ND | ND | ND | [98] |
| Male sheep | SeNps | + | - | - | - | + | ND | ND | + | [75] |
| Male sheep | SeY | ND | ND | ND | ND | + | ND | ND | ND | [29] |
| Rams | SeY | + | - | ND | + | ND | ND | +- | ND | [25] |
| Rams | SS | + | ND | ND | ND | ND | ND | ND | ND | [76] |
| Lactating goats | SS and SeMet | ND | ND | ND | ND | + | ND | ND | ND | [95] |
| Cashmere goats | SS | ND | ND | ND | ND | 0 | ND | ND | ND | [103] |
| Goats | SeY | + | ND | - | ND | ND | ND | ND | ND | [82] |
| Goats | SeY | ND | ND | ND | ND | + | ND | ND | ND | [91] |
| Male goats | SeY | + | 0 | - | ND | ND | ND | ND | ND | [81] |
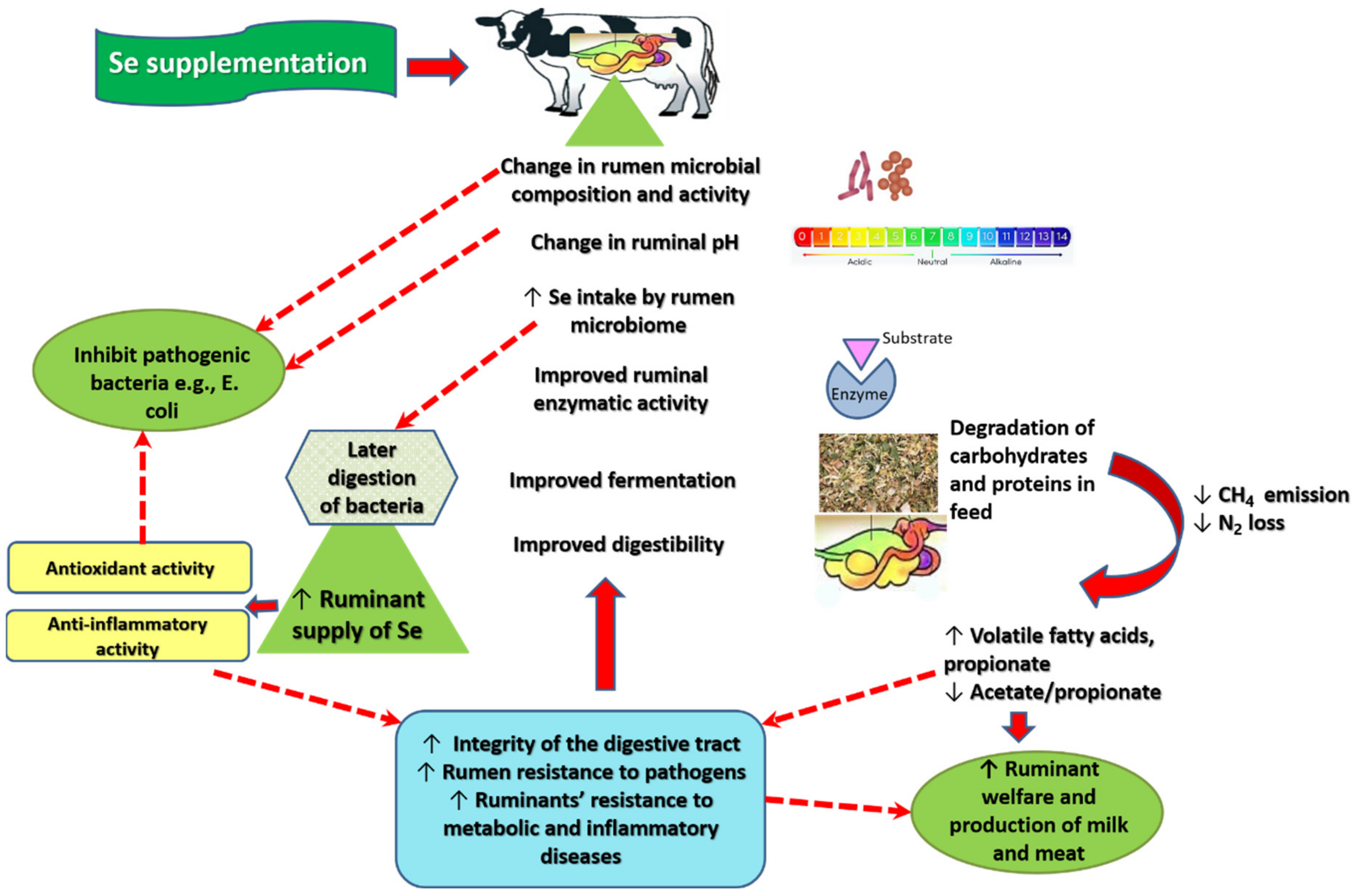
3. Mechanism of Action of Se in Ruminal Fermentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A to P ratio | Acetate to propionate ratio |
| ADF | Acid detergent fiber |
| AGEs | Advanced glycation end-products |
| BVDV2 | Bovine viral diarrhea virus |
| BW | Body weight |
| C. albicans | Candida albicans |
| CD4+ | Cluster of differentiation antigens positive cells 4 |
| CK | Creatine kinase |
| CP | Crude protein |
| DE | Digestive energy |
| DM | Dry matter |
| E. coli | Escherichia coli |
| FOXO | Forkhead Box O transcription factor |
| GSH-Px | Glutathione peroxidase |
| GE | Gross energy |
| LAB | Lactic acid bacteria |
| LDH | Lactate dehydrogenase |
| LPS | Lipopolysaccharide |
| ME | Metabolize energy |
| NDF | Neutral detergent fiber |
| NF-κB | Nuclear factor kappa light chain enhancer of activated B cells |
| OM | Organic matter |
| PD | Purine derivatives |
| ROS | Reactive oxygen species |
| Se | Selenium |
| SeMet | Selenomethionine |
| SeNps | Se nanoparticles |
| TNF-α | Tumor necrosis factor-α |
| VFA | Volatile fatty acids |
References
- Kobayashi, Y.; Oh, S.; Myint, H.; Koike, S. Use of Asian selected agricultural byproducts to modulate rumen microbes and fermentation. J. Anim. Sci. Biotechnol. 2016, 7, 70. [Google Scholar] [CrossRef]
- Gruninger, R.J.; Ribeiro, G.O.; Cameron, A.; McAllister, T.A. Invited review: Application of meta-omics to understand the dynamic nature of the rumen microbiome and how it responds to diet in ruminants. Animal 2019, 13, 1843–1854. [Google Scholar] [CrossRef]
- Doyle, N.; Mbandlwa, P.; Kelly, W.J.; Attwood, G.; Li, Y.; Ross, R.P.; Stanton, C.; Leahy, S. Use of Lactic Acid Bacteria to Reduce Methane Production in Ruminants, a Critical Review. Front. Microbiol. 2019, 10, 2207. [Google Scholar] [CrossRef] [PubMed]
- Zeineldin, M.; Barakat, R.; Elolimy, A.; Salem, A.Z.M.; Elghandour, M.M.Y.; Monroy, J.C. Synergetic action between the rumen microbiota and bovine health. Microb. Pathog. 2018, 124, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Jin, W.; Feng, P.F.; Liu, J.H.; Mao, S.Y. High-grain diet feeding altered the composition and functions of the rumen bacterial community and caused the damage to the laminar tissues of goats. Animal 2018, 12, 2511–2520. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Carr, M.A.; Edrington, T.S.; Anderson, R.C.; Nisbet, D.J. Diet, Escherichia coli O157:H7, and cattle: A review after 10 years. Curr. Issues Mol. Biol. 2009, 11, 67–80. [Google Scholar] [PubMed]
- Ali, A.M.; Kunugi, H. Royal jelly as an intelligent anti-aging—A focus on cognitive aging and Alzheimer’s disease: A review. Antioxidants 2020, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Apitherapy for age-related skeletal muscle dysfunction (sarcopenia): A review on the effects of royal jelly, propolis, and bee pollen. Foods 2020, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Intermittent fasting, dietary modifications, and exercise for the control of gestational diabetes and maternal mood dysregulation: A review and a case report. Int. J. Environ. Res. Public Health 2020, 17, 9379. [Google Scholar] [CrossRef] [PubMed]
- Miyai, S.; Hendawy, A.O.; Sato, K. Gene expression profile of peripheral blood mononuclear cells in mild to moderate obesity in dogs. Vet. Anim. Sci. 2021, 13, 100183. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. The effects of royal jelly acid, 10-hydroxy-trans-2-decenoic acid, on neuroinflammation and oxidative stress in astrocytes stimulated with lipopolysaccharide and hydrogen peroxide. Immuno 2021, 1, 212–222. [Google Scholar] [CrossRef]
- Hassan, A.A.; Yousif, M.H.; Abd-Elkhaliq, H.M.M.; Wahba, A.K.A.; El-Hamaky, A.M.a. The antimicrobial potential of selenium nanoparticles singly and in combination with cinnamon oil against fungal and bacterial causes of diarrhea in buffaloes. Adv. Anim. Vet. Sci. 2021, 9, 1238–1248. [Google Scholar] [CrossRef]
- Hendawy, A.O.; Shirai, M.; Takeya, H.; Sugimura, S.; Miyanari, S.; Taniguchi, S.; Sato, K. Effects of 5-aminolevulinic acid supplementation on milk production, iron status, and immune response of dairy cows. J. Dairy Sci. 2019, 102, 11009–11015. [Google Scholar] [CrossRef] [PubMed]
