Abstract
Global demand for renewable and sustainable energy is increasing, and one of the most common biofuels is ethanol. Most ethanol is produced by Saccharomyces cerevisiae (yeast) fermentation of either crops rich in sucrose (e.g., sugar cane and sugar beet) or starch-rich crops (e.g., corn and starchy grains). Ethanol produced from these sources is termed a first-generation biofuel. Yeast fermentation can yield a range of additional valuable co-products that accumulate during primary fermentation (e.g., protein concentrates, water soluble metabolites, fusel alcohols, and industrial enzymes). Distillers’ solubles is a liquid co-product that can be used in animal feed or as a resource for recovery of valuable materials. In some processes it is preferred that this fraction is modified by a second fermentation with another fermentation organism (e.g., lactic acid bacteria). Such two stage fermentations can produce valuable compounds, such as 1,3-propanediol, organic acids, and bacteriocins. The use of lactic acid bacteria can also lead to the aggregation of stillage proteins and enable protein aggregation into concentrates. Once concentrated, the protein has utility as a high-protein feed ingredient. After separation of protein concentrates the remaining solution is a potential source of several known small molecules. The purpose of this review is to provide policy makers, bioethanol producers, and researchers insight into additional added-value products that can be recovered from ethanol beers. Novel products may be isolated during or after distillation. The ability to isolate and purify these compounds can provide substantial additional revenue for biofuel manufacturers through the development of marketable co-products.
1. Introduction
The demand for fuel ethanol continues to grow, with global production projected to surpass 140 billion litres/year [1]. Sugarcane and maize continue to be dominant feedstocks for bioethanol production. As such, bioethanol production is projected to consume 25% and 14% of global sugarcane and maize by 2029 [1]. Bioethanol is typically produced using a range of fermentation processes, each specialized to utilize a narrow range of inputs. The inputs are classified based on feedstock types (e.g., sucrose based, starch based, lignocellulosic, and algal), as belonging to a specific generation [2]. The type of feedstock, nutrients, and fermentation conditions affect bioethanol yield, and the nature of co-products [2,3,4]. Fermentation co-products can also affect bioethanol yield, as some act as both yeast nutrients and antinutrients. While the yield of ethanol is a primary driver for ethanol production, the identification and valorization of fermentation co-products add value to ethanol production.
Yeasts, and particularly Saccharomyces cerevisiae, used in ethanol production are most efficient in warm (e.g., 20–30 °C) and acidic (pH between 4.5–6.5) environments [5]. During microbial fermentation, yeasts produce glycerol, as both an osmoprotectant [6,7,8] and for metabolic recovery of NAD+. Yeast also acidifies their environment to promote growth through proton secretion, secretion of organic acids (discussed below), removal of buffering agents, and dissipation of carbon dioxide [5]. The most common yeast used in alcoholic fermentation, Saccharomyces cerevisiae, is preferred for rapid and efficient conversion of sugar solutions to solutions with correspondingly high ethanol concentrations. The latter solutions are readily distilled and dehydrated to yield products suitable for blending with gasoline. Dehydration processes to produce anhydrous ethanol include heterogeneous azeotropic distillation using solvents (e.g., benzene), extractive distillation with solvents and entrainers (e.g., salts), adsorption using molecular sieves, and pervaporation membranes (e.g., zeolite, silica, etc.) [9]. During fermentation with yeast, value-added co-products (e.g., α-glycerylphosphorylcholine) can accumulate along with ethanol, while fusel alcohols can be recovered during distillation and are often added to ethanol used in fuel applications. Common lower value co-products can also accumulate (e.g., acetic acid, succinic acid, and glycerol) that are more difficult to valorize, and nuisance coproducts can accumulate (methanol, hydrogen sulfide, and methyl mercaptan). A portion of the volatile co-products are volatile, toxic, and/or contribute to odors that can be co-distilled with the ethanol product. Multi-stage distillation and other purification technologies are required to remove these compounds. A group of alcohols and aldehydes (e.g., aldehydes, butanols, propanols, etc.) are naturally synthesized from amino acids through the Ehrlich pathway [10] and simple sugars during fermentation [11,12]. In addition to alcohols, organic acids can also accumulate (e.g., acetic acid, succinic acid, and lactic acid) [3,13,14,15] because of yeast metabolic processes and even metabolism by adventitious bacteria present during ethanolic fermentation. Secondary fermentation (two-stage fermentation) of stillage can affect the contents of these compounds.
Upon the completion of ethanolic fermentation, the distillers’ grain waste by-product can be further upgraded and enriched (e.g., secondary fermentation) to produce additional added-value compounds (e.g., 1,3-propanediol), as well as a highly concentrated protein that can be utilized as feed for domestic animals [14,15]. Altogether, the production of these co-products and others can add considerable value to ethanol coproducts (Table 1). Therefore, to maximize the utilization of grain crops, it is beneficial to identify valuable co-products produced during the fermentation process. This review will examine several valuable co-products that accumulate during alcoholic fermentation (α-glycerylphosphorylcholine, and fusel alcohols), followed by those produced during secondary fermentation (e.g., organic acids) of the distillers’ grain co-products (whole and thin stillage), which are primarily from first generation ethanol production. The recovery and purification of these compounds can further add value and provide opportunities for increased utilization of grain crops and provide ethanol producers access to new markets.

Table 1.
Market size of coproduct solutes from alcoholic fermentation [16,17,18,19,20,21,22].
2. Thin Stillage and Distillers’ Grains
Following ethanolic fermentation and distillation processes, the by-product stillage contains much of the protein, oil, fiber, and non-starch carbohydrate that were not available to the yeast during fermentation. A common process for using these components starts with the separation of whole stillage into a liquid portion with suspended solids (thin stillage) and wet solids (distillers’ wet grains), using centrifugation, vibratory separation, or a press [23,24]. These by-products can then be further processed (i.e., drying and fractionation) or extracted into specific components.
Without any further processing, all or a portion of thin stillage can be returned or backset to the next fermentation. This practice replaces some of the water required to soak incoming feedstocks intended for fermentation [25]. Protein is a major nutrient remaining in the stillage, and many methods have been developed to recover stillage proteins. Grain thin stillage contains approximately 37% protein (w/w dry basis) [26], and research has been conducted to develop stillage protein concentrates. Physical clarification techniques using additives [27,28], gas flotation [29,30], centrifugation [31], and filtration [32,33] have been tested as approaches to produce protein-enriched solids from thin stillage. Another approach for improving stillage protein quality and concentration is through a two-stage fermentation strategy [15,34], where it is also possible to upgrade glycerol to higher value compounds. Where thin stillage is not suited for use as a feed, it may be used as a nitrogen-rich fertilizer for crops [35]. Another strategy is to pair thin stillage valorization with a protein extraction process. Protein extraction from oilseed meal requires the use of large volumes of solution to dissolve proteins before precipitation. Thin stillage has some dissolved protein, but it can be used as a solution for protein extraction from oilseed meal [36]. An economical use of thin stillage that avoids the need for evaporation while providing the benefit of the stillage as a nutrient solution involves simply providing stillage in the water for cattle. In this way, the stillage becomes a nutrient-rich water source [37].
Once thin stillage is separated by dewatering distillers’ wet grains [38,39], the thin stillage can then be dried to a concentrated syrup called distillers’ solubles (DS), which is useful as an animal feed component. Remaining solids or distillers’ wet grains (DWG) can be dried to produce distillers’ dried grains (DDG) for storage and shipping. An alternative practice is to add DS to the grains as they dry to produce dried distillers’ grains with solubles (DDGS), a product that is commonly used with cattle feed [40]. Compared to wet feed products, DDG and DDGS have extended shelf-life and are more easily shipped. The sale of fermentation by-products for use as cattle feed generally provides 10–20% of the total revenue of ethanol production facilities [41] while avoiding revenue losses that would be incurred if co-product disposal was necessary. Fractionation of the DDGS can concentrate protein and generate fractions with high fiber contents to produce additional protein and fiber products [42]. In some rations, the higher fiber content of DDGS is undesirable. Producing a higher protein- and fat-content feed ingredient can improve the value of this product stream. In addition to the use of stillage products in animal feed, a portion DDGS proteins can be more readily solubilized and extracted for a wide variety of industrial uses (e.g., biopolymer production) [43].
DDG, produced during first-generation biofuels processes, can be used as a substrate for a second fermentation after pre-treatment that converts unhydrolyzed and unprocessed cellulose into fermentable sugars [44]. Pre-treatment conditions are like those employed in second-generation biofuel processes and can include physical (e.g., milling), chemical (e.g., alkaline treatments), physicochemical (e.g., steam or CO2 explosion), or biological processes (e.g., enzymatic hydrolysis) [2]. Nonetheless, variability of DDG composition could affect uniform enzymatic digestibility, and fermentability. Modification of the overall process design to accommodate new processing steps, or variable input materials would affect the economics of biofuel production [44].
Alternatively, separation of fiber and germ, prior to fermentation, can enhance the value of non-starch/sugar nutrients in grain [45]. This can be accomplished through a combination of processes, including soaking, grinding, enzymatic hydrolysis, and sieving [45]. Wet milling ethanol production facilities can also implement front-end fractionation using conventional hydrocyclone systems [41], to further separate starch, protein, and fibre [46], prior to alcoholic fermentation.
Finally, oil is another product of bioethanol production that is often poorly utilized. For corn, methods of oil extraction from thin stillage have been patented [47], and other oil extraction methods from corn DDGS have been developed [48]. However, due to the lower oil content of wheat and other grains, oil recovery techniques have remained largely undeveloped. In addition to fractionating fermentation products, other high-value compounds can also be extracted during the initial and two-stage fermentation processes.
