Abstract
Butanol was produced commercially from cornstarch and sugarcane molasses (renewable resources) until 1983, when production of these plants was forced to cease because of unfavorable economics of production caused in part by escalating prices of these feedstocks. During recent years, the focus of research has been on the use of economically available agricultural biomass and residues and cutting-edge science and technology to make butanol production a commercially viable process again. In this study, we produced butanol from sweet sorghum bagasse (SSB) by employing high concentrations of SSB solids and integrated process technology through which simultaneous saccharification, fermentation, and recovery (SSFR) were conducted as one unit operation. The concentrated SSB (16–22% dry wt. basis or 160–220 gL−1) was used to reduce reactor size and potentially reduce fixed and operational costs. Indeed, ABE productivity and yield of 0.21 gL−1h−1 and 0.39 were obtained, respectively, when 160 gL−1 SSB (16%, dry wt.) was used in the SSFR process. In nonintegrated systems, use of >90 gL−1 solid loading is improbable and has not been done until this study.
1. Introduction
High oil prices, lack of energy security, and concerns about climate change have triggered immense interest in the production of energy from cellulosic biomass sources. Energy security is threatened by political instability in some oil-supplying regions, which also results in high gasoline prices. The consequences of climate change are also clear, and they include melting of glaciers on the north and south poles, often severe tornadoes, hurricanes, long droughts, and unexpected heavy rains and floods. As a result of the melting of glaciers, the sea level has been rising, threatening the coastline population. One of the solutions to these problems is the use of zero or overall low-carbon-emission fuels that can be produced from renewable cellulosic biomass [1]. Another solution is to develop and market electric or hybrid transportation vehicles [2], which is seen as a partial solution, as these vehicles are not yet cost competitive with gasoline-powered transportation counterparts. Prior to interest in the development of cellulosic biomass-derived fuels, starch and sugar producing crops such as corn and sugarcane molasses have been used [3]. Additionally, feedstocks such as whey permeate have also been used [4,5]. In the United States, corn is used to produce renewable biofuel; however, use of corn for this application has reached its upper limit. In 2019, 16.9 billion gallons, and in 2020, 13.8 billion gallons of ethanol were produced from corn in the US [6,7]. Use of corn above this level is likely to start food and feed vs. fuel competition thus increasing food and feed prices.
To avoid the aforementioned competition, renewable biofuels can be produced from cellulosic biomass. During the last 3–4 decades, significant advances have been made in this direction [8]. Another biofuel, known as butanol, can be produced from cellulosic biomass [9,10,11,12]. Butanol is a superior biofuel to ethanol, as it has many favorable gasoline-blending properties and contains 33% more energy on a gallon basis than ethanol [13]. Production of butanol on a commercial scale has historically come second to that of ethanol, as there were butanol production plants during World Wars I and II [3,14]. Additionally, the microbial strains that produce butanol can use all cellulosic sugars, such as glucose, xylose, arabinose, mannose, and galactose, in batch reactors. In recent studies, butanol has been produced from numerous cellulosic biomass feedstocks including wheat straw, corn stover, barley straw, sugarcane bagasse, energy crops [12], and yellow top presscake [15]. Some years ago, it was projected that at least 1 billion tons of biomass would be available for biofuel production in the US [16]. In the United States, sweet sorghum bagasse (SSB) is a biomass residue from sweet sorghum that can be used to produce biofuels. Sweet sorghum is being promoted by the USDA–ARS (United States Department of Agriculture–Agricultural Research Service), as this crop can be cultivated in tropical and arid conditions and has worldwide interest [17,18,19]. It should be noted that cellulosic biomass feedstocks require pretreatment and enzymatic hydrolysis prior to fermentation to butanol. In our bioreactors, we were successful in using 80–90 gL−1 cellulosic biomass hydrolysates [20]. This concentration of biomass is low and results in the requirement for large bioreactors and dilute product concentration after fermentation due to butanol toxicity to the culture. In order to improve the economics of butanol production from cellulosic substrate such as SSB and compete with gasoline, the following research studies should be conducted: (i) low-cost cellulosic biomass such as SSB should be used rather than cornstarch or molasses (SSB is more economical than corn); (ii) the cellulosic biomass should be pretreated hydrothermally rather than using chemicals such as dilute acid or alkali (use of chemicals is neither environmentally friendly nor economical); (iii) concentrated biomass should be used to reduce reactor size; (iv) process steps such as pretreatment, enzymatic saccharification, fermentation, and simultaneous product recovery should be integrated; and (v) product should be recovered using energy-efficient techniques. It has been indicated that until toxic product is removed simultaneously, process integration is not possible. Details of these product removal techniques have been reported in the literature [12,21].
