Production of Bioethanol—A Review of Factors Affecting Ethanol Yield
Abstract
1. Introduction
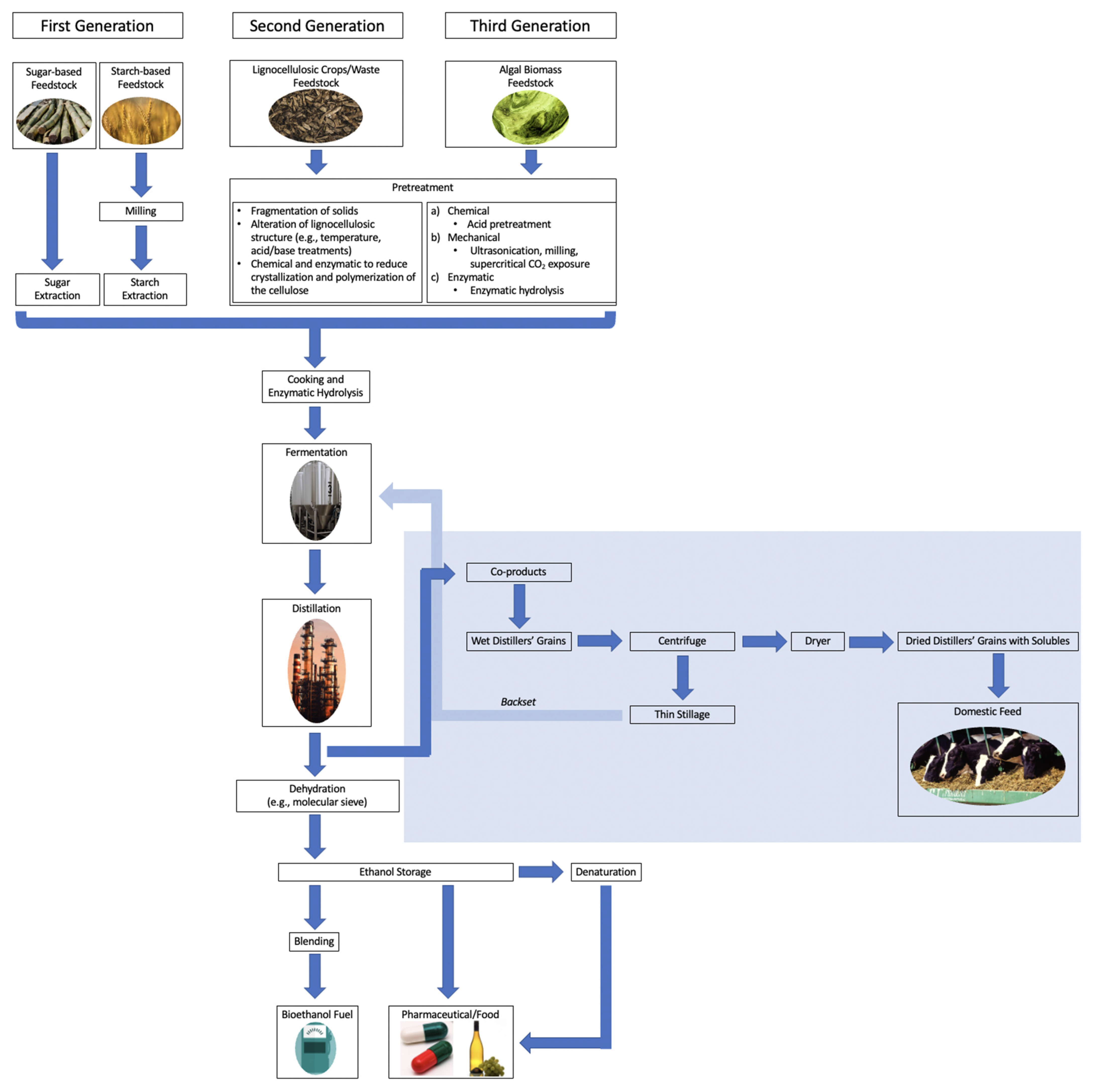
2. Bioethanol Production Processes
3. Very High Gravity, Solid-State, and Submerged/Liquid Fermentation
3.1. Submerged/Liquid State Fermentation
3.2. Solid-State Fermentation
3.3. Very High Gravity Fermentation
4. Batch, Fed-Batch, and Continuous Fermentation Modes
5. Yeast Stress
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Li, M.; Ni, T. Feedstock for bioethanol production from a technological paradigm perspective. BioResources 2015, 10, 6285–6304. [Google Scholar] [CrossRef]
- Stoeglehner, G.; Narodoslawsky, M. How sustainable are biofuels? Answers and further questions arising from an ecological footprint perspective. Bioresour. Technol. 2009, 100, 3825–3830. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2015–2024; OECD Publishing: Paris, France, 2015. [Google Scholar] [CrossRef]
- Renewable Fuels Association (RFA). Annual Fuel Ethanol Production. 2020. Available online: https://ethanolrfa.org/statistics/annual-ethanol-production/ (accessed on 22 April 2021).
- Huang, H.; Qureshi, N.; Chen, M.-H.; Liu, W.; Singh, V. Ethanol production from food waste at high solids content with vacuum recovery technology. J. Agric. Food Chem. 2015, 63, 2760–2766. [Google Scholar] [CrossRef]
- Malakar, S.; Paul, S.K.; Pou, K.R.J. Biotechnological Interventions in Beverage Production. In Biotechnological Progress and Beverage Consumption; Academic Press: New York, NY, USA, 2020; pp. 1–37. [Google Scholar] [CrossRef]
- Jambo, S.A.; Abdulla, R.; Azhar, S.H.M.; Marbawi, H.; Gansau, J.A.; Ravindra, P. A review on third generation bioethanol feedstock. Renew. Sustain. Energy Rev. 2016, 65, 756–769. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Shen, J.; Beattie, A.D.; Reaney, M.J.T. Saccharomyces cerevisiae fermentation of 28 barley and 12 oat cultivars. Fermentation 2021, 7, 59. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. Biofuels Explained—Ethanol and Biomass-BASED Diesel. 2020. Available online: https://www.eia.gov/energyexplained/biofuels/ (accessed on 4 August 2021).
