Abstract
Coffee is one of the most consumed beverages in the world, and its popularity has prompted the necessity to constantly increase the variety and improve the characteristics of coffee as a general commodity. The popularity of coffee as a staple drink has also brought undesired side effects, since coffee production, processing and consumption are all accompanied by impressive quantities of coffee-related wastes which can be a threat to the environment. In this review, we integrated the main studies on fermentative yeasts used in coffee-related industries with emphasis on two different directions: (1) the role of yeast strains in the postharvest processing of coffee, the possibilities to use them as starting cultures for controlled fermentation and their impact on the sensorial quality of processed coffee, and (2) the potential to use yeasts to capitalize on coffee wastes—especially spent coffee grounds—in the form of eco-friendly biomass, biofuel or fine chemical production.
1. Introduction
Along with tea, coffee is one of the most important habitual and social beverages in the modern world. Coffee represents an essential worldwide commodity and the major export commodity of around 70 tropical and subtropical countries [1]. In December 2020, the United States Department of Agriculture forecast the world coffee production for 2020/2021 at 175.5 million bags (60 kg per bag), which represents an increase of 7 million bags compared to the previous year [2]. The world market is dominated by two species of coffee: Coffea arabica (Arabica) and Coffeea canephora (Robusta). Arabica is native to Ethiopia, while Robusta is native to Central Africa. As suggested by its name, Robusta grows in dry areas, having a bitter taste and approximately double caffeine content compared to Arabica [3]. Although its caffeine concentration is lower, Arabica is considered to have higher quality, due to its organoleptic qualities [4]. Even so, both species are cultivated almost in equal amounts, Arabica and Robusta accounting for 56.6% and 43.4% of world production, respectively [2]. The subspecies of Robusta include Comilon, Kouilloi, Niaouli and Uganda, while the Arabica varieties include Moka, Maragogype, Bourbon, Mundo Novo, Caturra, Icatu, Catuai and Catimorand Creole [4].
Due to the high content of caffeine and of other valuable nutraceuticals such as caffeic acid, chlorogenic acid and a plethora of other polyphenols with antioxidant proprieties, coffee has important and well documented pharmacological significance. The chemical composition (organic acids, sugars, aromas including heterocyclic compounds) determines the strength, the taste and the flavor, but the quality of the coffee also depends on physical factors (size, color and defective beans) [5]. There are also numerous other factors which influence the quality of the final product, which include geographical origin and climate, temperature variations, altitude, species, harvesting methods, processing procedures and storage [6]. As for the end product, the coffee quality is evaluated in terms of acidity, sweetness, bitterness and aroma [7].
Yeasts, especially from the genus Saccharomyces, have been used since ancient times for baking, brewing, distilling, winemaking and other fermented beverage production (e.g., sake, palm wine) [8]. S. cerevisiae is one of the most studied microorganisms in both fundamental and industrial research, and the study of S. cerevisiae under wine fermentation conditions is one of the oldest and most representative research in biotechnology [9]. The natural habitats of yeasts include fruits, cacti, the bark of trees, soil, etc. A multitude of strains have been isolated from different sources and environments (industrial, laboratory, wild isolates); among numerous sources, yeasts were also isolated from unroasted coffee [10]. S. cerevisiae is highly used in the fermentation processes involved in coffee production or valorization of coffee waste residue. Besides S. cerevisiae, numerous yeast species from different genera have been detected in the coffee processing steps, which include Pichia, Candida, Saccharomyces and Torulaspora [11,12]. The coffee’s aroma is the result of a mix of over 800 volatile chemical compounds and the main chemical classes of compounds generated in coffee during and after fermentation with yeasts are: acids (citric, malic, succinic, chlorogenic and quinic acid), volatile compounds (alcohols, aldehydes and esters), furans, furanones, pyrans, and pyrones, ketones, lactones; phenols, pyrazines; pyridines, pyrroles and thiophene [12].
This mini review aims to offer a picture of the utility of yeasts in coffee processing, with a special focus on the studies revealing the influence of this fermentative microorganism on both the quality and chemical content of coffee-based beverages but also on its potential for the transformation of coffee residues in a value-added product.
2. Yeasts and Coffee Processing
The ripe coffee fruit used to obtain the coffee beans is called coffee cherry or coffee berry. The bean itself is the seed/pit inside the cherry, and—when roasted and ground—the source of the coffee beverage. The steps followed from cherry harvesting to obtaining the final product (roasted coffee) are presented in Figure 1a. The structure of the coffee cherry consists of the outer skin (pericarp), silverskin (perisperm) and coffee seed (endosperm) (Figure 1b, left). The pericarp consists of three layers, which from outside are: the exocarp (pulp), mesocarp (mucilage) and endocarp (parchment) [4].
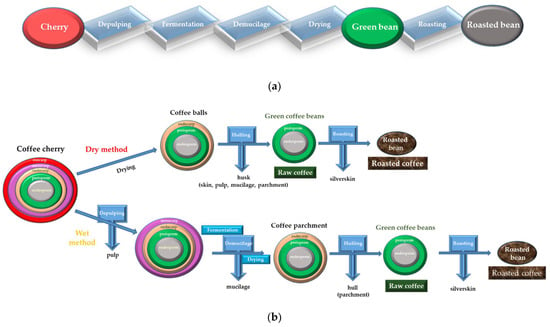
Figure 1.
