Enhanced Energy Recovery from Food Waste by Co-Production of Bioethanol and Biomethane Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Enzymatic Hydrolysis
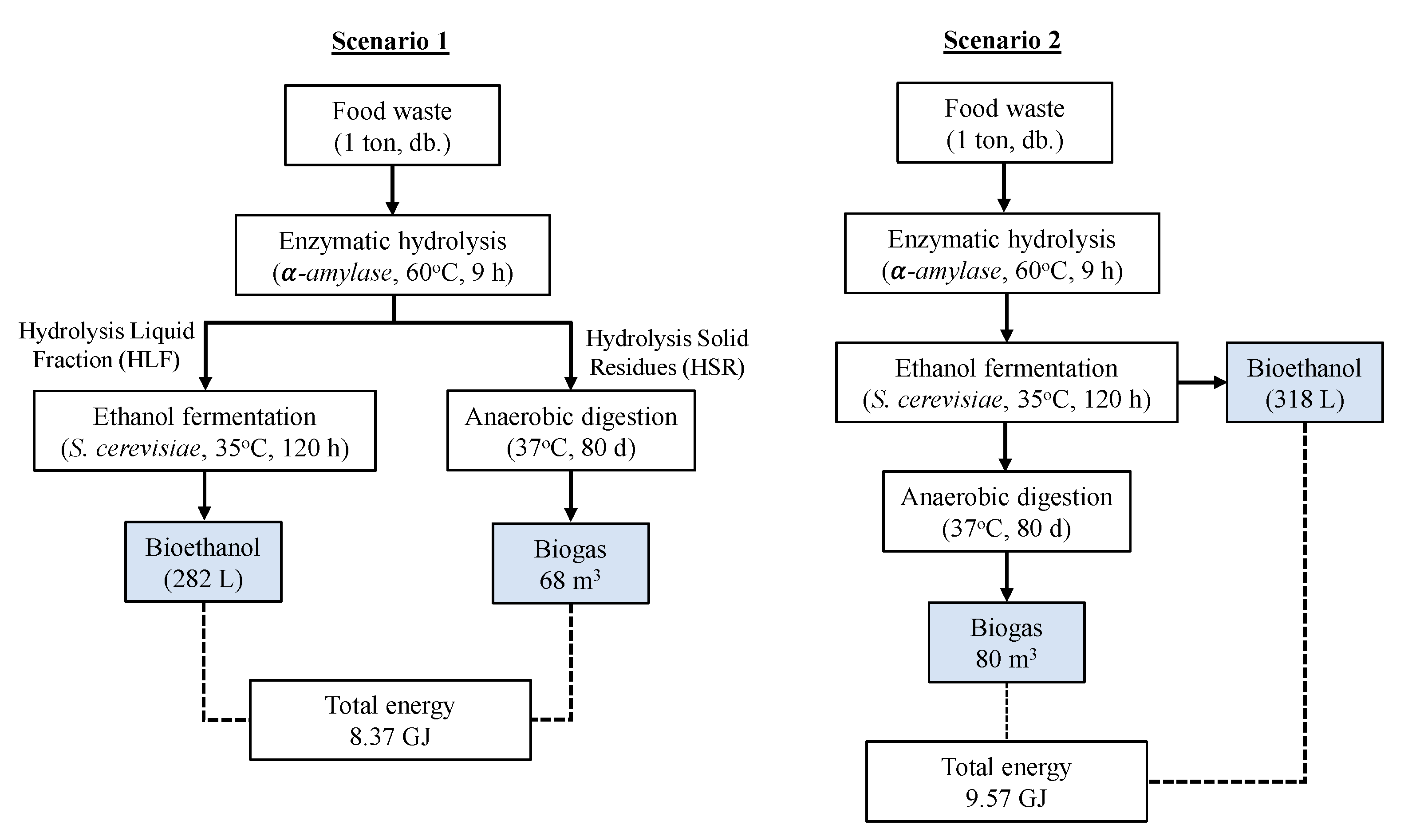
2.3. Bioethanol and Biomethane Production
- Scenario 1: In the ethanol fermentation, only the liquid fractions from the hydrolysis were used, and the solid residues were separated for use in the production of biomethane.