3. α-Glycerylphosphorycholine
α-Glycerylphosphorylcholine (α-GPC) (Figure 1) is a yeast metabolite and precursor involved in the synthesis of acetylcholine and membrane phospholipids [49]. This compound has garnered interest in the natural products, medical, pharmaceutical, food, athletic performance, and cosmetic industries [50,51]. As α-GPC is a source of choline, it has broad potential applications in foods related to health and performance. It is commonly marketed as a nootropic, due to its ability improve cognitive recovery and neurological function in healthy individuals, maintain neurological function after brain injury or mitigate deterioration of those affected by brain disease [49,52]. For example, α-GPC has been used to improve the learning and memory abilities in stroke patients [53] and is also being investigated as a nootropic in treating psychiatric and neurological conditions (e.g., Alzheimer’s, dementia, schizophrenia, etc.) [53,54,55,56,57]. Furthermore, relating to its applicability as a nootropic supplement, α-GPC has been demonstrated to improve muscle strength [58]. With Alzheimer’s disease predicted to increase three-fold, to affect 131.5 million people, by 2050 [58], the demand for α-GPC could increase correspondingly, in addition to its use for muscle therapy. Collectively, the global market projections for nootropic supplements are predicted to surpass $10 billion by 2025 [59], making this nootropic an attractive value-added product if it can be recovered efficiently.
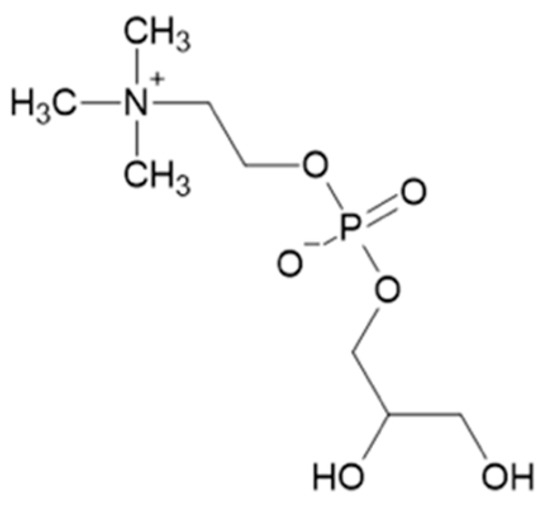
Figure 1.
Chemical structure of α-glycerylphosphorylcholine.
Synthesis of α-Glycerylphosphorylcholine
α-GPC can be produced chemically or with the use of enzymes. Chemical methods typically involve either hydrolysis of phosphatidylcholine (PC) or condensation of glycerol derivatives with phosphocholine donors using basic catalysts [60,61,62]. The chemical processes can produce toxic fumes and require the use of strong acids and harmful or undesirable solvents [62,63]. The use of toxic substrates can produce α-GPC that is not safe for use in food and, thus, not marketable. Alternatively, α-GPC has been produced by enzymatic hydrolysis of PC in aqueous media [52,57,64,65,66], employing phospholipases (Figure 2) [64]. Enzymatic production of α-GPC is advantageous, as the amounts of chemical reagents can be reduced, thereby making a comparably inexpensive product that is suited for use in food and cosmetic products [64]. However, enzymatic production of α-GPC can also be difficult due to the limited solubility of PC in aqueous phases and long reaction times (e.g., low activity) for phospholipases [67,68,69]. Surfactants can be used to improve α-GPC production, while being environmentally friendly [69].
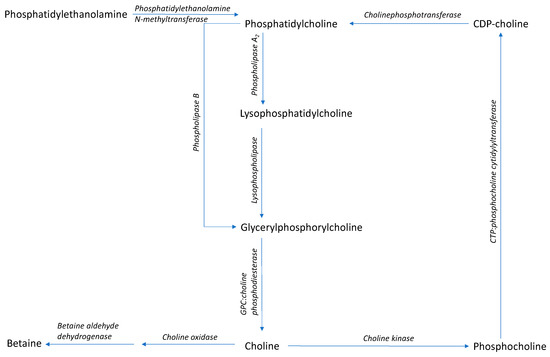
Figure 2.
Synthesis and metabolism pathway of α-glycerylphosphorylcholine (Adapted from Li and Vance 2008 [70]; Gallazzini and Burg 2009 [71]). The blue area represents processes of the Kennedy Pathway, and the green area represents the synthesis of phosphatidycholine via methylation of phosphatidylethanolamine.
Fermentation of ginger feedstocks using Schizosaccharomyces pombe yeast [72] has also resulted in the production of a nootropic compound (e.g., 6-paradol) [72]; however, these fermentations are often slower and produce by-products [73,74] that can make purification costly. Nonetheless, the ability to isolate and purify these nootropic coproducts can add significant revenue for bioethanol producers, by providing ethanol producers with new products that meet the needs of other markets. For example, nootropics (e.g., α-GPC) had a value of 7.21 billion USD in 2020, and they are expected to grow with a CAGR of 80% until 2028 [75]. Accumulation of α-GPC in the mash has been observed during alcoholic fermentation of cereal crops, such as wheat, barley, and oat, although the concentration produced can differ among cultivars [3,13]. For example, fermentation of 28 barley and 12 oat cultivars resulted in the accumulation of between 0.84 g/L to 1.81 g/L for barley and 0.62 g/L to 0.88 g/L for oat, depending on cultivar [3]. Meanwhile, fermentation of wheat resulted in α-GPC accumulation of approximately 1.68 g/L [13]. Oyeneye et al. also found that treatment of the grain with phospholipase A1, an enzyme that readily hydrolyses phosphatidyl choline and lysophosphatidyl choline, produced a beer with higher α-GPC than other treatments. This treatment effect strongly suggests that α-GPC might accumulate as a result of phosphatidyl choline hydrolysis. Pre-treatment of the feedstock (e.g., soaking, germination, incubation temperature, etc.) can also influence α-GPC yield (unpublished data). Therefore, alcoholic fermentation of cereal feedstocks can be highly advantageous due to the inexpensive processes involved and because α-GPC can be concurrently produced with ethanol during fermentation. α-GPC is a naturally produced endogenous choline derivative; however, it is rarely found at high concentrations in nature. Therefore, there is great potential in developing alternative, inexpensive, and sustainable means for commercial production to supply this compound.
4. Fusel Alcohols
A series of primary alcohols are found as natural co-products, generated during the fermentation process. These compounds are largely produced by metabolic processes, called the Ehrlich pathway in yeast, that recover nitrogen required for growth and metabolism. Enzymes of the pathway catalyze the transfer of amines between amino acids and ketones and the decarboxylation of α-keto acids to produce aldehydes, and they reduce aldehydes to form fusel alcohols (Figure 3). Products from the Ehrlich pathway can be influenced by the presence of certain amino acid intermediates (Table 2).
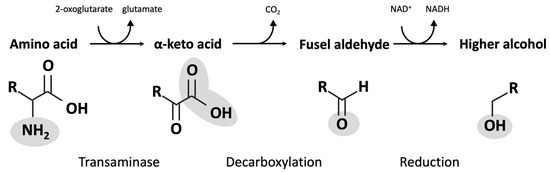
Figure 3.
Ehrlich pathway conversion of amino acids into fusel alcohols.

Table 2.
Amino acids intermediates and products of the Ehrlich pathway. Reconstructed from Hazelwood et al., (2008) [10].
Fusel alcohols are somewhat volatile, and as such, distillation enables their separation from fermented mash. These are a mixture of primarily alcohols, including active amyl alcohol (2-methyl-1-butanol), isoamyl alcohol, isobutyl alcohol, and, in lesser amounts, n-amyl alcohol, n-butyl alcohol, and methionol. Less volatile alcohols are also present in the mash and are poorly extracted by distillation, including phenethyl alcohol and tyrosol. Some of these alcohols are aromatic and are associated with strong tastes and pungent odours. Used sparingly, fusel alcohols and esters can contribute positively to foods and beverage flavours, but at higher concentrations, they are associated with unpleasant flavours and “hangover” symptoms [76]. These compounds can be detrimental to yeast growth; therefore, their removal is essential in maintaining efficient fermentation [10,76,77,78]. When these materials are extracted from backset or continuous distillation processes, the fermentation efficiency is increased, and a valuable co-product can be isolated.
During ethanol distillation, fusel alcohols concentrate in the distillation column as they are less volatile than the ethanol water azeotrope. The removal of these compounds is not required for fuel production but is essential for ethanol destined for food or pharmaceutical applications. If these higher alcohols are not removed, they can reach their limit of solubility and increase vapour pressures in the column. In turn, the increase in vapour pressure can cause boiling and flooding and thus interfere with distillation. In bioethanol plants with continuous distillation processes, the fusel alcohols are distributed in the distillation or rectifier column, where the product at the top of the column is purified alcohol (~95% by wt.), and at the bottom is mostly water. Fusel alcohols are most volatile at lower ethanol concentrations and tend to collect in the intermediate region where ethanol concentration is approximately 45% (v/v) [76]. In continuous distillation columns, fusel alcohol removal is accomplished by drawing a solution of water, ethanol, and fusel alcohols from the center of the rectifier (Figure 4).
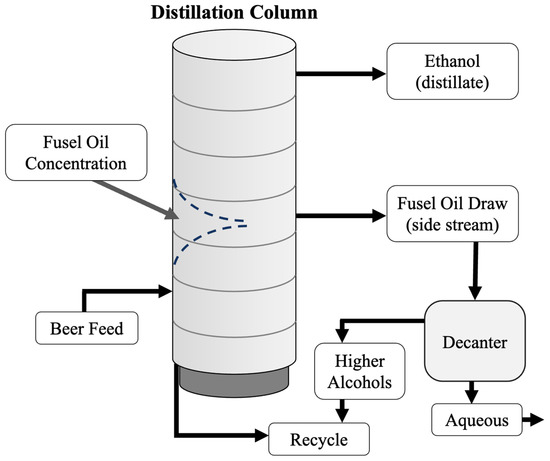
Figure 4.