From coffee cherry to coffee bean. (a) The main steps followed to obtain coffee beans. (b) The fate of coffee fruit on the road to roasted beans. The structure of coffee cherry consists of the outer skin (pericarp), silverskin (perisperm) and coffee seed (endosperm). The pericarp represents the outer three layers of the fruit: the exocarp (pulp), mesocarp (mucilage) and endocarp (parchment) [4]. In the dry method (top) commonly used for Robusta varieties, the coffee cherries are sun-dried until the moisture content is between 10% and 12%. The dried cherries are called coffee balls. In the wet method (bottom), applied mainly for Arabica varieties, the cherries are de-pulped to remove the skin and pulp. The resulting beans remain covered with a gluey texture similar to honey, the mucilage, which is subsequently removed by fermentation. After washing and drying to moisture content 10% to 12%, the coffee parchment is obtained, which represents the coffee beans surrounded by their endocarp (parchment) and perisperm (silver skin). The hulling is the process where the hull (parchment, in the wet method) of the coffee parchment or husk of the coffee ball (in the dry method) is mechanically removed [24]. The green coffee beans are obtained after hulling, and in the next step, the green beans are sorted and roasted [25].
2.1. Drying Methods
After harvesting, the ripe cherries are processed using three methods—wet, dry and semi-dry—which are crucial for the quality of the final product, as these methods influence the chemical composition of the end-product and determine the sensory characteristic of the coffee beverage [13]. The oldest and least sophisticated processing approach is the dry method, in which the coffee beans (the pit inside the cherry, and the source of the coffee beverage) are fermented and dried in the sun. The coffee cherries are washed and spread on platforms (or ground, concrete or tarmac) and laid to sun dry for 10 to 25 days until the beans’ moisture decreases to 11–12%. During this time, natural fermentation occurs, when enzymes from different microorganism are secreted, and the pulp and mucilage are degraded [14]. During this process, widely applied in Brazil and Ethiopia, the drying operation is the most important step because it affects the final coffee quality [15]. As expected, naturally-dried coffee is often associated with a high-quality product [16].
In the semi-dry method, the coffee beans are separated from the fruit by pulping. In this step, the exocarp and most of the mesocarp (also called mucilage) are removed, then the beans are allowed to sun dry until 10–12% moisture content is reached. Concomitantly with the drying step, the fermentation process occurs and the microorganisms degrade the mucilage [17]. This method is widely used in Brazil [14], and has its beginnings in the early 1990s [13,16].
Wet processing is widely used in some regions, including Colombia, Central America, and Hawaii [13]. In this method, the pulp and peel are mechanically removed from cherries, then the beans are placed in water tanks and submerged in water for 6–72 h to remove the mucilage [11,14,18]. This step also allows fermentation, and the remaining mucilage (comprising around 5% of the coffee cherries’ dry matter) that adheres to the coffee pulp is degraded [19]. The fruits are then spread over patios or dried mechanically [20]. The fermentation process ends when the mucilage layer is removed and the beans are allowed to sundry. A disadvantage of this method (applied mainly for Arabica species) is that huge amounts of water are necessary during the pulp removal and in the fermentation tanks [21,22]. The coffee husk and pulp have similar compositions: 7.5–15% protein, 2.0–7.0% fat and 21–32% carbohydrates [23].
2.2. Yeast as Starter Cultures in Coffee Processing
It is widely believed that coffee fermentation by inoculating different microorganisms, processed by different methods, increase the production of volatile compounds and consequently improve the flavor and aroma of the beverage [6,26,27]. The different fermentation methods used in the step of mucilage removal can change the chemical composition of green coffee through the action of microbial and/or endogenous coffee enzymes [28]. The mucilaginous layer is formed of polysaccharides (especially pectins). The pectinolytic enzymes (pectinases) from microorganisms (bacteria or yeasts) achieve the degradation of the pulp and mucilage to form alcohols and acids (acetic, malic, lactic, butyric and other long-chained carboxylic acids) [18]. The most important enzymes involved in coffee fermentation are polygalacturonase (PG), pectin lyase (PL) and pectin methylesterase (PME). The time required for this step is a function of the processing methods [29,30]. The selection of microorganism for the fermentation step is based on many criteria, such as PL secretion, production of volatile compounds, the physical and chemical changes and the lack of production of undesirable metabolites [20]. The starter cultures (selected as single or multiple strains) are used to assure better fermentation control and predictability of the final product [31]. The yeasts used as starter cultures influence the types of the compounds produced during fermentation and after roasting, which are correlated with the sensorial characteristics perceived during the cupping test. In this regard, the starter yeasts modulate the sensory characteristics of the beverage and their utilization represents an alternative for sensorial differentiation of coffee.
Different sensorial profiles of coffee increase the final value of the product [6]. Yeast metabolism triggers the hydrolysis of macromolecules yielding reducing sugars, amino acids and chlorogenic acids [32], which are important precursors of aroma. The sensorial perceptions are correlated with chemical compounds identified and compared with the procedures without microbial starters [33] and it was demonstrated that the starter cultures have a great impact on coffee fermentation and directly affect the quality of the final beverage, because there is a causal relationship between yeast cultures, organic acids and volatile profiles [6].
The fact that yeasts possess both high pectinolytic activity and an important role in the demucilaging of coffee was known since early studies, when it was revealed that pectinolytic yeasts such as Saccharomyces marxianus, S. bayanus, S. cerevisiae var. ellipsoideus and Schizosaccharomyces spp. have a role in the degradation of the mucilage layer of Robusta coffee [34]. In fact, it was shown that yeasts are involved from the very start of the process, as they lay on the cherry surface, taking part in the natural fermentation of coffee. Later, by adding a mixture of selected pectinolytic yeasts to the normal fermentation, the mucilage-layer degradation is accelerated. Especially, a mixture of Saccharomyces species was remarkably effective leading to the complete elimination of pectic substances within 7 to 8 h [34].
