Anti-Inflammatory Effect on Colitis and Modulation of Microbiota by Fermented Plant Extract Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented Plant Extract
2.2. Animals
2.3. Induction of Colitis
2.4. IL-6 and TNF-α Assays
2.5. Dietary Supplementation and Fecal Collection
2.6. Microbial DNA Extraction
2.7. 16S rRNA Gene Sequencing
2.8. Statistical Analysis
3. Results and Discussion
3.1. Anti-Colitis Effect of FPE in Mice
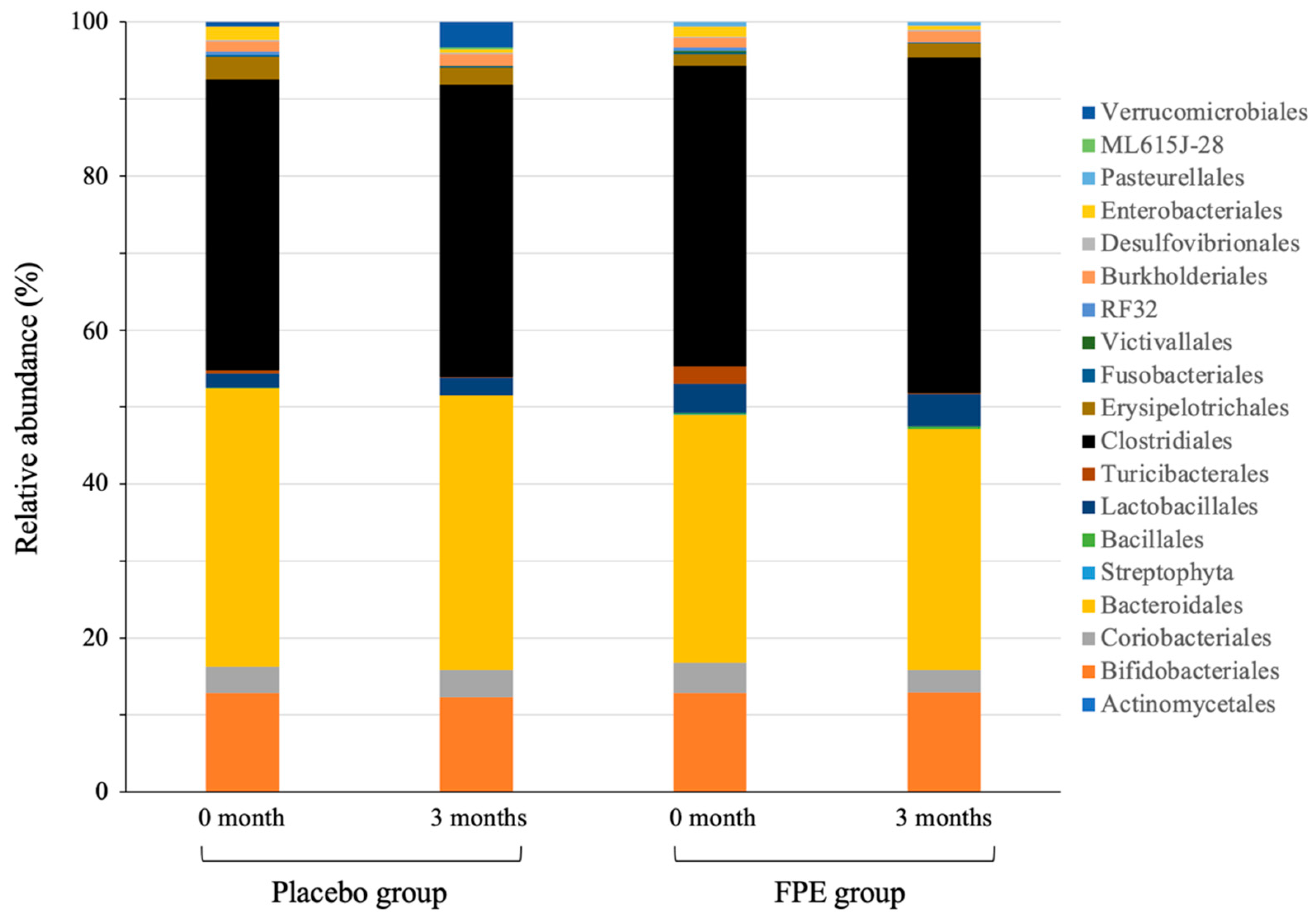
3.2. Change of Gut Microbiota Composition in Human
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Okabayashi, S.; Kobayashi, T.; Hibi, T. Inflammatory bowel disease in Japan—Is it similar to or different from Westerns? J. Anus Rectum Colon 2020, 4, 1–13. [Google Scholar] [CrossRef]
- Japan Intractable Diseases Information Center. [Ulcerative Colitis (Designated Intractable Disease No. 97)]. Available online: https://www.nanbyou.or.jp/entry/62 (accessed on 5 April 2021). (In Japanese).