In view of the above recommendations, the objectives of the present study were to produce butanol or ABE (acetone, butanol, ethanol) from concentrated SSB solids. The process integration mentioned in the above paragraph was expected to address the substrate and product inhibition problems that plague this fermentation. It should be noted that the use of concentrated solids is not possible until saccharification, fermentation, and product recovery are performed simultaneously. In our previous studies, a solid concentration of 80–90 gL−1 (8–9% dry weight biomass; approximately 55 gL−1 sugars) was used in a batch reactor [20]. For the present study, the SSB was pretreated hydrothermally and used at high concentrations (16–22% dry wt. basis; approximately 160–220 gL−1) in the reactor. The saccharification of the sugar polymers in SSB, fermentation to butanol, and product removal were combined into a single-step process performed in one bioreactor. This study was novel and has not been previously studied for the SSB feedstock at such a high biomass loading for butanol production in integrated systems.
2. Materials and Methods
2.1. Sweet Sorghum Bagasse (SSB) Pretreatment and Presaccharification
SSB was obtained from the Eastern Regional Research Center (ERRL; Nhuan P Nghiem), U.S. Department of Agriculture, Wyndmoor, PA. The composition of SSB is presented in Table 1 [22]. In the present study, the SSB was pretreated at 190 °C for zero min (as soon as this temperature was reached, cooling was started) in a 200 mL-capacity stainless-steel reactor (Labomat BFA-12, Mathis USA Inc., Concord, NC, USA). In order to pretreat cellulosic biomass, 11 g SSB and 39 g distilled water were transferred to the reactor (220 gL−1, biomass to water ratio 22:78) and mixed well, after which the reactor was tightly closed with the cap by hand. This was followed by transferring the reactors to the oven for heat treatment (190 °C). This temperature was suitable because it was effective for pretreatment and did not generate fermentation inhibitors. The rate of heating was 2.6 °C min−1. After heat treatment, the reactors were cooled to 25 °C at a rate of 6 °C min−1. Then, the contents were transferred to a 250 mL screw-capped PyrexTM glass bottle in which pH was adjusted to 5.0 using 5 M NaOH solution. This was followed by adding 1.53 mL of each of three enzyme solutions (Celluclast 1.5 L (cellulase: enzyme activity 751 ± 35 U mL−1; Sigma Chemicals St. Louis, MO, USA), β-glucosidase (activity 380 ± 19 U mL−1; Novozyme 188, Sigma Chemicals), and xylanase (activity 9837 ± 190 U mL−1, Viscostar 150 L; Dyadic Corporation Jupiter, FL, USA); [20]) to the bottle and transferring the bottle to an incubator (New Brunswick Scientific, New Brunswick, NJ, USA) for presaccharification at 45 °C and 150 rpm for 30 h. The pretreatment and presaccharification were performed in three reactors and three bottles, respectively. After biomass presaccharification, the contents were pooled into one 500 mL PyrexTM glass bottle. The initial reaction mixture volume was 177 mL (after inoculation).

Table 1.
Composition of sweet sorghum bagasse (SSB) on dry basis [22].
For 160 gL−1 SSB, 8 g of biomass and 42 g of distilled water were used (biomass to water ratio 16:84), and pretreatment and presaccharification were performed as described above. In this case (160 gL−1 biomass), 1.12 mL of each enzyme solution was used. Since biomass concentration was lower than 220 gL−1, presaccharification took 15 h, which is half of the time required for 220 gL−1. The reactor size and reactor mixture volume were the same as above.
2.2. Microbial Strain and Inoculum Development
Clostridium beijerinckii P260 was a generous gift from Professor David Jones from University of Otago, Dunedin, New Zealand. The spores of the strain were stored at 4 °C in sterile distilled water. In order to activate the spores, 100 µL of spore suspension in a 1.5 mL sterile polypropylene microcentrifuge tube (BIC Plas Inc. San Fafael, CA, USA) was heat shocked on a heating block (Cole-ParmerTM, Vernon Hills, IL, USA) at 75 °C for 2 min followed by transferring 20 µL of the spore suspension to sterilized and cooled (25 °C) Cooked Meat Medium (CMM; DifcoTM: Becton-Dickinson and Company, Sparks, MD, USA). The concentration of the spore suspension prior to inoculation was 0.03 gL−1 (dry weight). The CMM was prepared by transferring 3.5 g of CMM (DifcoTM) dry pellets and 0.60 g of glucose (Fisher Scientific, Fair Lawn, NJ, USA) into a 50 mL screw-capped PyrexTM glass bottle that contained 35 mL of distilled water. The pellets were soaked for 15 min prior to sterilization at 121 °C for 15 min. After sterilization, the CMM was cooled to 25 °C. Upon inoculation with the heat-shocked spores, the bottle was transferred to an anaerobic jar (BBL GasPakTM, Sparks, MD, USA) in which anaerobic condition was generated using BD GasPakTM EZ envelopes (Sigma Chemicals, St. Louis, MO, USA) and the contents were incubated at 35 °C for 14–16 h or until gassing was observed. This was termed as stage I inoculum.