2.3. Selection of Yeasts as Starter Cultures in Wet, Dry and Semi-Dry Processing
To identify the most suitable strains to be used as starters, natural isolates were initially studied. In one of the early works, fifteen yeasts and bacteria isolated from dry and semi-dry coffee processing were evaluated for their ability to produce pectinolytic enzymes and organic compounds [20]. The strains cultivated on coffee peel and pulp media in a single culture or two-by-two mixed inocula had different behavior concerning PME, PL and PG [20], with the yeast strains showing a better behavior comparatively with bacterial strains, regarding both fermentation speed the quality of a dry fermentation process. Very good strains to be used as starter cultures evaluated for their ability to ferment the coffee pulp were identified as strains of S. cerevisiae, Pichia guilliermondii and Candida parapsilosis [20].
To identify the best yeast strains suitable for the wet-processing of coffee cherries, eight different yeast strains belonging to species Wickerhamomy cesanomalus, Saccharomycopsis fibuligera, Papiliotrema flavescens, Pichia kudriavzevii, and S. cerevisiae were isolated, identified and characterized and subsequently selected based on the ability to produce pectinases. All strains had yielded good results on two growth media: coffee pulp media and synthetic pectin media, but the S. cerevisiae strains produced superior pectin methylesterases [30]. Additionally for the wet method, the performance of yeasts was evaluated concerning the sensory quality and compounds profile, using S. cerevisiae and Torulaspora delbrueckii strains on Mundo Novo and Catuaí coffee varieties. The study demonstrated that 18 volatile compounds were detected in green coffee, and 75 volatile compounds in roasted coffee. This difference was attributed to chemical reactions such as the nonenzymatic browning, including Maillard reaction, and also to Strecker degradation of proteins and other degradation reactions of sugars and polysaccharides. Both yeast inoculations improved the sensory quality of the Catuaí and Mundo Novo variety of coffee, and two important contributors to the coffee aroma (2-furanmethanol propanoate and 2-ethyl-3,5-dimethylpyrazine) were identified. Nevertheless, T. delbrueckii was better adapted and presented more promising results in relation to the sensorial analysis [35].
In a different study made on Arabica, variety Catuaí Vermel, three yeasts (including S. cerevisiae) and six bacterial starters were inoculated in coffee beans pre-sterilized by autoclaving, followed by wet fermentation. Scanning electron microscopy was used to observe microbial adhesion, revealing that adhesion of inoculated microbial cells reached a maximum after 48 h of fermentation. As expected, the microorganism influenced the chemical composition of the coffee, especially the precursors of the flavored markers, such as heptadecanol, 4-hydroxy-2-methylacetophenone, 7-methyl-4-octanol and guaiacol. In yeast-treated coffee, heptadecanol, 4-hydroxy-2-methylacetophenone, 4-ethenyl-1,2-dimethoxybenzene were found, while 2-methyl,1-butanol was found only in bacterial-treated beans. At the same time, guaiacol was found only in the coffee inoculated with Bacillus subtilis as a starter. The study reconfirms the possibility of starter’s selection for a targeted quality of coffee, and that yeast starters ensure volatile alcohol production, while the bacterial starters ensure good production of acids. For yeasts, heptadecanol and 4-hydroxy-2-methylacetophenone (important for anticancer and antioxidant proprieties [36]) can be used as markers, while for bacteria, 7-methyl-4-octanol can be used to prove their presences and their metabolically active status during fermentation and guaiacol can be used only for B. subtilis strains [37].
Also using the wet method, a study on a strain of Pichia fermentans strain was shown that the yeast can be used as a starter culture to conduct controlled coffee bean fermentation. Using P. fermentans inoculum leads to an increased concentration of volatile compounds responsible for specific aroma (ethanol, acetaldehyde, ethyl acetate and isoamyl acetate) and a decreased production of lactic acid during the fermentation process. [38].
In a study on 144 yeasts isolated from spontaneously fermenting coffee beans, 9 isolates were evaluated for their potential to produce aromatic compounds in a coffee pulp simulation medium and for their pectinolytic activity. It was observed that one S. cerevisiae strain had the best pectinase activity, while a strain of Pichia fermentans produced the highest concentrations of flavor-active ester compounds (ethyl acetate and isoamyl acetate). In the wet fermentation experiments, using P. fermentans YC5.2 as inoculum, single or in combination with Saccharomyces sp. YC9.15, significant enhancement of the volatile aroma compounds was noted, with higher sensory scores for fruity, buttery and fermented aroma. The research indicated the starter inocula can indeed improve the coffee quality, at the same time giving the possibility to control and standardize the fermentation process towards final products with novel and desirable flavor profiles [38,39]. In a similar study involving wet fermentation of Australian coffee beans, 6 yeast and 17 bacterial species were identified, the yeasts having a major role in decreasing of pH and in producing compounds linked to superior coffee quality. Among the yeast species, Hanseniaspora uvarum and Pichia kudriavzevii were predominant, exhibiting enzymatic activities relevant for coffee bean fermentation [40].