- Hendawy, A.; Mansour, M.; El-Din, A.N. Effects of medicinal plants on haematological indices, colostrum, and milk composition of ewes. J. Vet. Med. Anim. Sci. 2019, 2, 1008. [Google Scholar]
- Hurst, J.J.; Wallace, J.S.; Aga, D.S. Method development for the analysis of ionophore antimicrobials in dairy manure to assess removal within a membrane-based treatment system. Chemosphere 2018, 197, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.D.S.; Cooke, R.F. Effects of ionophores on ruminal function of beef cattle. Animals 2021, 11, 2871. [Google Scholar] [CrossRef]
- Novilla, M.N. Chapter 78—Ionophores. In Veterinary Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1073–1092. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Hypoproteinemia predicts disease severity and mortality in COVID-19: A call for action. Diagn. Pathol. 2021, 16, 31. [Google Scholar] [CrossRef]
- Reichhardt, C.C.; Messersmith, E.M.; Brady, T.J.; Motsinger, L.A.; Briggs, R.K.; Bowman, B.R.; Hansen, S.L.; Thornton, K.J. Anabolic implants varying in hormone type and concentration influence performance, feeding behavior, carcass Characteristics, plasma trace mineral concentrations, and liver trace mineral concentrations of angus sired steers. Animals 2021, 11, 1964. [Google Scholar] [CrossRef]
- Hirpessa, B.B.; Ulusoy, B.H.; Hecer, C. Hormones and hormonal anabolics: Residues in animal source food, potential public health impacts, and methods of analysis. J. Food Qual. 2020, 2020, 5065386. [Google Scholar] [CrossRef]
- Kolok, A.S.; Ali, J.M.; Rogan, E.G.; Bartelt-Hunt, S.L. The fate of synthetic and endogenous hormones used in the US beef and dairy industries and the potential for human exposure. Curr. Environ. Health Rep. 2018, 5, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Hendawy, A.O.; Khattab, M.S.; Sugimura, S.; Sato, K. Effects of 5-aminolevulinic acid as a supplement on animal performance, iron status, and immune response in farm animals: A review. Animals 2020, 10, 1352. [Google Scholar] [CrossRef]
- Nabi, F.; Arain, M.A.; Hassan, F.; Umar, M.; Rajput, N.; Alagawany, M.; Syed, S.F.; Soomro, J.; Somroo, F.; Liu, J. Nutraceutical role of selenium nanoparticles in poultry nutrition: A review. Worlds Poult. Sci. J. 2020, 76, 459–471. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Yang, W.Z.; Dong, Q.; Yang, X.M.; He, D.C.; Zhang, P.; Dong, K.H.; Huang, Y.X. Effects of selenium yeast on rumen fermentation, lactation performance and feed digestibilities in lactating dairy cows. Livest. Sci. 2009, 126, 239–244. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Z.; Tan, Y.; Chang, S.; Zheng, H.; Wang, H.; Yan, T.; Guru, T.; Hou, F. Selenium yeast dietary supplement affects rumen bacterial population dynamics and fermentation parameters of tibetan sheep (Ovis aries) in Alpine meadow. Front. Microbiol. 2021, 12, 663945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.D.; Wang, C.; Du, H.S.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L. Effects of sodium selenite and coated sodium selenite on lactation performance, total tract nutrient digestion and rumen fermentation in Holstein dairy cows. Anim. Int. J. Anim. Biosci. 2020, 14, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Naderi, M.; Puar, P.; Zonouzi-Marand, M.; Chivers, D.P.; Niyogi, S.; Kwong, R.W.M. A comprehensive review on the neuropathophysiology of selenium. Sci. Total Environ. 2021, 767, 144329. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Tan, Y.H.; Cui, X.X.; Chang, S.H.; Xiao, X.; Yan, T.H.; Wang, H.; Hou, F.J. Effect of different levels of selenium yeast on the antioxidant status, nutrient digestibility, selenium balances and nitrogen metabolism of Tibetan sheep in the Qinghai-Tibetan Plateau. Small Rumin. Res. 2019, 180, 63–69. [Google Scholar] [CrossRef]
- Flores-Mateo, G.; Navas-Acien, A.; Pastor-Barriuso, R.; Guallar, E. Selenium and coronary heart disease: A meta-analysis. Am. J. Clin. Nutr. 2006, 84, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Ravn-Haren, G. Absorption, distribution, metabolism and excretion (ADME) of oral selenium from organic and inorganic sources: A review. J. Trace Elem. Med. Biol. 2021, 67, 126801. [Google Scholar] [CrossRef]
- Galbraith, M.L.; Vorachek, W.R.; Estill, C.T.; Whanger, P.D.; Bobe, G.; Davis, T.Z.; Hall, J.A. Rumen microorganisms decrease bioavailability of inorganic selenium supplements. Biol. Trace Elem. Res. 2016, 171, 338–343. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhao, J.; Wang, L.; Liu, Q.; Fan, Y.; Li, B.; Yu, Y.-L.; Chen, C.; Li, Y.-F. Using nano-selenium to combat Coronavirus Disease 2019 (COVID-19)? Nano Today 2021, 36, 101037. [Google Scholar] [CrossRef]
- Seale, L.A.; Torres, D.J.; Berry, M.J.; Pitts, M.W. A role for selenium-dependent GPX1 in SARS-CoV-2 virulence. Am. J. Clin. Nutr. 2020, 112, 447–448. [Google Scholar] [CrossRef]
