Abstract
An increasing interest in novel wine productions is focused on non-Saccharomyces yeasts due to their potential in improving sensory profiles. Although Kluyveromyces marxianus has been originally isolated from grapes and its enzymatic activities are used in oenology, rarely it has been used as co-starter. The K. marxianus Km L2009 strain has been characterized here and selected as a co-starter both at laboratory- and winery-scale fermentation. The Km L2009 strain showed growth of up to 40 (mg/L) of sulfites and 6% (v/v) of ethanol. Gas chromatographic analysis demonstrates that wines produced by mixed fermentation contain remarkably higher quantities of free monoterpenes and aliphatic esters than wines produced only by commercial strains of Saccharomyces cerevisiae. Differences in the volatile organic compound composition produced sensorially distinct wines. In light of these results, it is possible to state that even within the K. marxianus species it is possible to select strains capable of improving the aromatic quality of wines.
1. Introduction
Over the past three decades, non-Saccharomyces yeasts partially replaced their role from spoilage agents to quality improvers of wines. Several laboratories reported on the role of non-Saccharomyces yeasts to reduce the alcohol content, increase the concentration of glycerol, modulate the acid content, produce pectinolytic and proteolytic activities, influence the concentration of polysaccharides, and increase the aromatic content of wines [1,2,3,4,5]. Nowadays non-Saccharomyces yeasts represent a biodiversity pool to operate accurate selections of strains with good oenological characteristics [6,7,8]. Due to low alcohol tolerance of non-Saccharomyces yeasts, the subsequent growth of Saccharomyces is mandatory to carry out alcoholic fermentation [4,9,10,11]. Actually, the application of non-Saccharomyces yeasts in winemaking is increasing. This phenomenon is demonstrated by the commercial availability of several strains belonging to Lachancea thermotolerans, Metschnikowia pulcherrima, Torulaspora delbrueckii, Pichia kluyveri, and Schizosaccharomyces pombe [2].
Originally isolated on grapes by Marx and described for the first time by Hansen in 1888 [12], Kluyveromyces marxianus was later found in other fruits, decaying plant tissues, insects, and in naturally fermented milk-based products [13]. Since the 70s, several studies have been conducted on enzymes produced by K. marxianus, such as inulinase, β-galactosidase, β-glucosidase, endopolygalacturonidases, protein phosphatases, carboxypeptidases, and aminopeptidases [14]. Nowadays, among non-Saccharomyces species, K. marxianus is widely investigated for numerous biotechnological applications [15].
Specific studies have shown the ability of K. marxianus to contribute to the sensory profile of foods and drinks producing higher alcohols, particularly 2-phenylethanol, associated with a rose odor, and esters, such as 2-phenylethyl acetate, isoamyl acetate and ethyl acetate, responsible of floral and/or fruity sensory attributes [16,17]. Due to low pathogenicity, sensitivity to antimycotics [18] and qualified presumption of safety [19], the use of K. marxianus in the agro-food industry shows guarantees for the safety of operators and consumers.
In spite of benefits of oenological use of several K. marxianus enzymes [20,21,22], the application of K. marxianus species as a wine co-starter is still rare. To our knowledge, one thermotolerant strain of K. marxianus was used as immobilized cells in in-vitro semisweet winemaking [23]. Vigentini et al. [24] found the highest amount of certain fermentation flavors in 20 L trials of a Georgian white wine produced with a strain of K. marxianus. Finally, Rollero et al. [25] used a K. marxianus strain in 10 L trials of red wines produced with pre-fermentative cold maceration and significant amounts of pectinase, phenylethanol and phenylethyl acetate were found, as well as methanol.
Previously we reported on the isolation of K. marxianus Km L2009 strain in the island of Linosa (Italy) and on its ability to produce β-glucosidase [26]; this enzyme increases the release of monoterpenols from their flavorless, non-volatile glycosidic precursors, thus helping to increase wine aromatic content [27].
In the present research, further investigation into the Km L2009 strain has been reported with an aim to: (i) characterize the Km L2009 strain for its oenological traits; (ii) select the strain as potential co-starter for fermenting white grape must; and (iii) evaluate the effect of the Km L2009 strain inoculum in large-scale fermentation and the sensory quality of bottled products.
2. Materials and Methods
2.1. Yeast Strains
Kluyveromyces marxianus Km L2009 was isolated from grapes during 2009 vintage in the Linosa Island [26]. S. cerevisiae Fermol Arome Plus, Davis 522 and Super 16 are marketed by AEB (Brescia, Italy); S. cerevisiae SIHA 7 by Eaton (Nettersheim, Germany); S. cerevisiae EZFERM 44 by Esseco (San Martino di Trecate, Italy); S. cerevisiae CK S102 and UCLM S325 by Fermentis (Marcq-en-Barœul, France); S. cerevisiae Actiflore® F33 and Zymaflore® ST, VL1, VL3, X5, X16 by Laffort Oenologie (Bordeaux, France); S. cerevisiae Cross EvolutionTM, Lalvin BA11, EC-1118, ICV K1, QA23 and Uvaferm DV10 by Lallemand (Montreal, QC, Canada); S. cerevisiae PDM by Maurivin (Toowoomba, Australia); S. cerevisiae FR-WP by Ferrari (Verona, Italy).
2.2. Technological Screening of Strain Km L2009
2.2.1. Killer Activity Assay
Killer activity was measured, according to Regodón et al. [28] in 4.7 MB medium (5 g/L yeast extract, 10 g/L peptone, 20 g/L glucose, 20 g/L agar, Oxoid, Basingstoke, Hampshire, England, 0.03 g/L methylene blue, 0.1 M sodium citrate, Sigma-Aldrich, St. Louis, MO, USA), by overlaying 10 μL stationary phase cells of K. marxianus Km L2009 on lawns of the commercial S. cerevisiae strains: Fermol Arome Plus, Davis 522, Super 16 (AEB, Brescia, Italy), SIHA 7 (Eaton, Nettersheim, Germany), EZFERM 44 (Esseco, San Martino di Trecate, Italy), CK S102, UCLM S325 (Fermentis, Marcq-en-Barœul, France), Actiflore® F33, Zymaflore® ST, VL1, VL3, X5, X16 (Laffort Oenologie, Bordeaux, France), Cross EvolutionTM, Lalvin BA11, EC-1118, ICV K1, QA23, Uvaferm DV10 (Lallemand, Montreal, QC, Canada), PDM (Maurivin, Toowoomba, Australia); FR-WP (Ferrari, Verona, Italy). Km L2009 produced a clear halo of killing in sensitive lawns.
2.2.2. Sulfite Tolerance Assay
The assay was carried out according to Caridi et al. [29] by inoculating 5 mL of 48 h preculture of the strain K. marxianus Km L2009 in flasks with 100 mL of sterile white must (20°Bx, pH 3.0) with different amounts of SO2 (0, 20, 40, and 70 mg/L, added as potassium metabisulfite, Esseco, Trecate, Italy) and topped with 10 mL of liquid paraffin (Sigma-Aldrich, St. Louis, MO, USA). The release of CO2 was measured as weight loss, daily. Uninoculated must and must containing 100 mg/L of SO2 inoculated with the strain S. cerevisiae Zymaflore®X5 (Laffort Oenologie, Bordeaux, France) were used as negative and positive controls, respectively. Two independent assays, with each thesis in duplicate and measurements in triplicate, were performed.
2.2.3. Alcohol Tolerance Assay
The assay was carried as a sulfite tolerance assay, by inoculating the strain K. marxianus Km L2009 in sterile white must (20°Bx, pH 3.0) with increasing amounts of ethyl alcohol (0%, 2%, 4%, and 8% v/v, Sigma-Aldrich, St. Louis, MO, USA) and measuring, as weight loss, the amount of released CO2. Uninoculated must was used as a negative control; must with 8% (v/v) of ethyl alcohol and inoculated with the strain S. cerevisiae Zymaflore®X5 (Laffort Oenologie, Bordeaux, France) was used as a positive control. Two independent assays, with each thesis in duplicate and measurements in triplicate, were performed.