2.4. Role of Yeasts in Controlling the Production of Non-Desired Compounds
The pharmacological effects of coffee have been intensively studied, and some of the endogenous compounds of coffee were shown to be associated with gastric irritation or increased blood cholesterol. These compounds are caffeine [41], chlorogenic acids, β-N-alkanoyl-5-hydroxytryptamides (C-5HTs) [42,43] furokaurane, diterpenes, cafestol and kahweol [44]. To decrease the content of these potentially toxic compounds, the producers use different approaches, such as steam treatment (for caffeine and chlorogenic acid removal) and wax removal (for β-N-alkanoyl-5-hydroxytryptamides) from green coffee beans [44]. In a study on the coffee fermentation by three S. cerevisiae strains as starter cultures (bakery, white and sparkling wines yeasts), an important decrease of C-5HTs and of diterpenes cafestol and kahweol was recorded. Importantly, the fermentation did not affect the caffeine or 5-caffeoylquinic acid (5-CQA) contents, monitored in the green coffee beans. Moreover, the use of starter cultures during the wet phase of processing had a positive influence on the content of some important health-related compounds, at the same time improving the sensory quality of the beverage [44].
A study of the microbiota associated with the semi-dry process [13] revealed that normally in the early stages of the fermentation bacteria prevail, while in the later stages the yeasts are predominant, simultaneously with the decreasing of the moisture content. Delays during the semi-dry fermentation and drying process can trigger the outgrowth of filamentous fungi, potentially leading to food safety risk and decreased beverage quality [45]. Yeasts have an essential role in preventing oxygenic filamentous fungi growth and boosting the production of pectinolytic enzymes, which helps the degradation of coffee mucilage and pulp [18]. In this regards, a good selection of a starter for fermentation is important to prevent the growth of filamentous fungi, especially producers of ochratoxin A (OTA) [31].
2.5. Role of Yeasts in Obtaining Specialty Coffees
S. cerevisiae strains used in the semi-dry coffee fermentation process were shown to have a special effect on chemical and sensorial properties of the final beverage by influencing the carbohydrate composition towards a good yield of glucose and fructose, also showing good adaptability to the fermentation process, and affording citric and succinic acids which are determinant for the good quality of the coffee [46]. To control the effect of yeast on the coffee flavor, the direct fermentation of sterilized green coffee beans is preferable; such an evaluation was done in the semi-dry process using monocultures of S. cerevisiae and P. kluyveri and assessing modifications on the final roasted coffee flavor. Using this approach, it was seen that yeasts were necessary for enhanced production of volatile compounds, also modulating the pathway of pyrolytic reactions during roasting [47].
2.6. Influence of Yeast Inoculation Methods on Chemical Composition of Coffee
Two methods of inoculating starter cultures were applied for different strains of S. cerevisiae, Candida parapsilosis and Torulaspora delbrueckii in a study using the semi-dry process: direct and bucket inoculation. The study revealed the benefit of the bucket method, which allowed multiplication of cells during coffee processing, especially in the case of S. cerevisiae [27].
Another method utilizing yeasts as starter cultures employed inoculation by directly spraying the cherries with yeast suspensions before the drying process. The study was achieved on three different yeasts (S. cerevisiae, Candida parapsilosis and Torulospora delbrueckii) using Arabica var. Cataui Amarelo processed by the dry method. The spray inoculation method was done in comparison to the buckets inoculation method and it was found that the former method influenced the concentration of caffeine, chlorogenic acid and trigonelline, while the concentration of citric and malic acids was high in both cases. After roasting, the coffee inoculated with S. cerevisiae by the spraying method had an extra caramel flavor, while the coffee inoculated with C. aparapsilosis had a fruity flavor (apple, cherry). In all samples, furfural (caramel, toasted odor), 2-heptanone (banana, fruity) and pyrazine-methyl (nutty, almond, sweet) were identified, with different intensities [48].
2.7. Yeast, Coffee and Climate
It was shown that coffees cultivated on soils at higher altitude have more expressive notes of flavor, aroma and sweetness than coffees from warmer regions [11]. Beside the microorganisms present in the coffee plants and in the post-harvest material, the high temperatures negatively influence the final coffee quality. In a study involving four methods of coffee processing and plants from six different altitude strata, it was found that on high altitude areas, the method of spontaneous water-washed fermentations or spontaneous semi-dry fermentations can positively influence the quality of the final product [49]. On the other hand, for plants cultivated in hot areas at lower altitude, the aroma of coffee was woodier, while plants grown at higher altitude, provide final products with a wider variation of aroma [49]. To improve the quality of coffee obtained from plants grown in the less favored climatic areas, biotechnological approaches using yeasts as starter cultures in the fermentation phase, especially S. cerevisiae, can be applied to gain quality [12]. At the same time, it must not be overlooked that spontaneous fermentation is more favorable for Arabica coffee grown at higher altitudes, while S. cerevisiae can improve the quality of the coffee grown at lower altitude [49]. In a study that evaluated the dynamic behavior of yeasts inoculated in coffee from different geographical origins, it was noticed that inoculation with S. cerevisiae and T. delbrueckii leads to a differentiation of chemical coffee composition and also to a superior sensorial profile, both yeast inoculation increasing the coffee beverage scores [50,51].
2.8. Special Compounds in Yeast-Fermented Coffee
In unprocessed coffee, fruits contain citric, malic and succinic acids, which are maintained during fermentation. Together with chlorogenic and quinic acids, they are the main acids in green coffee beans, favoring the sensorial characteristics of coffee [11]. At the initial fermentation time, citric, malic, succinic, lactic, oxalic, isobutyric and isovaleric acids prevail, together with numerous volatiles compounds. After fermentation, the main compounds are acids (citric, oxalic and especially lactic acid), alcohols (2,3-butanediol, 2-heptanol, phenylethyl alcohol and benzyl alcohol) and aldehydes. In the batches inoculated with S. cerevisiae and using the natural processing method, the presence of lactic, isobutyric and isovaleric acids were not detected, in contrast with the coffee processed using the pulped natural method. The utilization of selected yeasts proved to be an alternative for sensorial differentiation [6].