- Natural Resources Canada. Ethanol. 2020. Available online: https://www.nrcan.gc.ca/energy-efficiency/transportation-alternative-fuels/alternative-fuels/biofuels/ethanol/3493 (accessed on 4 August 2021).
- Ribeiro, B.E. Beyond commonplace biofuels: Social aspects of ethanol. Energy Policy 2013, 57, 355–362. [Google Scholar] [CrossRef]
- Havlík, P.; Schneider, U.A.; Schmid, E.; Böttcher, H.; Fritz, S.; Skalský, R.; Aoki, K.; De Cara, S.; Kindermann, G.; Kraxner, F.; et al. Global land-use implications of first and second generation biofuel targets. Energy Policy 2011, 39, 5690–5702. [Google Scholar] [CrossRef]
- Arifin, Y.; Tanudjaja, E.; Dimyati, A.; Pinontoan, R. A second generation biofuel from cellulosic agricultural by-product fermentation using clostridium species for electricity generation. Energy Procedia 2014, 47, 310–315. [Google Scholar] [CrossRef][Green Version]
- Kim, S.; Dale, B.E. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Gulsunoglu, Z.; Aravind, S.; Bai, Y.; Wang, L.; Kutcher, R.A.; Tanaka, T. Deoxynivalenol (DON) accumulation and nutrient recovery in black soldier fly larvae (Hermetia illucens) fed wheat infected Fusarium spp. Fermentation 2019, 5, 83. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Cernauskas, D.; Vidmantiene, D.; Basinskiene, L.; Bartkiene, E.; Bakutis, B.; Baliukoniene, V. Combined fermentation for increasing efficiency of bioethanol production from Fusarium sp. contaminated barley biomass. Catal. Today 2014, 223, 108–114. [Google Scholar] [CrossRef]
- Mohr, A.; Raman, S. Lessons from first generation biofuels and implications for the sustainability appraisal of second generation biofuels. Energy Policy 2013, 63, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Govumoni, S.P.; Koti, S.; Kothagouni, S.Y.; Venkateshwar, S. Evaluation of pretreatment methods for enzymatic saccharification of wheat straw for bioethanol production. Carbohydr. Polym. 2013, 91, 646–650. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Dahadha, S.; Amin, Z.; Lakeh, A.A.B.; Elbeshbishy, E. Evaluation of different pretreatment processes of lignocellulosic biomass for enhanced biomethane production. Energy Fuels 2017, 31, 10335–10347. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Raud, M.; Orupõld, K.; Kikas, T. Second-generation bioethanol production: A review of strategies for waste valorisation. Agron. Res. 2017, 15, 830–847. [Google Scholar]
- Carriquiry, M.A.; Du, X.; Timilsina, G.R. Second generation biofuels: Economics and policies. Energy Policy 2011, 39, 4222–4234. [Google Scholar] [CrossRef]
- Singh, A.; Olsen, S.I. A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels. Appl. Energy 2011, 88, 3548–3555. [Google Scholar] [CrossRef]
- Harun, R.; Yip, J.W.S.; Thiruvenkadam, S.; Ghani, W.A.W.A.K.; Cherrington, T.; Danquah, M.K. Algal biomass conversion to bioethanol—A step-by-step assessment. Biotechnol. J. 2014, 9, 73–86. [Google Scholar] [CrossRef]
- Hargreaves, P.I.; Barcelos, C.A.; da Costa, A.C.A. Production of ethanol 3G from Kappaphycus alvarezii: Evaluation of different process strategies. Bioresour. Technol. 2013, 134, 257–263. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.S.; Um, B.H.; Oh, K. Pretreatment of Laminaria japonica for bioethanol production with extremely low acid concentration. Renew Energy 2013, 54, 196–200. [Google Scholar] [CrossRef]
- Lee, J.Y.; Li, P.; Ryu, H.J.; Oh, K. Ethanol production from Saccharina japonica using an optimized extremely low acid pretreatment followed by simultaneous saccharification and fermentation. Bioresour. Technol. 2013, 127, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.W.; Quinn, M.; Van Wychen, S.; Hyman, D.; Laurens, L.M. Separation and quantification of microalgal carbohydrates. J. Chromatogr. A 2012, 1270, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Kligerman, D.C.; Bouwer, E.J. Prospects for biodiesel production from algae-based wastewater treatment in Brazil: A review. Renew. Sustain. Energy Rev. 2015, 52, 1834–1846. [Google Scholar] [CrossRef]
- Scown, C.D.; Baral, N.R.; Yang, M.; Vora, N.; Huntington, T. Technoeconomic analysis for biofuels and bioproducts. Curr. Opin. Biotechnol. 2021, 67, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Sadia, S.; Bakhtawar, J.; Irfan, M.; Shakir, H.A.; Khan, M.; Ali, S. Role of substrate to improve biomass to biofuel production technologies. In Bioprocessing for Biofuel Production; Springer: Berlin/Heidelberg, Germany, 2021; pp. 127–156. [Google Scholar] [CrossRef]
- Macrelli, S.; Mogensen, J.; Zacchi, G. Techno-economic evaluation of 2nd generation bioethanol production from sugar cane bagasse and leaves integrated with the sugar-based ethanol process. Biotechnol. Biofuels 2012, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Rajoli, S.; Taherzadeh, M.J. Techno-economic analysis of integrating first and second-generation ethanol production using filamentous fungi: An industrial case study. Energies 2016, 9, 359. [Google Scholar] [CrossRef]
- Rincón, L.E.; Jaramillo, J.J.; Cardona, C.A. Comparison of feedstocks and technologies for biodiesel production: An environmental and techno-economic evaluation. Renew. Energy 2014, 69, 479–487. [Google Scholar] [CrossRef]
- Ziolkowska, J.R. Biofuels technologies. In Biofuels for a More Sustainable Future; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Oh, Y.-K.; Hwang, K.-R.; Kim, C.; Kim, J.R.; Lee, J.-S. Recent developments and key barriers to advanced biofuels: A short review. Bioresour. Technol. 2018, 257, 320–333. [Google Scholar] [CrossRef]
- Sher, F.; Pans, M.A.; Sun, C.; Snape, C.; Liu, H. Oxy-fuel combustion study of biomass fuels in a 20 kWth fluidized bed combustor. Fuel 2018, 215, 778–786. [Google Scholar] [CrossRef]
- Natural Resources Canada. Near-Zero Emissions Oxy-Fuel Combustion. 2016. Available online: https://www.nrcan.gc.ca/our-natural-resources/energy-sources-distribution/clean-fossil-fuels/coal-co2-capture-storage/carbon-capture-storage/near-zero-emissions-oxy-fuel-combustion/4307 (accessed on 18 June 2021).