- Scenario 2: The entire hydrolysate was used in ethanol fermentation, followed by the extraction of fermented solid residues for additional anaerobic digestion.
3. Results and Discussion
3.1. Food Waste Characteristic
3.2. Enzymatic Hydrolysis
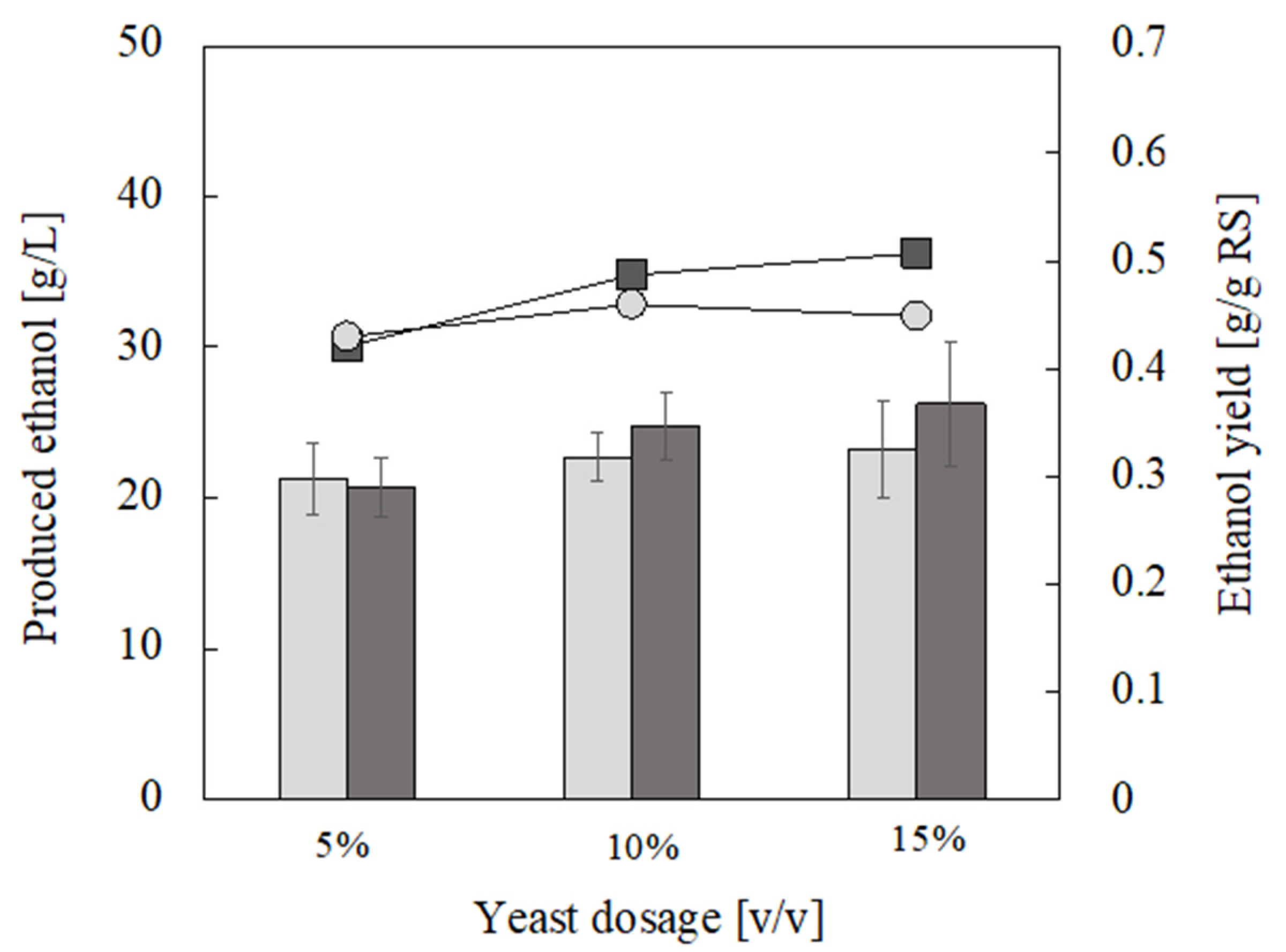
3.3. Ethanol Production from Food Waste Hydrolysate