Some terpenes—such as 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one, DDMP—described as an antioxidant compound [52], are metabolites formed by biosynthesis during S. cerevisiae fermentations, conferring special characteristics to the coffee (sweet, caramel and nut characteristics). Yeast-fermented coffees yield another special compound named Cicloteno (2-hydroxy-1-methyl cyclopentene-3-one), an alcohol formed by the Maillard reaction from the lactic aldehyde resulted from oxidation and/or degradation of fructose, responsible for maple or caramel-like odor [32,48,53].
Many alcohols are produced by yeasts from intermediates of amino acid metabolism, such as oxy-acids, subsequently subjected to formation of esters through an esterification reaction between fatty acids and a molecule of alcohol. Ester formation may contribute to floral and fruity sensory notes of coffees [54], and the use of yeasts, which have the potential to increase the esters contents, may have a positive impact on the quality of the coffee [12,20,32].
As can be easily observed, the role of the starter cultures in the coffee fermentation process is to decrease the fermentation time, to improve the process control, to minimize the growth of toxigenic fungi and to increase the sensory quality by the production of metabolites that offer a special aroma to the final coffee product [15]. The main acids and volatile compounds associated with flavor identified in green or roasted coffee from a number of studies presented in this review are listed in Table 1.

Table 1.
Yeast strains used as starter inoculum in bean fermentation of coffee varieties and the main compounds in coffee associated with yeast fermentation.
3. Yeasts as Toolboxes for Capitalization of Coffee Wastes
The general consumption of coffee generates huge quantities of waste material all over the world and spent coffee grounds (SCG) represent the main byproduct of the coffee industry. It is considered that more than 50% of beans are removed in the form of SCG after the extraction of the final product or instant coffee making process [55]. Additionally, coffee residue wastes are generated by cafeterias (coffee shops, restaurants, etc.) and coffee factories. Numerically, 1 ton of green coffee generates about 650 kg of SCG and about 2 kg of wet SCG are obtained from each 1 kg of instant coffee produced [56]. In the coffee-producing countries, there is a critical need for improving the technologies used for waste coffee treatment in order to prevent ecological damage [57], and new strategies are considered to convert the coffee wastes into useful products. The coffee waste materials contain high amounts of hemicelluloses and lignin [55] that could generate high-value products including biofuels (e.g., biodiesel, bioethanol) and fine chemicals (lactic acid, polyhydroxy alkanoate) [58].
3.1. Coffee Wastes and the Environment
The huge amounts of residue generated after processing of the raw coffee, which are disposed by dumping into nature, cause serious environmental problems, including contamination of soil and groundwater due to the leaching [59]. Coffee pulp usually consists of approximately 50% carbohydrates, 20% fibers, 10% protein, 2.5% fat, 1.3% caffeine, tannins and other phenolic compounds (in dry weight) [60,61,62], but the normal utilization which applies to waste biomass (fertilizer, livestock feed, compost, etc.) is rather limited in the case of coffee pulp residues due to the high contents of caffeine and polyphenols [19]. The attempts to detoxify the coffee pulp for superior application in agriculture, and to use it for producing several added-value-products such as enzymes, flavor, aroma compounds, organic acids, etc., revealed the potential of the SCG [19]. There are numerous industrial processes used to release the phenols bound to the plant cell walls: fermentation, enzymatic extraction, alkaline and acid hydrolyzes [61] occasionally assisted by ultrasound or microwave [63]. Because these compounds are in high concentrations in the pulp and they are considered toxic to the environment, alcoholic fermentation could be used as a pretreatment to detoxify the coffee pulp prior to reuse in agriculture. At the same time, the recovered compounds can be used in other industries due to their functional properties [6].
In the wet method of coffee processing, a tremendous amount of waste is generated, in the form of pulp and contaminated waters. The wastewater is acidic and has a high amount of organic matter content with a high chemical oxygen demand, representing an important environmental hazard; therefore, new approaches need to be considered [57]. S. cerevisiae is often used to increase the number of hydroxycinnamic acids in coffee pulp extract through the breakage of ester bonds between these molecules and the pulp cell wall, allowing detoxification of the pulp and, at the same time, a decrease of the disposable residue, leading to a less harsh environmental impact and at the same time providing a new source of potential applications [59].
3.2. Spent Coffee Grounds and Biofuels
The bioenergy sectors, continuously concerned with achieving sustainable living conditions, use the agro-industrial wastes for bioethanol production by hydrolysis of the biomass followed by fermentation; the value of such wastes is considerable due to their non-competitiveness with foodstuffs, abundance and low cost [64]. Bioethanol is a byproduct of the sugar fermentation by yeast species, notably S. cerevisiae, most commonly used for ethanol production. It is well known that some yeast species ferment only hexoses, whereas others (e.g., Pichia stipitis) consume both hexoses and pentoses [63]. S. cerevisiae is widely used in the biotechnology industry, but this yeast does not naturally ferment xylose. In this regard, metabolic engineering represents a possibility to obtain strains designed for targeted aims. For example, a three-plasmid yeast expression system was developed utilizing the portable small ubiquitin-like modifier (SUMO) vector set combined with the efficient endogenous yeast protease Ulp1 to produce soluble proteins that allow xylose utilization by the engineered S. cerevisiae strain [65]. The same group of researchers reported that using recombinant technology, metabolically engineered S. cerevisiae cells can be used for expression of xylose isomerase and xylulokinase that enhance the xylose utilization [66]. The use of the waste resulted in coffee production as feedstock for biorefineries is increasingly interesting and various methodologies have been proposed, including gradual steps of bioconversion using different microorganisms sequentially. One example is the 4-step conversion: first, bioconversion consists of sugar fermentation by a thermotolerant strain (e.g., Kluyveromyces marxianus); in the second step, the resulting sugar-depleted solids are treated with the yeast Yarrowia lipolytica, then the lignocellulosic fraction of the waste was treated with a recombinant strain of S. cerevisiae, to have the residual solids treated with an engineered strain of Rhodotorula glutinis [65] to be further processed into renewable gasoline from coffee and other crop wastes [57].