2.3. Laboratory-Scale Fermentations
Forty eight-hour cultured strains of S. cerevisiae Zymaflore®X5 (Laffort Oenologie, Bordeaux, France) and K. marxianus Km L2009 in filter sterilized white must (12°Bx, pH 3.4) were centrifuged, washed in sterile 0.1% peptone (Oxoid, Basingstoke, Hampshire, UK), resuspended in a small volume of sterile 0.1% peptone (Oxoid, Basingstoke, Hampshire, UK), counted in Bürker chamber (Assistent®, Glaswarenfabrik Karl Hecht GmbH & Co, Sondheim vor der Rhön, Germany), inoculated in 450 mL of filter sterilized Muscat of Alexandria must (sugars 202.80 ± 0.01 g/L, pH 3.68 ± 0.01, total acidity 3.04 ± 0.02 g/L) and left to ferment at 18 °C for 21 days. The inoculum concentrations were 7.8 ± 3.1 × 105 cells/mL for strain Zymaflore®X5 (Laffort Oenologie, Bordeaux, France), and 8.1 ± 2.2 × 105 cells/mL for strain Km L2009. In sequential inoculum, must was inoculated with Km L2009 (1.2 ± 0.8 × 106 cells/mL) and, after 7 days of fermentation at 18 °C, with 5.3 ± 1.4 × 106 cells/mL of the strain Zymaflore®X5 (Laffort Oenologie, Bordeaux, France) and left to ferment for a further 14 days. Two independent assays, with each thesis in duplicate, were performed. Glucose concentration was determined by Keto-Diabur test®5000 (Roche Diagnostic, Mannheim, Germany), other analyses were carried out as described in Section 2.4.
2.4. Wine Production and Monitoring
2.4.1. Vinification
Wines were produced at the winery “G. Dalmasso” in Marsala, Italy. Approximately 1700 L of Muscat of Alexandria must and 900 L of Grillo must were supplemented with 20 mg/L of SO2 and with 20 mg/L of pectolytic enzymes Zym 1000S (Esseco, Trecate, Italy). Cold static clarification was carried out at 5 °C for 48 h. Six aliquots of must were used for each vinification (Muscat of Alexandria: 220 L each, 18.35°Bx, pH 3.33 ± 0.00, total acidity 4.06 ± 0.01 g/L; Grillo: 100 L each, 21.00°Bx, pH 3.21 ± 0.01, total acidity 6.43 ± 0.03 g/L). These were enriched with thiamine (0.6 mg/L, Esseco, Trecate, Italy), and with enough diammonium phosphate (Esseco, Trecate, Italy) to reach a Yeast Available Nitrogen concentration of 200 mg/L (measured according to Gump et al. [30]). For each vinification, two aliquots were left for spontaneous fermentation; two aliquots were inoculated according to the manufacturer’s instructions with active dry yeast (S. cerevisiae Lalvin QA23, Lallemand, Montreal, QC, Canada) at 1.8 ± 0.6 × 106 cfu/mL (Muscat of Alexandria) and 7.5 ± 0.6 × 106 cfu/mL (Grillo); two aliquots were inoculated with the strain K. marxianus Km L2009 (fresh yeast produced by Grape Ltd., Alba, Italy) at 2.1 ± 0.5 × 106 cfu/mL (Muscat of Alexandria) and 3.0 ± 0.2 × 106 cfu/mL (Grillo), letting Saccharomyces yeasts grow spontaneously. Musts fermented at 16 ± 1 °C. Muscat of Alexandria fermentations inoculated with S. cerevisiae took 13 days; all the others took 16 days. At the end of alcoholic fermentation, wines were racked and supplemented with 30 mg/L of SO2. After protein and tartaric stabilizations [31], wines were bottled six months after the end of the alcoholic fermentation.
2.4.2. Microbiological Analyses
Samples of fermenting must were serially diluted into sterile 0.1% peptone and plated on WL Nutrient Agar, Lysine Agar and WL Differential Agar (Oxoid, Basingstoke, Hampshire, UK). WL Nutrient Agar allows the growth of all yeasts but with different colony morphology as a function of the genus or species [26,32,33,34,35]. Lysine Agar allows growth of all yeast except Saccharomyces [36]. WL Differential Agar is WL Nutrient Agar with 10 mg/L cycloheximide (Sigma-Aldrich, St. Louis, MO, USA): at this concentration S. cerevisiae yeasts do not grow, according to Di Maio et al. [37], but K. marxianus Km L2009 still grows well, showing its typical colony morphology [26]: the concentration of all other non-Saccharomyces yeasts was calculated as the difference between the concentration of all non-Saccharomyces yeasts (detected on Lysine Agar) and the concentration of K. marxianus (detected on WL Nutrient Agar and/or on WL Differential Agar). To check the absence of microbial species able to alter wine characteristics, further microbiological analyses were performed on WL Nutrient Agar, Lysine Agar, MRS Agar and Tomato Juice Agar (Oxoid, Basingstoke, Hampshire, UK) before and after bottling (data not shown). All analyses were performed in triplicate.
2.4.3. Molecular Analyses
To verify that Km L2009 was the only K. marxianus strain present, we diluted and plated on WL Nutrient Agar (Oxoid, Basingstoke, Hampshire, UK) must samples on the fifth day of the vinifications inoculated with this strain, when the highest concentration of this species was recorded. Fifty colonies with the K. marxianus morphology were then isolated for each fermenting must and, according to Belloch et al. [38], mitochondrial DNA Restriction Fragment Length Polymorphism (mt-DNA RFLP) assays were performed with the restriction endonuclease Hinf I (Thermo Fisher Scientific, Waltham, Massachusetts, USA) following the procedure reported by Querol et al. [39] and analyzed on 0.7% (w/v) agarose gel (Euroclone, Pero, Italy) in 0.5 × TBE buffer (40 mM Tris-Cl, pH 8.3, 45 mM boric acid, 1 mM EDTA, Mallinckrodt Baker BV, Deventer, The Netherlands).
To analyze the mt-DNA of the yeasts present at the end of vinifications, the protocol described in Di Maio et al. [40] was followed: 100 µL of lees were diluted in 1 mL of YPD (Yeast extract 10 g/L, Peptone 20 g/L, Dextrose 20 g/L, Oxoid, Basingstoke, Hampshire, UK) supplemented with tetracycline to prevent bacterial growth (30 mg/L, Sigma-Aldrich, St. Louis, MO, USA) and grown at 28 °C for 24–48 h. The mt-DNA of the yeast cells was then prepared, digested and analyzed as previously described.
2.4.4. Chemical Analyses
For the determination of alcohol content, pH, total acidity, total dry extract, methanol, free and total SO2, we used the OIV (International Organization of Vine and Wine) official methods (OIV-MA-AS312-01A; OIV-MA-AS313-15; OIV-MA-AS313-01; OIV-MA-AS2-03B; OIV-MA-AS312-03B; OIV-MA-AS323-04B [41]). Glucose + fructose, glycerol, acetic acid, malic acid, lactic acid, citric acid, and tartaric acid concentrations were determined enzymatically by monitoring the changes in absorbance using an Enotech apparatus (Steroglass, San Martino in Campo, Italy). The Folin-Ciocalteu method and the p-(dimethylamino)cinamaldehyde (p-DMACA) method [42] were used respectively for the determination of the total phenolics and the total catechins of wines. All measurements were performed in triplicate. We used reagents produced by Carlo Erba Reagents (Cornaredo, Italy), Mallinckrodt Baker BV (Deventer, The Netherlands), Sigma-Aldrich (St. Louis, MO, USA).