- Fakhrolmobasheri, M.; Nasr-Esfahany, Z.; Khanahmad, H.; Zeinalian, M. Selenium supplementation can relieve the clinical complications of COVID-19 and other similar viral infections. Int. J. Vitam. Nutr. Res. 2021, 91, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.A.; Ebeid, H.M.; Hassan, F.-U. Revisiting the effects of different dietary sources of selenium on the health and performance of dairy animals: A review. Biol. Trace Elem. Res. 2021, 199, 3319–3337. [Google Scholar] [CrossRef]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef]
- Manzanares, W.; Moreira, E.; Hardy, G. Pharmaconutrition revisited for critically ill patients with coronavirus disease 2019 (COVID-19): Does selenium have a place? Nutrition 2021, 81, 110989. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Wise, L.A. Environmental Selenium and Human Health: An Update. Curr. Environ. Health Rep. 2018, 5, 464–485. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Tang, J.; Jia, G.; Liu, G.; Chen, X.; Cai, J.; Shang, H.; Zhao, H. Pancreatic atrophy caused by dietary selenium deficiency induces hypoinsulinemic hyperglycemia via global down-regulation of selenoprotein encoding genes in broilers. PLoS ONE 2017, 12, e0182079. [Google Scholar] [CrossRef] [PubMed]
- Kiełczykowska, M.; Kocot, J.; Paździor, M.; Musik, I. Selenium—A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention systematic review and meta-analysis. JAMA 2007, 297, 842–857. [Google Scholar] [CrossRef]
- Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef]
- Yin, H.; Qi, Z.; Li, M.; Ahammed, G.J.; Chu, X.; Zhou, J. Selenium forms and methods of application differentially modulate plant growth, photosynthesis, stress tolerance, selenium content and speciation in Oryza sativa L. Ecotoxicol. Environ. Saf. 2019, 169, 911–917. [Google Scholar] [CrossRef]
- Hofstee, P.; McKeating, D.R.; Bartho, L.A.; Anderson, S.T.; Perkins, A.V.; Cuffe, J.S.M. Maternal selenium deficiency in mice alters offspring glucose metabolism and thyroid status in a sexually dimorphic manner. Nutrients 2020, 12, 267. [Google Scholar] [CrossRef]
- Lin, S.-L.; Wang, C.-W.; Tan, S.-R.; Liang, Y.; Yao, H.-D.; Zhang, Z.-W.; Xu, S.-W. Selenium deficiency inhibits the conversion of thyroidal thyroxine (T4) to triiodothyronine (T3) in chicken thyroids. Biol. Trace Elem. Res. 2014, 161, 263–271. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Gowda, S.; Mundkur, L. An exploratory study of selenium status in healthy individuals and in patients with COVID-19 in a south Indian population: The case for adequate selenium status. Nutrition 2020, 82, 111053. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.F.; Fleming, H.R.; Cogan, T.; Hodgson, C.; Davies, D.R. Assessing the ability of silage lactic acid bacteria to incorporate and transform inorganic selenium within laboratory scale silos. Anim. Feed Sci. Technol. 2019, 253, 125–134. [Google Scholar] [CrossRef]
- Hudman, J.F.; Glenn, A.R. Selenium uptake by Butyrivibrio fibrisolvens and Bacteroides ruminicola. FEMS Microbiol. Lett. 1985, 27, 215–220. [Google Scholar] [CrossRef]
- Mansour, M.M.; Hendawy, A.O.; Zeitoun, M.M. Effect of mastitis on luteal function and pregnancy rates in buffaloes. Theriogenology 2016, 86, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Mandour, A.S.; Samir, H.; El-Beltagy, M.A.; Abdel-Daim, M.M.; Izumi, W.; Ma, D.; Matsuura, K.; Tanaka, R.; Watanabe, G. Effect of supra-nutritional selenium-enriched probiotics on hematobiochemical, hormonal, and Doppler hemodynamic changes in male goats. Environ. Sci. Pollut. Res. 2020, 27, 19447–19460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Teng, Z.; Wang, L.; Wang, L.; Huang, T.; Zhang, X. Dietary selenium deficiency and excess accelerate ubiquitin-mediated protein degradation in the muscle of rainbow trout (Oncorhynchus mykiss) via Akt/FoxO3a and NF-κB signaling pathways. Biol. Trace Elem. Res. 2021. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Physical frailty/sarcopenia as a key predisposing factor to coronavirus disease 2019 (COVID-19) and its complications in older adults. BioMed 2021, 1, 11–40. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Skeletal muscle damage in COVID-19: A call for action. Medicina 2021, 57, 372. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Approaches to nutritional screening in patients with Coronavirus Disease 2019 (COVID-19). Int. J. Environ. Res. Public Health 2021, 18, 2772. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Screening for sarcopenia (physical frailty) in the COVID-19 era. Int. J. Endocrinol. 2021, 2021, 5563960. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Van Saun, R.J.; Bobe, G.; Stewart, W.C.; Vorachek, W.R.; Mosher, W.D.; Nichols, T.; Forsberg, N.E.; Pirelli, G.J. Organic and inorganic selenium: I. Oral bioavailability in ewes1. J. Anim. Sci. 2012, 90, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Żarczyńska, K.; Baumgartner, W.; Sobiech, P. Coagulology, biochemical profile and muscle pathology in calves diagnosed with nutritional muscular dystrophy. Pol. J. Vet. Sci. 2017, 20, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Ali, E.M.; Ahmed, M.S.; Hendawy, A.O. Targeting gut microbiome and the recovery of muscle loss associated with cancer (cachexia): An overview of the possible effect of bee products. Med. Legal Update 2021, 21, 163–171. [Google Scholar] [CrossRef]