Distillation column with a fusel alcohol draw near the middle of the column. The fusel alcohols can be removed from the distillation process and decanted, reusing the higher alcohols and removing the aqueous phase. Without a fusel alcohol draw, the fusel alcohols will fall in the column and form a two-phase system. This can interfere with the distillation efficiency.
In batch distillation, fusel alcohols form azeotropes with water that can accumulate at the bottom of the column to become concentrated late distilling fractions called “tails” that evaporate when the column temperature rises at the end of a distillation.
The accumulation of fusel alcohol in commercial fermentations can vary from 1–11 mL/L of ethanol produced [79]. With global biofuel production capacity increasing, the capacity for the production of substantial amounts of fusel alcohol is possible. For example, the US Energy Information Administration announced that the total US biofuels plant production capacity reached 21 billion gallons per year as of January 2021 [80]. If fusel oils were separated from fuel ethanol, hundreds of millions of litres of fusel alcohols could be recovered annually. Ultimately, it is beneficial to investigate additional uses for these compounds to promote sustainability and discover new market values for these co-products. Typically, fusel alcohols are blended with ethanol to make a product that is suited for fuel applications. Separated fusel oils can also be used as an energy source, but this is not an ideal fuel, as negative environmental impacts can outweigh the value of energy recovered when combusting these materials [81]. Despite some of the drawbacks in fermentations that accumulate fusel alcohols, they have utility as gasoline and diesel additives that improve fuel properties and combustion [5,10,76,77,78,82]. In particular, fusel alcohol gasoline blends do not exhibit any phase separation, and engines operating on these blends can achieve higher compression ratios and performance than possible with gasoline alone.
Fusel alcohols can also be purified and esterified to yield a range of valuable esters. Isoamyl alcohol is the primary component in fusel alcohols and can be used in the production of organic esters for industrial purposes. For example, isoamyl acetate is formed by esterification of isoamyl alcohol with acetic acid, and it has applications in commercial products such lacquers and as a food flavouring additive, with a banana-, caramel-, or pear-like flavour profile [76,77,78]. As such, the recovery of isoamyl alcohol can add significant value to the bioethanol producer, as this compound has three-times the market value of fuel ethanol [81]. Unfortunately, isolating and purifying isoamyl alcohol from fusel alcohols is challenging, due to the formation of heterogenous azeotropes of several alcohols with water during distillation. Multiple decanters have been used to collect fusel alcohol draws at alternating positions in the distillation column, to separate and purify fusel alcohols, although these methods typically do not afford products that are sufficiently pure for commercial application [76,79,81]. Esterification of fusel alcohols, a process called reactive distillation, has also been proposed and demonstrated successfully at a bench-scale to simultaneously obtain highly enriched alcohol esters [76,77]. Another method to aid in fusel alcohol separation is the addition of water to promote phase separation of higher alcohols from the aqueous phase (containing methanol, ethanol, propanol, and isopropanol). The organic phase can then be further distilled to enrich isoamyl alcohol [76,79].
Enrichment and purification of individual fusel alcohols and their esters can produce value-added compounds, suitable for inclusion in flavours and fragrances. The accumulation of these alcohols depends greatly on yeast genetics [76,77,78] and fermentation medium components (e.g., sugar source) [76,77]. For example, the production of isopropanol can be manipulated through the addition of exogenous acetone to the mash [76,77]. Yeast genetics determines the production of volatile compounds, such as fusel alcohols, that contribute to the product flavours and aromas [77,83,84]. Identifying yeasts that appropriately influence the flavour profiles of the final product is of great importance for the distillation of materials such as beers, ciders, and spirits. Alteration of fusel compounds is possible, although the complete suppression of fusel alcohol formation by selection of yeast is not, due to the biosynthesis of the α-keto acid present in the Ehrlich pathway (Figure 3) [83,85,86]. Overall, modifications can influence the production of alcohol by-products, and they can add considerable value to the bioethanol producer if these compounds can be further isolated and purified.
5. Two-Stage Fermentation of Thin Stillage
In additional to physical processing, stillage can be fermented by a range of organisms. The biorefinery of thin stillage has been accomplished via fermentation with a consortium of LAB (e.g., Lactobacilli) selectively recovered from a stillage storage tank (Figure 5) [14,15]. Fermentation was effective in modifying the stillage, allowing the efficient separation of a protein-rich fraction. The consortium organisms belong to species that are classified as Generally Recognized as Safe (GRAS) and are routinely used in the food industry. Clarification of thin stillage via LAB fermentation may result from a combination of gas production that leads to anoxic gas flotation [87], production of exopolysaccharides that cause particle aggregation [14], and/or allowing sufficient time for settling/floatation of aggregated particles or separation of the particles from the solution with decanting and desludging centrifuges. The resulting protein-rich slurry contains much of the original thin stillage protein, as well as proteins produced by the LAB. Drying the slurry creates a concentrate of up to 60% protein [88]. The bacteria-rich protein concentrate can then serve as a probiotic animal feed supplement [89]. Furthermore, LAB are capable of utilizing and thriving on complex carbohydrates as their carbon source. Two-stage fermentation of wheat-based thin stillage using LAB produced succinic acid (>2.0 g/L), lactic acid (>4.5 g/L), and acetic acid (>4.5 g/L) within 72 h at 37 °C [15].

Figure 5.
Wheat thin stillage before (left) and after (right) 48 h of secondary fermentation by lactic acid bacteria. The initially homogenous solution separates into an upper layer composed of, mostly, clear liquid and a protein rich slurry in the bottom layer.
5.1. Organic Acids
Many of the organic acids (e.g., acetic acid, succinic acid, and lactic acid) produced by yeast are acids that typically arise from glycolysis (e.g., acetic acid and lactic acid) and the citric acid cycle (e.g., succinic acid) [90]. These compounds are typically produced commercially via fermentation microbial processes [90,91,92] for use in the food, beverage, and manufacturing industries. The efficiency of production of these organic acids is dependent on the microorganisms, feedstock, fermenter productivity, and development of efficient recovery processes [93,94,95].
Due to its pH tolerance and simple nutrient requirements, Saccharomyces cerevisiae has been investigated and genetically modified for organic acid production [96]. Organic acids are not traditionally produced by Saccharomyces cerevisiae fermentation. The production of these organic acids could produce considerable additional value (Table 1), but a portion of the organic acids (e.g., acetic acid and lactic acid) seen in commercial ethanol beers are typically attributable to nuisance organisms present during bioethanol production. At modest concentrations, these organic acids can inhibit fermentation [5,97,98]. For example, acetic acid present in commercial Saccharomyces cerevisiae fermentations is undesirable, with concentrations > 0.4 g/L signifying bacterial contamination and concentrations > 0.6 g/L leading to impaired fermentations [95,99]. Thus, during ethanolic fermentation, it is often preferred to minimize production of these compounds. However, after the completion of primary ethanolic fermentation, the resulting thin stillage by-product can be used as culture media for lactic acid bacteria (LAB) fermentation (two-stage fermentation; discussed below) for producing additional succinic acid, lactic acid, and acetic acid [14,15]. For example, fermentation (72 h at 37 °C) on wheat-based thin stillage with an initial glycerol content of 10 g/L using Lactobacilli resulted in the accumulation of succinic acid (>2.0 g/L), lactic acid (>4.5 g/L), and acetic acid (>4.5 g/L) in the stillage medium [15].
5.2. Conversion of Glycerol to 1,3-Propanediol
In addition to the production of organic acids, during fermentation, glycerol is also produced by yeast to protect cells against lysis and regenerate NAD+ needed for glycolysis [6,7,8]. Interestingly, glycerol in the fermentation mash can be further upgraded through its conversion to 1,3-propanediol using Lactobacilli [14,15]. This compound is useful for the production of textiles, carpets, adhesives, moldings, etc. [100,101]. The conversion of glycerol to 1,3-propanediol is catalyzed via a two-step reaction. First, glycerol is converted to 3-hydroxypropionaldehyde by glycerol dehydratase, and subsequently, it is converted to 1,3-propanediol by 1,3-propanediol oxidoreductase [101,102]. Glycerol dehydratase and 1,3-propanediol oxidoreductase activity require cobalamin (aka vitamin B12) as a cofactor for catalysis [101,102]. Furthermore, cobalamin is an essential nutrient for DNA synthesis and cellular energy production. Some lactic acid bacteria (e.g., Lactobacillus reuteri) can produce this essential nutrient [103,104,105,106]. A two-stage fermentation of wheat-based thin stillage with a consortium of Lactobacilli converted most glycerol (10 g/L) present to 1,3-propanediol (6.1 g/L) [14,15] within 72 h. Genetic sequencing of members of the consortium identified Lactobacilli gene sequences that encoded for proteins that likely produce cobalamin [15]. The inoculum size for this study was 0.01% (v/v), and thus, it could be easily implemented at other bioethanol facilitate, to facilitate the production of these valuable compounds and vitamins.
5.3. Bacteriocins
Bacteriocins are another potential value-added product that could be purified after a second two-stage fermentation of ethanol stillage with Lactobacillus. Bacteriocins are antimicrobial proteins produced by most bacteria [107,108]. This broad class of compounds inhibits the growth of competing bacteria. Typically, the bacteria that produce bacteriocins simultaneously produce immunity proteins [109]. Some bacteriocins exhibit broad-spectrum antagonistic effects. For example, bacteriocins produced by Gram-negative bacteria typically affect closely related species, whereas Gram-positive bacteria can produce bacteriocins that exhibit a broader spectrum of activity [110]. These antimicrobial compounds might have utility as natural food preservatives or for pharmaceutical applications [110,111]. Bacteriocins derived from Generally Recognized as Safe (GRAS) organisms would have greater potential for such applications (e.g., Lactobacilli). Genes encoding known bacteriocin proteins were present in a consortium of Lactobacilli that was capable of two-stage fermentation of wheat-based thin stillage [15]. It is not known if the identification of bacteriocins was complete, as most bacteriocins and their sequences remain unidentified, and only a few have been investigated for their utility in foods as antagonistic compounds [112]. Furthermore, growth media composition [113], fermentation temperature [114], and pH [115] can also affect bacteriocin production and yield.