2.4.5. Volatile Compound Analyses
Volatile compounds were determined following the methods of Corona [43]. Aliquots of 25 mL of wine, charged with 1-Heptanol as an internal standard (0.25 mL of 40 mg/L hydro alcoholic solution), diluted to 75 mL with distilled water, were passed through a 1 g C18 cartridge (Isolute, SPE Columns, Uppsala, Sweden, part n° 221-0100-C) previously activated with 3 mL of methanol followed by 4 mL of distilled water. After washing with 30 mL of distilled water, volatiles were recovered by elution with 12 mL dichloromethane, dehydrated and evaporated to 0.5 mL prior to injection into the gas chromatograph (PerkinElmer Autosystem XL, Milan, Italy) and GC-MS (Agilent 6890 Series GC system, Agilent 5973 Net Work Mass Selective Detector, Milan, Italy), both equipped with a DB-WAX column (Agilent Technologies, 30 m, 0.250 mm i.d., film thickness 0.25 μm, part n° 122-7032). Oven temperatures: 40 °C for 2 min (during splitless injection), from 40 to 60 °C, 40 °C/min, 60 °C for 2 min, from 60 to 190 °C, 2 °C/min, from 190 to 230, 5 °C/min, 230 °C for 15 min; injector 250 °C, Fid 250 °C, transfer line 230 °C, carrier helium 1 mL/min.; EM. 70 eV. Volatile organic compounds (VOC) were identified by comparison of the mass spectra and GC retention times with those of the pure commercial standard compounds or others prepared in our laboratory and by comparing their mass spectra with those within the NIST/EPA/NIH Mass Spectral Library database (Version 2.0d, build 2005). Data are reported as averages ± standard deviations of measurements in triple of fermentations realized in duplicate. Volatiles classification is in agree with Ilc et al. [44]
2.4.6. Sensory Analyses
Three months after bottling, the sensory evaluation of wines by duo-trio test (UNI ISO 10399 [45]) and by a paired comparison test (UNI ISO 5495 [46]) was carried out. We followed the ISO guidelines (UNI ISO 8589 [47]) with a panel of 23 or 25 tasters (10 females and 13–15 males). The panel was composed of technicians and students of the Degree Course in Viticulture and Oenology of the University of Palermo (Palermo, Italy), which regularly perform sensory analysis and have experience with the evaluation of wines and with the methodology and the technical aspects. For the test, we used amber glasses in order to evaluate the wines according to the smell and taste components and the presentation of the samples was random. We evaluated the significance according to Roessler et al. [48]
2.4.7. Statistical and Explorative Multivariate Analyses
Analysis of Variance (ANOVA) and Tukey’s Honestly Significant Difference (HSD) test were used to calculate significant differences between oenological parameters and the volatile compounds of different wines. All tests were performed using the statistical program SPSS (v. 13, IBM, Armonk, NY, USA).
In order to graphically represent the values and distribution of VOC concentration with significant differences among samples, a heat map clustered analysis (HMCA), based on a double hierarchical dendrogram with a heat map plot, was employed to represent the individual content values contained in the data matrix as colors, according to Martorana et al. [49] The relative concentration of VOCs were depicted by color intensity from yellow (lowest concentration) to red (highest concentration). Heat map analysis of the VOC levels was performed using the autoscaled data. Statistical analyses were performed using XLStat software v. 7.5.2 (Addinsoft, NY, USA) for Excel.
3. Results and Discussion
3.1. Technological Screening of Strain Km L2009
Since it is known that K. marxianus shows killer activity against other yeasts [50], the ability of K. marxianus Km L2009 strain to inhibit the growth of commercial S. cerevisiae strains was tested (Table 1). Only 7 strains out of the 21 tested were not inhibited in their growth by the strain Km L2009. The fact that the Km L2009 strain is, at least in the conditions of this assay, incompatible with two thirds of the tested commercial S. cerevisiae strains, could limit its use in sequential fermentations involving sensitive commercial starters. On the other hand, the compatibility with the remaining one third and the large number of commercial S. cerevisiae available on the market [51] still make it possible to use it in various combinations with a significant number of S. cerevisiae strains. Then, although any use of the Km L2009 strain with commercial strains must be preceded by a careful study of its compatibility with the S. cerevisiae strains to be used in mixed fermentations, we can assume that a number of compatible strains can be found with some ease, useful for completing the fermentation of the main types of wine. The S. cerevisiae Zymaflore®X5 (Laffort Oenologie, Bordeaux, France) later used in sequential fermentations was chosen from the seven compatible strains.

Table 1.
Killer activity of the strain Kluyveromyces marxianus Km L2009 against commercial yeast strains.
Then we assessed the ability of the K. marxianus Km L2009 strain to tolerate sulfites, preservatives widely used in wine production. Figure 1a shows that SO2 reduces the fermentative activity of the strain at a concentration of 40 mg/L and stops it at 70 mg/L, but it does not produce any adverse effect at 20 mg/L.

Figure 1.
(a) Sulfite tolerance. Fermentative activity of the strain K. marxianus Km L2009 in musts without sulfites (—□—), or with 20 mg/L (—◊—), 40 mg/L (—∆—), 70 mg/L (—○—) sulfites; not inoculated must (- – -). (b) Ethanol tolerance. Fermentative activity of the strain K. marxianus Km L2009 in musts without ethanol (—□—), or with 2% (—◊—), 4% (—∆—), 6% (—○—) and 8% (—×—) ethyl alcohol (v/v); not inoculated must (- – -).
These results indicate that the eventual use of this strain in wine production will be directly possible only for wines with low or no sulfites, which, however, are enjoying a growing interest from an audience of consumers increasingly attentive to health issues [52].
Because the increase in ethanol concentration is responsible for the decline of non-Saccharomyces yeasts during spontaneous fermentations [1], the fermentative activity of the strain Km L2009 at different concentrations of ethyl alcohol was assessed (Figure 1b). Though ethanol adversely affected the growth of the strain Km L2009 at concentrations of 4% (v/v), this yeast still showed fermentative activity at alcohol concentrations of 6% (v/v). It is therefore reliable that its growth is possible only in the first phase of fermentation: to exhaust the sugars of a must, the subsequent action of S. cerevisiae is then necessary. The Km L2009 strain therefore does not differ from many other reported cases, with the use of non-Saccharomyces species entailing the sequential inoculation of a S. cerevisiae strain [6] or the development of a spontaneous population of Saccharomyces able to complete the alcoholic fermentation [11].
3.2. Laboratory-Scale Fermentations
To complete the preliminary oenological investigation of the strain K. marxianus Km L2009, micro-fermentations of sterile Muscat of Alexandria must, a variety rich of aromatic terpenes and of their non-volatile glycosidic complexes [53], were performed. Musts that received only the inoculum of strain Km L2009 or only the commercial strain S. cerevisiae (Zimaflore®X5, Laffort Oenologie, Bordeaux, France), and those inoculated with strain Km L2009 first and, after seven days, with the same commercial S. cerevisiae strain, were monitored for three weeks. The daily microbiological controls showed that in fermentations where the strain Km L2009 was inoculated alone (Figure 2a), after a slight initial decrease, its growth reached the highest level (7.4 ± 1.1 × 108 cells/mL) in 6–7 days and progressively decreased afterward. In single strain fermentations (Figure 2b), S. cerevisiae reached 3.0 ± 0.3 × 108 cells/mL after 5 days and then maintained a plateau phase at 2 − 3 × 108 cells/mL throughout the rest of the fermentation. When sequential fermentations were performed (Figure 2c), the trend of growth of the strain Km L2009 was similar to the one observed in the single strain fermentation, except for a faster and greater decrease in the second part of the fermentation; the growth of the S. cerevisiae strain increased continuously up to the fifteenth day (reaching 2.9 ± 0.4 × 107 cells/mL) and then slowly decreased until the end of the assay.