- Corbin, K.R.; Byrt, C.S.; Bauer, S.; DeBolt, S.; Chambers, D.; Holtum, J.A.M.; Karem, G.; Henderson, M.; Lahnstein, J.; Beahan, C.T.; et al. Prospecting for energy-rich renewable raw materials: Agave leaf case study. PLoS ONE 2015, 10, e0135382. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The State of Food and Agriculture 2008. Biofuels: Prospects, Risks and Opportunities. 2008. Available online: http://www.fao.org/3/i0100e/i0100e.pdf (accessed on 5 August 2021).
- Nwakaire, J.N.; Ezeoha, S.L.; Ugwuishiwu, B.O. Production of cellulosic ethanol from wood sawdust. Agric. Eng. Int. CIGR J. 2013, 15, 136–140. [Google Scholar]
- Mussatto, S.I.; Dragone, G.; Guinarães, P.M.R.; Silva, J.P.A.; Carneiro, L.M.; Roberto, I.C.; Vicente, A.; Dominques, L.; Teixeira, J.A. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol. Adv. 2010, 28, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, T.V.; Hebbale, D. Bioethanol from macroalgae: Prospects and challenges. Renew. Sustain. Energy Rev. 2020, 117, 109147. [Google Scholar] [CrossRef]
- Benedetti, M.; Vecchi, V.; Barera, S.; Dall’Osto, L. Biomass from microalgae: The potential of domestication towards sustainable biofactories. Microb. Cell Factories 2018, 17, 173. [Google Scholar] [CrossRef] [PubMed]
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A boon for production of bioactive compounds by processing of food industries waste (by-produts). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef] [PubMed]
- Ratanapariyanuch, K.; Shin, Y.Y.; Emami, S.; Reaney, M.J.T. Production of protein concentrate and 1,3-propanediol by wheat-based thin stillage fermentation. J. Agric. Food Chem. 2017, 65, 3858–3867. [Google Scholar] [CrossRef] [PubMed]
- Tse, T.J.; Shen, J.; Shim, Y.Y.; Reaney, M.J.T. Changes in bacterial populations and their metabolism over 90 sequential cultures on wheat-based thin stillage. J. Agric. Food Chem. 2020, 68, 4717–4729. [Google Scholar] [CrossRef]
- Bhargav, S.; Panda, B.P.; Ali, M.; Javed, S. Solid-state fermentation: An overview. Chem. Biochem. Eng. Q 2008, 22, 49–70. [Google Scholar]
- Lizardi-Jiménez, M.A.; Hernández-Martínez, R. Solid state fermentation (SSF): Diversity of applications to valorize waste and biomass. 3 Biotech 2017, 7, 44. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Farinas, C.S. Developments in solid-state fermentation for the production of biomass degrading enzymes for the bioenergy sector. Renew. Sustain. Energy Rev. 2015, 52, 179–188. [Google Scholar] [CrossRef]
- Soccol, C.R.; Scopel, E.; da Costa, F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; Vandenberghe, L.P. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Belewu, M.A.; Babalola, F.T. Nutrient enrichment of some waste agricultueal residues after solid state fermentation using Rhizopus oligosporus. J. Appl. Biosci. 2009, 13, 695–699. [Google Scholar]
- Soccol, C.R.; Faraco, V.; Karp, S.; Vandenberghe, L.P.S.; Thomaz-Soccol, V.; Woiciechowski, A.; Pandey, A. Lignocellulosic bioethanol: Current status and future perspectives. In Biofuels; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 101–122. [Google Scholar] [CrossRef]
- Marulanda, V.A.; Gutierrez, C.D.B.; Alzate, C.A.C. Thermochemical, biological, biochemical, and hybrid conversion methods of bioderived molecules into renewable fuels. In Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts; Woodhead Publishing: Sawston, UK, 2019; pp. 59–81. [Google Scholar]
- Hsu, C.-L.; Chang, K.-S.; Lai, M.-Z.; Chang, T.-C.; Chang, Y.-H.; Jang, H.-D. Pretreatment and hydrolysis of cellulosic agricultural wastes with a cellulase-producing Streptomyces for bioethanol production. Biomass Bioenergy 2011, 35, 1878–1884. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Prasad, S.; Singh, A.; Joshi, H.C. Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour. Conserv. Recycl. 2007, 50, 1–39. [Google Scholar] [CrossRef]
- Philippidis, G.P.; Smith, T.K. Limiting factors in the simultaneous saccharification and fermentation process for conversion of cellulosic biomass to fuel ethanol. Appl. Biochem. Biotechnol. 1995, 51, 117–124. [Google Scholar] [CrossRef]
- Ingale, S.; Joshi, S.J.; Gupte, A. Production of bioethanol using agricultural waste: Banana pseudo stem. Braz. J. Microbiol. 2014, 45, 885–892. [Google Scholar] [CrossRef]
- Botella, C.; Diaz, A.B.; Wang, R.; Koutinas, A.; Webb, C. Particulate bioprocessing: A novel process strategy for biorefineries. Process Biochem. 2009, 44, 546–555. [Google Scholar] [CrossRef]
- Horita, M.; Kitamoto, H.; Kawaide, T.; Tachibana, Y.; Shinozaki, Y. On-farm solid state simultaneous saccharification and fermentation of whole crop forage rice in wrapped round bale for ethanol production. Biotechnol. Biofuels 2015, 8, 9. [Google Scholar] [CrossRef]