To prepare inoculum for SSB fermentation, 3 g of glucose (Fisher Scientific, Fair Lawn, NJ, USA) and 0.1 g of yeast extract (BactoTM Yeast Extract: Becton Dickinson and Company) were dissolved in 100 mL distilled water and transferred to a 150 mL screw-capped glass bottle. Then, the bottle was autoclaved at 121 °C for 15 min and cooled to room temperature (25 °C). To this solution, 1 mL of each stock solution was added. The composition of stock solutions (in gL−1) was as follows: buffer (KH2PO4, 50; K2HPO4, 50; Ammonium acetate, 220), mineral (MgSO4.7H2O, 20.0; MnSO4.7H2O, 1.0; FeSO4.7H2O, 1.0; NaCl, 1.0), and vitamin (Para-amino-benzoic acid, 0.10; Thiamine, 0.10; Biotin, 0.001). These solutions were filter sterilized and stored separately at 4 °C until needed. One milliliter of each of these was added to 100 mL medium. This was followed by inoculating the medium with 7 mL of stage I inoculum and incubating the culture in an anaerobic jar (BBL GasPakTM) using anaerobic gas-generating envelopes (BD GasPakTM EZ) at 35 °C for approximately 10–12 h. Upon cell growth, this culture was termed as stage II inoculum and was used to inoculate SSB medium for butanol production.
2.3. Simultaneous SSB Saccharification, Fermentation, and Product Recovery
To the presaccharified suspension, 7.5 mL of presterilized yeast extract solution (from 40 gL−1; previously prepared and sterilized at 121 °C for 15 min) and 2.0 mL of each stock solution (buffer, mineral, and vitamin) were added. Then, the pH of the pooled presaccharified SSB solution/suspension was adjusted to 6.2 using 5 M NaOH solution. A pH of 6.2 is preferred for cell growth, and a pH of 5.0–5.4 is optimum for ABE production. This was followed by making the solution anaerobic by sparging oxygen-free nitrogen gas at a flow rate of 50–100 mL min−1 for 15 min. Soon after this, the bottle was inoculated with 10 mL of stage II inoculum. The initial dry weight cell concentration (soon after inoculation) in the fermentation medium was calculated to be 0.17 gL−1. During growth and fermentation, cell concentration in the SSB fermentation medium could not be measured or controlled because of the presence of biomass fibrous particles that interfered with optical density measurement. However, it is anticipated that it was approximately 3 gL−1 because in glucose-based fermentation medium with same amount of yeast extract and stock solutions, it is 3 gL−1. The culture was incubated at 35 °C, and product was recovered using a vacuum (oil-free vacuum pump at 28.5 inches of Hg gauge pressure; Gist Manufacturing Inc., Bent Harbor, MI, USA, model DAA) for 30 min at specific timepoints during fermentation. During this 30 min time period, the reactor temperature dropped to 33.5 from 35 °C. In order to control reactor temperature, the reactor was placed in a heating water bath (Julabo, GmbH, Seelbach, Germany). Usually, the product recovery was initiated when ABE concentration was 4.0 or >4.0 gL−1 in the reactor. The reduced pressure or vacuum at fermentation temperature (35 °C) made the reaction mixture boil off [21]. As a result, ABE/butanol was carried and condensed in the condenser (1 °C), collected in the flask or condensate receiver, and then removed by a condensate removal pump. Figure 1 shows a simplified schematic diagram of simultaneous SSB saccharification, fermentation to butanol, and product recovery. The simultaneous saccharification, fermentation, and recovery (SSFR) were performed in the same reactor. During the ABE/butanol recovery process, excessive foam was observed. The foam was controlled by adding 10–20 µL of 100-fold diluted antifoam (Antifoam 204; Sigma Chemicals, St. Louis, MO, USA).
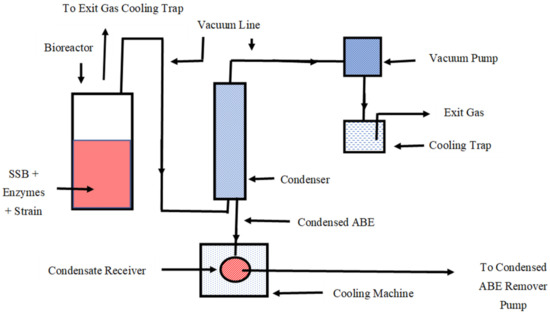
Figure 1.
A schematic diagram of production of butanol from concentrated sweet sorghum bagasse. For simultaneous product recovery, a vacuum was used.
At the end of fermentation, 50 mL of homogeneous suspension was withdrawn from the reactor and transferred to a 150 mL PyrexTM bottle, to which 0.6 mL each of three hydrolytic enzyme (cellulase, β-glucosidase, and xylanase) solutions was added, followed by adjustment of the pH to 5.0 with 5 M NaOH. Then, the bottle was incubated at 45 °C with 150 rpm for 72 h in an incubator (New Brunswick Scientific, NJ, USA), after which samples were taken to analyze the sugar content. This was performed to test if any unhydrolyzed sugars were present in the SSB.