Currently, there are five recognized classes of bacteriocins that are segregated primarily on their molecular size and properties [34]. Unlike traditional antibiotics, which typically act as enzyme inhibitors [116], bacteriocins elicit adverse effects by inhibiting bacterial cell growth, by disrupting essential functions (e.g., translation and transcription) [117], and by targeting the cell surface and altering membrane permeability [110,117,118,119,120,121] (e.g., formation of membrane channels). Bacteriocin-producing organisms simultaneously express bacteriocin-immunity proteins that protect producing organisms from their own toxins [122,123]. Bacteria can acquire or lose immunity against specific bacteriocins through horizontal gene transfer [124,125,126,127,128,129,130].
Although the applications for bacteriocins as a food preservative and for pharmaceutical use are promising, the purification of bacteriocins can be difficult. This is likely due to their complex molecular structure, physicochemical properties, and heterogeneity [110]; therefore, specific purification processes might be required for individual bacteriocins [110]. These difficulties and current separation and approaches to bacteriocins purification are further reviewed in Tse and Reaney (2020) [34].
6. Spent Yeast
Spent yeast is another by-product from the brewing and biofuels industry. The spent yeast is typically removed at the end of the fermentation, although a small amount can be retained for subsequent fermentation batches [131]. Due to its protein content, discarded yeast is typically used as inexpensive animal feed materials [131]. However, spent yeast also contains valuable nutrients, such as vitamins (e.g., vitamins B1, B2, B3, B6, B9, and B12) [132,133,134], minerals (e.g., Na, K, Ca, Mg, Fe, Mn, Zn, Cu, Se, Cr, and Mo) [134,135], proteins (e.g., mannoproteins and hydrolysates) [135], carbohydrates (e.g., β-glucans) [135], antioxidants (e.g., glutathione) [136], and phenolic compounds (e.g., gallic acid and (±)-catechin) [132]. Retrieval of these compounds can have added value in animal feeds, nutritional supplements, and functional foods (e.g., flavor enhancers) and non-food additives (e.g., cosmetics) [131,137]. Hydrolysis of spent yeast can yield a complex mixture of oligopeptides, peptides, and free amino acids, also known as hydrolysates [137]. Total solids recovery of hydrolysates, proteins, and α-amino nitrogen content in dried spent yeast was reported to be 50%, 55.9%, and 4.8%, respectively [137]. However, the composition of the spent yeast (e.g., the presence of contaminating bacteria) during fermentation can have direct implications on the yeast extract and concentration of nutrients in these fractions [138]. Furthermore, as yeast are living cells with vigorous metabolism, they have a high ratio of RNA to other nutrients. Consumption of large amounts of nucleic acids can impart detrimental health effects to foods. Consumption of nucleic acid-rich materials can lead to uric acid accumulating in tissues and the consequent symptoms of gout [131]. Therefore, processing of spent yeast should include RNA degradation processes, prior to its application in food supplements.
7. Enzymes and Pharmaceuticals Products Produced via Microbial Fermentation
Hydrolytic enzymes have a market value close to a $1 billion/year, and they play an important role in many industries, including in the preparation of pharmaceutics, cosmetics, medicines, nutritional supplements, chemicals, beverages, and foods (Table 3) [139]. Although hydrolytic enzymes and pharmaceutical products may be recovered from microbial fermentation, other methods are more effective, so they are not described in detail here. Solid-state and submerged fermentations have been employed for enzyme production [140,141,142,143,144,145,146], with the former method demonstrating advantages for certain enzymes’ production (e.g., invertase, pectinases, and tannases from Aspergillus sp.), rather than liquid-based fermentation systems [145,147]. Solid-state fermentation can also be more productive than liquid systems while being simpler technically and requiring lower capital investment, lower energy input, lower water requirement, better product recovery, and a lack of foam build up when compared to submerged fermentations [148]. Improved enzyme production has also been demonstrated using co-cultivation methods [145]. Nonetheless, both submerged and solid-state fermentation technologies are being investigated and improved upon to increase industrial enzymes production.

Table 3.
Examples of industrial enzymes and the microbial producers. Reconstructed from Ventura-Sobreville et al., 2015 [139]; de Souza Vandenberghe et al., 2016 [149]; Singh et al., 2019 [150], and citations therein.
8. Conclusions
In conclusion, microbial fermentation is often utilized to produce a wide variety of valuable compounds used in several industries. First-generation biofuel producers utilizing Saccharomyces cerevisiae for grain fermentation can potentially generate substantial revenue through the production, isolation, and purification of numerous added-value co-products. These products can be co-produced during initial ethanolic fermentation (e.g., ethanol and α-GPC), distillation (e.g., fusel alcohols), and two-stage fermentation processes (e.g., 1,3-propanediol, organic acids, essential nutrients, and high protein domestic feed). The application of two-stage fermentation using LAB opens the possibility of not only upgrading and enriching thin stillage products, but it is also safe for feed production, due to the GRAS status of known Lactobacilli. Fortunately, these technologies have demonstrated success in producing value-added products, and they can be rapidly implemented into existing facilities, and downstream LAB fermentation utilizes minimal inoculant (0.01% v/v). However, production yields of these products can vary depending on the composition of the feedstock, the fermentation medium, microorganisms present, and fermentation conditions. In addition, complications can arise in the development of isolation and purification for these compounds. Nonetheless, the co-production of these added-value compounds will not only increase the value and utilization of grain crops, but it can also provide ethanol producers with significant additional revenue and new market entries for these fermentation co-products.
Author Contributions
Conceptualization, T.J.T.; writing—original draft, T.J.T., D.J.W., F.C.; writing—review and editing, T.J.T., D.J.W., F.C., S.K.P., M.J.T.R.; supervision, M.J.T.R.; funding acquisition, M.J.T.R. All authors have read and agreed to the published version of this manuscript.
Funding
This work was supported by the Saskatchewan Agricultural Development Fund (20190155, 20190154, 20180281, 20180248, 20180255, 20170133), the National Sciences and Engineering Research Council of Canada Discovery Grant (RGPIN-2018-06631), and Mitacs (IT19122, IT16156).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Fina B. Nelson for providing photos for the two-stage fermentation of wheat-based thin stillage.
Conflicts of Interest
Martin J. T. Reaney is the founder of, and has an equity interest in, Prairie Tide Diversified Inc. (PTD), Saskatoon, SK, Canada: previous company name is Prairie Tide Chemicals Inc.).
References
- OECD; FAO. OECD-FAO Agricultural Outlook 2020–2029; FAO: Rome, Italy; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of bioethanol—A review of factors affecting ethanol yield. Fermentation 2021. Accepted. [Google Scholar]
- Tse, T.J.; Wiens, D.J.; Shen, J.; Beattie, A.D.; Reaney, M.J.T. Saccharomyces cerevisiae fermentation of 28 barley and 12 oat cultivars. Fermentation 2021, 7, 59. [Google Scholar] [CrossRef]
- McCallum, B.D.; Depauw, R.M. A review of wheat cultivars grown in the Canadian prairies. Can. J. Plant Sci. 2008, 88, 649–677. [Google Scholar] [CrossRef]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- André, L.; Hemming, A.; Adler, L. Osmoregulation in Saccharomyces cerevisiae. Studies on the osmotic induction of glycerol production and glycerol-3-phosphate dehydrogenase (NAD+). FEBS Lett. 1991, 286, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Larsson, K.; Ansell, R.; Eriksson, P.; Adler, L. A gene encoding sn-glycerol 3-phosphate dehydrogenase (NAD+) complements an osmosensitive mutant of Saccharomyces cerevisiae. Mol. Microbiol. 1993, 10, 1101–1111. [Google Scholar] [CrossRef]
- Ansell, R.; Granath, K.; Hohmann, S.; Thevelein, J.M.; Adler, L. The two isoenzymes for yeast NAD+-depedent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 hav distinct roles in osmoadaptation and redox regulation. EMBO. J. 1997, 16, 2179–2187. [Google Scholar] [CrossRef] [Green Version]
- Gil, I.D.; Gómez, J.M.; Rodríguez, G. Control of an extractive distillation process to dehydrate ethanol using glycerol as entrainer. Comput. Chem. Eng. 2012, 39, 129–142. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.-M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, R.J. Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [Green Version]
- Sentheshanmuganathan, S. The mechanism of the formation of higher alcohols from amino acids by Saccharomyces cerevisiae. Biochem. J. 1960, 74, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Janssen, P.H. Propanol as an end product of threonine fermentation. Arch. Microbiol. 2004, 182, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Oyeneye, A.; Shen, J.; Shim, Y.Y.; Tse, T.J.; Reaney, M.J.T. Production of α—Glycerylphosphorylcholine and other compounds from wheat fermentation. ACS Omega 2020, 5, 12486–12494. [Google Scholar] [CrossRef]
- Ratanapariyanuch, K.; Shin, Y.Y.; Emami, S.; Reaney, M.J.T. Production of protein concentrate and 1,3-propanediol by wheat-based thin stillage fermentation. J. Agric. Food. Chem. 2017, 65, 3858–3867. [Google Scholar] [CrossRef]
- Tse, T.J.; Shen, J.; Shim, Y.Y.; Reaney, M.J.T. Changes in bacterial populations and their metabolism over 90 sequential cultures on wheat-based thin stillage. J. Agric. Food. Chem. 2020, 68, 4717–4729. [Google Scholar] [CrossRef]
- Grand View Research. Ethanol Market Size, Share & Trends Analysis Report by Source (Second Generation, Grain-Based), by Purity (Denatured, Undenatured), by Application (Beverages, Fuel & Fuel Additives), and Segment Forecasts, 2020–2027; Grand View Research: San Francisco, CA, USA, 2020. [Google Scholar]
- Grand View Research. Acetic Acid Market Size, Share and Trends Analysis Report by Application (Vinyl Acetate Monomer, Purified Terephthalic Acid, Acetate Esters, Ethanol), by Region, and Segment Forecasts, 2020–2027; Grand View Research: San Francisco, CA, USA, 2020. [Google Scholar]
- Grand View Research. Succinic Acid Market Size, Share & Trends Analysis Report by Application, by Region (North America, Europe, Asia Pacific, Row), and Segment Forecasts, 2015–2022; Grand View Research: San Francisco, CA, USA, 2016. [Google Scholar]
- Grand View Research. Lactic Acid Market Size, Share and Trends Analysis Report by Raw Material (Sugarcane, Corn, Cassava), by Application (Pla, Food & Beverages), by Region, and Segment Forecasts, 2021–2028; Grand View Research: San Francisco, CA, USA, 2021. [Google Scholar]
- Grand View Research. Glycerol Market Size, Share & Trends Analysis Report by Source (Biodiesel, Fatty Acids, Fatty Alcohols, Soap), by Type (Crude, Refined) by End Use (Food & Beverage, Pharmaceutical), by Region, and Segment Forecasts, 2020–2027; Grand View Research: San Francisco, CA, USA, 2020. [Google Scholar]
- Market Watch. Nootropics Market Size Rising at CAGR of 12.5% during 2021-2028: Global Industry Brief Analysis of Top Countries Data, Trends and Drivers with Top Key Players. Available online: https://www.marketwatch.com/press-release/nootropics-market-size-rising-at-cagr-of-125-during-2021-2027-global-industry-brief-analysis-of-top-countries-data-trends-and-drivers-with-top-key-players-2021-07-22 (accessed on 4 October 2021).