The bioethanol production can be enhanced using popping pretreated-coffee residue waste subjected to enzymatic hydrolysis performed at high pressure, followed by simultaneous saccharyfication and fermentation by S. cerevisiae [58]. Among the sugars present in the coffee waste, mannose has the highest concentration (20–25% of the total carbohydrate content) [57], making this wastes an excellent mannose source for its eventual use in food industry, biological research and pharmaceutical applications [63]. However, mannose occurs mainly in the form of galactomannans, which are bound to arabinogalactans and cellulose to form polymers that are highly recalcitrant and hydrophilic; thus, the recovery of mannose from coffee waste by enzymatic hydrolysis is very difficult [58]. A biorefinery process for the production of D-mannose and ethanol from coffee residue waste was proposed, using strains of S. cerevisiae and Pichia stipites which allow simultaneous separation of mannose following hydrolysis and ethanol production under various fermentation conditions [61].
Another method to obtain ethanol and biodiesel from SCG involved ultrasound-assisted solvent extraction of SCG oil, although the procedure’s drawback was the high acidity of the SCG oil which did not allow direct transesterification; this problem can be overcome by processing oil-free SCG hydrolyzed by sulfuric to be used directly in fermentation with S. cerevisiae [67], providing better yield comparative with ethanol productivity from whole coffee husks [68].
Triglycerides and free fatty acids present in the SCG sometimes hinder the enzymatic saccharyfication, leading to a low conversion to ethanol. To overcome this problem, a biorecovery method was proposed to convert the crude lipids extracted from SCG into fatty acid methyl esters and fatty acid ethyl esters via the non-catalytic biodiesel transesterification reaction [64]. This procedure afforded high yields of bioethanol calculated against consumed sugar and lipids extracted from SCG. Moreover, obtaining fatty acid ethyl esters rather than fatty acid methyl esters increased the overall renewability [69]. Another approach to valorize the SCG introduced a combination atmospheric air plasma and FeCl3, in order to create a superior pretreatment that involve Fenton chemistry for a more effective delignification prior to enzymatic treatment and bioethanol production by S. cerevisiae [70]. Using S. cerevisiae, bioethanol was also obtained from other wastes such as coffee juice and mucilage. The technique proposed involved acid hydrolysis to increase the fermentable sugars content in the must, followed by application of the inoculum for the efficient performance of the fermentation [71].
Production of coffee pulp extract for further use in the ethanol production can be improved by the addition of molasses or sugarcane juice. Using a S. cerevisiae strain, it was observed that the best procedure for obtaining coffee pulp extract was grinding at room temperature followed by manual pressing. With this procedure, the total amount of sugars did not vary, but lower concentrations of pectin and phenolic compounds were obtained. From different fermentation assays (fermentation media with sugarcane juice, molasses, coffee pulp extract, and mixtures of the substances), mixing of coffee pulp with sugarcane juice or molasses increased the yield of ethanol production [72].
3.3. Spent Coffee Grounds and Fine Chemicals
Some possible utilization of SGC to obtain various organic chemicals is presented in Figure 2. The valorization of wastes in the form of lactic acid received increased attention due to intensive use of lactic acid in different industries—cosmetic, chemical (polylactic acid a biodegradable plastic), pharmaceutical and even in food production [73].
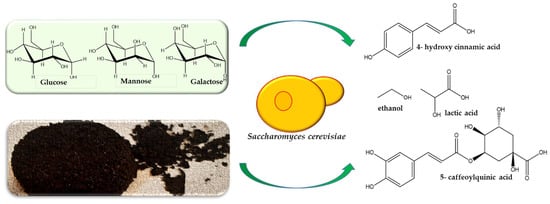
Figure 2.
Capitalization of spent coffee waste. Appling different methods of treatment, the spent coffee waste (rich in glucose, mannose or galactose) can be turned into industrial by-products such as bioethanol, lactic acid or 5-cafeoylquinic acid.
Lactic acid is a primary product of bacterial fermentation. Because the lactic acid bacteria have low tolerance to toxic compounds, S. cerevisiae—whose tolerance to toxic compounds is higher—is often preferred [68]. SCG as a substrate for lactic acid production through fermentation by S. cerevisiae is possible only by engineered strains expressing heterologous lactate dehydrogenase [74]. The ability of the engineered S. cerevisiae strain to generate lactic acid and ethanol from SCG was assayed comparatively with the whole slurry containing different types of fermentable sugars and it was revealed that SCG with a substantial amount of hemicelluloses could be transformed into lactic acid and ethanol, and that lactic acid production was promoted by xylose addition. [74].