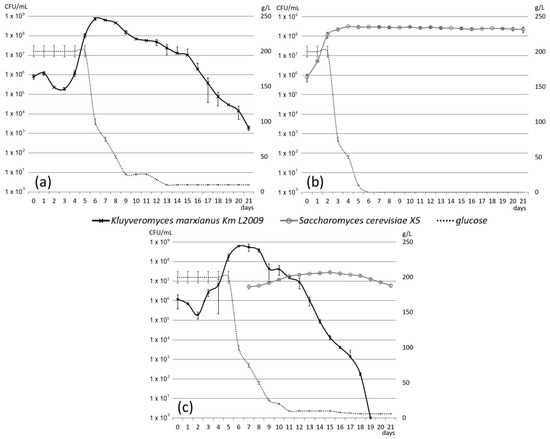
Figure 2.
Growth in sterile must of K. marxianus strain Km L2009 alone (a), of S. cerevisiae strain Zimaflore® X5 alone (b) and of the two strains inoculated sequentially (c). Each graph shows the days of fermentation on the horizontal axis, the CFU/mL of the yeast strains on the left vertical axis and the g/L of glucose on the right vertical axis.
Wines produced by the different types of inoculum show differences in several parameters (Table 2), with the most marked differences in residual sugars, higher in fermentations with K. marxianus, and the consequent differences in alcohol and total dry extract values. Although for the fermentation with K. marxianus alone this is an expected result, the presence of residual sugars in mixed fermentation results from a limitation in the development of S. cerevisiae, as indicated by the maximum concentration reached by this species (Figure 2c), 10 times lower compared to the fermentation of S. cerevisiae alone (Figure 2b). Since the Km L2009 strain does not seem to inhibit the strain Zimaflore®X5 (Laffort Oenologie, Bordeaux, France) used (see Table 1), the most likely explanation for this is that the growth in the first phase of fermentation of a very high number of K. marxianus cells exhausted the availability in the must of essential nutrients such as vitamins and nitrogen sources. This detail was taken into account in the subsequent use of the Km L2009 strain in the winery.

Table 2.
The main chemical-physical parameters and statistical data analysis of the laboratory-scale fermentations. Data are reported as average values ± standard deviations of two different vinifications, each with measurements in triplicate.
Further differences were found in the values of citric acid and of pH, but no difference was found for methanol. In our opinion, this latest result is important because previously the oenological use of K. marxianus was associated with an increased production of methanol [25], a compound well known for its harmful effects on human health [54]. The character of low methanol production, imputable to this strain, is added to the other aforementioned characteristics of the species [18,19], which provides consumer safety in case of the oenological use of the Km L2009 strain.
Table 3 shows the volatile organic compounds (VOCs) found at the end of these fermentations. With the limitations deriving from the differences in residual sugars in the various fermentations, statistically significant differences were observed between wines produced using only S. cerevisiae and those produced by K. marxianus. In particular, in fermentations by only K. marxianus or mixed by K. marxianus with S. cerevisiae, we found a higher amount of linalool and of total free terpenes, consistently with the reported production of β-glucosidase by strain Km L2009 [26]. This could affect the overall quality of the wines, even in the case of compounds that are present at concentrations below their perception threshold, because of their synergistic effect; in fact it is known that terpenes interact in such a way that a component can increase the aroma of another, and that a mixture is more aromatic than the single most aromatic component of this mixture [55]. In musts fermented by K. marxianus, we also found higher quantities of isoamyl acetate and 2-phenylethyl acetate, which are responsible for fruity and floral odors, particularly appreciated in white wines [56].

Table 3.
Free volatile compounds (in µg/L) found in lab-scale fermentations. Odor thresholds and sensory descriptions according to [31,56,60,61,62,63,64,65,66,67,68,69].
Figure 3 shows the graphical representation of the VOC amount only for chemicals with statistically different concentrations among the experimental fermentations. The hierarchical dendrogram combined with the heat map plot shows that each type of fermentation significantly affected the distribution and concentrations of VOCs among trials. The HMCA clearly separated both fermentations carried out with K. marxianus from that with only S. cerevisiae. Furthermore, the highest concentration of most VOCs was found in musts fermented by K. marxianus, alone or together with S. cerevisiae. These results show that, at least in lab-scale sterile must fermentations, Km L2009 is able to produce wines characterized by a richer and clearly distinguishable volatile component when compared with wines obtained from the same must but by only Saccharomyces: it is then possible to add K. marxianus to the large group of non-Saccharomyces yeasts able to differentiate the aromatic profile of wines [57,58,59].
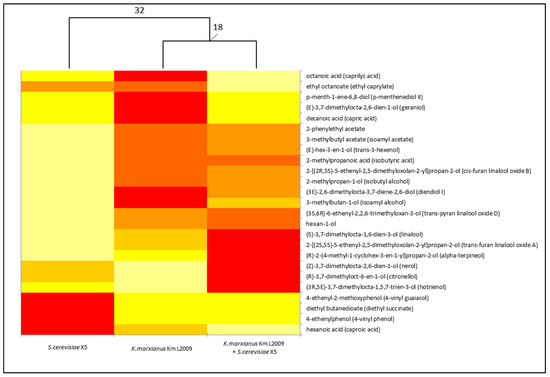
Figure 3.
VOCs distribution among different fermentations. The dendrogram is based only on VOCs of Table 3 showing concentrations with statistically significant differences. The heat map plot depicts the relative quantity of each VOC (variables clustering on the X-axis) within each type of fermentation (Y-axis clustering). The values for VOC concentrations are depicted by color intensity from light yellow (lowest concentration) to red (highest concentration). Numbers indicate the distances between the clustered columns calculated as Euclidean distances.
3.3. Wine Production and Monitoring
Two white grape cultivars, Muscat of Alexandria, rich in free and glycosidically bound terpenes [53], and Grillo, with low amounts of these compounds [70], were used. For each cultivar, two aliquots of the same must, adequately supplemented with vitamins and nitrogen sources (see materials and methods, Section 2.4.1), were left to spontaneous fermentation; two aliquots were inoculated with a commercial wine yeast; two aliquots were inoculated with the strain K. marxianus Km L2009, letting then Saccharomyces yeasts grow spontaneously: this last procedure was preferred to a sequential inoculum with a commercial S.cerevisiae strain, because in a previous similar experimentation [11], we found that, in mixed fermentations realized in the high contaminated environment of a real winery, wild Saccharomyces take over anyway during the first days and complete fermentation. In spontaneous fermentations of both cultivars (Figure 4a), non-Saccharomyces yeasts increased in the first five–six days (≤6.5 ± 2.4 × 105 cfu/mL in Muscat of Alexandria; ≤6.1 ± 0.0 × 106 cfu/mL in Grillo) and then decreased in coincidence with the growth of Saccharomyces spp. (≤38 ± 4 × 106 cfu/mL in Muscat of Alexandria; ≤82 ± 14 × 106 cfu/mL in Grillo). Musts inoculated with commercial S. cerevisiae strain (Figure 4b) always showed the lag, exponential and stationary phases, with the highest growth levels reaching 44 ± 1 × 106 cfu/mL in the Muscat of Alexandria and 94 ± 4 × 106 cfu/mL in Grillo. In these fermentations, non-Saccharomyces amounted to ≤2.9 ± 0.2 × 105 cfu/mL in Muscat of Alexandria, and ≤5.8 ± 0.1 × 106 cfu/mL in Grillo. In sequential fermentations (Figure 4c), daily microbiological tests showed that K. marxianus was mostly present in the first five–six days of fermentation (≤9.9 ± 3.3 × 106 cfu/mL in Muscat of Alexandria; ≤8.7 ± 0.8 × 106 cfu/mL in Grillo), until it was replaced by Saccharomyces (≤37 ± 4 × 106 cfu/mL in Muscat of Alexandria; ≤44 ± 18 × 106 cfu/mL in Grillo). Molecular assays showed that the multiplying K. marxianus strain was actually Km L2009 (Supplementary Figure S1). Saccharomyces remained in the stationary phase until the end of alcoholic fermentation, while K. marxianus decreased gradually. In these vinifications, other non-Saccharomyces yeasts were present always at lower concentrations than K. marxianus and/or Saccharomyces (≤4.0 ± 1.1× 105 cfu/mL in Muscat of Alexandria, and ≤ 3.4 ± 0.1 × 106 cfu/mL in Grillo).