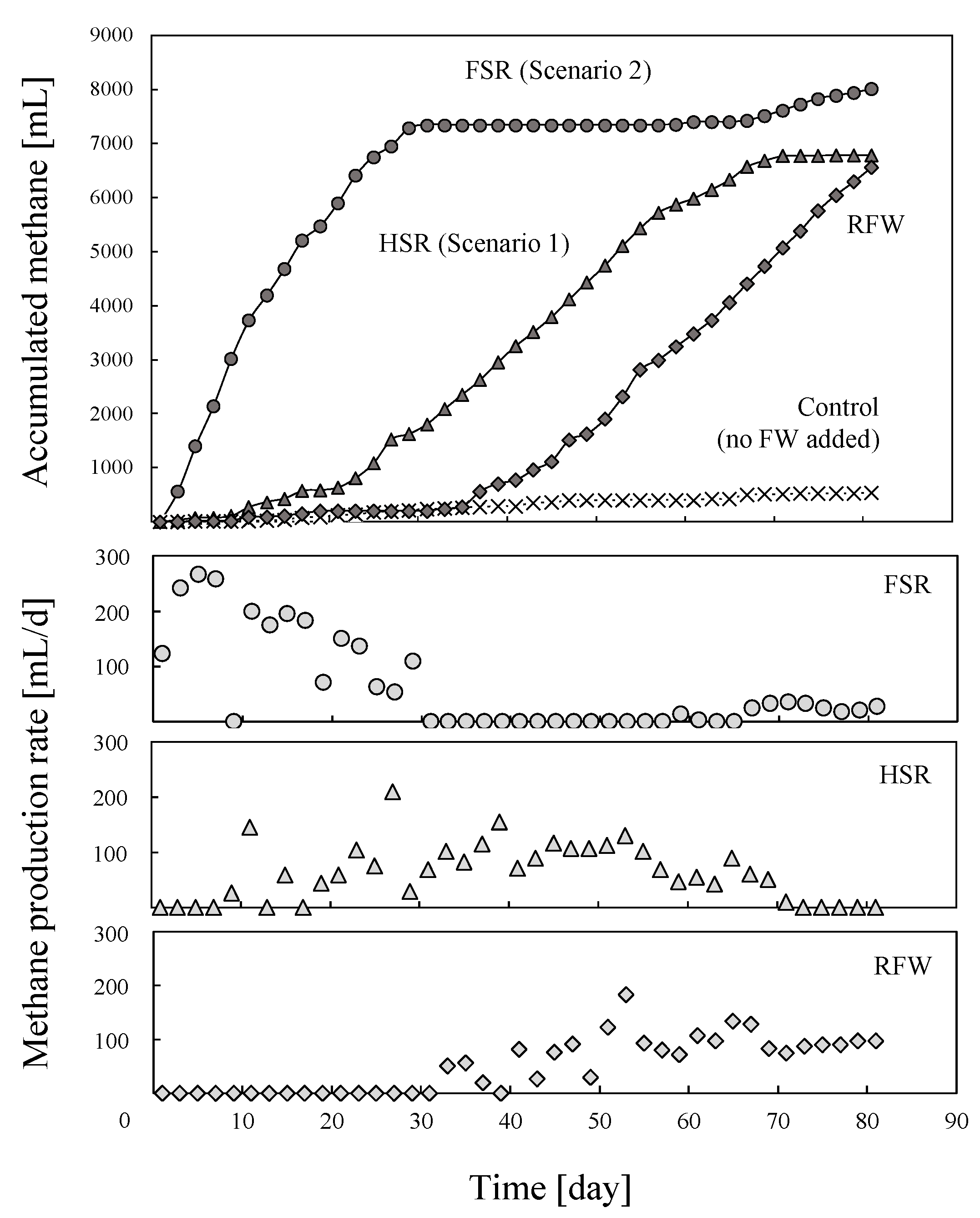
3.4. Biomethane Production
3.5. Gross Energy Output
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IEA. Global Energy Review 2021. 2021. Available online: https://www.iea.org/reports/global-energy-review-2021 (accessed on 1 September 2021).