- Olofsson, K.; Winman, M.; Lidén, G. Controlled feeding of cellulases improves conversion of xylose in simultaneous saccharification and co-fermentation for bioethanol production. J. Biotechnol. 2010, 145, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Sarks, C.; Gunawan, C.; Bice, B.D.; Simonettt, S.P.; Narasimhan, R.A.; Willis, L.B.; Dale, B.E.; Balan, V.; Sato, T.K. Phenotypic selection of a wild Saccharomyces cerevisiae strain for simultaneous saccharification and co-fermentation of AFEXTM pretreated corn stover. Biotechnol. Biofuels 2013, 6, 108. [Google Scholar] [CrossRef]
- Ha, S.-J.; Galazka, J.M.; Kim, S.R.; Choi, J.-H.; Yang, X.; Seo, J.-H.; Glass, N.L.; Cate, J.H.D.; Jin, Y.-S. Engineered Saccharomyces cerevisiae capable of simualtaneous cellobiose and xylose fermentation. Proc. Natl. Acad. Sci. USA 2011, 108, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, M.; Qi, Q.; Gao, C.; Lin, C.S.K. Mixed food waste as renewable feedstock in succinic acid fermentation. Appl. Biochem. Biotechnol. 2014, 174, 1822–1833. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Venus, J. Potential role of sequential solid-state and submerged-liquid fermentations in a circular bioeconomy. Fermentation 2021, 7, 76. [Google Scholar] [CrossRef]
- Gomes, D.; Cruz, M.; de Resende, M.; Ribeiro, E.; Teixeira, J.; Dominques, L. Very high gravity bioethanol revisited: Main challenges and advances. Fermentation 2021, 7, 38. [Google Scholar] [CrossRef]
- Bai, F.W.; Anderson, W.A.; Moo-Young, M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Deesuth, A.; Laopaiboon, P.; Klanrit, P.; Laopaiboon, L. Improvement of ethanol production from sweet sorghum juice under high gravity and very high gravity conditions: Effect of nutrient supplementation and aeration. Ind. Crop. Prod. 2015, 74, 95–102. [Google Scholar] [CrossRef]
- Rendleman, C.M.; Shapouri, H. New Technologies in Ethanol Production; No. 1473-2021-026; AgEcon Search USDA: Washington, DC, USA, 2007. [Google Scholar]
- Puligundla, P.; Smogrovicova, D.; Obulam, V.S.R.; Ko, S. Very high gravity (VHG) ethanolic brewing and fermentation: A research update. J. Ind. Microbiol. Biotechnol. 2011, 38, 1133–1144. [Google Scholar] [CrossRef]
- Ishmayana, S.; Learmonth, R.P.; Kennedy, U.J. Fermentation performance of the yeast Saccharomyces cerevisiae in media with high sugar concentration. In Proceedings of the 2nd International Seminar on Chemistry, Bandung, Indonesia, 24–25 November 2011; Padjadjaran University: Jawa Barat, Indonesia, 2011; pp. 379–385. [Google Scholar]
- Yang, Y.; Sha, M. A Beginner’s Guide to Bioprocess Modes—Batch, Fed-Batch, and Continuous Fermentation; Eppendorf Application Note 408; Eppendorf: Hamburg, Germany, 2019. [Google Scholar]
- Wang, F.-S.; Li, C.-C.; Lin, Y.-S.; Lee, W.-C. Enhanced ethanol production by continuous fermentation in a two-tank system with cell cycling. Process Biochem. 2013, 48, 1425–1428. [Google Scholar] [CrossRef]
- Schuler, M.L.; Kargi, F. Bioprocess Engineering Basic Concepts, 2nd ed.; Pearson: London, UK, 2002. [Google Scholar]
- Vazquez, A. Overflow Metabolism: From Yeast to Marathon Runners, 1st ed.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Nicol, D.A. Rum. In Fermented Beverage Production, 2nd ed.; Lea, A.G.H., Piggot, J.R., Eds.; Springer: New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Zabed, H.; Faruq, G.; Sahu, J.N.; Azirun, M.S.; Hashim, R.; Boyce, A.N. Bioethanol production from fermentable sugar juice. Sci. World J. 2014, 2014, 957102. [Google Scholar] [CrossRef]
- Díaz-Montaño, D.M. Continuous Agave Juice Fermentation for Producing Bioethanol. In Biomass Now—Sustainable Growth and Use; Intechopen: London, UK, 2013; pp. 209–230. [Google Scholar] [CrossRef][Green Version]
- Krantz, M.; Nordlander, B.; Valadi, H.; Johansson, M.; Gustafsson, L.; Hohmann, S. Anaerobicity prepares Saccharomyces cerevisiae cells for faster adaptation to osmotic shock. Eukaryot. Cell 2004, 3, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Claassen, P.A.M.; van Lier, J.B.; Lopez Contreras, A.M.; van Niel, E.W.J.; Sijtsma, L.; Stams, A.J.M.; de Vries, S.S.; Weusthuis, R.A. Utilisation of biomass for the supply of energy carriers. Appl. Microbiol. Biotechnol. 1999, 52, 741–755. [Google Scholar] [CrossRef]
- Walker, G.M.; Basso, T.O. Mitigating stress in industrial yeasts. Fungal Biol. 2020, 124, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Cray, J.A.; Stevenson, A.; Ball, P.; Bankar, S.B.; Eleutherio, E.C.; Ezeji, T.C.; Singhal, R.S.; Thevelein, J.M.; Timson, D.J.; Hallsworth, J.E. Chaotropicity: A key factor in product tolerance of biofuel-producing microorganisms. Curr. Opin. Biotechnol. 2015, 33, 228–259. [Google Scholar] [CrossRef] [PubMed]
- Deparis, Q.; Claes, A.; Foulquié-Moreno, M.R.; Thevelein, K.M. Engineering tolerance to industrially relevant stress factors in yeast cell factories. FEMS Yeast Res. 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Erten, H.; Tanguler, H.; Cakiroz, H. The effect of pitching rate on fermentation and Flavour compounds in high gravity brewing. J. Inst. Brew. 2007, 113, 75–79. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Le, V.V.M. Using high pitching rate for improvement of yeast fermentation performance in high gravity brewing. Int. Food Res. J. 2009, 16, 547–554. [Google Scholar]