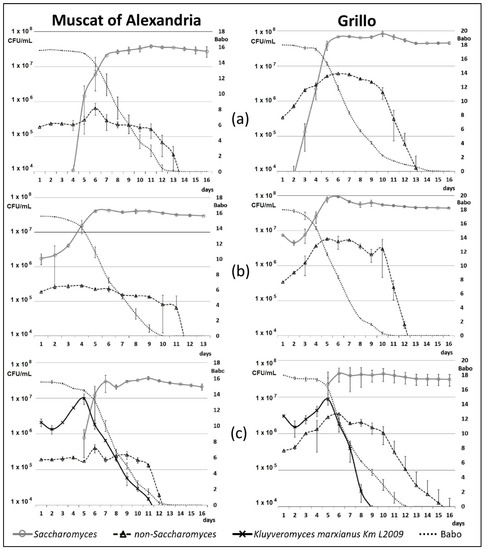
Figure 4.
Growth curve of Kluyveromyces marxianus strain Km L2009, Saccharomyces and other non-Saccharomyces yeasts in vinifications of Muscat of Alexandria (left) and Grillo (right) musts, with no starter inoculum (a), with Saccharomyces cerevisiae strain QA23 (b), and with K. marxianus Km L2009 (c) inoculum.
Molecular assays were carried out in order to control the Saccharomyces strains taking part to the fermentations. The commercial S. cerevisiae strain (Figure 5, lanes 1–3) was shown to successfully proliferate until the end of fermentation. Similar tests carried out in spontaneous and K. marxianus vinifications revealed that one different Saccharomyces strain, probably a resident strain of the winery, took over and was the only one detectable at the end of these fermentations (Figure 5, lanes 4–7).
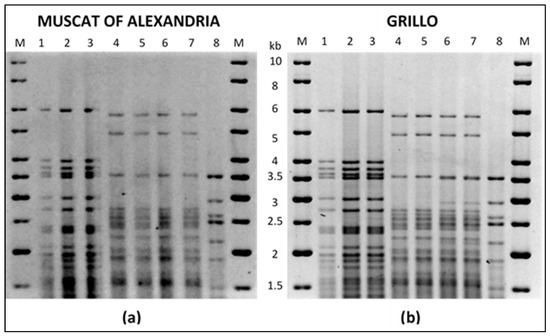
Figure 5.
mt-DNA RFLP of yeasts present at the end of fermentations of Muscat of Alexandria (a) and Grillo (b) musts. Strain S. cerevisiae Lalvin QA23 (lanes 1); yeast lees from vinifications inoculated with S. cerevisiae Lalvin QA23 (lanes 2 and 3); yeast lees from spontaneous vinifications (lanes 4 and 5); yeast lees from vinifications inoculated with K. marxianus Km L2009 (lanes 6 and 7); strain K. marxianus Km L2009 (lane 8). Molecular weight marker (lanes M).
All wines produced by the different types of fermentation do not contain any residual sugars (Supplementary Tables S1 and S2), demonstrating that, in the presence of adequate nutrients, Saccharomyces has no difficulty in completing a fermentation started by the Km L2009 strain. Still, wines show differences in some main oeno-chemical parameters, but again not for methanol: these results confirm what was already observed in lab-scale fermentations and lead to the presumption that methanol production, previously reported as high in the oenological use of K. marxianus [25], is a strain-specific character. Spontaneous fermentations show a tendency to lower total dry extract; fermentations with S. cerevisae QA23 show lower values of alcohol and higher values of glycerol and residual hexoses, while wines fermented by K. marxianus show lower values of malic, lactic and citric acid and higher pH values. Although these differences are statistically significant, their values are actually very small and it is likely that they do not produce substantial differences between the different types of wine, also in the case of acetic acid, a compound that receives a lot of attention from winemakers. The enological use of the Km L2009 strain in mixed fermentations has no influence on the general chemical-physical characteristics of the produced wines, such as alcohol content, quantity of glycerol and concentration of the different acids: K. marxianus therefore seems to behave differently compared to other species, such as Starmerella bacillaris (syn., Candida zemplinina), known for producing wines richer in glycerol [11,71], or Lachancea thermotolerans, used to increase the acidity of wines [72].
Instead, we found greater differences when considering volatile compounds. In the Muscat of Alexandria wines (Table 4), we found significant and substantial differences in the amount of free terpenes: among the compounds present well above their odor threshold, linalool is more abundant in wines made with K. marxianus and, on the whole, free terpenes are 50% more abundant in these wines compared to those produced with only S. cerevisiae; again, this is consistent with the ability of the strain to produce β-glucosidase as previously reported [26]. In wines produced with the strain Km L2009, aliphatic esters as a whole are one and a half times more abundant than in wines made by spontaneous fermentation and twice as much if compared to wines made by the commercial S. cerevisiae strain.

Table 4.
Free volatile compounds (in µg/L) found in Muscat of Alexandria wines. Odor thresholds and sensory descriptions according to [31,56,60,61,62,63,64,65,66,67,68,69].
As observed in laboratory fermentations, the most important difference was found for isoamyl acetate, present in double quantities in the mixed fermentations compared to those realized with the commercial S. cerevisiae. Again, in the wines obtained by mixed fermentation, we found the greatest amount of 2-phenylethyl acetate (50–60% more than in the other fermentations), although in this case differences between wines are smaller than in laboratory scale fermentations and are probably not perceptible if we consider the corresponding range of odor threshold. Finally, in wines produced by K. marxianus, we found higher amounts of some compounds characterized by negative descriptors, as some aliphatic acids (hexanoic and octanoic) and volatile phenols: in this case, however, the compounds would be barely or not perceptible if we consider the ranges of their odor thresholds. Since in laboratory fermentations (performed using sterile musts; Table 3) these compounds are present in much lower concentrations and far below their odor threshold, it is possible that their presence in the Muscat of Alexandria wines was due to other microbial species, proliferated during the alcoholic fermentation under the winery conditions.
Table 5 shows the volatile compounds found in the Grillo wines, essentially free of terpenes. Again, we found higher quantities (up to double) of different aliphatic esters, and especially of isoamyl acetate, in the wine produced with the strain Km L2009. No statistically significant differences were instead found in Grillo wines made using the commercial S. cerevisiae strain or K. marxianus Km L2009 for other compounds such as 2-phenylethyl acetate (characterized by positive descriptors), or hexanoic and octanoic acids and 4-vinyl phenol (characterized by negative descriptors).

Table 5.
Free volatile compounds (in µg/L) found in Grillo wines. Odor thresholds and sensory descriptions according to [31,56,60,61,62,63,64,65,66,67,68,69].
Figure 6 shows the graphical representation of the VOC concentrations with significant differences in distinct types of vinifications of Muscat of Alexandria (top) and Grillo (bottom).