- Silva, F.M.; Oliveira, L.B.; Mahler, C.F.; Bassin, J. Hydrogen production through anaerobic co-digestion of food waste and crude glycerol at mesophilic conditions. Int. J. Hydrogen Energy 2017, 42, 22720–22729. [Google Scholar] [CrossRef]
- Jarunglumlert, T.; Prommuak, C.; Putmai, N.; Pavasant, P. Scaling-up bio-hydrogen production from food waste: Feasibilities and challenges. Int. J. Hydrogen Energy 2018, 43, 634–648. [Google Scholar] [CrossRef]
- Westerholm, M.; Liu, T.; Schnürer, A. Comparative study of industrial-scale high-solid biogas production from food waste: Process operation and microbiology. Bioresour. Technol. 2020, 304, 122981. [Google Scholar] [CrossRef]
- Shamurad, B.; Sallis, P.; Petropoulos, E.; Tabraiz, S.; Ospina, C.; Leary, P.; Dolfing, J.; Gray, N. Stable biogas production from single-stage anaerobic digestion of food waste. Appl. Energy 2020, 263, 114609. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Dimou, A.; Fakas, S.; Diamantopoulou, P.; Philippoussis, A.; Galiotou-Panayotou, M.; Aggelis, G. Biotechnological conversion of waste cooking olive oil into lipid-rich biomass using Aspergillus and Penicillium strains. J. Appl. Microbiol. 2011, 110, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Omar, W.N.N.W.; Amin, N.A.S. Optimization of heterogeneous biodiesel production from waste cooking palm oil via response surface methodology. Biomass Bioenergy 2011, 35, 1329–1338. [Google Scholar] [CrossRef]
- Sunthitikawinsakul, A.; Sangatith, N. Study on the quantitative fatty acids correlation of fried vegetable oil for biodiesel with heating value. Procedia Eng. 2012, 32, 219–224. [Google Scholar] [CrossRef][Green Version]
- Huang, H.; Singh, V.P.; Qureshi, N. Butanol production from food waste: A novel process for producing sustainable energy and reducing environmental pollution. Biotechnol. Biofuels 2015, 8, 1–12. [Google Scholar] [CrossRef]
- Yeshanew, M.M.; Frunzo, L.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Production of biohythane from food waste via an integrated system of continuously stirred tank and anaerobic fixed bed reactors. Bioresour. Technol. 2016, 220, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, O.; Mohan, S.V. Pre-aeration of food waste to augment acidogenic process at higher organic load: Valorizing biohydrogen, volatile fatty acids and biohythane. Bioresour. Technol. 2017, 242, 68–76. [Google Scholar] [CrossRef]
- UNEP. Food Waste Index Report 2021. 2021. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 10 September 2021).