- Wang, C.; Lovell, R.T. Organic selenium sources, selenomethionine and selenoyeast, have higher bioavailability than an inorganic selenium source, sodium selenite, in diets for channel catfish (Ictalurus punctatus). Aquaculture 1997, 152, 223–234. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Q.; Zhan, T.; Han, Y.; Tang, C.; Zhang, J. Effect of different selenium sources on growth performance, tissue selenium content, meat quality, and selenoprotein gene expression in finishing pigs. Biol. Trace Elem. Res. 2020, 196, 463–471. [Google Scholar] [CrossRef]
- Ibrahim, D.; Kishawy, A.T.Y.; Khater, S.I.; Hamed Arisha, A.; Mohammed, H.A.; Abdelaziz, A.S.; Abd El-Rahman, G.I.; Elabbasy, M.T. Effect of dietary modulation of selenium form and level on performance, tissue retention, quality of frozen stored meat and gene expression of antioxidant status in ross broiler chickens. Animals 2019, 9, 342. [Google Scholar] [CrossRef]
- Diniz, W.J.S.; Bobe, G.; Klopfenstein, J.J.; Gultekin, Y.; Davis, T.Z.; Ward, A.K.; Hall, J.A. Supranutritional maternal organic selenium supplementation during different trimesters of pregnancy affects the muscle gene transcriptome of newborn beef calves in a time-dependent manner. Genes 2021, 12, 1884. [Google Scholar] [CrossRef]
- Arce-Cordero, J.A.; Monteiro, H.F.; Lelis, A.L.; Lima, L.R.; Restelatto, R.; Brandao, V.L.N.; Leclerc, H.; Vyas, D.; Faciola, A.P. Copper sulfate and sodium selenite lipid-microencapsulation modifies ruminal microbial fermentation in a dual-flow continuous-culture system. J. Dairy Sci. 2020, 103, 7068–7080. [Google Scholar] [CrossRef]
- Lee, M.R.F.; Fleming, H.R.; Whittington, F.; Hodgson, C.; Suraj, P.T.; Davies, D.R. The potential of silage lactic acid bacteria-derived nano-selenium as a dietary supplement in sheep. Anim. Prod. Sci. 2019, 59, 1999–2009. [Google Scholar] [CrossRef]
- Wei, J.Y.; Wang, J.; Liu, W.; Zhang, K.Z.; Sun, P. Short communication: Effects of different selenium supplements on rumen fermentation and apparent nutrient and selenium digestibility of mid-lactation dairy cows. J. Dairy Sci. 2019, 102, 3131–3135. [Google Scholar] [CrossRef] [PubMed]
- Romero-Pérez, A.; García-García, E.; Zavaleta-Mancera, A.; Ramírez-Bribiesca, J.E.; Revilla-Vázquez, A.; Hernández-Calva, L.M.; López-Arellano, R.; Cruz-Monterrosa, R.G. Designing and evaluation of sodium selenite nanoparticles in vitro to improve selenium absorption in ruminants. Vet. Res. Commun. 2010, 34, 71–79. [Google Scholar] [CrossRef]
- Milewski, S.; Sobiech, P.; Błażejak-Grabowska, J.; Wójcik, R.; Żarczyńska, K.; Miciński, J.; Ząbek, K. The efficacy of a long-acting injectable selenium preparation administered to pregnant ewes and lambs. Animals 2021, 11, 1076. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Propolis, bee honey, and their components protect against coronavirus disease 2019 (Covid-19): A review of in silico, in vitro, and clinical studies. Molecules 2021, 26, 1232. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.J.; Shi, L.G.; Yue, W.B.; Zhang, C.X.; Ren, Y.S.; Liu, Q. Effect of high-dose nano-selenium and selenium-yeast on feed digestibility, rumen fermentation, and purine derivatives in sheep. Biol. Trace Elem. Res. 2012, 150, 130–136. [Google Scholar] [CrossRef]
- Ibrahim, E.M.; Mohamed, M.Y. Effect of different dietary selenium sources supplementation on nutrient digestibility, productive performance and some serum biochemical indices in sheep. Egypt. J. Nutr. Feeds 2018, 21, 53–64. [Google Scholar] [CrossRef]
- Shi, L.G.; Xun, W.J.; Yue, W.B.; Zhang, C.X.; Ren, Y.S.; Liu, Q.A.; Wang, Q.A.; Shi, L. Effect of elemental nano-selenium on feed digestibility, rumen fermentation, and purine derivatives in sheep. Anim. Feed Sci. Technol. 2011, 163, 136–142. [Google Scholar] [CrossRef]
- Hidiroglou, M.; Lessard, J.R. The effect of selenium or vitamin E supplementation on volatile fatty acid content of rumen liquor in sheep fed a purified diet. Int. J. Vitam. Nutr. Res. 1976, 46, 458–463. [Google Scholar]