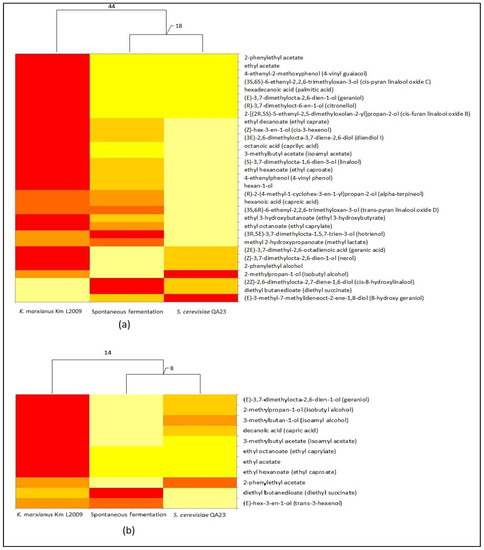
Figure 6.
VOC distribution in different Muscat of Alexandria (a) and Grillo (b) wines. Dendrograms are based only on VOCs, respectively of Table 4 and Table 5, showing concentrations with statistically significant differences. The heat maps depict the relative quantity of each VOC (variables clustering on X-axis) within each type of vinification (Y-axis clustering). The values for VOC concentrations are depicted by color intensity from light yellow (lowest concentration) to red (highest concentration). Numbers indicate the distances between the clustered columns calculated as Euclidean distances.
Each type of fermentation is characterized by a different distribution of VOC concentrations, with the highest of most of them detected in wines produced by K. marxianus Km L2009. We find it particularly interesting that, although the genotypic analyses of microbial populations demonstrated the presence of the same strain of S. cerevisiae at the end of spontaneous and K. marxianus vinifications (see Figure 5), spontaneous fermentation produced wines with a VOCs distribution more similarly to that produced by the commercial strain S. cerevisiae QA23, showing a greater distinctiveness of the wines produced by the strain Km L2009 and confirming the results obtained in lab-scale fermentations (see Figure 3).
We completed, with sensory analysis, the comparison of the experimental wines, performing duo-trio tests and paired comparison tests. Wines obtained from the same must and with the same type of inoculum never resulted in a difference. On the other hand, wines obtained from the same grapes, but by different kinds of inoculum, resulted always in a significant difference, except for Muscat of Alexandria wines obtained by spontaneous fermentation and by the K. marxianus strain (Table 6); this could be due to a greater difficulty of judges to perceive differences in wines very rich in flavors, such as Muscat, in combination with the growth of the same strain of Saccharomyces in the two types of fermentation (see Figure 5). Anyhow, in most cases, the differences in the distribution of VOC concentration produces sensory differences actually perceptible to the taster, at least nine months after the end of alcoholic fermentation, with the last three in the bottle.

Table 6.
Results of duo-trio test performed with Muscat of Alexandria (top) and Grillo (bottom) wines produced by spontaneous fermentation (Spontaneous), by inoculum with the Saccharomyces cerevisiae commercial strain QA23 (S. cerevisiae) and with Kluyveromyces marxianus Km L2009 strain (Km L2009). p: p-value; α: significance level; n.s.: not significant.
When the judges were also asked to express a preference (Table 7), only the Grillo wine fermented with K. marxianus was preferred over the wine produced from the same must but with the commercial S. cerevisiae strain: in this case, judges justified their choice by defining the wine produced with the strain Km L2009 to be more complex, fruity and aromatic. Therefore, different types of fermentation often produced sensory differences; however, frequently such differences did not lead to a preference toward one type of wine or another. This is especially evident in the case of the Muscat of Alexandria, where the large amount of flavors could make more difficult the expression of a preference by the judges.

Table 7.
Results of the paired comparison test performed with Muscat of Alexandria (top) and Grillo (bottom) wines produced by spontaneous fermentation (Spontaneous), by the Saccharomyces cerevisiae commercial strain QA23 (S. cerevisiae) and by Kluyveromyces marxianus Km L2009 strain (Km L2009). p: p-value; α: significance level; n.s.: not significant.
4. Conclusions
In the present work, we tried to enrich the very limited scientific knowledge on the role of K. marxianus yeast as a potential co-starter for wine production. Throughout a polyphasic approach, it was possible to show that K. marxianus strain Km L2009 is able to produce white wines with remarkably different characteristics compared to wines produced from the same grapes by spontaneous fermentation or by a commercial S. cerevisiae strain. These wines can be produced by the initial inoculum of the K. marxianus strain Km L2009, followed by the subsequent spontaneous proliferation of Saccharomyces yeasts. The wines obtained with this type of fermentation showed a significant amount of total esters, mainly the isoamyl acetate. The inoculum of strain Km L2009 into must rich in glycosidically bound terpenes allowed the production of wines with high free terpene concentrations. Sensory results also confirmed differences among wines.
This study is part of a larger analyses of yeast populations in Sicily (Italy) [26,34,40], also with the aim of selecting new yeast strains for the regional wine industry [11,73,74]: based on the results described in this paper, we believe it is possible to use the Km L2009 strain to diversify the production of local wines. Further studies are instead necessary to understand a possible wider use of this strain. Preliminary results of experiments conducted in our laboratory demonstrate the ability of this strain to acquire a greater tolerance to sulfites if grown progressively in the presence of increasing quantities of this preservative, as is also known for other species [75]; therefore, in the future, its use could expand also to the production of wines with higher quantities of sulfites. During the last harvest, the further use of the Km L2009 strain in five other Sicilian wineries, for the first commercial productions with different varieties of white grapes, confirmed the ability of the strain to multiply in the first phase of fermentation and the full completion of fermentation by Saccharomyces in the second one. The analysis of these wines, even repeated several times, will be useful to understand whether any sensory differences are repeatable and lasting. Further uses of the Km L2009 strain for the production of red wines remain to be investigated, but on the basis of the data available today, it seems promising for the production of a wide range of wines. Future studies of this and other new strains of Kluyveromyces marxianus will let us understand to what extent this species can be useful in wine production.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/fermentation7020079/s1, Figure S1: mt-DNA RFLP analysis of the K. marxianus yeasts isolated from Km L2009 vinifications; Table S1: Main chemical-physical data of Muscat of Alexandria wines; Table S2: Main chemical-physical data of Grillo wines.
Author Contributions
Conceptualization, D.O.; methodology, D.O.; validation, D.O.; formal analysis, D.O., O.C. and N.F.; investigation, E.B., G.P., P.G., M.S., T.F., V.G., F.A., M.M.; resources, D.O., O.C. and N.F.; data curation, D.O.; writing—original draft preparation, D.O. and N.F.; writing—review and editing, D.O.; visualization, D.O.; supervision, D.O.; project administration, D.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Acknowledgments
Authors wish to thank Rocco Di Stefano and Graziana Grassini for their personal evaluation of wines and the provided useful advice.
Conflicts of Interest
Under Italian law, the industrial property of the K. marxianus strain Km L2009 described in this paper and any resulting economic benefits belong to D.O. and to the Istituto Regionale del Vino e dell’Olio.