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Wang, J.; Linnernbrink, M.; Künzel, S.; Fernandes, R.; Nadeau, M.J.; Rosenstiel, P.; Baines, J.F. Dietary history contributes to enterotype-like clustering and functional metagenomic content in the intestinal microbiome of wild mice. Proc. Natl. Acad. Sci. USA 2014, 111, 2703–2710. [Google Scholar] [CrossRef]
- Willing, B.; Vörös, A.; Roos, S.; Jones, C.; Jansson, A.; Lindberg, J.E. Changes in faecal bacteria associated with concentrate and forage-only diets fed to horses in training. Equine Vet. J. 2009, 41, 908–914. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced infammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Cani, P.D. Gut microbiota and the pathogenesis of insulin resistance. Curr. Diab. Rep. 2011, 11, 154–159. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, C.A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and infammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Fecteau, M.E.; Pitta, D.; Vecchiarelli, B.; Indugu, N.; Kumar, S.; Gallagher, S.C.; Fyock, T.L.; Sweeney, R.W. Dysbiosis of the Fecal Microbiota in Cattle Infected with Mycobacterium avium subsp. paratuberculosis. PLoS ONE 2016, 11, e0160353. [Google Scholar]
- Ley, R.E.; Bäckhed, R.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Backhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Grajek, W.; Olejnik, A.; Sip, A. Probiotics, prebiotics and antioxidants as functional foods: A review. Acta Biochim. Pol. 2005, 52, 665–671. [Google Scholar] [CrossRef]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Manson, J.M.; Rauch, M.; Gilmore, M.S. The commensal microbiology of the gastrointestinal tract. Adv. Exp. Med. Biol. 2008, 635, 15–28. [Google Scholar]
- McCracken, V.J.; Lorenz, R.G. The gastrointestinal ecosystem: A precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 2001, 3, 1–11. [Google Scholar] [CrossRef]
- Lievin-Le Moal, V.; Servin, A.L. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: Mucins, antimicrobial peptides, and microbiota. Clin. Microbiol. Rev. 2006, 19, 315–337. [Google Scholar] [CrossRef]
- Furusawa, F.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Ganzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Bell, V.; Ferrao, J.; Fernandes, T. Nutritional guidelines and fermented food frameworks. Foods 2017, 6, 65. [Google Scholar] [CrossRef]
- Moussa, L.; Bézirard, V.; Salvador-Cartier, C.; Bacquié, V.; Lencina, C.; Lévêque, M.; Braniste, V.; Ménard, S.; Théodorou, V.; Houdeau, E. A low dose of fermented soy germ alleviates gut barrier injury, hyperalgesia and faecal protease activity in a rat model of inflammatory bowel disease. PLoS ONE 2012, 7, e49547. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Nordlund, E.; Mattila, I.; Katina, K.; Aura, A.M.; Kolehmainen, M.; Orešič, M.; Mykkänen, H.; Poutanen, K. Postprandial differences in the plasma metabolome of healthy Finnish subjects after intake of a sourdough fermented endosperm rye bread versus white wheat bread. Nutr. J. 2011, 10, 116. [Google Scholar] [CrossRef]
- Mueller, T.; Voigt, W. Fermented wheat germ extract—Nutritional supplement or anticancer drug? Nutr. J. 2011, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.P.; Heuberger, A.L.; Weir, T.L.; Barnett, B.; Broeckling, C.D.; Prenni, J.E. Rice bran fermented with Saccharomyces boulardii generates novel metabolite profiles with bioactivity. J. Agric. Food. Chem. 2011, 59, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.; Ruvio, S.; Granlund, L.; Salminen, S.; Viitanen, M.; Ouwehand, A.C. Probiotics and immunosenescence: Cheese as a carrier. FEMS Immunol. Med. Microbiol. 2010, 59, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Ariefdjohan, M.W.; Savaiano, D.A.; Nakatsu, C.H. Comparison of DNA extraction kits for PCR-DGGE analysis of human intestinal microbial communities from fecal specimens. Nutr. J. 2010, 9, 23. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Kuwaki, S.; Nakajima, N.; Tanaka, H. Plant-based paste fermented by lactic acid bacteria and yeast: Functional analysis and possibility of application to functional foods. Biochem. Insights 2012, 5, 21–29. [Google Scholar] [CrossRef]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Ze, X.; David, Y.B.; Laverde-Gomez, J.A.; Dassa, B.; Sheridan, P.O.; Duncan, S.H.; Louis, P.; Henrissat, B.; Juge, N.; Koropatkin, N.M.; et al. Unique organization of extracellular amylases into amylosomes in the resistant starch-utilizing human colonic Firmicutes bacterium Ruinococcus Bromii. mBio 2015, 6, e01058-15. [Google Scholar] [CrossRef]