- Mahmoodi, P.; Karimi, K.; Taherzadeh, M.J. Hydrothermal processing as pretreatment for efficient production of ethanol and biogas from municipal solid waste. Bioresour. Technol. 2018, 261, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Christakopoulos, P. Ethanol production from enzymatically treated dried food waste using enzymes produced on-site. Sustainability 2015, 7, 1446–1458. [Google Scholar] [CrossRef]
- Ntaikou, I.; Antonopoulou, G.; Lyberatos, G. Sustainable second-generation bioethanol production from enzymatically hydrolyzed domestic food waste using pichia anomala as biocatalyst. Sustainabiltiy 2020, 13, 259. [Google Scholar] [CrossRef]
- Mihajlovski, K.; Radovanović, Ž.; Carević, M.; Dimitrijević-Branković, S. Valorization of damaged rice grains: Optimization of bioethanol production by waste brewer’s yeast using an amylolytic potential from the Paenibacillus chitinolyticus CKS1. Fuel 2018, 224, 591–599. [Google Scholar] [CrossRef]
- Bibra, M.; Rathinam, N.K.; Johnson, G.R.; Sani, R.K. Single pot biovalorization of food waste to ethanol by Geobacillus and Thermoanaerobacter spp. Renew. Energy 2020, 155, 1032–1041. [Google Scholar] [CrossRef]
- Dhiman, S.S.; David, A.; Shrestha, N.; Johnson, G.R.; Benjamin, K.M.; Gadhamshetty, V.; Sani, R.K. Simultaneous hydrolysis and fermentation of unprocessed food waste into ethanol using thermophilic anaerobic bacteria. Bioresour. Technol. 2017, 244, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Meneses, L.; Raud, M.; Orupõld, K.; Kikas, T. Second-generation bioethanol production: A review of strategies for waste valorisation. Agron. Res. 2017, 15, 830–847. [Google Scholar]
- Jarunglumlert, T.; Prommuak, C. Net energy analysis and techno-economic assessment of co-production of bioethanol and biogas from cellulosic biomass. Fermentation 2021, 7, 229. [Google Scholar] [CrossRef]
- Li, W.; Ghosh, A.; Bbosa, D.; Brown, R.C.; Wright, M.M. Comparative techno-economic, uncertainty and life cycle analysis of lignocellulosic biomass solvent liquefaction and sugar fermentation to ethanol. ACS Sustain. Chem. Eng. 2018, 6, 16515–16524. [Google Scholar] [CrossRef]
- Gubicza, K.; Nieves, I.U.; Sagues, W.; Barta, Z.; Shanmugam, K.; Ingram, L.O. Techno-economic analysis of ethanol production from sugarcane bagasse using a liquefaction plus simultaneous saccharification and co-fermentation process. Bioresour. Technol. 2016, 208, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Joelsson, E.; Galbe, M.; Wallberg, O.; Dienes, D.; Kovacs, K. Combined production of biogas and ethanol at high solids loading from wheat straw impregnated with acetic acid: Experimental study and techno-economic evaluation. Sustain. Chem. Process. 2016, 4, 22. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Xu, J.; Ou, X.; Chang, S.; Wu, M. Techno-economic analysis of bioethanol production from lignocellulosic biomass in China: Dilute-acid pretreatment and enzymatic hydrolysis of corn stover. Energies 2015, 8, 4096–4117. [Google Scholar] [CrossRef]
- Shafiei, M.; Karimi, K.; Taherzadeh, M. Techno-economical study of ethanol and biogas from spruce wood by NMMO-pretreatment and rapid fermentation and digestion. Bioresour. Technol. 2011, 102, 7879–7886. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Aden, A.; Elander, R.T.; Pallapolu, V.R.; Lee, Y.; Garlock, R.J.; Balan, V.; Dale, B.E.; Kim, Y.; Mosier, N.S.; et al. Process and technoeconomic analysis of leading pretreatment technologies for lignocellulosic ethanol production using switchgrass. Bioresour. Technol. 2011, 102, 11105–11114. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of Structural Carbohydrates and Lignin in Biomass; NREL: Golden, CO, USA, 2008. [Google Scholar]
- Chiang, L.-C.; Gong, C.-S.; Chen, L.-F.; Tsao, G.T. D-xylulose fermentation to ethanol by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1981, 42, 284–289. [Google Scholar] [CrossRef]