References
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A. Enological Repercussions of Non-Saccharomyces Species. Fermentation 2019, 5, 68. [Google Scholar] [CrossRef]
- Morata, A. Enological Repercussions of Non-Saccharomyces Species in Wine Biotechnology. Fermentation 2019, 5, 72. [Google Scholar] [CrossRef]
- Morata, A. Enological Repercussions of Non-Saccharomyces Species 2.0. Fermentation 2020, 6, 110. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Borneman, A.R. Yeasts found in vineyards and wineries. Yeast 2016, 34, 111–128. [Google Scholar] [CrossRef]
- Mateo, J.J.; Maicas, S. Application of non-Saccharomyces yeasts to wine-making process. Fermentation 2016, 2, 14. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F. Non-Saccharomyces wine yeasts have a promising role in biotechnological approaches to winemaking. Ann. Microbiol. 2011, 61, 25–32. [Google Scholar] [CrossRef]
- Romani, C.; Lencioni, L.; Biondi Bartolini, A.; Ciani, M.; Mannazzu, I.; Domizio, P. Pilot Scale Fermentations of Sangiovese: An Overview on the Impact of Saccharomyces and Non-Saccharomyces Wine Yeasts. Fermentation 2020, 6, 63. [Google Scholar] [CrossRef]
- Giaramida, P.; Ponticello, G.; Di Maio, S.; Squadrito, M.; Genna, G.; Barone, E.; Scacco, A.; Corona, O.; Amore, G.; Di Stefano, R.; et al. Candida zemplinina for production of wines with less alcohol and more glycerol. S. Afr. J. Enol. Vitic. 2013, 34, 204–211. [Google Scholar] [CrossRef][Green Version]
- Lodder, J.; Kreger-van Rij, N.J.W. The Yeasts: A Taxonomic Study; North-Holland Publishing, Co.: Amsterdam, The Netherlands, 1952; pp. 155–158. [Google Scholar]
- Lachance, M.A. Kluyveromyces van der Walt (1971). In The Yeasts, A Taxonomic Study, 5th ed.; Kurtzman, C.P., Fell, J.W., Boekhout, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 471–482. [Google Scholar]
- Fonseca, G.G.; Heinzle, E.; Wittmann, C.; Gombert, A.K. The yeast Kluyveromyces marxianus and its biotechnological potential. Appl. Microbiol. Biotechnol. 2008, 79, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.P.; Etschmann, M.M.W.; Schrader, J.; de Billerbeck, G.M. Cell factory applications of the yeast Kluyveromyces marxianus for the biotechnological production of natural flavour and fragrance molecules. Yeast 2015, 32, 3–16. [Google Scholar] [CrossRef]
- Reyes-Sánchez, F.; Páez-Lerma, J.B.; Rojas-Contreras, J.A.; López-Miranda, J.; Soto-Cruz, N.Ó.; Reinhart-Kirchmayr, M.R. Study of the Enzymatic Capacity of Kluyveromyces marxianus for the Synthesis of Esters. J. Mol. Microbiol. Biotechnol. 2019, 29, 1–9. [Google Scholar] [CrossRef]
- Papon, N.; Courdavault, V.; Clastre, M.; Bennett, R.J. Emerging and emerged pathogenic Candida species: Beyond the Candida albicans paradigm. PLoS Pathog. 2013, 9, e1003550. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA (2017–2019). EFSA J. 2020, 18, e05966. [Google Scholar] [CrossRef]
- Piemolini-Barreto, L.T.; Zacaria, J.; Delamare, A.P.; Antonio, R.V.; Echeverrigaray, S. Variation in phenolic compounds, anthocyanins, and color in red wine treated with enzymatic extract of Kluyveromyces marxianus. World J. Microbiol. Biotechnol. 2014, 30, 1541–1547. [Google Scholar] [CrossRef]
- Piemolini-Barreto, L.T.; Antonio, R.V.; Echeverrigaray, S. Comparison of a pectinoltytic extract of Kluyveromyces marxianus and a commercial enzyme preparation in the production of Ives (Vitis labrusca) grape juice. World J. Microbiol. Biotechnol. 2015, 31, 755–762. [Google Scholar] [CrossRef]
- Sieiro, C.; Villa, T.G.; da Silva, A.F.; García-Fraga, B.; Vilanova, M. Albariño wine aroma enhancement through the use of a recombinant polygalacturonase from Kluyveromyces marxianus. Food Chem. 2014, 145, 179–185. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; McErlean, C.; Kanellaki, M.; Hack, C.J.; Marchant, R.; Banat, I.M.; Koutinas, M.M. High-temperature wine making using the thermotolerant yeast strain Kluyveromyces marxianus IMB3. Appl. Biochem. Biotechnol. 2004, 112, 25–35. [Google Scholar] [CrossRef]
- Vigentini, I.; Maghradze, D.; Petrozziello, M.; Bonello, F.; Mezzapelle, V.; Valdetara, F.; Failla, O.; Foschino, R. Indigenous Georgian wine associated-yeasts and grape cultivars to edit the wine quality in a precision oenology perspective. Front. Microbiol. 2016, 7, 352. [Google Scholar] [CrossRef] [PubMed]
- Rollero, S.; Zietsman, A.J.J.; Buffetto, F.; Schückel, J.; Ortiz-Julien, A.; Divol, B. Kluyveromyces marxianus Secretes a Pectinase in Shiraz Grape Must That Impacts Technological Properties and Aroma Profile of Wine. J. Agric. Food Chem. 2018, 66, 11739–11747. [Google Scholar] [CrossRef] [PubMed]
- Polizzotto, G.; Barone, E.; Ponticello, G.; Fasciana, T.; Barbera, D.; Corona, O.; Amore, G.; Giammanco, A.; Oliva, D. Isolation, identification and oenological characterization of non-Saccharomyces yeasts in a Mediterranean island. Lett. Appl. Microbiol. 2016, 63, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S.; Mateo, J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Regodón, J.A.; Peréz, F.; Valdés, M.E.; De Miguel, C.; Ramìrez, M. A simple and effective procedure for selection of wine yeast strains. Food Microbiol. 1997, 14, 247–254. [Google Scholar] [CrossRef]
- Caridi, A.; Cufari, A.; Ramondino, D. Isolation and clonal pre-selection of enological Saccharomyces. J. Gen. Appl. Microbiol. 2002, 48, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Gump, B.H.; Zoecklein, B.W.; Fugelsang, K.C.; Whiton, R.S. Comparison of analytical methods for prediction of prefermentation nutritional status of grape juice. Am. J. Enol. Vitic. 2002, 53, 325–329. [Google Scholar]
- Ribérau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology. Volume 2. The Chemistry of Wine: Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 206–209, 301–331. [Google Scholar]
- Hall, J.F. Detection of wild yeasts in the brewery. J. Inst. Brew. 1971, 77, 513–516. [Google Scholar] [CrossRef]
- Pallmann, C.L.; Brown, J.A.; Olineka, T.L.; Cocolin, L.; Mills, D.A.; Bisson, L.F. Use of WL Medium to profile native flora fermentations. Am. J. Enol. Vitic. 2001, 52, 198–203. Available online: https://www.ajevonline.org/content/52/3/198 (accessed on 15 May 2021).
- Romancino, D.; Di Maio, S.; Muriella, R.; Oliva, D. Analysis of non Saccharomyces yeast populations isolated from grape must from Sicily (Italy). J. Appl. Microbiol. 2008, 105, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Liu, A.; Xue, B.; Liu, Y. Yeast species associated with spontaneous wine fermentation of Cabernet Sauvignon from Ningxia, China. World J. Microbiol. Biotechnol. 2011, 27, 2475–2482. [Google Scholar] [CrossRef]
- Fowell, R.R. The identification of wild yeast colonies on Lysine Agar. J. Appl. Bacteriol. 1965, 28, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, S.; Polizzotto, G.; Planeta, D.; Oliva, D. A method to discriminate between the Candida stellata and Saccharomyces cerevisiae in mixed fermentation on WLD and Lysine Agar Media. S. Afr. J. Enol. Vitic. 2011, 32, 35–41. [Google Scholar] [CrossRef][Green Version]
- Belloch, C.; Barrio, E.; Uruburu, F.; Garcia, M.D.; Querol, A. Characterisation of four species of the genus Kluyveromyces by mitochondrial DNA restriction analysis. System. Appl. Microbiol. 1997, 20, 397–408. [Google Scholar] [CrossRef]
- Querol, A.; Barrio, E.; Huerta, T.; Ramón, D. Molecular monitoring of wine fermentations conducted by active dry yeast strains. Appl. Envir. Microbiol. 1992, 58, 2948–2953. [Google Scholar] [CrossRef]
- Di Maio, S.; Polizzotto, G.; Di Gangi, E.; Foresta, G.; Genna, G.; Verzera, A.; Scacco, A.; Amore, G.; Oliva, D. Biodiversity of indigenous Saccharomyces populations from old wineries of south-eastern Sicily (Italy): Preservation and economic potential. PLoS ONE 2012, 7, e30428. [Google Scholar]
- The International Organisation of Vine and Wine: Compendium of International Methods of Analysis of Wines and Musts. Available online: http://www.oiv.int/en/technical-standards-and-documents/methods-of-analysis/compendium-of-international-methods-of-analysis-of-wines-and-musts-2-vol (accessed on 3 March 2021).