2.4. Analyses
ABE and acetic and butyric acids (HAc and HBu) were measured by gas chromatography (GC: 6890N, Agilent Technologies, Wilmington, DE, USA). The GC glass column was packed with 80/100 chromosorb WAW, and the column dimensions were 6 ft × 0.25 in × 2 mm (Supelco, Belfonte, PA, USA). The GC was equipped with an autosampler and injector. Prior to injecting samples, each sample was diluted 4-fold using distilled water. One milliliter of diluted sample and 100 µL of internal standard (5 gL−1 n-propanol) were mixed, and 1 µL of the mixture was injected into the GC device. The GC oven’s initial temperature was 100 °C and was ramped up at a rate of 40 °C.min−1 to 180 °C, at which it was held for 4 min. The injector and detector temperatures were 225 and 250 °C, respectively. Sugars (glucose, xylose, and arabinose) in the samples were measured by high-performance liquid chromatography (HPLC; Agilent Technologies, Santa Clara, CA, USA) using an Amines HPX-87P 300 mm column (Bio-rad, Hercules, CA, USA). Deionized water was used as the mobile phase at a flow rate of 0.60 mL.min−1 and a column temperature of 85 °C. Prior to injection, the samples were diluted 20-fold with distilled water in 1.5 mL Eppendorf centrifuge tubes, after which they were centrifuged at 13,000 rpm (Eppendorf 5417C) for 20 min. The clear liquid (1 mL) was transferred to an HPLC vial. A 10 µL sample was injected into the HPLC device. The standard solution contained 10 gL−1 each of glucose, xylose, and arabinose. Productivity was calculated as total ABE produced divided by fermentation time, expressed as gL−1h−1. Total fermentation time was the time period between inoculation and the cessation of fermentation. Yield was defined as total ABE produced divided by total sugars utilized.
3. Results and Discussion
It was essential to presaccharify the SSB for fermentation, as prior to presaccharification, there was no free liquid in the reactor for cell growth. As soon as SSB was presaccharified and presence of liquid was observed, the bottle was removed from the incubator, and a sample was taken to measure sugar concentration. The presaccharified SSB medium (SSB 220 gL−1) contained 34.32 gL−1 glucose, 17.52 gL−1 xylose, and 2.02 gL−1 arabinose (data not presented in Table or Figure). In the presaccharified medium, the total released sugar concentration was 53.86 gL−1, or 35.66% of that available in 220 gL−1 (151.01 gL−1 sugar) SSB. This sugar concentration was calculated using the SSB composition provided in Table 1. The mixture also contained 5.28 gL−1 acetic acid. Presaccharification prepared the SSB for simultaneous saccharification and fermentation, and hence, the medium was inoculated with 10 mL of actively growing C. beijerinckii P260 (~6%, v/v). The fermentation was run for 72 h, and the ABE concentrations are shown in Figure 2A. The culture produced 0.56 gL−1 acetone, 0.00 gL−1 butanol, and 1.04 gL−1 ethanol. It was observed that the culture became acidogenic rather than solventogenic and accumulated 7.75 gL−1 acetic acid (of this 5.28 gL−1 was from the SSB) and 0.93 gL−1 butyric acid, thus totaling 8.68 gL−1 acids. At the end of fermentation, the total residual sugar concentration was 42.85 gL−1 (Figure 2B). The culture utilized 6.61 gL−1 total sugar and produced 1.60 gL−1 acetone and ethanol and 3.40 gL−1 total acids. The total acids and solvents were 5.0 gL−1. Because the concentration of butanol in the reactor was 0.00 gL−1 (though acetone and ethanol in the reactor was 1.60 gL−1), no product recovery was performed.
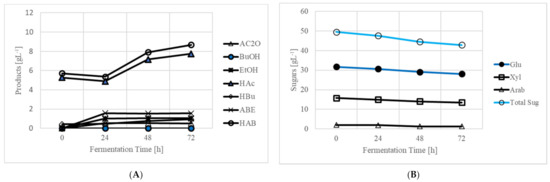
Figure 2.
Production of ABE from 220 gL−1 (22% dry wt. basis) SSB in an integrated process of simultaneous saccharification, fermentation, and recovery. (A) Products (acetone butanol ethanol or ABE). ABE and acid abbreviations as in Table 2; (B) sugars. Glu—glucose, Xyl—xylose, Arab—arabinose, Tot. Sug.—total sugar.
The possible reasons for arrest of fermentation include: (i) lack of nutrients, (ii) high concentration of sugars, and (iii) high concentration of solids. To investigate if lack of nutrients was the reason for arrest of fermentation, reactor contents were centrifuged, and clear liquid was divided into two parts. Half was fermented without adding any nutrients (after pH adjustment to 6.2 with 5M NaOH and developing anaerobic environment). After 72 h of fermentation, the culture produced 1.89 gL−1 acetone, 1.30 gL−1 butanol, and 0.53 gL−1 ethanol. The total concentration of ABE was 3.72 gL−1. To the other part, 2 gL−1 yeast extract solution and 10 mL L−1 stock solutions (buffer, minerals, and vitamins) were added (pH adjusted and anaerobic conditions created prior to inoculation). After fermentation, the culture produced 2.19 gL−1 acetone, 1.02 gL−1 butanol, and 0.66 gL−1 ethanol (total ABE 3.87 gL−1). Comparison of these two fermentations suggested that the culture was not deficient in nutrients. Reason (ii) was disregarded, as this culture can tolerate and grow in concentrated sugar solutions (up to 200 gL−1 [20]). In the present experiment, the initial sugar concentration was 49.46 gL−1 (Figure 2B), which was much lower than this culture can tolerate. Reason number (iii) was considered, and hence, solid concentration was reduced to 160 gL−1.