In a study on the possibility to use yeasts for capitalization of coffee pulp, eight yeast strains cultivated in a mixture were analyzed in a semi-dry process. It was observed that a strain of Hanseniaspora uvarum showed high potential for fermentation of coffee residues with good yield of ethanol production, also yielding an important number of valuable compounds (higher alcohols, acetates, terpenes, and aldehyde) [75]. Rhodotorula mucilaginosa CCMA 0156, a pigmented yeast, able to grow on husk and pulp coffee, was proved to be a strong candidate to produce higher biomass and in the same time, carotenoids, especially β-carotene. The carotenoids from coffee pulp had antioxidant and antimicrobial characteristics, adding valuable proprieties to those substances, mainly used as colorants [76].
4. Concluding Remarks
As coffee consumption constantly increased all over the world, the quality of coffee became an important aim of food product research. In this review, we presented the most representative studies set to reveal the importance of various yeast strains used in the fermentative steps of postharvest coffee processing, with a focus on how the interplay between yeast fermentative traits, strain selection and variation of processing methods can influence the quality of the final product. A significant number of studies presented in this review revealed the importance of yeast starters in influencing the sensory characteristics of coffee. Special attention was paid to yeast fermentation processes which afforded extracellular enzymes secretion and production of various valuable organic compounds which contribute to the organoleptic traits of coffee products. This review also presented studies dealing with the involvement of yeasts in biotechnologies related to processing of the ever-increasing coffee waste residues and how natural or engineered yeasts can be used to process the coffee wastes to produce valuable products such as processed biomass, bioethanol or biodiesel, or valuable chemicals such as reducing sugars, lactic acid, polyphenolic antioxidants, etc. In conclusion, the use of yeasts in the coffee-related industries is relevant and has a dualistic valence: contributing to improve and diversify the sensorial quality of coffee and, at the same time, emerging as a tool for capitalization of cumbersome coffee wastes, such as spent coffee grounds.
Author Contributions
Conceptualization, L.L.R. and I.C.F. investigation, L.L.R.; resources, I.C.F.; writing—original draft preparation, L.L.R.; writing—review and editing, L.L.R. and I.C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/3/a-i4985e.pdf (accessed on 17 December 2020).
- USDA. United States Department for Agriculture. Available online: https://apps.fas.usda.gov/psdonline/circulars/coffee.pdf (accessed on 17 December 2020).
- Martín, M.L.; Pablos, F.; González, A.G. Discrimination between arabica and robusta green coffee varieties according to their chemical composition. Talanta 1998, 46, 1259–1264. [Google Scholar] [CrossRef]
- Pérez, S.B.Y.; Saldaña-Trinidad, S. Chemistry and biotransformation of coffee by products to biofuels. In The Question of Caffeine; Latosinska, J.N., Latosinska, M., Eds.; InTech: London, UK, 2017; pp. 143–161. [Google Scholar] [CrossRef]
- Cheng, B.; Smyth, H.E.; Furtado, A.; Henry, R.J. Slower development of lower canopy beans produces better coffee. J. Exp. Bot. 2020, 71, 4201–4214. [Google Scholar] [CrossRef] [PubMed]
- Bressani, A.P.P.; Martinez, S.J.; Sarmento, A.B.I.; Borém, F.M.; Schwan, R.F. Organic acids produced during fermentation and sensory perception in specialty coffee using yeast starter culture. Food Res. Int. 2020, 128, 108773. [Google Scholar] [CrossRef] [PubMed]
- Sunarharum, W.B.; Williams, D.J.; Smyth, H.E. Complexity of coffee flavor: A compositional and sensory perspective. Food Res. Int. 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Camarasa, C.; Sanchez, I.; Brial, P.; Bigey, F.; Dequin, S. Phenotypic landscape of Saccharomyces cerevisiae during wine fermentation: Evidence for origin-dependent metabolic traits. PLoS ONE 2011, 6, e25147. [Google Scholar] [CrossRef]
- Ruta, L.L.; Farcasanu, I.C. Anthocyanins and anthocyanin-derived products in yeast-fermented beverages. Antioxidants 2019, 8, 182. [Google Scholar] [CrossRef]
- Ludlow, C.L.; Cromie, G.A.; Garmendia-Torres, C.; Sirr, A.; Hays, A.; Field, C.; Jeffery, E.W.; Fay, J.C.; Dudle, A.D. Independent origins of yeast associated with coffee and cacao fermentation. Curr. Biol. 2016, 26, 965–971. [Google Scholar] [CrossRef]
- De Bruyn, F.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuyst, L. Exploring the impacts of postharvest processing on the microbiota and metabolite profiles during green coffee bean production. Appl. Environ. Microbiol. 2016, 83, e02398-16. [Google Scholar] [CrossRef]
- Evangelista, S.R.; Miguel, M.G.; de Souza Cordeiro, C.; Silva, C.F.; Pinheiro, A.C.; Schwan, R.F. Inoculation of starter cultures in a semi-dry coffee (Coffea arabica) fermentation process. Food Microbiol. 2014, 44, 87–95. [Google Scholar] [CrossRef]
- Vilela, D.M.; Pereira, G.V.; Silva, C.F.; Batista, L.R.; Schwan, R.F. Molecular ecology and polyphasic characterization of the microbiota associated with semi-dry processed coffee (Coffea arabica L.). Food Microbiol. 2010, 27, 1128–1135. [Google Scholar] [CrossRef]