- Di Stefano, R.; Cravero, M.C.; Gentilini, N. Metodi per lo studio dei polifenoli dei vini. L’Enotecnico 1989, 25, 83–89. [Google Scholar]
- Corona, O. Wine-making with protection of must against oxidation in a warm semi-arid terroir. S. Afr. J. Enol. Vitic. 2010, 31, 58–63. [Google Scholar] [CrossRef]
- Ilc, T.; Werck-Reichhart, D.; Navrot, N. Meta-Analysis of the Core Aroma Components of Grape and Wine Aroma. Front. Plant Sci. 2016, 7, 1472. [Google Scholar] [CrossRef]
- International Organization for Standardization, Geneva, Switzerland. UNI ISO 10399 (2010). Sensory Analysis—Methodology—Duo-Trio Test. Available online: https://www.iso.org/standard/74219.html (accessed on 3 March 2021).
- International Organization for Standardization, Geneva, Switzerland. UNI ISO 5495 (2005). Sensory Analysis—Methodology—Paired Comparison Test. Available online: https://www.iso.org/standard/31621.html (accessed on 5 May 2021).
- International Organization for Standardization, Geneva, Switzerland. UNI ISO 8589 (2007). Sensory Analysis—General Guidance for the Design of Test Rooms. Available online: https://www.iso.org/standard/36385.html (accessed on 3 March 2021).
- Roessler, E.B.; Pangborn, R.M.; Sidel, J.L.; Stone, H. Expanded statistical tables for estimation significance in paired-preference, paired-difference, duo-trio and triangle tests. J. Food Sci. 1978, 43, 940–943. [Google Scholar] [CrossRef]
- Martorana, A.; Alfonzo, A.; Gaglio, R.; Settanni, L.; Corona, O.; La Croce, F.; Vagnoli, P.; Caruso, T.; Moschetti, G.; Francesca, N. Evaluation of different conditions to enhance the performances of Lactobacillus pentosus OM13 during industrial production of Spanish-style table olives. Food Microbiol. 2017, 61, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Sangorrín, M.P.; Zajonskovsky, I.E.; Lopes, C.A.; Rodríguez, M.E.; Giraudo de van Broock, M.R.; Caballero, A.C. Killer behaviour in wild wine yeasts associated with Merlot and Malbec type musts spontaneously fermented from northwestern Patagonia (Argentina). J. Basic Microbiol. 2001, 41, 105–113. [Google Scholar] [CrossRef]
- Borneman, A.R.; Forgan, A.H.; Kolouchova, R.; Fraser, J.A.; Schmidt, S.A. Whole Genome Comparison Reveals High Levels of Inbreeding and Strain Redundancy Across the Spectrum of Commercial Wine Strains of Saccharomyces cerevisiae. G3 Genes Genomes Genet. 2016, 6, 957–971. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.Á.; Monagas, M.; Bartolomé, B.; Moreno-Arribas, M.V. Wine features related to safety and consumer health: An integrated perspective. Crit. Rev. Food Sci. Nutr. 2012, 52, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Hjelmeland, A.K.; Zweigenbaum, J.; Ebeler, S.E. Profiling monoterpenol glycoconjugation in Vitis vinifera L. cv. Muscat of Alexandria using a novel putative compound database approach, high resolution mass spectrometry and collision induced dissociation fragmentation analysis. Anal. Chim. Acta 2015, 887, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Hodson, G.; Wilkes, E.; Azevedo, S.; Battaglene, T. Methanol in wine. BIO Web Conf. 2017, 9, 02028. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the aroma of grapes and wines: A review. S. Afr. J. Enol. Vitic. 1983, 4, 49–58. [Google Scholar] [CrossRef]
- Bartowsky, E.J.; Pretorius, I.S. Microbial formation and modification of flavor and off-flavor compounds in wine. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 209–231. [Google Scholar] [CrossRef]
- Varela, C. The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Boekhout, T.; Gojkovic, Z.; Katz, M. Evaluation of non-Saccharomyces yeasts in the fermentation of wine, beer and cider for the development of new beverages. J. Inst. Brew. 2018, 124, 389–402. [Google Scholar] [CrossRef]
- Gamero, A.; Dijkstra, A.; Smit, B.; de Jong, C. Aromatic Potential of Diverse Non-Conventional Yeast Species for Winemaking and Brewing. Fermentation 2020, 6, 50. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W. Food Chemistry, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 857–859. [Google Scholar] [CrossRef]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma—A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Mateo, J.J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Ferrari, G.; Lablanquie, O.; Cantagrel, R.; Ledauphin, J.; Payot, T.; Fournier, N.; Guichard, E. Determination of key odorant compounds in freshly distilled cognac using GC-O, GC-MS, and sensory evaluation. J. Agric. Food Chem. 2004, 52, 5670–5676. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Dragone, G.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Characterization of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 2009, 112, 929–935. [Google Scholar] [CrossRef]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Teixeira, J.A.; de Almeida e Silva, J.B.; Schwan, R.F. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jaboticaba and umbu. LWT Food Sci. Technol. 2010, 43, 1564–1572. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Masa, A.; Oliveira, J.M. Correlation between volatile composition and sensory properties in Spanish Albariño wines. Microchem. J. 2010, 95, 240–246. [Google Scholar] [CrossRef]
- Sànchez-Palomo, E.; García-Carpintero, E.G.; Gómez Gallego, M.Á.; Gonzàlez Viñas, M.Á. The aroma of rojal red wines from La Mancha region—Determination of key odorants. In Gas Chromatography in Plant Science, Wine Technology, Toxicology and Some Specific Applications; Salih, B., Çelikbıçak, Ö., Eds.; Intech: Rijeka, Croatia, 2012; pp. 147–170. [Google Scholar] [CrossRef]
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar] [CrossRef]
- Scafidi, P.; Pisciotta, A.; Patti, D.; Tamborra, P.; Di Lorenzo, R.; Barbagallo, M.G. Effect of artificial shading on the tannin accumulation and aromatic composition of the Grillo cultivar (Vitis vinifera L.). BMC Plant Biol. 2013, 13, 175. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Torchio, F.; Rolle, L.; Gerbi, V.; Cocolin, L. Exploitation of the non-Saccharomyces yeast Starmerella bacillaris (synonym Candida zemplinina) in wine fermentation: Physiological and molecular characterizations. Int. J. Food Microbiol. 2015, 199, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef] [PubMed]
- Scacco, A.; Oliva, D.; Di Maio, S.; Polizzotto, G.; Genna, G.; Tripodi, G.; Lanza, C.M.; Verzera, A. Indigenous Saccharomyces cerevisiae strains and their influence on the quality of Cataratto, Inzolia and Grillo white wines. Food Res. Int. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Di Maio, S.; Genna, G.; Gandolfo, V.; Amore, G.; Ciaccio, M.; Oliva, D. Presence of Candida zemplinina in Sicilian Musts and Selection of a Strain for Wine Mixed Fermentations. S. Afr. J. Enol. Vitic. 2012, 33, 80–87. [Google Scholar] [CrossRef]
- Nardi, T.; Corich, V.; Giacomini, A.; Blondin, B. A sulphite-inducible form of the sulphite efflux gene SSU1 in a Saccharomyces cerevisiae wine yeast. Microbiology 2010, 156, 1686–1696. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).