Pretreatment and presaccharification using 160 gL−1 solids (16% w/v) were performed as described in the materials and methods section above. This was followed by simultaneous saccharification, fermentation, and recovery. During the first 23 h of fermentation, 2.40 gL−1 acetone, 0.49 gL−1 butanol, and 1.26 gL−1 ethanol were produced, thus totaling 4.15 gL−1 ABE (Figure 3A). At the time of inoculation, 0.43 gL−1 butyric acid was present, which increased to 3.23 gL−1 (Figure 3B). The initial pH (at the time of inoculation) was adjusted to 6.2 which decreased to 5.40 at 23 h. During the period from 7 to 29 h, vigorous gassing was observed. At 29 h, product was recovered by vacuum. After recovery, 0.09 gL−1 acetone, 0.03 gL−1 butanol, and 0.08 gL−1 ethanol were present in the bioreactor. Soon after recovery, fermentation was vigorous, and at 94 h, 3.92 gL−1 acetone, 2.71 gL−1 butanol, and 0.80 gL−1 ethanol had accumulated in the bioreactor. At this time, the total product concentration was 7.43 gL−1. At 94 h, product was recovered, and after recovery, 3.70 gL−1 ABE were present in the bioreactor. At 107 h, ABE concentration increased to 6.09 gL−1. At this time, product was recovered, and vigorous fermentation was observed. At 119 h, ABE concentration was 4.20 gL−1 in the bioreactor, which decreased to 1.98 gL−1 after ABE recovery. At 132 h, although ABE concentration was 1.59 gL−1, product was recovered. The sugar concentrations in the bioreactor at various fermentation times are shown in Figure 3C. After 120 h, although there was some gassing, the residual sugar (xylose) concentration was 7.78 gL−1. Soon after recovery, the fermentation was stopped, as there was no gas production. During recovery periods, excessive foaming was observed, which was controlled by adding antifoam. In the entire process, 1 mL (diluted volume) or 0.01 g of antifoam was used. It is not known whether antifoam was used by the culture for cell growth or butanol production. Even if it was utilized, the use of such a small amount (0.01 g) was disregarded for butanol production calculations, as it would not result in any yield variation.
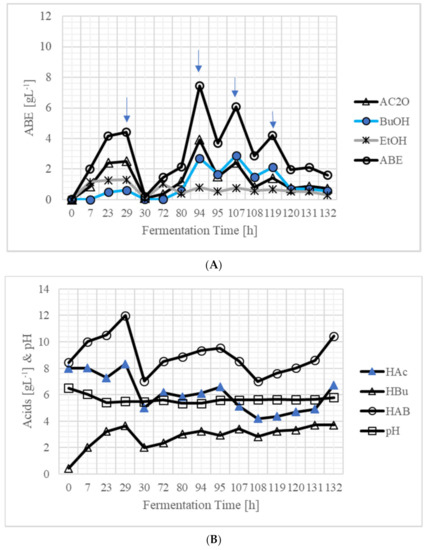
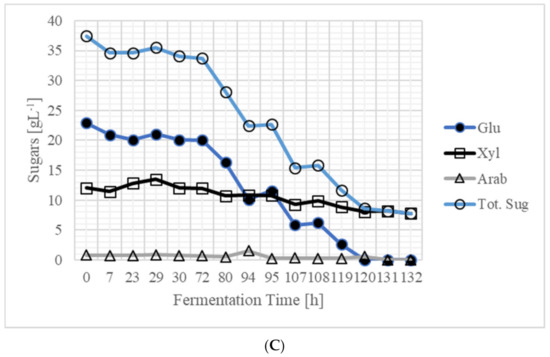
Figure 3.
Production of ABE from 160 gL−1 (16% dry wt. basis) SSB in an integrated process of simultaneous saccharification, fermentation, and recovery: (A) ABE (abbreviations as in Table 2)—arrows indicate the points at which a vacuum was applied for 30 min to recover ABE. During 30 min of vacuum, the ABE were recovered, and ABE concentrations below inhibitory levels were reached in the reactor; (B) acids; and (C) sugars. Throughout the fermentation, vigorous gassing was observed. After recovery at 119 h, 3 mL of yeast extract (YE, from 40 gL−1) solution was added as a nutrient solution. The initial reaction volume (when the reactor was inoculated) was 177 mL, and the final volume, when fermentation ceased at 132 h, was 150 mL.
Based on material balance, 160 gL−1 SSB contained 109.82 gL−1 sugars of which 37.46 gL−1 were released during presaccharification process that represents 34.11% of the total sugars in the SSB. At the end of fermentation 7.78 gL−1 xylose (the only residual sugar) was present in the fermentation broth and 2.58 gL−1 sugars remained unhydrolyzed. During enzymatic saccharification, fermentation, and recovery, 69.78 gL−1 (63.54%; ((109.82 − (37.46 + 2.58))/109.82)) of the sugars present in SSB were simultaneously saccharified.