- Market Watch. DDGS Market Size in 2021: 5.2% CAGR with Top Countries Data, Global Forecast to 2026 by Trends, Product Type, Future Growth, Leading Key Players, Demand Forecast and Revenue Analysis. 2021. Available online: https://www.marketwatch.com/press-release/ddgs-market-size-in-2021-52-cagr-with-top-countries-data-global-forecast-to-2026-by-trends-product-type-future-growth-leading-key-players-demand-forecast-and-revenue-analysis-updated-117-pages-report-2021-08-18 (accessed on 4 October 2021).
- Ham, G.A.; Stock, R.A.; Klopfenstein, T.J.; Larson, E.M.; Shain, D.H.; Hanke, H.E. Wet corn distillers byproducts compared with dried corn distillers’ grains with solubles as a source of protein and energy for ruminants. J. Anim. Sci. 1994, 72, 3246–3257. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.V.; Sexson, K.R.; Lagoda, A.A. Protein-rich residue from wheat alcohol distillation: Fractionation and characterization. Cereal Chem. 1984, 61, 423–427. [Google Scholar]
- Kwiatkowski, J.R.; McAloon, A.J.; Taylor, F.; Johnston, D.B. Modeling the process and costs of fuel ethanol production by the corn dry-grind process. Ind. Crops Prod. 2006, 23, 288–296. [Google Scholar] [CrossRef]
- Mustafa, A.F.; McKinnon, J.J.; Ingledew, M.W.; Christensen, D.A. The nutritive value for ruminants of thin stillage and distillers’ grains derived from wheat, rye, triticale and barley. J. Agric. Food Chem. 2000, 80, 607–613. [Google Scholar] [CrossRef]
- Castellari, M.; Versari, A.; Fabiani, A.; Parpinello, G.P.; Galassi, S. Removal of ochratoxin A in red wines by means of adsorption treatments with commercial fining agents. J. Agric. Food Chem. 2001, 49, 3917–3921. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. Water Treatment: Principles and Design, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Chadwick, T.H.; Schroeder, E.D. Characterization and treatability of pomace stillage. J Water Pollut. Control Fed. 1973, 45, 1978–1984. [Google Scholar]
- Burke, D.A. Application of the AGF (Anoxic Gas Flotation) Process. Paper Presented at the 4th International Conference on Flotation in Water and Waste Water Treatment; Finnish Water and Wastewater Works Association: Helsinki, Finland, 2000. [Google Scholar]
- Macfarlane, A.; Prestidge, R.; Farid, M.; Chen, J. Dissolved air flotation: A novel approach to recovery of organosolv lignin. Chem. Eng. J. 2009, 148, 15–19. [Google Scholar] [CrossRef]
- Arora, A.; Dien, B.S.; Belyea, R.L.; Singh, V.; Tumbleson, M.E.; Rausch, K.D. Nutrient recovery from the dry grind process using sequential micro and ultrafiltration of thin stillage. Bioresour. Technol. 2010, 101, 3859–3863. [Google Scholar] [CrossRef] [PubMed]
- Bento, J.M.A.; Fleming, H.L. Membrane-Based Process for the Recovery of Lactic Acid and Glycerol from a “Corn Thin Stillage” Stream. U.S. Patent 5,250,182A, 14 January 1994. [Google Scholar]
- Tse, T.J.; Reaney, M.J.T. Enrichment and Utilization of Thin Stillage By-products. In Food Wastes and By-Products: Nutraceutical and Health Potential; Campos-Vega, R., Oomah, B.D., Vergara-Castañeda, H.A., Eds.; John Wiley & Son Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Alotaibi, K.D.; Schoenau, J.J.; Hao, X. Fertilizer potential of thin stillage from wheat-based ethanol production. Bioenerg. Res. 2014, 7, 1421–1429. [Google Scholar] [CrossRef]
- Ratanapariyanuch, K.; Tyler, R.T.; Shim, Y.Y.; Reaney, M.J.T. Biorefinery process for protein extraction from oriental mustard (Brassica juncea (L.) Czern.) using ethanol stillage. AMB Express. 2012, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Lapišová, K.; Vlček, R.; Klozová, J.; Rychtera, M.; Melzoch, K. Separation techniques for distillery stillage treatment. Czech J. Food Sci. 2006, 24, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Scheimann, D.W. Method of Dewatering Grain Stillage Solids. U.S. Patent 7,566,469 B2, 14 May 2009. [Google Scholar]
- Ingledew, W.M.; Austin, G.D.; Kelsall, D.R.; Kluhspies, C. The alcohol industry: How has it changed and matured. In The Alcohol Textbook, 5th ed.; Ingledew, W.M., Kelsall, D.R., Austin, G.D., Kluhspies, C., Eds.; Nottingham University Press: Nottingham, UK, 2009. [Google Scholar]
- Bhadra, R.; Muthukumarappan, K.; Rosentrater, K.A.; Kannadhason, S. Drying kinetics of Distillers Wet Grains (DWG) under varying Condensed Distillers Solubles (CDS) and temperature levels. Cereal Chem. 2011, 88, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Rosentrater, K.A.; Ileleji, K.; Johnston, D.B. Manufacturing of Fuel Ethanol and Distillers Grains—Current and Evolving Processes. In Distillers Grains—Production, Properties, and Utilization; Liu, K.S., Rosentrater, K.A., Eds.; AOCS Publishing: Boca Raton, FL, USA, 2012. [Google Scholar]
- Singh, V.; Moreau, R.A.; Hicks, K.B.; Belyea, R.L.; Staff, C.H. Removal of Fiber from Distillers Dried Grains with Solubles (DDGS) to Increase Value. Trans. ASAE 2002, 45, 389–392. [Google Scholar] [CrossRef]
- Villegas-Torres, M.F.; Ward, J.M.; Lye, G.J. The protein fraction from wheat-based dried dis-tiller’s grain with solubles (DDGS): Extraction and valorization. New Biotechnol. 2015, 32, 606–611. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R.; Bals, B.; Balan, V.; Dale, B.E.; Dien, B.S.; Cotta, M.A. Effect of compositional variability of distillers’ grains on cellulosic ethanol production. Bioresour. Technol. 2010, 101, 5385–5393. [Google Scholar] [CrossRef]
- Rausch, K.D.; Belyea, R.L. The Future of Coproducts From Corn Processing. Appl. Biochem. Biotechnol. 2006, 128, 047–086. [Google Scholar] [CrossRef]
- Rausch, K.D.; Hummel, D.; Johnson, L.A.; May, J.B. Wet Milling: The Basis for Corn Biorefineries. In Corn; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 501–535. [Google Scholar]
- Cantrell, D.F.; Winsness, D.J. Method of Recovering Oil from Thin Stillage. United. States Patent US 8,008,517 B2, 30 August 2011. [Google Scholar]
- Haas, M. Extraction and Use of DDGS Lipids for Biodiesel Production. In A Distillers Grains—Production, Properties, and Utilization; Liu, K.S., Rosentrater, K., Eds.; AOCS Publishing: Boca Raton, FL, USA, 2012. [Google Scholar]
- Sangiorgi, G.B.; Barbagallo, M.; Giordano, M.; Meli, M.; Panzarasa, R. Alpha-Glycerophosphocholine in the mental recovery of cerebral ischemic Attacks. Ann. N. Y. Acad. Sci. 1994, 717, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Shurtleff, W.; Aoyagi, A. History of Soy Lecithin. 2007. Available online: http://www.soyinfocenter.com/HSS/lecithin1.php (accessed on 13 October 2021).