- Du, H.S.; Wang, C.; Wu, Z.Z.; Zhang, G.W.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L. Effects of rumen-protected folic acid and rumen-protected sodium selenite supplementation on lactation performance, nutrient digestion, ruminal fermentation and blood metabolites in dairy cows. J. Sci. Food Agric. 2019, 99, 5826–5833. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Wang, C.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L.; Zhang, J. Effects of sodium selenite addition on ruminal fermentation, microflora and urinary excretion of purine derivatives in Holstein dairy bulls. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1719–1726. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhang, Z.D.; Dai, S.H.; Wang, Y.; Tian, X.F.; Zhao, J.H.; Wang, C.; Liu, Q.; Guo, G.; Huo, W.J. Effects of sodium selenite and coated sodium selenite addition on performance, ruminal fermentation, nutrient digestibility and hepatic gene expression related to lipid metabolism in dairy bulls. Livest. Sci. 2020, 237, 104062. [Google Scholar] [CrossRef]
- Zhang, G.W.; Wang, C.; Du, H.S.; Wu, Z.Z.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Zhang, Y.L.; Pei, C.X.; et al. Effects of folic acid and sodium selenite on growth performance, nutrient digestion, ruminal fermentation and urinary excretion of purine derivatives in Holstein dairy calves. Livest. Sci. 2020, 231, 103884. [Google Scholar] [CrossRef]
- Abbasi, B.; Malhi, M.; Siyal, F.; Arain, M.A.; Bhutto, Z.A.; Soomro, S.; Rui, R. Influence of dietary selenium yeast on fermentation pattern mucosal growth and glutathione peroxidase (gsh-px) activity in colon of goat. J. Dairy Vet. Anim. Res. 2018, 7, 253–259. [Google Scholar] [CrossRef]
- Shahid, A.; Moolchand, M.; Soomro, S.A.; Giasuddin, S.M.; Kalhoro, N.H.; Kaka, A.; Mal, R.; Soomro, M.A.; Samo, S.P.; Sanjrani, M.N. Influence of dietary selenium yeast supplementation on fermentation pattern, papillae morphology and antioxidant status in rumen of goat. Pak. J. Zool. 2020, 52, 65–571. [Google Scholar] [CrossRef]
- Zhu, A.; Wang, F.; Feng, X.; Nie, H.; Li, F.; Wang, J.; Wang, H.; Zhu, G.; Wang, Z. Effects of different dietary selenium supplementation on growth, selenium retention in tissues and rumen fermentation in growing Hu sheep. J. Nanjing Agric. Univ. 2017, 40, 718–724. [Google Scholar]
- Miltko, R.; Rozbicka-Wieczorek, J.A.; Wiesyk, E.; Czauderna, M. The influence of different chemical forms of selenium added to the diet including carnosic acid, fish oil and rapeseed oil on the formation of volatile fatty acids and methane in the rumen, and fatty acid profiles in the rumen content and muscles of lambs. Acta Vet. 2016, 66, 373–391. [Google Scholar] [CrossRef]
- Naziroglu, M.; Aksakal, M. Effects of vitamin E and selenium on rumen protozoa in lambs. Turk. J. Vet. Anim. Sci. 1997, 21, 81–90. [Google Scholar]
- Liu, Q.; Wang, C.; Huang, Y.X.; Miao, C.H.; Gao, D.H. Effects of Sel-Plex on rumen fermentation and purine derivatives of urine in Simmental steers. J. Anim. Feed Sci. 2007, 16, 133–138. [Google Scholar] [CrossRef]
- Ferreira, A.V.D.; Cominotte, A.; Ladeira, M.M.; Casagrande, D.R.; Teixeira, P.D.; van Cleef, E.; Ezequiel, J.; Castagnino, P.; Neto, O.R.M. Feedlot diets with soybean oil, selenium and vitamin E alters rumen metabolism and fatty acids content in steers. Anim. Feed Sci. Technol. 2020, 260, 114362. [Google Scholar] [CrossRef]
- Del Razo-Rodriguez, O.E.; Ramirez-Bribiesca, J.E.; Lopez-Arellano, R.; Revilla-Vazquez, A.L.; Gonzalez-Munoz, S.S.; Cobos-Peralta, M.A.; Hernandez-Calva, L.M.; McDowell, L.R. Effects of dietary level of selenium and grain on digestive metabolism in lambs. Czech J. Anim. Sci. 2013, 58, 253–261. [Google Scholar] [CrossRef]
- Del Claro, G.R.; Zanetti, M.A.; Netto, A.S.; Vilela, F.G.; Melo, M.P.; Correa, L.B.; Freitas, J.E. The effects of copper and selenium supplementation in the diet of Brangus steers on performance and rumen fermentation. Arq. Brasil. Med. Vet. E Zootecn. 2013, 65, 255–261. [Google Scholar] [CrossRef]
- Alimohamady, R.; Aliarabi, H.; Bahari, A.; Dezfoulian, A.H. Influence of different amounts and sources of selenium supplementation on performance, some blood parameters, and nutrient digestibility in lambs. Biol. Trace Elem. Res. 2013, 154, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Samo, S.P.; Malhi, M.; Gadahi, J.; Lei, Y.; Kaciwal, A.B.; Soomro, S.A. Effect of organic selenium supplementation in diet on gastrointestinal tract performance and meat quality of goat. Pak. J. Zool. 2018, 50, 995–1003. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Lou, S.; Wanapat, M.; Wang, Z.; Zhu, W.; Hou, F. Selenium supplementation improves nutrient intake and digestibility, and mitigates CH(4) emissions from sheep grazed on the mixed pasture of alfalfa and tall fescue. J. Anim. Physiol. Anim. Nutr. 2021, 105, 611–620. [Google Scholar] [CrossRef]
- Mudgal, V.; Garg, A.K.; Dass, R.S. Effect of dietary selenium and copper supplementation on growth and nutrient utilization in buffalo (Bubalus bubalis) calves. Anim. Nutr. Feed Technol. 2007, 7, 79–88. [Google Scholar]