- Cutaia, A.J. Malt beverages and brewing materials: Gas chromatographic determination of ethanol in beer. J. Assoc. Off. Anal. Chem. 1984, 67, 192–193. [Google Scholar] [CrossRef]
- Hong, Y.S.; Yoon, H.H. Ethanol production from food residues. Biomass Bioenergy 2011, 35, 3271–3275. [Google Scholar] [CrossRef]
- Kim, Y.S.; Jang, J.Y.; Park, S.J.; Um, B.H. Dilute sulfuric acid fractionation of Korean food waste for ethanol and lactic acid production by yeast. Waste Manag. 2018, 74, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Han, G.H.; Oh, B.R.; Chun, Y.N.; Eom, C.-Y.; Kim, S.W. Volumetric scale-up of a three stage fermentation system for food waste treatment. Bioresour. Technol. 2008, 99, 4394–4399. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.A.; Wang, Q.; Jin, Y.; Yue, S.; Ma, H. Assessment of bioethanol fermentation performance using different recycled waters of an integrated system based on food waste. BioResources 2019, 14, 3717–3730. [Google Scholar]
- Yu, M.; Wu, C.; Wang, Q.; Sun, X.; Ren, Y.; Li, Y.-Y. Ethanol prefermentation of food waste in sequencing batch methane fermentation for improved buffering capacity and microbial community analysis. Bioresour. Technol. 2018, 248, 187–193. [Google Scholar] [CrossRef]
- Nagao, N.; Tajima, N.; Kawai, M.; Niwa, C.; Kurosawa, N.; Matsuyama, T.; Yusoff, F.M.; Toda, T. Maximum organic loading rate for the single-stage wet anaerobic digestion of food waste. Bioresour. Technol. 2012, 118, 210–218. [Google Scholar] [CrossRef]
- Taheri, M.E.; Salimi, E.; Saragas, K.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Effect of pretreatment techniques on enzymatic hydrolysis of food waste. Biomass Convers. Biorefinery 2021, 11, 219–226. [Google Scholar] [CrossRef]
- Cekmecelioglu, D.; Uncu, O.N. Kinetic modeling of enzymatic hydrolysis of pretreated kitchen wastes for enhancing bioethanol production. Waste Manag. 2013, 33, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Theodoropoulos, C.; Yousuf, A. Techno-economic evaluation of heat integrated second generation bioethanol and furfural coproduction. Biochem. Eng. J. 2019, 144, 89–103. [Google Scholar] [CrossRef]
- Saeed, M.A.; Ma, H.; Yue, S.; Wang, Q.; Tu, M. Concise review on ethanol production from food waste: Development and sustainability. Environ. Sci. Pollut. Res. 2018, 25, 28851–28863. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.C.; Pak, D. Feasibility of producing ethanol from food waste. Waste Manag. 2011, 31, 2121–2125. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Liu, Y.; Xu, X.; Huang, J.; He, H.; Chen, L.; Qiu, S.; Tang, J.; Hou, P. Bioethanol production from waste hamburger by enzymatic hydrolysis and fermentation. J. Clean. Prod. 2020, 264, 121658. [Google Scholar] [CrossRef]
- Han, W.; Xu, X.; Gao, Y.; He, H.; Chen, L.; Tian, X.; Hou, P. Utilization of waste cake for fermentative ethanol production. Sci. Total Environ. 2019, 673, 378–383. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Q.; Xiang, J.; Yu, M.; Chang, Q.; Gao, M.; Sonomoto, K. Enhanced productions and recoveries of ethanol and methane from food waste by a three-stage process. Energy Fuels 2015, 29, 6494–6500. [Google Scholar] [CrossRef]
- Uncu, O.N.; Cekmecelioglu, D. Cost-effective approach to ethanol production and optimization by response surface methodology. Waste Manag. 2011, 31, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Kiran, E.U.; Liu, Y. Bioethanol production from mixed food waste by an effective enzymatic pretreatment. Fuel 2015, 159, 463–469. [Google Scholar] [CrossRef]
- Moreno, A.D.; Magdalena, J.A.; Oliva, J.M.; Greses, S.; Lozano, C.C.; Latorre-Sánchez, M.; Negro, M.J.; Susmozas, A.; Iglesias, R.; Llamas, M.; et al. Sequential bioethanol and methane production from municipal solid waste: An integrated biorefinery strategy towards cost-effectiveness. Process. Saf. Environ. Prot. 2021, 146, 424–431. [Google Scholar] [CrossRef]