- Van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schettini, G.; Ventra, C.; Florio, T.; Grimaldi, M.; Meucci, O.; Scorziello, A.; Postiglione, A.; Marino, A. Molecular mechanisms mediating the effects of l-α-glycerylphosphorylcholine, a new cognition-enhancing drug, on behavioral and biochemical parameters in young and aged rats. Pharmacol. Biochem. Behav. 1992, 43, 139–151. [Google Scholar] [CrossRef]
- Grimm, M.O.W.; Grösgen, S.; Riemenschneider, M.; Tanila, H.; Grimm, H.S.; Hartmann, T. From brain to food: Analysis of phosphatidylcholins, lyso-phosphatidylcholins and phospha-tidylcholin—plasmalogens derivates in Alzheimer ’ s disease human post mortem brains and mice model via mass spectrometry. J. Chromatogr. A 2011, 1218, 7713–7722. [Google Scholar] [CrossRef]
- Moreno, M.D.J.M. Cognitive improvement in mild to moderate Alzheimer’s dementia after treatment with the acetylcholine precursor choline alfoscerate: A multicenter, double-blind, randomized, placebo-controlled trial. Clin. Ther. 2003, 25, 178–193. [Google Scholar] [CrossRef]
- Parnetti, L.; Amenta, F.; Gallai, V. Choline alphoscerate in cognitive decline and in acute cerebrovascular disease: An analysis of published clinical data. Mech. Ageing Dev. 2001, 122, 2041–2055. [Google Scholar] [CrossRef]
- Amenta, F.; Parnetti, L.; Gallai, V.; Wallin, A. Treatment of cognitive dysfunction associated with Alzheimer’s disease with cholinergic precursors. Ineffective treatments or inappropriate approaches? Mech. Ageing Dev. 2001, 122, 2025–2040. [Google Scholar] [CrossRef]
- Bellar, D.; Leblanc, N.R.; Campbell, B. The effect of 6 days of alpha glycerylphosphorylcholine on isometric strength. J. Int. Soc. Sports Nutr. 2015, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- Wimo, A.; Ali, G.-C.; Guerchet, M.; Prince, M.; Wu, Y.-T. World Alzheimer Report 2015 The Global Impact of Dementia. Alzheimer’s Disease International. 2016. Available online: https://www.alzint.org/resource/world-alzheimer-report-2015/ (accessed on 3 October 2021).
- Wood, L. Global Brain Health Supplements Market, 2017 to 2025—ResearchAndMarkets.com. Available online: https://www.businesswire.com/news/home/20180404005510/en/Global-Brain-Health-Supplements-Market-2017-2025 (accessed on 3 October 2021).
- Sonkar, K.; Ayyappan, V.; Tressler, C.; Adelaja, O.; Cai, R.; Cheng, M.; Glunde, K. Focus on the glycerophosphocholine pathway in choline phospholipid metabolism of cancer. NMR Biomed. 2018, 32, e4112. [Google Scholar] [CrossRef]
- Brockerhoff, H.; Yurkowski, M. Simplified Preparation of L-α-Glyceryl Phosphoryl Choline. Can. J. Biochem. 1965, 43, 1777. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, Y.S.; Song, E.S.; Kang, D.S.; Song, I.W.; Kang, P.G.; Oh, S.S.; Moon, S.C.; Lee, B.G. A Process for Preparation of I-Alpha-Glycerophosphoeyl Choline. W.O. Patent 2007145476 A1, 21 December 2007. [Google Scholar]
- PubChem Compound Summary for CID 1146, Trimethylamine. 2020. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Trimethylamine (accessed on 25 November 2020).
- Kim, J.; Song, Y.; Lee, S.J.; Lee, J.E.; Chung, M.; Kim, I.; Kim, B.H. Enzymatic preparation of food-grade l -α-glycerylphosphorylcholine from soy phosphatidylcholine or fractionated soy lecithin. Biotechnol. Prog. 2020, 36, e2910. [Google Scholar] [CrossRef]
- Uziel, M.; Hanahan, D.J. An enzymatic route to L-alpha-glycerylphosphorylcholine. J. Biol. Chem. 1956, 220, 1–7. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Liu, Y. Aqueous medium enzymatic preparation of l-alpha glycerylphosphorylcholine optimized by response surface methodology. Eur. Food Res. Technol. 2012, 234, 485–491. [Google Scholar] [CrossRef]
- Bang, H.-J.; Kim, I.-H.; Kim, B.H. Phospholipase A 1-catalyzed hydrolysis of soy phosphatidylcholine to prepare l -α-glycerylphosphorylcholine in organic-aqueous media. Food Chem. 2016, 190, 201–206. [Google Scholar] [CrossRef]
- Yang, Y.R.; Jang, H.-J.; Ryu, S.H.; Suh, P.-G. Phospholipases in Health and Disease. In Phospholipases in Health and Disease; Springer International Publishing: Berlin/Heidelberg, Germany, 2014; Volume 10, pp. 3–38. [Google Scholar]
- Lu, Y.; Zhang, A.; Wang, X.; Hao, N.; Chen, K.; Ouyang, P. Surfactant enhanced l-α-glycerylphosphorylcholine production from phosphatidylcholine using phospholipase A1 in the aqueous phase. Biocatal. Biotransform. 2019, 37, 361–366. [Google Scholar] [CrossRef]
- Li, Z.; Vance, D.E. Thematic Review Series: Glycerolipids. Phosphatidylcholine and choline homeostasis. J. Lipid Res. 2008, 49, 1187–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallazzini, M.; Burg, M.B. What’s New About Osmotic Regulation of Glycerophosphocholine. Physiology 2009, 24, 245–249. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.W.; Park, H.-Y.; Oh, M.S.; Yoo, H.H.; Lee, S.-H.; Ha, S.K. Neuroprotective effect of 6-paradol enriched ginger extract by fermentation using Schizosaccharomyces pombe. J. Funct. Foods 2017, 31, 304–310. [Google Scholar] [CrossRef]
- Minnaar, P.; Jolly, N.; Paulsen, V.; Du Plessis, H.; Van Der Rijst, M. Schizosaccharomyces pombe and Saccharomyces cerevisiae yeasts in sequential fermentations: Effect on phenolic acids of fermented Kei-apple (Dovyalis caffra L.) juice. Int. J. Food Microbiol. 2017, 257, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Miljić, U.; Puškaš, V.; Vučurović, V.; Muzalevski, A. Fermentation Characteristics and Aromatic Profile of Plum Wines Produced with Indigenous Microbiota and Pure Cultures of Selected Yeast. J. Food Sci. 2017, 82, 1443–1450. [Google Scholar] [CrossRef]
- Grand View Research. Brain Health Supplements Market Size, Share & Trends Analysis Report By Product (Natural Molecules, Herbal Extract), By Application (Memory Enhancement, Depression & Mood), By Region, And Segment Forecasts, 2021–2028. 2021. Available online: https://www.grandviewresearch.com/industry-analysis/brain-health-supplements-market (accessed on 8 October 2021).
- Hori, H.; Fujii, W.; Hatanaka, Y.; Suwa, Y. Effects of Fusel Oil on Animal Hangover Models. Alcohol. Clin. Exp. Res. 2003, 27, 37S–41S. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, F. Über die Bedingungen der Fuselölbildung und über ihren Zusammenhang mit dem Eiweißaufbau der Hefe. Eur. J. Inorg. Chem. 1907, 40, 1027–1047. [Google Scholar] [CrossRef] [Green Version]
- Pires, E.J.; Teixeira, J.; Brányik, T.; Vicente, A. Yeast: The soul of beer’s aroma—a review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Pedroza, J.D.J.; Sánchez-Ramírez, E.; Segovia-Hernández, J.G.; Hernández, S.; Orjuela, A. Recovery of alcohol industry wastes: Revaluation of fusel oil through intensified processes. Chem. Eng. Process. Process. Intensif. 2021, 163, 108329. [Google Scholar] [CrossRef]
- US Energy Information Administration. 2021; EIA Releases Plant-Level U.S. Biofuels Production Capacity Data. 13 September 2021. Available online: Eia.gov/todayinenergy/detail.php?id=49516#:~:text=Fuel%20ethanol%20production%20capacity%20was,since%20the%20beginning%20of%202020 (accessed on 2 October 2021).