- Ibrahim, E. The impact of dietary supplementation of iodine and selenium on nutrients digetibility and productive performance of ewes and their suckling lambs. Egypt. J. Nutr. Feeds 2016, 19, 255–263. [Google Scholar] [CrossRef][Green Version]
- Taheri, Z.; Karimi, S.; Mehrban, H.; Moharrery, A. Supplementation of different selenium sources during early lactation of native goats and their effects on nutrient digestibility, nitrogen and energy status. J. Appl. Anim. Res. 2018, 46, 64–68. [Google Scholar] [CrossRef]
- Ganie, A.A.; Baghel, R.P.S.; Mudgal, V.; Sheikh, G.G. Effect of selenium supplementation on growth and nutrient utilization in buffalo heifers. Anim. Nutr. Feed Technol. 2010, 10, 255–259. [Google Scholar]
- Nicholson, J.W.G.; McQueen, R.E.; Bush, R.S. Response of growing cattle to supplementation with organically bound or inorganic sources of selenium or yeast cultures. Can. J. Anim. Sci. 1991, 71, 803–811. [Google Scholar] [CrossRef]
- Kumar, N.; Garg, A.K.; Mudgal, V.; Dass, R.S.; Chaturvedi, V.K.; Varshney, V.P. Effect of different levels of selenium supplementation on growth rate, nutrient utilization, blood metabolic profile, and immune response in lambs. Biol. Trace Elem. Res. 2008, 126 (Suppl. S1), S44–S56. [Google Scholar] [CrossRef]
- Faccenda, A.; Zambom, M.A.; de Avila, A.S.; Schneider, C.R.; Werle, C.H.; Anschau, F.A.; Almeida, A.R.E.; Lange, M.J.; dos Santos, G.T. Performance and milk composition of Holstein cows fed with dried malt bagasse and selenium-enriched Saccharomyces cerevisiae. Livest. Sci. 2020, 238, 104081. [Google Scholar] [CrossRef]
- Skrivanova, E.; Marounek, M.; De Smet, S.; Raes, K. Influence of dietary selenium and vitamin E on quality of veal. Meat Sci. 2007, 76, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Garg, A.K.; Dass, R.S. Effect of dietary supplementation of inorganic and organic selenium on intake and utilization of nutrients in lambs. Anim. Nutr. Feed Technol. 2009, 9, 253–260. [Google Scholar]
- Shinde, P.L.; Dass, R.S.; Garg, A.K.; Bhadane, K.P. Effect of vitamin E and selenium supplementation on growth, nutrient utilization and their balance in male buffalo calves. Anim. Nutr. Feed Technol. 2008, 8, 157–165. [Google Scholar]
- Qin, F.; Zhu, X.P.; Zhang, W.; Zhou, J.P.; Zhang, S.W.; Jia, Z.H. Effects of dietary iodine and selenium on nutrient digestibility, serum thyroid hormones, and antioxidant status of liaoning cashmere goats. Biol. Trace Elem. Res. 2011, 143, 1480–1488. [Google Scholar] [CrossRef]
- Mihalikova, K.; Gresakova, L.; Boldizarova, K.; Faix, S.; Leng, L.; Kisidayova, S. The effects of organic selenium supplementation on the rumen ciliate population in sheep. Folia Microbiol. 2005, 50, 353–356. [Google Scholar] [CrossRef]
- Faixova, Z.; Piesova, E.; Makova, Z.; Cobanova, K.; Faix, S. Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on ruminal enzyme activities and blood chemistry in sheep. Acta Vet. Brno 2016, 85, 185–194. [Google Scholar] [CrossRef]
- Faixová, Z.; Faix, Š.; Leng, Ľ.; Váczi, P.; Maková, Z.; Szabóová, R. Haematological, blood and rumen chemistry changes in lambs following supplementation with Se-yeast. Acta Vet. Brunensis 2007, 76, 3–8. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Rasmussen, M.A.; Cray, W.C., Jr.; Casey, T.A.; Whipp, S.C. Rumen contents as a reservoir of enterohemorrhagic Escherichia coli. FEMS Microbiol. Lett. 1993, 114, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Soldavini, J.; Kaunitz, J.D. Pathobiology and potential therapeutic value of intestinal short-chain fatty acids in gut inflammation and obesity. Digest. Dis. Sci. 2013, 58, 2756–2766. [Google Scholar] [CrossRef]
- Eslick, S.; Thompson, C.; Berthon, B.; Wood, L. Short-chain fatty acids as anti-inflammatory agents in overweight and obesity: A systematic review and meta-analysis. Nutr. Rev. 2021, nuab059. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, J.; Wu, X.; Yang, C.S.; Zhang, J. Selenium nanoparticles act as an intestinal p53 inhibitor mitigating chemotherapy-induced diarrhea in mice. Pharmacol. Res. 2019, 149, 104475. [Google Scholar] [CrossRef] [PubMed]
- Bittar, J.H.J.; Palomares, R.A.; Hurley, D.J.; Hoyos-Jaramillo, A.; Rodriguez, A.; Stoskute, A.; Hamrick, B.; Norton, N.; Adkins, M.; Saliki, J.T.; et al. Immune response and onset of protection from Bovine viral diarrhea virus 2 infection induced by modified-live virus vaccination concurrent with injectable trace minerals administration in newly received beef calves. Vet. Immunol. Immunopathol. 2020, 225, 110055. [Google Scholar] [CrossRef] [PubMed]