- Suihko, M.L.; Vilpola, A.; Linko, M. Pitching rate in high gravity brewing. J. Inst. Brew. 1993, 99, 341–346. [Google Scholar] [CrossRef]
- Casey, G.P.; Magnus, C.A.; Ingledew, W.M. High gravity brewing: Nutrient enhanced production of high concentrations of ethanol by brewing yeast. Biotechnol. Lett. 1983, 5, 429–434. [Google Scholar] [CrossRef]
- Casey, G.P.; Magnus, C.A.; Ingledew, W.M. High-gravity brewing: Effects of nutrition on yeast composition, fermentative ability, and alcohol production. Appl. Environ. Microbiol. 1984, 48, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Dragone, G.; Silva, D.P.; de Almeida e Silva, J.B.; de Almeida Lima, U. Improvement of the ethanol productivity in a high gravity brewing at pilot plant scale. Biotechnol. Lett. 2003, 25, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.L.; Doan, N.H.D.; Le, V.V.M. Using fed-batch fermentation in very high gravity brewing: Effects of Tween 80 and ergosterol supplementation on fermentation performance of immobilized yeast in calcium alginate gel. Int. Food Res. J. 2010, 17, 995–1002. [Google Scholar]
- Blieck, L.; Toye, G.; Dumortier, F.; Verstrepen, K.J.; Delvaux, F.R.; Thevelein, J.M.; Van Dijck, P. Isolation and characterization of brewer’s yeast variants with improved fermentation performance under high gravity conditions. Appl. Environ. Microbiol. 2007, 73, 815–824. [Google Scholar] [CrossRef]
- Norton, S.; D’Amore, T. Physiological effects of yeast cell immobilization: Applications for brewing. Enzym. Microb. Technol. 1994, 16, 365–375. [Google Scholar] [CrossRef]
- Norton, S.; Watson, K.; D’Amore, T. Ethanol tolerance of immobilized brewers’ yeast cells. Appl. Microbiol. Biotechnol. 1995, 43, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Patkova, J.; Smogrovicova, D.; Domeny, Z.; Bafrncova, P. Very high gravity wort fermentation by immobilised yeast. Biotechnol. Lett. 2000, 22, 1173–1177. [Google Scholar] [CrossRef]
- Smogrovicova, D.; Pátková, J.; Dömény, Z.; Navratil, M. Improvement in beer fermentation under very high gravity conditions by entrapped yeast. Minerva Biotecnol. 2000, 12, 331–336. [Google Scholar]
- Virkajärvi, I.; Vainikka, M.; Virtanen, H.; Home, S. Productivity of immobilized yeast reactors with very-high-gravity worts. J. Am. Soc. Brew. Chem. 2002, 60, 188–197. [Google Scholar] [CrossRef]
- Debourg, A. Yeast management and high gravity fermentation. Cerevisia 2010, 35, 16–22. [Google Scholar] [CrossRef]
- Ceccato-Antonini, S.R. Conventional and nonconventional strategies for controlling bacterial contamination in fuel ethanol fermentations. World J. Microbiol. Biotechnol. 2018, 34, 80. [Google Scholar] [CrossRef] [PubMed]
- Abee, T. Pore-forming bacteriocins of Gram-positive bacteria and self-protection mechanisms of producer organisms. FEMS Microbiol. Lett. 1995, 129, 1–9. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.P.; do Prado, C.D.; Eleutherio, E.C.A.; Bonatto, D.; Malavazi, I.; da Cunha, A.F. Improvement of Brazilian bioethanol production—Challenges and perspectives on the identification and genetic modification of new strains of Saccharomyces cerevisiae yeasts isolated during ethanol processes. Fungal Biol. 2018, 122, 582–591. [Google Scholar] [CrossRef]
- André, L.; Hemming, A.; Adler, L. Osmoregulation in Saccharomyces cerevisiae. Studies on the osmotic induction of glycerol production and glycerol-3-phosphate dehydrogenase (NAD+). FEBS Lett. 1991, 286, 13–17. [Google Scholar] [CrossRef]
- Larsson, K.; Ansell, R.; Eriksson, P.; Adler, L. A gene encoding sn-glycerol 3-phosphate dehydrogenase (NAD+) complements an osmosensitive mutant of Saccharomyces cerevisiae. Mol. Microbiol. 1993, 10, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Ansell, R.; Granath, K.; Hohmann, S.; Thevelein, J.M.; Adler, L. The two isoenzymes for yeast NAD+-depedent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation. EMBO J. 1997, 16, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Nissen, T.L.; Hamann, C.W.; Kielland-Brandt, M.C.; Nielsen, J.; Villadsen, J. Anaerobic and aerobic batch cultivations of Saccharomyces cerevisiae mutants impaired in glycerol synthesis. Yeast 2000, 16, 463–474. [Google Scholar] [CrossRef]
- Pagliardini, J.; Hubmann, G.; Alfenore, S.; Nevoigt, E.; Bideaux, C.; Guillouet, S.E. The metabolic costs of improving ethanol yield by reducing glycerol formation capacity under anaerobic conditions in Saccharomyces cerevisiae. Microb. Cell Fact. 2013, 12, 29. [Google Scholar] [CrossRef]
- Berthels, N.J.; Otero, R.R.C.; Bauer, F.F.; Pretorius, I.S.; Thevelein, J.M. Correlation between glucose/fructose discrepancy and hexokinase kinetic properties in different Saccharomyces cerevisiae wine yeast strains. Appl. Microbiol. Biotechnol. 2008, 77, 1083–1091. [Google Scholar] [CrossRef]