- Ferreira, M.C.; Meirelles, A.J.A.; Batista, E.A.C. Study of the Fusel alcohol Distillation Process. Ind. Eng. Chem. Res. 2013, 52, 2336–2351. [Google Scholar] [CrossRef]
- Patil, A.G.; Koolwal, S.; Butala, H.D. Fusel alcohol: Composition, removal and potential utilization. Int. Sugar J. 2002, 104, 51–58. [Google Scholar]
- Smit, B.A.; Engels, W.J.; Smit, G. Branched chain aldehydes: Production and breakdown pathways and relevance for flavour in foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, C.M.E.; Pietrowski, G.D.A.M.; Braga, C.; Rossi, M.J.; Ninow, J.; Dos Santos, T.P.M.; Wosiacki, G.; Jorge, R.M.M.; Nogueira, A. Apple Aminoacid Profile and Yeast Strains in the Formation of Fusel Alcohols and Esters in Cider Production. J. Food Sci. 2015, 80, 1170. [Google Scholar] [CrossRef]
- Schoondermark-Stolk, S.A.; Tabernero, M.; Chapman, J.; Ter Schure, E.G.; Verrips, C.T.; Verkleij, A.J.; Boonstra, J. Bat2p is essential in Saccharomyces cerevisiae for fusel alcohol production on the non-fermentable carbon source ethanol. FEMS Yeast Res. 2005, 5, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Eden, A.; Van Nedervelde, L.; Drukker, M.; Benvenisty, N.; Debourg, A. Involvement of branched-chain amino acid aminotransferases in the production of fusel alcohols during fermentation in yeast. Appl. Microbiol. Biotechnol. 2001, 55, 296–300. [Google Scholar] [CrossRef]
- Kang, T.S.; Korber, D.R.; Tanaka, T. Metabolic engineering of a glycerol-oxidative pathway in Lactobacillus panis PM1 for utilization of bioethanol thin stillage: Potential to produce platform chemicals from glycerol. Appl. Environ. Microbiol. 2014, 80, 7631–7639. [Google Scholar] [CrossRef] [Green Version]
- Ratanapariyanuch, K.; Shim, Y.Y.; Wiens, D.J.; Reaney, M.J.T. Grain Thin Stillage Protein Utilization: A Review. J. Am. Oil Chem. Soc. 2018, 95, 933–942. [Google Scholar] [CrossRef]
- Pedersen, C.; Jonsson, H.; Lindberg, J.E.; Roos, S. Microbiological Characterization of Wet Wheat Distillers’ Grain, with Focus on Isolation of Lactobacilli with Potential as Probiotics. Appl. Environ. Microbiol. 2004, 70, 1522–1527. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Redden, H.; Alper, H.S. Frontiers of yeast metabolic engineering: Diversifying beyond ethanol and Saccharomyces. Curr. Opin. Biotechnol. 2013, 24, 1023–1030. [Google Scholar] [CrossRef]
- Yang, L.; Lübeck, M.; Lübeck, P.S. Aspergillus as a versatile cell factory for organic acid production. Fungal Biol. Rev. 2017, 31, 33–49. [Google Scholar] [CrossRef]
- Liaud, N.; Giniés, C.; Navarro, D.; Fabre, N.; Crapart, S.; Gimbert, I.H.-; Levasseur, A.; Raouche, S.; Sigoillot, J.-C. Exploring fungal biodiversity: Organic acid production by 66 strains of filamentous fungi. Fungal Biol. Biotechnol. 2014, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.H.; Jang, Y.-S.; Lee, S.Y. Production of succinic acid by metabolically engineering microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–66. [Google Scholar] [CrossRef]
- Raab, A.M.; Lang, C. Oxidative versus reductive succinic acid production in the yeast saccharomyces cerevisiae. Bioeng. Bugs 2011, 2, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Ferone, M.; Raganati, F.; Ercole, A.; Olivieri, G.; Salatino, P.; Marzochella, A. Continuoous succinic acid fermentation by Actinobacillus succinogenes in a packed-bed biofilm reactor. Biotechnol. Biofuels. 2018, 11, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, D.A.; Zelle, R.; Pronk, J.; Van Maris, A.J.A. Metabolic engineering of Saccharomyces cerevisiae  for production of carboxylic acids: Current status and challenges. FEMS Yeast Res. 2009, 9, 1123–1136. [Google Scholar] [CrossRef] [Green Version]
- Pielech-Przybylska, K.; Balcerek, M.; Ciepielowski, G.; Pacholczyk-Sienicka, B.; Albrecht, Ł.; Dziekońska-Kubczak, U.; Bonikowski, R.; Patelski, P. Effect of Co-Inoculation with Saccharomyces cerevisiae and Lactic Acid Bacteria on the Content of Propan-2-ol, Acetaldehyde and Weak Acids in Fermented Distillery Mashes. Int. J. Mol. Sci. 2019, 20, 1659. [Google Scholar] [CrossRef] [Green Version]
- Sousa, M.J.; Ludovico, P.; Rodrigues, F.; Leão, C.; Côrte-Real, M. Stress and cell death in yeast induced by acetic acid. In Cell Metabolism—Cell Homeostasis and Stress Response; Bubulya, P., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Thoukis, G.; Ueda, M.; Wright, D. The formation of succinic acid during alcoholic fermentation. Am. J. Enol. Vitic. 1965, 16, 1–8. [Google Scholar]
- Kaur, G.; Srivastava, A.; Chand, S. Advances in biotechnological production of 1,3-propanediol. Biochem. Eng. J. 2012, 64, 106–118. [Google Scholar] [CrossRef]
- Kraus, G.A. Synthetic Methods for the Preparation of 1,3-Propanediol. CLEAN—Soil Air Water 2008, 36, 648–651. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, S.; Wang, Y.; Fang, B. Key enzymes catalyzing glycerol to 1,3-propanediol. Biotechnol. Biofuels 2016, 9, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.; Vera, J.L.; van der Heijden, R.; Valdez, G.; de Vos, W.M.; Sesma, F.; Hugenholtz, J. The complete coenzyme B12 synthesis gene cluster of Lactobacillus reuteri CRL1098. Microbiology 2008, 154, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Martín, R.; Olivares, M.; Marín, M.L.; Xaus, J.; Fernandez, L.; Fernández, J.M. Characteriza-tion of a reuterin-producing Lactobacillus coryniformis strain isolated from a goat’s milk cheese. Int. J. Food Microbiol. 2005, 104, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Madhu, A.N.; Giribhattanavar, P.; Narayan, M.S.; Prapulla, S.G. Probiotic lactic acid bacte-rium from kanjika as a potential source of vitamin B12: Evidence from LC-MS, immunological and microbiological techniques. Biotechnol. Lett. 2010, 32, 503–506. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Bottacini, F.; Fosso, B.; Kelleher, P.; Calasso, M.; Di Cagno, R.; Ventura, M.; Picardi, E.; Van Sinderen, U.; Gobbetti, M. Lactobacillus rossiae, a Vitamin B12 Producer, Represents a Metabolically Versatile Species within the Genus Lactobacillus. PLoS ONE 2014, 9, e107232. [Google Scholar] [CrossRef] [Green Version]
- Klaenhammer, T.R. Bacteriocins of lactic acid bacteria. Biochimie 1988, 70, 337–349. [Google Scholar] [CrossRef]
- Riley, M.A. Molecular mechanisms of bacteriocin evolution. Annu. Rev. Genet. 1998, 32, 255–278. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Lin, C.; Sung, C.T.; Fang, J. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [CrossRef] [Green Version]
- De Vuyst, L.; Vandamme, E.J. Antimicrobial Potential of Lactic Acid Bacteria. In Bacteriocins of Lactic Acid Bacteria; Springer International Publishing: Berlin/Heidelberg, Germany, 1994; pp. 91–142. [Google Scholar]
- Elayaraja, S.; Annamalai, N.; Mayavu, P.; Balasubramanian, T. Production, purification and characterization of bacteriocin from Lactobacillus murinus AU06 and its broad antibacterial spectrum. Asian Pac. J. Trop. Biomed. 2014, 4, S305–S311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L. The use of biological agents in processing: Antimicrobials, fermentation and antagonistic control. In Packaging for Nonthermal Processing of Food, 2nd ed.; Pascall, M.A., Jung, H.H., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- De Vuyst, L.; Vandamme, E.J. Influence of the carbon source on nisin production in Lacto-coccus lactis subsp. lactis batch fermentations. J. Gen. Microbiol. 1992, 138, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Rayman, K.; Hurst, A. Nisin: Properties, biosynthesis and fermentation. In Biotechnology of Industrial Antibiotics; Vandamme, E.J., Ed.; Marcel Dekker: New York, NY, USA, 1984. [Google Scholar]
- Biswas, S.R.; Ray, P.; Johnson, M.C.; Ray, B. Influence of growth conditions on the production of a bacteriocin, pediocin AcH, by Pediococcus acidilactici H. Appl. Environ. Microbiol. 1991, 57, 1265–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Oscáriz, J.C.; Pisabarro, A.G. Classification and mode of action of membrane active bacteriocins produced by gram-positive bacteria. Int. Microbiol. 2001, 4, 13–19. [Google Scholar] [CrossRef]
- Gänzle, M.G. Reutericyclin: Biological activity, mode of action, and potential applications. Appl. Microbiol. Biotechnol. 2004, 64, 326–332. [Google Scholar] [CrossRef]
- Tagg, J.R.; Dajani, A.S.; Wannamaker, L.W. Bacteriocins of Gram-positive bacteria. Bacteriol. Rev. 1976, 40, 722–756. [Google Scholar] [CrossRef] [PubMed]
- Héchard, Y.; Sahl, H.-G. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie 2002, 84, 545–557. [Google Scholar] [CrossRef]
- Moll, G.N.; Konings, W.N.; Driessen, A.J.M. Bacteriocins: Mechanism of membrane insertion and pore formation. Lact. Acid Bact. Genet. Metab. Appl. 1999, 76, 185–198. [Google Scholar] [CrossRef]
- Parada, J.L.; Caron, C.R.; Medeiros, A.B.P.; Soccol, C.R. Bacteriocins from lactic acid bacteria: Purification, properties and use as biopreservatives. Braz. Arch. Biol. Technol. 2007, 50, 521–542. [Google Scholar] [CrossRef] [Green Version]
- Todorov, S.D. Bacteriocins from Lactobacillus plantarum production, genetic organization and mode of action: Produção, organização genética e modo de ação. Braz. J. Microbiol. 2009, 40, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Vossen, J.M.; Herwijnen, M.H.; Leer, R.J.; Brink, B.T.; Pouwels, P.H.; Huis in `t Veld, J.H.J. Production of acidocin B, a bacteriocin of Lactobacillus acidophilus M46 is a plasmid-encoded trait: Plasmid curing, genetic marking by in vivo plasmid integration, and gene transfer. FEMS Microbiol. Lett. 1994, 116, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Vescovo, M.; Morelli, L.; Bottazzi, V.; Gasson, M.J. Conjugal Transfer of Broad-Host-Range Plasmid pAMbeta1 into Enteric Species of Lactic Acid Bacteria. Appl. Environ. Microbiol. 1983, 46, 753–755. [Google Scholar] [CrossRef] [Green Version]