- Mynhardt, H.; van Ryssen, J.B.J.; Coertze, R.J. The effect of the heat processing of soybean seed on the metabolism of its selenium in lambs. Anim. Feed Sci. Technol. 2006, 128, 122–134. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; He, X.; Lian, S.; Liang, J.; Yu, D.; Sun, D.; Wu, R. Selenium deficiency induces duodenal villi cell apoptosis via an oxidative stress-induced mitochondrial apoptosis pathway and an inflammatory signaling-induced death receptor pathway. Met. Integr. Biometal Sci. 2018, 10, 1390–1400. [Google Scholar] [CrossRef]
- Liao, S.F. Invited review: Maintain or improve piglet gut health around weanling: The fundamental effects of dietary Amino acids. Animals 2021, 11, 1110. [Google Scholar] [CrossRef]
- Ahmed, Z.; Malhi, M.; Soomro, S.; Gandahi, J.; Arijo, A.; Bhutto, B.; Qureshi, T. Dietary selenium yeast supplementation improved some villi morphological characteristics in duodenum and jejunum of young goats. J. Anim. Plant Sci. 2016, 26, 382–387. [Google Scholar]
- Cheng, L.; Shu, Y.; He, M.; Xia, X.; Li, Y.; Feng, S.; Wang, X.; Wu, J. Effects of selenium-enriched yeast and Bacillus subtilis on intestinal mucosal morphology and rectum microflora of Hu lambs. Chin. J. Anim. Nutr. 2018, 30, 4660–4669. [Google Scholar]
- Panousis, N.; Roubies, N.; Karatzias, H.; Frydas, S.; Papasteriadis, A. Effect of selenium and vitamin E on antibody production by dairy cows vaccinated against Escherichia coli. Vet. Rec. 2001, 149, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Palomares, R.A.; Hurley, D.J.; Bittar, J.H.J.; Saliki, J.T.; Woolums, A.R.; Moliere, F.; Havenga, L.J.; Norton, N.A.; Clifton, S.J.; Sigmund, A.B.; et al. Effects of injectable trace minerals on humoral and cell-mediated immune responses to Bovine viral diarrhea virus, Bovine herpes virus 1 and Bovine respiratory syncytial virus following administration of a modified-live virus vaccine in dairy calves. Vet. Immunol. Immunopathol. 2016, 178, 88–98. [Google Scholar] [CrossRef]
- Wallace, L.G.; Bobe, G.; Vorachek, W.R.; Dolan, B.P.; Estill, C.T.; Pirelli, G.J.; Hall, J.A. Effects of feeding pregnant beef cows selenium-enriched alfalfa hay on selenium status and antibody titers in their newborn calves. J. Anim. Sci. 2017, 95, 2408–2420. [Google Scholar] [CrossRef]
- Krausova, G.; Kana, A.; Vecka, M.; Hyrslova, I.; Stankova, B.; Kantorova, V.; Mrvikova, I.; Huttl, M.; Malinska, H. In Vivo Bioavailability of Selenium in Selenium-Enriched Streptococcus thermophilus and Enterococcus faecium in CD IGS Rats. Antioxidants 2021, 10, 463. [Google Scholar] [CrossRef]
- Zhou, M.; Zheng, X.; Zhu, H.; Li, L.; Zhang, L.; Liu, M.; Liu, Z.; Peng, M.; Wang, C.; Li, Q.; et al. Effect of Lactobacillus plantarum enriched with organic/inorganic selenium on the quality and microbial communities of fermented pickles. Food Chem. 2021, 365, 130495. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, J.; Yang, K.; Liu, M.; Qi, Y.; Zhang, T.; Fan, M.; Wei, X. Antibacterial activity of selenium-enriched lactic acid bacteria against common food-borne pathogens in vitro. J. Dairy Sci. 2018, 101, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H.; Abdelmageed, H.A.; Mandour, A.S.; Ahmed, M.E.; Ahmad, S.; Hendawy, A.O. Vitamin K in COVID-19—Potential anti-COVID-19 effects of vitamin K antagonists (VKA) and fermented milk fortified with bee honey as a natural source of vitamin K and probiotics. Fermentation 2021, 7, 202. [Google Scholar] [CrossRef]
- Thanh, N.T.; Loh, T.C.; Foo, H.L.; Hair-Bejo, M.; Azhar, B.K. Effects of feeding metabolite combinations produced by Lactobacillus plantarum on growth performance, faecal microbial population, small intestine villus height and faecal volatile fatty acids in broilers. Br. Poult. Sci. 2009, 50, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Apitherapy for Parkinson’s disease: A focus on the effects of propolis and royal jelly. Oxid. Med. Cell. Longev. 2020, 2020, 1727142. [Google Scholar] [CrossRef]
- Alexander, T.W.; Yanke, L.J.; Topp, E.; Olson, M.E.; Read, R.R.; Morck, D.W.; McAllister, T.A. Effect of subtherapeutic administration of antibiotics on the prevalence of antibiotic-resistant Escherichia coli bacteria in feedlot cattle. Appl. Environ. Microbiol. 2008, 74, 4405–4416. [Google Scholar] [CrossRef]
- Lovtsova, L.; Guliy, O.; Larionova, O.; Zabelina, M.; Uskov, K.; Lovtsov, I. Effect of a nanomodified antibiotic on field strains of E. coli and Enterobacter cloacae. IOP Conf. Ser. Earth Environ. Sci. 2021, 723, 022071. [Google Scholar] [CrossRef]
- Pereira, A.M.; Pinna, C.; Biagi, G.; Stefanelli, C.; Maia, M.R.G.; Matos, E.; Segundo, M.A.; Fonseca, A.J.M.; Cabrita, A.R.J. Supplemental selenium source on gut health: Insights on fecal microbiome and fermentation products of growing puppies. FEMS Microbiol. Ecol. 2020, 96, fiaa212. [Google Scholar] [CrossRef]
- Acres, S.D. Enterotoxigenic Escherichia coli infections in newborn calves: A review. J. Dairy Sci. 1985, 68, 229–256. [Google Scholar] [CrossRef]