During the fermentation, the culture used 1.22 gL−1 (8.00 − 6.78 gL−1; Figure 3B) acetic acid and produced 3.23 gL−1 butyric acid. Although, the culture utilized acetic acid, it was not used for material balance calculations. After 220 gL−1 SSB presaccharification, 5.28 gL−1 acetic acid was measured, whereas at the end of 160 gL−1 presaccharification, 8.00 gL−1 acetic acid was present. At this stage, the reason for this discrepancy is not clear. In the course of fermentation, ABE were recovered by applying a vacuum four times, as shown in Figure 3A. The volatiles in the gas phase were recovered, and ABE-rich condensates were obtained. Acetone concentration ranged from 2.17 to 16.75 gL−1, and butanol concentration ranged from 1.54 to 20.38 gL−1 (Table 2). Ethanol concentration in the condensate was low and varied from 0.62 to 1.78 gL−1. The total ABE concentration in the condensates varied from 7.91 to 32.38 gL−1. Further separation of ABE from this concentrated stream would be economical, as the condensate did not contain medium residues such as lignin that complicate the recovery process. As presented in Table 2, both acetic and butyric acids were also partially recovered. In ABE fermentation, the carbon source or substrate is converted to acetic and butyric acids before these acids are assimilated in product formation. The maximum ABE concentration is strain specific; however, for most strains, it does not exceed 20 gL−1. The strain used in the present fermentation could produce up to 29 gL−1 ABE in batch reactors. There is another strain (Clostridium beijerinckii BA101) that can accumulate 25–30 gL−1 ABE in batch systems [11]. For these strains, studies on tolerance to acids is scarce; however, it is anticipated that they can tolerate 10–12 gL−1 acetic and butyric acids.

Table 2.
Concentrations of ABE in the recovered condensates at various fermentation times during simultaneous SSB saccharification, fermentation, and recovery.
In this system, 9.07 gL−1 ABE was produced from 99.46 gL−1 sugars, thus resulting in a solvent (ABE) yield and productivity of 0.09 and 0.07 gL−1h−1, respectively. These parameters are low as compared to those obtained using glucose as a carbon source [20]. We routinely obtained yield and productivity of 0.38–0.40 and 0.44 gL−1h−1, respectively, in our batch fermentations fed with glucose. The only possible reasons for such a low ABE yield and productivity include loss of solvents during recovery through the connecting rubber tubing and with exiting fermentation gases. Attempts were made to capture exit-line solvents by bubbling exiting fermentation gases through water (300 mL) kept at 10 °C. By using 10 °C chilled water to capture ABE, we were able to condense 0.09 g solvents (Table 3), which was 8.74% of the total ABE condensed in the condenser. It is suggested that a lower condensation temperature be used to condense all ABE. Additionally, in large-scale plants, loss of ABE by diffusion can be prevented by using steel pipes rather than rubber pipelines. ABE do not diffuse through metal pipes.

Table 3.
Concentrations and amounts of condensed ABE in the exit gases (vacuum pump and reactor).
By material balance, the sugars present in the SSB should be equal to the sugars used for the ABE and acid production, cell growth, and maintenance, and residual sugar (residual sugar 7.78 + 2.58 = 10.36 gL−1). The bioreactor was fed with 160 gL−1 SSB, which contained 109.82 gL−1 monomeric sugars. Of this, 99.46 gL−1 (109.82 gL−1 (initial sugar) − 10.36 gL−1 (residual)) was utilized. This sugar was converted to ABE, acids, cell growth, and cell maintenance [25]. In this system, 2.01 gL−1 acids was produced, which utilized 5.15 gL−1 sugar (acid yield 0.39). Based on a cell yield of 0.20 [25,26], the culture utilized 15.0 gL−1 sugar for cell growth (3 gL−1 cell concentration) [9]. For maintenance energy, the culture used 0.02 g sugars/(g biomass.h) [25], thus resulting in the utilization of 7.92 g sugar in 132 h of operation. This resulted in total sugar utilization of 28.07 gL−1 for acid production, cell growth, and maintenance energy, thus leaving behind 71.39 gL−1 (99.46 gL−1 (total sugar for conversion) − 28.07 gL−1 (cell growth, acid production, and maintenance)) sugar for ABE production. Based on a theoretical ABE yield of 0.39 [23], the culture produced 27.84 gL−1 ABE. Since it was a closed system (no bleed), the only way ABE could leave was either by diffusion through the rubber tubing or with the exit gas. This suggested that 18.77 gL−1 ABE was lost by diffusion, indicating that the actual ABE productivity was 0.21 gL−1h−1. This productivity is close to or higher than productivity in many nonintegrated systems based on use of commercial feedstocks such as whey permeate (0.14 gL−1h−1) [12].