- Ceapa, C.; Davids, M.; Ritari, J.; Lambert, J.; Wels, M.; Douillard, F.P.; Smokvina, T.; de Vos, W.M.; Knol, J.; Kleerebezem, M. The variable regions of Lactobacillus rhamnosus genomes reveal the dynamic evolution of metabolic and host-adaptation repertoires. Genome Biol. Evol. 2016, 8, 1889–1905. [Google Scholar] [CrossRef] [Green Version]
- Baugher, J.L.; Durmaz, E.; Klaenhammer, T.R. Spontaneously Induced Prophages in Lactobacillus gasseri Contribute to Horizontal Gene Transfer. Appl. Environ. Microbiol. 2014, 80, 3508–3517. [Google Scholar] [CrossRef] [Green Version]
- Mercanti, D.J.; Rousseau, G.M.; Capra, M.L.; Quiberoni, A.; Tremblay, D.M.; Labrie, S.; Moineau, S. Genomic Diversity of Phages Infecting Probiotic Strains of Lactobacillus paracasei. Appl. Environ. Microbiol. 2016, 82, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Tannock, G.W.; Luchansky, J.B.; Miller, L.; Connell, H.; Thode-Andersen, S.; Mercer, A.; Klaenhammer, T.R. Molecular Characterization of a Plasmid-Borne (pGT633) Erythromycin Resistance Determinant (ermGT) from Lactobacillus reuteri 100-63. Plasmid 1994, 31, 60–71. [Google Scholar] [CrossRef]
- Egervärn, M.; Roos, S.; and Lindmark, H. Identification and characterization of antibiotic resistance genes in Lactobacillus reuteri and Lactobacillus plantarum. J. Appl. Microbiol. 2009, 107, 1658–1668. [Google Scholar] [CrossRef]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.; Ferreira, I.M. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.-J. Yeast extract production using spent yeast from beer manufacture: Influence of industrially applicable disruption methods on selected substance groups with biotechnological relevance. Eur. Food Res. Technol. 2019, 245, 1169–1182. [Google Scholar] [CrossRef]
- Pinto, L.; Lopes, M.; Filho, C.C.; Alves, L.; Benevides, C. Determinação do valor nutritivo de derivados de levedura de cervejaria (Saccharomyces spp.). Rev. Bras. de Prod. Agroind. 2013, 15, 7–17. [Google Scholar] [CrossRef]
- Pinto, M.; Coelho, E.; Nunes, A.; Brandão, T.; Coimbra, M.A. Valuation of brewers spent yeast polysaccharides: A structural characterization approach. Carbohydr. Polym. 2015, 116, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Hwang, C.-F.; Liao, C.-C. Medium optimization for glutathione production by Saccharomyces cerevisiae. Process. Biochem. 1999, 34, 17–23. [Google Scholar] [CrossRef]
- Bayarjargal, M.; Munkhbat, E.; Ariunsaikhan, T.; Odonchimeg, M.; Uurzaikh, T.; Gan-Erdene, T.; Regdel, D. Utilization of spent brewer’s yeast Saccharomyces cerevisiae for the production of yeast enzymatic hydrolysate. Mong. J. Chem. 2014, 12, 88–91. [Google Scholar] [CrossRef]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.-J. Spent Yeast from Brewing Processes: A Biodiverse Starting Material for Yeast Extract Production. Fermentation 2019, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Ventura-Sobrevilla, J.; Villa, D.B.; Rodriguez, R.; Martinez-Hernandez, J.; Aguilar, C.N. Microbial biosynthesis of enzymes for food applications. In Improving and Tailoring Enzymes for Food Quality and Functionality; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 85–99. [Google Scholar]
- Chutmanop, J.; Chuichulcherm, S.; Chisti, Y.; Srinophakun, P. Protease production byAspergillus oryzae in solid-state fermentation using agroindustrial substrates. J. Chem. Technol. Biotechnol. 2008, 83, 1012–1018. [Google Scholar] [CrossRef]
- Hansen, G.H.; Lübeck, M.; Frisvad, J.; Lübeck, P.S.; Andersen, B. Production of cellulolytic enzymes from ascomycetes: Comparison of solid state and submerged fermentation. Process. Biochem. 2015, 50, 1327–1341. [Google Scholar] [CrossRef]
- Pandey, A.; Selvakumar, P.; Soccol, C.R.; Nigam, P. Solid state fermentation for the production of industrial enzymes. Curr. Sci. 1999, 77, 149–162. [Google Scholar]
- Sivaramakrishnan, S.; Gangadharan, D.; Nampoothiri, K.M.; Soccol, C.R.; Pandey, A. Alpha amylase production by Aspergillus oryzae employing solid-state fermentation. J. Sci. Ind. Res. 2007, 66, 621–626. [Google Scholar]
- Sandhya, C.; Sumantha, A.; Szakacs, G.; Pandey, A. Comparative evaluation of neutral protease production by Aspergillus oryzae in submerged and solid-state fermentation. Process. Biochem. 2005, 40, 2689–2694. [Google Scholar] [CrossRef]
- Hu, H.; Brink, J.V.D.; Gruben, B.; Wösten, H.; Gu, J.-D.; de Vries, R. Improved enzyme production by co-cultivation of Aspergillus niger and Aspergillus oryzae and with other fungi. Int. Biodeterior. Biodegrad. 2011, 65, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Couri, S.; Terzi, S.D.C.; Pinto, G.; Freitas, S.; da Costa, A.C.A. Hydrolytic enzyme production in solid-state fermentation by Aspergillus niger 3T5B8. Process. Biochem. 2000, 36, 255–261. [Google Scholar] [CrossRef]
- Viniegra-González, G.; Favela-Torres, E.; Aguilar, C.N.; Rómero-Gomez, S.D.J.; Díaz-Godínez, G.; Augur, C. Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem. Eng. J. 2003, 13, 157–167. [Google Scholar] [CrossRef]
- De Souza, P.M.; de Oliveira, P. Application of microbial α-amylase in industry—a review. Braz. J. Microbiol. 2011, 41, 850–861. [Google Scholar] [CrossRef]
- Vandenberghe, L.D.S.; de Carvalho, J.; Libardi, N.; Rodrigues, C.; Soccol, C.R. Microbial Enzyme Factories. In Agro-Industrial Wastes as Feedstock for Enzyme Production; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 1–22. [Google Scholar]
- Singh, R.S.; Singh, T.; Pandey, A. Microbial Enzymes—An Overview. In Advances in Enzyme Technology; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 1–40. [Google Scholar]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Optimization of Aspergillus niger Fermentation for the Production of Glucose Oxidase. Food Bioprocess Technol. 2008, 2, 344–352. [Google Scholar] [CrossRef]
- Saqib, S.; Akram, A.; Halim, S.A.; Tassaduq, R. Sources of β-galactosidase and its applications in food industry. 3 Biotech 2017, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Kandalai, L.; Kandalai, K.; Hameeda, B.; Reddy, G. Fermentative production of lactase from Lactobacillus amylophilus GV6. J. Sci. Ind. Res. 2013, 72, 548–552. [Google Scholar]
- Kazemi, S.; Khayati, G.; Faezi-Ghasemi, M. β-galactosidase production by Aspergillus niger ATCC 9142 using inexpensive substrates in solid-state fermentation: Optimization by orthogonal arrays design. Iran Biomed. J. 2016, 20, 287–294. [Google Scholar]
- Czinkóczky, R.; Németh, Á. Production of the enzyme cyclodextrin glycosyltransferase using different fermentation techniques. Hung. J. Ind. Chem. 2019, 47, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Maiorano, A.; Piccoli, R.A.M.; Silva, E.S.D.S.S.D.; Rodrigues, M.F.D.A. Microbial production of fructosyltransferases for synthesis of pre-biotics. Biotechnol. Lett. 2008, 30, 1867–1877. [Google Scholar] [CrossRef]
- Sangeetha, P.T.; Ramesh, M.N.; Prapulla, S.G. Production of fructosyl transferase by Aspergillus oryzae CFR 202 in solid-state fermentation using agricultural by-products. Appl. Microbiol. Biotechnol. 2004, 65, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, Y.; Chen, J. Microbial Transglutaminase Production: Understanding the Mechanism. Biotechnol. Genet. Eng. Rev. 2009, 26, 205–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FernandaVolken de Souza, C.; Flôres, S.H.; Ayub, M.A.Z. Optimization of medium composition for the production of transglutaminase by Bacillus circulans BL32 using statistical experimental methods. Process Biochem. 2006, 41, 1186–1192. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Ramteke, P.W.; Mishra, P.K. Synthetic Biology Strategy for Microbial Cellulases. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 229–238. [Google Scholar] [CrossRef]
- Felse, P.A.; Panda, T. Production of microbial chitinases—A revisit. Bioprocess Eng. 2000, 23, 127–134. [Google Scholar] [CrossRef]
- Chaduvula, A.I.R.; Pulipati, K.; Jetti, A. Production of Invertase by Aspergillus niger Under Solid State Fermentation Using Orange Fruit Peel as Substrate. Adv. Crop. Sci. Technol. 2016, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Dawood, A.; Ma, K. Applications of Microbial β-Mannanases. Front. Bioeng. Biotechnol. 2020, 8, 598630. [Google Scholar] [CrossRef]
- Shettigar, R.R.; Misra, N.M.; Naik, B.; Patel, K. Eco-friendly extreme pressure lubricants for water based drilling fluids. Int. Proc. Chem. Biol. Environ. Eng. 2015, 90, 8. [Google Scholar] [CrossRef]
- Falade, A.; Jaouani, A.; Mabinya, L.; Okoh, A.; Nwodo, U. Exoproduction and Molecular Characterization of Peroxidase from Ensifer adhaerens. Appl. Sci. 2019, 9, 3121. [Google Scholar] [CrossRef] [Green Version]
- Illanes, A.; Acevedo, F.; Gentina, J.; Reyes, I.; Torres, R.; Cartagena, O.; Ruiz, A.; Vásquez, M. Production of penicillin acylase from Bacillus megaterium in complex and defined media. Process. Biochem. 1994, 29, 263–270. [Google Scholar] [CrossRef]
- Rajendhran, J.; Krishnakumar, V.; Gunasekaran, P. Production of Penicillin G acylase from Bacillus sp.: Effect of medium components. World J. Microbiol. Biotechnol. 2003, 19, 107–110. [Google Scholar] [CrossRef]
- De León-Rodríguez, A.; Rivera-Pastrana, D.; Medina-Rivero, E.; Flores-Flores, J.L.; Estrada-Baltazar, A.; Ordóñez-Acevedo, L.G.; de la Rosa, A.P.B. Production of penicillin acylase by a recombinant Escherichia coli using cheese whey as substrate and inducer. Biomol. Eng. 2006, 23, 299–305. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).