- Ammerman, C.B.; Miller, S.M. Selenium in ruminant nutrition: A review. J. Dairy Sci. 1975, 58, 1561–1577. [Google Scholar] [CrossRef]
- Mehdi, Y.; Dufrasne, I. Selenium in cattle: A review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Juniper, D.T. Revisiting oxidative stress and the use of organic selenium in dairy cow nutrition. Animals 2019, 9, 462. [Google Scholar] [CrossRef] [PubMed]
- Genther, O.N.; Hansen, S.L. The effect of trace mineral source and concentration on ruminal digestion and mineral solubility. J. Dairy Sci. 2015, 98, 566–573. [Google Scholar] [CrossRef]
- Russell, J.B.; Wilson, D.B. Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH? J. Dairy Sci. 1996, 79, 1503–1509. [Google Scholar] [CrossRef]
- Kolver, E.S.; de Veth, M.J. Prediction of ruminal pH from pasture-based diets. J Dairy Sci 2002, 85, 1255–1266. [Google Scholar] [CrossRef]
- Dijkstra, J.; Tamminga, S. Simulation of the effects of diet on the contribution of rumen protozoa to degradation of fibre in the rumen. Br. J. Nutr. 1995, 74, 617–634. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A. Rumen microbes, enzymes and feed digestion—A review. Asian-Australas. J. Anim. Sci. 2002, 15, 1659–1676. [Google Scholar] [CrossRef]
- Nozière, P.; Ortigues-Marty, I.; Loncke, C.; Sauvant, D. Carbohydrate quantitative digestion and absorption in ruminants: From feed starch and fibre to nutrients available for tissues. Animal 2010, 4, 1057–1074. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.-P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Hidiroglou, M.; Heaney, D.P.; Jenkins, K.J. Metabolism of inorganic selenium in rumen bacteria. Can. J. Physiol. Pharmacol. 1968, 46, 229–232. [Google Scholar] [CrossRef]
- Surai, P.F. Selenium in Nutrition and Health; Nottingham University Press: Nottingham, UK, 2006. [Google Scholar]
- Vinceti, M.; Vicentini, M.; Wise, L.A.; Sacchettini, C.; Malagoli, C.; Ballotari, P.; Filippini, T.; Malavolti, M.; Rossi, P.G. Cancer incidence following long-term consumption of drinking water with high inorganic selenium content. Sci. Total Environ. 2018, 635, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G. Selenium. J. Toxicol. Clin. Toxicol. 1999, 37, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, M.R. Chronic selenium toxicity studies in sheep. Aust. Vet. J. 1966, 42, 442–448. [Google Scholar] [CrossRef]
- O’Toole, D.; Raisbeck, M.F. Pathology of experimentally induced chronic selenosis (alkali disease) in yearling cattle. J. Vet. Diagn. Investig. 1995, 7, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, J.; Shen, X. Effects of nano-selenium poisoning on immune function in the wumeng semi-fine wool sheep. Biol. Trace Elem. Res. 2021, 199, 2919–2924. [Google Scholar] [CrossRef]
- Dalia, A.M.; Loh, T.C.; Sazili, A.Q.; Samsudin, A.A. Influence of bacterial organic selenium on blood parameters, immune response, selenium retention and intestinal morphology of broiler chickens. BMC Vet. Res. 2020, 16, 365. [Google Scholar] [CrossRef]
- Ishii, T.; Shibata, K.; Kai, S.; Noguchi, K.; Hendawy, A.O.; Fujimura, S.; Sato, K. Dietary Supplementation with Lysine and Threonine Modulates the Performance and Plasma Metabolites of Broiler Chicken. J. Poult. Sci. 2019, 56, 204–211. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendawy, A.O.; Sugimura, S.; Sato, K.; Mansour, M.M.; Abd El-Aziz, A.H.; Samir, H.; Islam, M.A.; Bostami, A.B.M.R.; Mandour, A.S.; Elfadadny, A.; et al. Effects of Selenium Supplementation on Rumen Microbiota, Rumen Fermentation, and Apparent Nutrient Digestibility of Ruminant Animals: A Review. Fermentation 2022, 8, 4. https://doi.org/10.3390/fermentation8010004
Hendawy AO, Sugimura S, Sato K, Mansour MM, Abd El-Aziz AH, Samir H, Islam MA, Bostami ABMR, Mandour AS, Elfadadny A, et al. Effects of Selenium Supplementation on Rumen Microbiota, Rumen Fermentation, and Apparent Nutrient Digestibility of Ruminant Animals: A Review. Fermentation. 2022; 8(1):4. https://doi.org/10.3390/fermentation8010004
Chicago/Turabian StyleHendawy, Amin Omar, Satoshi Sugimura, Kan Sato, Mohamed Mohsen Mansour, Ayman H. Abd El-Aziz, Haney Samir, Md. Aminul Islam, A. B. M. Rubayet Bostami, Ahmed S. Mandour, Ahmed Elfadadny, and et al. 2022. "Effects of Selenium Supplementation on Rumen Microbiota, Rumen Fermentation, and Apparent Nutrient Digestibility of Ruminant Animals: A Review" Fermentation 8, no. 1: 4. https://doi.org/10.3390/fermentation8010004
APA StyleHendawy, A. O., Sugimura, S., Sato, K., Mansour, M. M., Abd El-Aziz, A. H., Samir, H., Islam, M. A., Bostami, A. B. M. R., Mandour, A. S., Elfadadny, A., Ragab, R. F., Abdelmageed, H. A., & Ali, A. M. (2022). Effects of Selenium Supplementation on Rumen Microbiota, Rumen Fermentation, and Apparent Nutrient Digestibility of Ruminant Animals: A Review. Fermentation, 8(1), 4. https://doi.org/10.3390/fermentation8010004