- Berthels, N.J.; Otero, R.R.C.; Bauer, F.F.; Thevelein, J.M.; Pretorius, I.S. Discrepancy in glucose and fructose utilization during fermentation by Saccharomyces cerevisiae wine yeast strains. FEMS Yeast Res. 2004, 4, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Tronchoni, J.; Gamero, A.; Arroyo-López, F.N.; Barrio, E.; Querol, A. Differences in the glucose and fructose consumption profiles in diverse Saccharomyces wine species and their hybrids during grape juice fermentation. Int. J. Food Microbiol. 2009, 134, 237–243. [Google Scholar] [CrossRef] [PubMed]
- De la Torre-González, F.J.; Narváez-Zapata, J.A.; López-y-López, V.E.; Larralde-Corona, C.P. Ethanol tolerance is decreased by fructose in Saccharomyces and non-Saccharomyces yeasts. LWT-Food Sci. Technol. 2016, 67, 1–7. [Google Scholar] [CrossRef]
- Bisson, L.F. Stuck and sluggish fermentations. Am. J. Enol. Vitic. 1999, 50, 107–119. [Google Scholar]
- Pina, C.; Goncalves, P.; Prista, C.; Loureiro-Dias, M.C. Ffz1, a new transporter specific for fructose from Zygosaccharomyces bailii. Microbiology 2004, 150, 2429–2433. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.J.; Miranda, L.; Côrte-Real, M.; Leão, C. Transport of acetic acid in Zygosaccharomyces bailii: Effects of ethanol and their implications on the resistance of the yeast to acidic environments. Appl. Environ. Microbiol. 1996, 62, 3152–3315. [Google Scholar] [CrossRef]
- Cavazza, A.; Poznanski, E.; Trioli, G. Restart of fermentation of simulated stuck wines by direct inoculation of active dry yeast. Am. J. Enol. Vitic. 2004, 55, 160–167. [Google Scholar]
- Varela, C.; Pizarro, F.; Agosin, E. Biomass content governs fermentation rate in nitrogen-deficient wine musts. Appl. Environ. Microbiol. 2004, 70, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Bisson, L.F.; Butzke, C.E. Diagnosis and rectification of stuck and sluggish fermentations. Am. J. Enol. Vitic. 2000, 51, 168–177. [Google Scholar]
- Thomas, K.C.; Ingledew, W.M. Production of 21% (v/v) ethanol by fermentation of very high gravity (VHG) wheat mashes. J. Ind. Microbiol. 1992, 10, 61–68. [Google Scholar] [CrossRef]
- Cot, M.; Loret, M.-O.; François, J.; Benbadis, L. Physiological behavior of Saccharomyces cerevisiae in aerated fed-batch fermentation for high level production of bioethanol. FEMS Yeast Res. 2006, 7, 22–32. [Google Scholar] [CrossRef][Green Version]
- Stanley, D.; Bandara, A.; Fraser, S.; Stanley, G.A. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010, 109, 13–24. [Google Scholar] [CrossRef]
- Stanley, G.A.; Hobley, T.J.; Pamment, N.B. Effect of acetaldehyde on Saccharomyces cerevisiae and Zymomonas mobilis subjected to environmental shocks. Biotechnol. Bioeng. 1997, 53, 71–78. [Google Scholar] [CrossRef]
- Birch, R.M.; Walker, G.M. Influence of magnesium ions on heat shock and ethanol stress responses of Saccharomyces cerevisiae. Enzyme. Microb. Technol. 2000, 26, 678–687. [Google Scholar] [CrossRef]
- Chandler, M.; Stanley, G.A.; Rogers, P.; Chambers, P. A genomic approach to defining the ethanol stress response in the yeast Saccharomyces cerevisiae. Ann. Microbiol. 2004, 54, 427–454. [Google Scholar]
- Hu, X.H.; Wang, M.H.; Tan, T.; Li, J.R.; Yang, H.; Leach, L.; Zhang, R.M.; Luo, Z.W. Genetic dissection ofethanol tolerance in the budding yeast Saccharomyces cerevisiae. Genetics 2007, 175, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Hallsworth, J.E.; Nomura, Y.; Iwahara, M. Ethanol-induced water stress and fungal growth. J. Ferment. Bioeng. 1998, 86, 451–456. [Google Scholar] [CrossRef]
- Plesset, J.; Palm, C.; McLaughlin, C.S. Induction of heat shock proteins and thermotolerance by ethanol in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 1982, 108, 1340–1345. [Google Scholar] [CrossRef]
- Lucero, P.; Peñalver, E.; Moreno, E.; Lagunas, R. Internal trehalose protects endocytosis from inhibition by ethanol in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2000, 66, 4456–4461. [Google Scholar] [CrossRef]
- Meaden, P.G.; Arneborg, N.; Guldfeldt, L.U.; Siegumfeldt, H.; Jakobsen, M. Endocytosis and vacuolar morphology in Saccharomyces cerevisiae are altered in response to ethanol stress or heat shock. Yeast 1999, 15, 1211–1222. [Google Scholar] [CrossRef]
- Alexandre, H.; Rousseaux, I.; Charpentier, C. Ethanol adaptation mechanisms in Saccharomyces cerevisiae. Biotechnol. Appl. Biochem. 1994, 20, 173–183. [Google Scholar] [PubMed]