It should be noted that the culture lost the ability to metabolize xylose under the present conditions, making xylose the only sugar present in the reactor at 132 h (Figure 3). At this stage, it is not clear why the culture lost its ability to use xylose after an extended period of fermentation. This study revealed that use of 160 gL−1 SSB in an integrated process in which SSB saccharification, fermentation and recovery were combined was possible. When fermentation ceased, the concentration of other sugars was zero gL−1 (except xylose) (Figure 3). After fermentation had ceased, 50 mL of suspension was hydrolyzed with new enzymes to check if the hydrolysis was complete. This experiment suggested that 97.65% of hydrolysis was complete during presaccharification and saccharification in the reactor. Successful fermentation of 160 gL−1 SSB and comparison with 220 gL−1 SSB fermentation suggested that 220 gL−1 SSB fermentation ceased because of the high solid-to-liquid ratio. In the medium, initial xylose concentration was 12.06 gL−1, and at the end of fermentation, the residual xylose was 7.78 gL−1. It is known that C. beijerinckii P260 utilizes xylose in batch fermentations that last up to 72 h [9]. In order to utilize xylose in continuous or extended fermentations, either the microbial strain should be genetically modified, or the bioreactor should be inoculated with new inoculum near the end of fermentation. In this study, our goal was to produce butanol from SSB pretreated hydrothermally and using integrated process in which simultaneous saccharification, fermentation, and recovery were combined successfully. Additionally, we were able to use high loadings of biomass in the bioreactor. For butanol fermentation, where >90 gL−1 biomass cannot be completely utilized because of product inhibition [20], 160 gL−1 is considered high. Application of process integration, such as this, reduces the number of unit operations and hence capital and operational costs, thus improving the economics of production of biofuels from cellulosic biomass. Furthermore, it also reduces water usage and fermentation effluents generation by 178%. This number was obtained by dividing the 160 gL−1 SSB biomass used in this reactor by the 90 gL−1 biomass used in batch reactors and multiplying by 100. It should be noted that SSB is a different feedstock and was not pretreated using chemicals such as dilute sulfuric acid as reported in previous studies on wheat straw [20]. A brief summary of results obtained in these investigations is provided in Table 4. The use of concentrated cellulosic biomass feedstock (SSB) and combination of the three unit operations (one pot) could be possible approaches to reduce the cost of butanol/ABE production from agricultural residues.

Table 4.
A summary of butanol/ABE production from 160 gL−1 SSB in an integrated process.
4. Conclusions
In this study, butanol or ABE was produced from concentrated SSB solids. C. beijerinckii P260 was unable to ferment 220 gL−1 SSB to ABE because the maximum solid concentration it could tolerate was exceeded. Approximately 160 gL−1 SSB appeared to be the maximum solid concentration C. beijerinckii P260 can handle during ABE fermentation. The process of ABE fermentation with cellulosic biomass requires pretreatment, saccharification, fermentation, and recovery. Three out of the four process steps (simultaneous saccharification, fermentation, and product recovery; SSFR) were successfully combined to produce butanol or ABE from concentrated SSB. In this study, the kinetic parameters of ABE yield and productivity were 0.39 and 0.21 gL−1h−1, respectively. The total ABE concentration in the recovered stream was 7.91–32.38 gL−1, which was expected to be concentrated economically, as medium components such as lignin and microbial cells that hinder product recovery were not present. This study on the use of concentrated biomass feedstock and simultaneous product recovery addressed substrate and product inhibition problems associated with this fermentation.
Author Contributions
N.Q. and B.C.S.—conceptualization, project administration, and resources; N.Q.—performing experiments and writing; N.N.N.—hardware, supervision, and review and editing; S.L. and T.C.E.—writing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
For these studies, financial support was provided by the United States Department of Agriculture–Agricultural Research Service (CRIS Number 5010-41000-163-00D).
Institutional Review Board Statement
Not applicable, as these studies did not involve humans or animals.
Informed Consent Statement
Not applicable, as these studies did not involve humans.
Data Availability Statement
Not applicable.
Acknowledgments
Q.N. would like to thank David Jones (retired from University of Otago, Dunedin, New Zealand) for his generous gift of Clostridium beijerinckii P260 and Greg Kennedy and Eric Hoecker for measuring sugar concentrations in samples using HPLC. Q.N. would also like to thank Bruce Dien (United States Department of Agriculture–Agricultural Research Service, National Center for Agricultural Utilization Research, Peoria, IL, USA) and Ana M. López-Contreras (Wageningen Food and Biobased Research, The Netherlands) for reviewing this manuscript and making exceptionally helpful comments that improved the quality of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Venkatesh, A.; Posen, I.D.; MacLean, H.L.; Chu, P.L.; Griffin, W.M.; Saville, B.A. Environmental Aspects of Biotechnology. Adv. Biochem. Eng. Biotechnol. 2020, 173, 77–119. [Google Scholar] [PubMed]
- Vertès, A.A. The industrial world in the twenty first century. In Green Energy to Sustainability: Strategies for Global Industries; Vertès, A.A., Qureshi, N., Blaschek, H.P., Yukawa, H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 613–648. [Google Scholar]
- Jones, D.R.; Woods, D.R. Acetone-butanol fermentation revisited. Microbiol. Rev. 1986, 50, 484–524. [Google Scholar] [CrossRef] [PubMed]
- Ennis, B.M.; Maddox, I.S. Use of Clostridium acetobutylicum P262 for production of solvents from whey permeate. Biotechnol. Lett. 1985, 7, 601–606. [Google Scholar] [CrossRef]
- Ennis, B.M.; Maddox, I.S.; Schoutens, G.H. Immobilized Clostridium acetobutylicum for continuous butanol production from whey permeate. N. Z. J. Dairy Sci. Technol. 1986, 21, 99–109. [Google Scholar]
- Anonymous. How much ethanol was produced in 2019 in the US? Available online: https://www.eia.gov/todayinenergy/detail.php?id=41393 (accessed on 2 November 2021).