- Zhao, N.; Yu, M.; Wang, Q.; Song, N.; Che, S.; Wu, C.; Sun, X. Effect of ethanol and lactic acid pre-fermentation on putrefactive bacteria suppression, hydrolysis, and methanogenesis of food waste. Energy Fuels 2016, 30, 2982–2989. [Google Scholar] [CrossRef]
- Refai, S.; Wassmann, K.; Deppenmeier, U. Short-term effect of acetate and ethanol on methane formation in biogas sludge. Appl. Microbiol. Biotechnol. 2014, 98, 7271–7280. [Google Scholar] [CrossRef] [PubMed]
- Prasertsan, P.; Leamdum, C.; Chantong, S.; Mamimin, C.; Kongjan, P.; O-Thong, S. Enhanced biogas production by co-digestion of crude glycerol and ethanol with palm oil mill effluent and microbial community analysis. Biomass Bioenergy 2021, 148, 106037. [Google Scholar] [CrossRef]
- Karimi, S.; Karimi, K. Efficient ethanol production from kitchen and garden wastes and biogas from the residues. J. Clean. Prod. 2018, 187, 37–45. [Google Scholar] [CrossRef]
- Papa, G.; Rodriguez, S.; George, A.; Schievano, A.; Orzi, V.; Sale, K.; Singh, S.; Adani, F.; Simmons, B. Comparison of different pretreatments for the production of bioethanol and biomethane from corn stover and switchgrass. Bioresour. Technol. 2015, 183, 101–110. [Google Scholar] [CrossRef]
- Bondesson, P.-M.; Galbe, M.; Zacchi, G. Ethanol and biogas production after steam pretreatment of corn stover with or without the addition of sulphuric acid. Biotechnol. Biofuels 2013, 6, 11. [Google Scholar] [CrossRef]
- Moshi, A.P.; Crespo, C.F.; Badshah, M.; Hosea, K.M.; Mshandete, A.M.; Elisante, E.; Mattiasson, B. Characterisation and evaluation of a novel feedstock, Manihot glaziovii, Muell. Arg, for production of bioenergy carriers: Bioethanol and biogas. Bioresour. Technol. 2014, 172, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Kang, X.; Wang, W.; Yang, G.; He, L.; Fan, Y.; Cheng, X.; Sun, Y.; Li, L. Assessment of coproduction of ethanol and methane from pennisetum purpureum: Effects of pretreatment, process performance, and mass balance. ACS Sustain. Chem. Eng. 2021, 9, 10771–10784. [Google Scholar] [CrossRef]
- Sondhi, S.; Kaur, P.S.; Kaur, M. Techno-economic analysis of bioethanol production from microwave pretreated kitchen waste. SN Appl. Sci. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Muhammad, N.I.S.; Rosentrater, K.A. Economic assessment of bioethanol recovery using membrane distillation for food waste fermentation. Bioengineering 2020, 7, 15. [Google Scholar] [CrossRef] [PubMed]


| Component of FW | Fraction |
|---|---|
| Moisture content (%) | 89.01 ± 0.61 |
| Total solids 1, TS (%) | 10.99 ± 0.61 |
| Volatile solids 1, VS (%) | 10.59 ± 0.58 |
| Ash 1 (%) | 0.40 ± 0.03 |
| Total carbon 2 (%) | 51.03 ± 0.75 |
| Total nitrogen 2 (%) | 2.11 ± 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarunglumlert, T.; Bampenrat, A.; Sukkathanyawat, H.; Prommuak, C. Enhanced Energy Recovery from Food Waste by Co-Production of Bioethanol and Biomethane Process. Fermentation 2021, 7, 265. https://doi.org/10.3390/fermentation7040265
Jarunglumlert T, Bampenrat A, Sukkathanyawat H, Prommuak C. Enhanced Energy Recovery from Food Waste by Co-Production of Bioethanol and Biomethane Process. Fermentation. 2021; 7(4):265. https://doi.org/10.3390/fermentation7040265
Chicago/Turabian StyleJarunglumlert, Teeraya, Akarasingh Bampenrat, Hussanai Sukkathanyawat, and Chattip Prommuak. 2021. "Enhanced Energy Recovery from Food Waste by Co-Production of Bioethanol and Biomethane Process" Fermentation 7, no. 4: 265. https://doi.org/10.3390/fermentation7040265
APA StyleJarunglumlert, T., Bampenrat, A., Sukkathanyawat, H., & Prommuak, C. (2021). Enhanced Energy Recovery from Food Waste by Co-Production of Bioethanol and Biomethane Process. Fermentation, 7(4), 265. https://doi.org/10.3390/fermentation7040265