- Kaur, A.; Chen, T.; Green, S.J.; Mutlu, E.; Martin, B.R.; Rumpagaporn, P.; Patterson, J.A.; Keshavarzian, A.; Hamaker, B.R. Physical inaccessibility of a resistant starch shifts mouse gut microbiota to butyrogenic Firmicutes. Mol. Nutr. Food Res. 2019, 63, e1801012. [Google Scholar] [CrossRef]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutl. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Vital, M.; Karch, A.; Pieper, D.H. Colonic butyrate-producing communities in humans: An overview using omics data. mSystems 2017, 2, e00130-17. [Google Scholar] [CrossRef]
- Kanauchi, O.; Andoh, A.; Iwanaga, T.; Fujiyama, T.; Mitsuyama, K.; Toyonaga, A.; Bamba, T. Germinated barley foodstuffs attenuate colonic mucosal damage and mucosal nuclear factor kappa B activity in a spontaneous colitis model. J. Gastroenterol. 1999, 14, 1173–1179. [Google Scholar] [CrossRef]
- Andoh, A.; Bamba, T.; Sasaki, M. Physiological and anti-inflammatory roles of dietary fiber and butyrate in intestinal functions. J. Parenter. Enteral. Nutr. 1999, 23, S70–S73. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading players in the maintenance of gut homeostasis. Gut Phathogens 2013, 5, 23. [Google Scholar] [CrossRef]
- Levi, A.J. Diet in the management of Crohn’s disease. Gut 1985, 26, 985–988. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Day after DSS Administration | Negative Group | Positive Group | FPE Group |
|---|---|---|---|
| 4 | - | - | - (BK) |
| 5 | - | + | - (BK) |
| 6 | - | ++ | - (BK) |
| 7 | - | ++ | - (BK) |
| Phyla | Placebo Group | FPE Group | ||
|---|---|---|---|---|
| 0 Month | 3 Months | 0 Month | 3 Months | |
| Actinobacteria | 16.3 ± 1.7 | 15.8 ± 1.7 | 16.8 ± 1.8 | 15.5 ±1.1 |
| Bacteroidetes | 36.1 ± 1.3 | 35.7 ± 1.5 | 32.2 ± 2.6 | 31.3 ± 1.2 |
| Cyanobacteria | 0 | 0 | 0.1 ± 0.1 | 0 |
| Firmicutes | 43.1 ± 1.5 | 42.6 ± 2.1 | 46.7 ± 1.5 | 50.2 ± 1.5 ** |
| Fusobacteria | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 |
| Lentisphaerae | 0 | 0.1 ± 0.1 | 0.3 ± 0.2 | 0 |
| Nitrospirae | 0 | 0 | 0 | 0 |
| Proteobacteria | 3.7 ± 1.6 | 2.2 ± 0.4 | 3.7 ± 0.9 | 2.7 ± 0.5 |
| Spirochaetes | 0 | 0 | 0 | 0 |
| Synergistetes | 0 | 0 | 0 | 0 |
| TM7 | 0 | 0 | 0 | 0 |
| Tenericutes | 0 | 0.3 ± 0.3 | 0 | 0 |
| Verrucomicrobia | 1.5 ± 1.4 | 3.2 ± 2.1 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugimoto, M.; Watanabe, T.; Takaoka, M.; Suzuki, K.; Murakami, T.; Murakami, N.; Sumikawa, S. Anti-Inflammatory Effect on Colitis and Modulation of Microbiota by Fermented Plant Extract Supplementation. Fermentation 2021, 7, 55. https://doi.org/10.3390/fermentation7020055
Sugimoto M, Watanabe T, Takaoka M, Suzuki K, Murakami T, Murakami N, Sumikawa S. Anti-Inflammatory Effect on Colitis and Modulation of Microbiota by Fermented Plant Extract Supplementation. Fermentation. 2021; 7(2):55. https://doi.org/10.3390/fermentation7020055
Chicago/Turabian StyleSugimoto, Manabu, Toshiro Watanabe, Motoko Takaoka, Kyoko Suzuki, Tadatoshi Murakami, Nobutada Murakami, and Shoichi Sumikawa. 2021. "Anti-Inflammatory Effect on Colitis and Modulation of Microbiota by Fermented Plant Extract Supplementation" Fermentation 7, no. 2: 55. https://doi.org/10.3390/fermentation7020055
APA StyleSugimoto, M., Watanabe, T., Takaoka, M., Suzuki, K., Murakami, T., Murakami, N., & Sumikawa, S. (2021). Anti-Inflammatory Effect on Colitis and Modulation of Microbiota by Fermented Plant Extract Supplementation. Fermentation, 7(2), 55. https://doi.org/10.3390/fermentation7020055