- Anonymous. How much ethanol was produced in 2020 in the US? Available online: https://www.statista.com/statistics/281494/us-fuel-ethanol-production/ (accessed on 2 November 2021).
- Blaschek, H.P.; Ezeji, T.C.; Scheffran, J. Biofuels from Agricultural Wastes and Byproducts; Blackwell Publishers: Ames, IA, USA, 2010. [Google Scholar]
- Dürre, P. Biobutanol: An attractive biofuel. Biotechnology 2007, 2, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Dürre, P. New insights and novel developments in clostridial acetone/butanol-isopropanol fermentation. Appl. Microbiol. Biotechnol. 1998, 49, 639–648. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Blaschek, H.P. Fermentation of dried distillers’ grains and soluble (DDGS) hydrolysates to solvents and value-added products by solventogenic clostridia. Bioresour. Technol. 2008, 99, 5232–5242. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N. Solvent (Acetone-Butanol: AB) production. In Reference Module in Life Sciences; Roitberg, B., Cotter, P., Eds.; Elsevier Inc.: Oxford, UK, 2017; pp. 1–20. [Google Scholar]
- Anonymous. Biobutanol on the horizon. Chem. Eng. Prog. 2007, 77, 23–35. [Google Scholar]
- Zverlov, V.V.; Berezina, O.; Velikodvorskaya, G.A.; Schwarz, W.H. Bacterial acetone and butanol production by industrial fermentation in the Soviet Union: Use of hydrolyzed agricultural waste for butanol biorefinery. Appl. Microbiol. Biotechnol. 2006, 71, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Saha, B.; Liu, S.; Harry-O’kuru, R. Production of acetone-butanol-ethanol (ABE) from concentrated yellow top presscake using Clostridium beijerinckii P260. J. Chem. Technol. Biotechnol. 2020, 95, 614–620. [Google Scholar] [CrossRef]
- Anonymous. 2016 Billion Ton Report. Department of Energy (United States). Available online: https://www.energy.gov/eere/bioenergy/2016-billion-ton-report (accessed on 3 November 2021).
- Gnansounou, E.; Dauriat, A.; Wayman, C.E. Refining sweet sorghum to ethanol and sugar: Economic trade-offs in the context of North China. Bioresour. Technol. 2005, 96, 985–1002. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, M.; Ai, N.; Yu, F.; Ji, J. Kinetic model analysis of dilute sulfuric acid-catalyzed hemicellulose hydrolysis in sweet sorghum bagasse for xylose production. Ind. Crops Prod. 2012, 38, 81–86. [Google Scholar] [CrossRef]
- Sipos, B.; Reczey, J.; Somorai, Z.; Kadar, Z.; Diemes, D.; Reczey, K. Sweet sorghum as feedstock for ethanol production: Enzymatic hydrolysis of steam-pretreated bagasse. Appl. Biochem. Biotechnol. 2009, 153, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Saha, B.C.; Cotta, M.A. Butanol production from wheat straw hydrolysate using Clostridium beijerinckii. Bioproc. Biosys. Eng. 2007, 30, 419–427. [Google Scholar] [CrossRef]
- Mariano, A.P.; Qureshi, N.; Filho, R.M.; Ezeji, T.C. Bioproduction of butanol in bioreactors: New insights from simultaneous in situ butanol recovery to eliminate product toxicity. Biotechnol. Bioeng. 2011, 108, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Nghiem, N.P.; Nguyen, C.M.; Walker, T.H. Sweet sorghum biorefinery for production of fuel ethanol and value-added co-products. Biol. Eng. Trans. 2013, 6, 143–155. [Google Scholar]
- Lee, H.K.; Lee, S.K.; Lee, J.; Kim, S.; Park, C.; Kim, S.W.; Yoo, H.Y. Improvement of enzymatic glucose conversion from chestnut shells through optimization of KOH pretreatment. Int. J. Environ. Res. Public Health 2021, 18, 3772. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. In Laboratory Analytical Procedure; Technical Report: NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Qureshi, N.; Paterson, A.H.J.; Maddox, I.S. Model for continuous production of solvents from whey permeate in a packed bed reactor using cells of Clostridium acetobutylicum immobilized by adsorption onto bonechar. Appl. Microbiol. Biotechnol. 1988, 29, 323–328. [Google Scholar] [CrossRef]
- Yerushalmi, L.; Volesky, B.; Leung, W.K.; Neufeld, R.J. Variations of solvent yield in acetone butanol fermentation. Eur. J. Appl. Microbiol. Biotechnol. 1983, 18, 279–286. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).