- Sajbidor, J.; Ciesarová, Z.; Smogrovicová, D. Influence of ethanol on the lipid content and fatty acid composition of Saccharomyces cerevisiae. Folia Microbiol. 1995, 40, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.V.; Okorokov, L.A. Increase of the anion and proton permeability of Saccharomyces carlsbergensis plasmalemma by n-alcohols as a possible cause of its de-energization. Yeast 1990, 6, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Leao, C.; van Uden, N. Effects of ethanol and other alkanols on the general amino acid permease of Saccharomyces cerevisiae. Biotechnol. Bioeng. 1984, 26, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, C.P.; Juroszek, J.; Beavan, M.J.; Ruby, F.M.S.; de Morais, S.M.F.; Rose, A.H. Ethanol dissipatesthe proton-motive force across the plasma membrane of Saccharomyces cerevisiae. J. Gen. Microbiol. 1986, 137, 369–377. [Google Scholar]
- Mishra, P.; Prasad, R. Relationship between ethanol tolerance and fatty acyl composition of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1989, 30, 294–298. [Google Scholar] [CrossRef]
- Walker, G.M.; Maynard, I.A. Accumulation of magnesium ions during fermentative metabolism in Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 1997, 18, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M. Magnesium as a stress-protectant for industrial strains of Saccharomyces cerevisiae. J. Am. Soc. Brew. Chem. 1998, 56, 109–113. [Google Scholar] [CrossRef]
- Trofimova, Y.; Walker, G.M.; Rapoport, A. Anhydrobiosis in yeast: Influence of calcium and magnesium ions on yeast resistance to dehydration-rehydration. FEMS Microbiol. Lett. 2010, 308, 55–61. [Google Scholar] [CrossRef]
- Lam, F.H.; Ghaderi, A.; Fink, G.R.; Stephanopoulos, G. Engineering alcohol tolerance in yeast. Science 2014, 346, 71–75. [Google Scholar] [CrossRef]
- Walker, G.M.; Birch, R.M. Environmental stress responses in industrial yeasts. In Proceedings of the 5th Aviemore Conference on Malting, Brewing & Distilling; Campbell, I., Ed.; Institute of Brewing: London, UK, 1999; pp. 195–197. [Google Scholar]
- Walker, G.M.; Walker, R.S.K. Enhancing yeast alcoholic fermentations. Adv. Appl. Microbiol. 2018, 105, 87–129. [Google Scholar] [CrossRef] [PubMed]
- Caspeta, L.; Chen, Y.; Nielsen, J. Thermotolerant yeasts selected by adaptive evolution express heat stress response at 30 °C. Sci. Rep. 2016, 6, 27003. [Google Scholar] [CrossRef] [PubMed]
- van Voorst, F.; Houghton-Larsen, J.; Jønson, L.; Kielland-Brandt, M.C.; Brandt, A. Genome-wide identification of genes required for growth of Saccharomyces cerevisiae under ethanol stress. Yeast 2006, 23, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Bamforth, C.W. (Eds.) Brewing Microbiology: Current Research, Omics and Microbial Ecology; Caister Academic Press: Norfolk, UK, 2017; p. 332. [Google Scholar]
- Jamal, M. (Ed.) The CRISPR/Cas System: Emerging Technology and Application; Caister Academic Press: Norfolk, UK, 2017; p. 112. [Google Scholar]
- Mapelli, V. Yeast Metabolic Engineering: Methods and Protocols; Springer: New York, NY, USA, 2014; p. 313. [Google Scholar]
- Swinnen, S.; Schaerlaekens, K.; Pais, T.; Claesen, J.; Hubmann, G.; Yang, Y.; Demeke, M.; Foulquié-Moreno, M.R.; Goovaerts, A.; Souvereyns, K.; et al. Identification of novel causative genes determining the complex trait of high ethanol tolerance in yeast using pooled-segregant whole genome sequence analysis. Genome Res. 2012, 22, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Meijnen, J.P.; Randazzo, P.; Foulquié-Moreno, M.R.; Vandecruys, P.; Stojiljkovic, M.; Dumortier, F.; Zalar, P.; Boekhout, T.; Gunde-Cimerman, N.; Janez Kokošar, J.; et al. Polygenic analysis and targeted improvement of the complex trait of high acetic acid tolerance in the yeast Saccharomyces cerevisiae. Biotechnol. Biofuels 2016, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Si, T.; Chao, R.; Min, Y.; Wu, Y.; Ren, W.; Zhao, H. Automated multiplex genome-scale engineering in yeast. Nat. Commun. 2017, 8, 15187. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Foulquié-Moreno, M.R.; Clement, L.; Erdei, E.; Tanghe, A.; Schaerlaekens, K.; Dumortier, F.; Thevelein, J.M. QTL analysis of high thermotolerance with superior and downgraded parental yeast strains reveals new minor QTLs and converges on novel causative alleles involved in RNA processing. PLoS Genet. 2013, 9, e1003693. [Google Scholar] [CrossRef]
- Argyros, D.A.; Stonehouse, E.A. Yeast stress and fermentation. In The Alcohol Textbook, 6th ed.; Walker, G.M., Abbas, C., Ingledew, W.M., Pilgrim, C., Eds.; Ethanol Technology Institute: Duluth, MN, USA, 2017; pp. 287–297. [Google Scholar]
- González-Ramos, D.; de Vries, A.R.G.; Grijseels, S.S.; van Berkum, M.C.; Swinnen, S.; van den Broek, M.; Nevoigt, E.; Daran, J.-M.G.; Pronk, J.T.; van Maris, A.J.A. A new laboratory evolution approach to select for constitutive acetic acid tolerance in Saccharomyces cerevisiae and identification of causal mutations. Biotechnol. Biofuels 2016, 9, 173. [Google Scholar] [CrossRef]
- Ingledew, W.M. Very high gravity (VHG) and associated new technologies for fuel alcohol production. In The Alcohol Textbook, 6th ed.; Walker, G.M., Abbas, C., Ingledew, W.M., Pilgrim, C., Eds.; Ethanol Technology Institute: Duluth, MN, USA, 2017; pp. 363–376. [Google Scholar]
- De Nicola, R.; Walker, G.M. Accumulation and cellular distribution of zinc by brewing yeast. Enzyme. Microb. Technol. 2009, 44, 210–216. [Google Scholar] [CrossRef]
- Raj, S.B.; Ramaswamy, S.; Plapp, B.V. Yeast alcohol dehydrogenase structure and catalysis. Biochemistry 2014, 53, 5791–5803. [Google Scholar] [CrossRef]
- Walker, G.M. Metals in yeast fermentation processes. Adv. Appl. Microbiol. 2004, 54, 197–230. [Google Scholar] [CrossRef] [PubMed]
- Udeh, H.O.; Kgatla, T.E. Role of magnesium ions on yeast performance during very high gravity fermentation. J. Brew. Distill. 2013, 4, 19–45. [Google Scholar] [CrossRef]
- Pisat, N.P.; Pandey, A.; MacDiarmid, C.W. MNR2 Regulates Intracellular Magnesium Storage in Saccharomyces cerevisiae. Genetics 2009, 183, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Thanonkeo, P.; Laopaiboon, P.; Sootsuwan, K.; Yamada, M. Magnesium ions improve growth and ethanol production of Zymomonas mobilis under heat or ethanol stress. Biotechnology 2007, 6, 112–119. [Google Scholar] [CrossRef]
- Slininger, P.J.; Dien, B.S.; Gorsich, S.W.; Liu, Z.L. Nitrogen source and mineral optimization enhance D-xylose conversion to ethanol by the yeast Pichia stipitis NRRL Y-7124. Appl. Microbiol. Biotechnol. 2006, 72, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.S.; Coburn, J.W. Magnesium the mimic/antagonistic of calcium. NEJM 1984, 310, 1253–1254. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M. The role of magnesium in Biotechnology. Crit. Rev. Biotechnol. 1994, 14, 311–354. [Google Scholar] [CrossRef]
- Gibson, R.B. 125th Anniversary review: Improvement of higher gravity brewery fermentation via wort enrichment and supplementation. J. Inst. Brew. 2011, 117, 268–284. [Google Scholar] [CrossRef]
| Bioethanol Generation | Biomass Source | Ethanol Yield (L/t) |
|---|---|---|
| First | Sugar beet | 110 (L/t) [40] |
| First | Sugar cane | 70–75 (L/t) [40] |
| First | Cassava | 137–180 (L/t) [40] |
| First | Maize | 400 (L/t) [40] |
| First | Rice | 430 (L/t) [40] |
| First | Wheat | 340 (L/t) [40] |
| Second | Corn stover | 362–456 (L/t) [39,41] |
| Second | Wheat straw | 406 (L/t) [39,41] |
| Second | Sugarcane bagasse | 318–500 (L/t) [39,41] |
| Second | Switchgrass | 392–457 (L/t) [39] |
| Second | Sorghum | 268–380 (L/t) [39,41] |
| Second | Poplar | 419–456 (L/t) [39] |
| Second | Agave | 347 (L/t) [39] |
| Second | Agave Americana | 347 (L/t) [39] |
| Second | Agave tequilana | 401 (L/t) [39] |
| Second | Agave tequilana leaves | 401 (L/t) [39] |
| Second | Juice from Agave americana leaves | 34 (L/t) [39] |
| Second | Juice from Agave tequilana leaves | 30 (L/t) [39] |
| Second | Corn grain | 470 (L/t) [39] |
| Second | Rice straw | 416 (L/t) [39] |
| Second | Cotton gin trash | 215 (L/t) [39] |
| Second | Forest thinnings | 308 (L/t) [39] |
| Second | Hardwood sawdust | 381 (L/t) [39] |
| Second | Mixed paper | 439 (L/t) [39] |
| Third | Microalgae | 167–501 (L/t) [42] * |
| Third | Brown seaweeds (macroalgae) | 12–1128 (L/t) [43] ** |
| Third | Seagrass (macroalgae) | 747 (L/t) [43] ** |
| Third | Green seaweeds (macroalgae) | 72–608 (L/t) [43] ** |
| Third | Red seaweeds (macroalgae) | 12–595 (L/t) [43] ** |
| Submerged/Liquid-State Fermentation | Solid-State Fermentation | Very High Gravity Fermentation |
|---|---|---|
|
|
|
| Agricultural Residues | Industrial Residues | |
|---|---|---|
| Field Residues | Process Residues | |
| Straw | Husks | Potato peels |
| Stalks | Seeds | Orange peels |
| Leaves | Bagasse | Cassava peels |
| Batch | Fed-Batch | Continuous |
|---|---|---|
| Microorganisms are provided with a fixed volume of medium (nutrients and other ingredients). Culture environment is consistently changing as nutrients are consumed. | Media is inoculated with microorganisms which then grow under a batch regime for a certain amount of time, then nutrients are added incrementally throughout the fermentation. | Fresh media is continuously added to the fermenter, replacing the consumed nutrients. Ethanol, used media, and toxic metabolites are continuously removed. |
| Advantages: | Advantages: | Advantages: |
|
|
|
| Disadvantages: | Disadvantages: | Disadvantages: |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of Bioethanol—A Review of Factors Affecting Ethanol Yield. Fermentation 2021, 7, 268. https://doi.org/10.3390/fermentation7040268
Tse TJ, Wiens DJ, Reaney MJT. Production of Bioethanol—A Review of Factors Affecting Ethanol Yield. Fermentation. 2021; 7(4):268. https://doi.org/10.3390/fermentation7040268
Chicago/Turabian StyleTse, Timothy J., Daniel J. Wiens, and Martin J. T. Reaney. 2021. "Production of Bioethanol—A Review of Factors Affecting Ethanol Yield" Fermentation 7, no. 4: 268. https://doi.org/10.3390/fermentation7040268
APA StyleTse, T. J., Wiens, D. J., & Reaney, M. J. T. (2021). Production of Bioethanol—A Review of Factors Affecting Ethanol Yield. Fermentation, 7(4), 268. https://doi.org/10.3390/fermentation7040268