- Silva, C.F.; Batista, L.R.; Abreu, L.M.; Dias, E.S.; Schwan, R.F. Succession of bacterial and fungal communities during natural coffee (Coffea arabica) fermentation. Food Microbiol. 2008, 25, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Vinícius de Melo Pereira, G.; Soccol, V.T.; Brar, S.K.; Neto, E.; Soccol, C.R. Microbial ecology and starter culture technology in coffee processing. Crit. Rev. Food Sci. Nutr. 2017, 57, 2775–2788. [Google Scholar] [CrossRef]
- Duarte, G.S.; Pereira, A.A.; Farah, A. Chlorogenic acids and other relevant compounds in Brazilian coffees processed by semi-dry and wet post-harvesting methods. Food Chem. 2010, 118, 851–855. [Google Scholar] [CrossRef]
- Batista, L.R.; Chalfoun, S.M.; Batista, C.F.S.; Schwan, R.F. Coffee: Types and production. In The Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P., Toldrá, F., Eds.; Academic Press: Oxford, UK; Cambridge, MA, USA, 2016; p. 244. [Google Scholar]
- Silva, C.F. Microbial activity during coffee fermentation. In Cocoa and Coffee Fermentations; Schwan, R.F., Fleet, G.H., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 368–423. [Google Scholar]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recy. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Silva, C.F.; Vilela, D.M.; de SouzaCordeiro, C.; Duarte, W.F.; Dias, D.R.; Schwan, R.F. Evaluation of a potential starter culture for enhances quality of coffee fermentation. World J. Microbiol. Biotechnol. 2013, 29, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Avallone, S.; Brillouet, J.M.; Guyot, B.; Olguin, E.; Guiraud, J.P. Involvement of pectinolytic microorganisms is coffee fermentation. Int. J. Food Sci. Technol. 2002, 37, 191–198. [Google Scholar] [CrossRef]
- Masoud, W.; Jespersen, L. Pectin degrading enzymes in yeasts involved in fermentation of Coffea arabica in East Africa. Int. J. Food Microbiol. 2006, 110, 291–296. [Google Scholar] [CrossRef]
- Ulloa Rojas, J.B.; Verreth, J.A.; Amato, S.; Huisman, E.A. Biological treatments affect the chemical composition of coffee pulp. Bioresour. Technol. 2003, 89, 267–274. [Google Scholar] [CrossRef]
- Bekalo, S.A.; Reinhardt, H. Fibers of coffee husk and hulls for the production of particleboard. Mater. Struct. 2010, 43, 1049–1060. [Google Scholar] [CrossRef]
- Aristizábal, V.M.; Chacón-Perez, Y.; Carlos, A.; Alzate, C. The biorefinery concept for the industrial valorization of coffee processing by-products. In Handbook of Coffee Processing by-Products; Galanakis, C.M., Ed.; Academic Press: Oxford, UK; Cambridge, MA, USA, 2017; pp. 63–92. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, M.K.; Lee, K.G. Effect of reversed coffee grinding and roasting process on physicochemical properties including volatile compound profiles. Innov. Food Sci. Emerg. Technol. 2017, 44, 97–102. [Google Scholar] [CrossRef]
- Martinez, S.J.; Bressani, A.P.P.; Miguel, M.G.D.C.P.; Dias, D.R.; Schwan, R.F. Different inoculation methods for semi-dry processed coffee using yeasts as starter cultures. Food Res. Int. 2017, 102, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Avallone, S.; Guyot, B.; Brillouet, J.M.; Olguin, E.; Guiraud, J.P. Microbiological and biochemical study of coffee fermentation. Curr. Microbiol. 2001, 42, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Haile, M.; Kang, W.H. The role of microbes in coffee fermentation and their impact on coffee quality. J. Food Qual. 2019, 2019, 4836709. [Google Scholar] [CrossRef]
- Haile, M.; Kang, W.H. Isolation, identification, and characterization of pectinolytic yeasts for starter culture in coffee fermentation. Microorganisms 2019, 7, 401. [Google Scholar] [CrossRef] [PubMed]
- Massawe, G.A.; Lifa, S.J. Yeasts and lactic acid bacteria coffee fermentation starter cultures. Int. J. Postharvest Technol. Innov. 2010, 2, 41–80. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Modulation of coffee aroma via the fermentation of green coffee beans with Rhizopusoligosporus: I. Green coffee. Food Chem. 2016, 211, 916–924. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Modulation of coffee aroma via the fermentation of green coffee beans with Rhizopusoligosporus: II. Effects of different roast levels. Food Chem. 2016, 211, 925–936. [Google Scholar] [CrossRef]
- Agate, A.D.; Bhat, J.V. Role of pectinolytic yeasts in the degradation of mucilage layer of Coffea robusta cherries. Appl. Microbiol. 1966, 14, 256–260. [Google Scholar] [CrossRef]
- Martins, P.M.M.; Ribeiro, L.S.; Miguel, M.G.D.C.P.; Evangelista, S.R.; Schwan, R.F. Production of coffee (Coffea arabica) inoculated with yeasts: Impact on quality. J. Sci. Food Agric. 2019, 99, 5638–5645. [Google Scholar] [CrossRef]
- Dandekar, R.; Fegade, B.; Bhaskar, V.H. GC-MS analysis of phytoconstituents in alcohol extract of Epiphyllumoxy petalum leaves. J. Pharmacogn. Phytochem. 2015, 4, 149–154. [Google Scholar]
- Martinez, S.J.; Bressani, A.P.P.; Dias, D.R.; Simão, J.B.P.; Schwan, R.F. Effect of bacterial and yeast starters on the formation of volatile and organic acid compounds in coffee beans and selection of flavors markers precursors during wet fermentation. Front. Microbiol. 2019, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- De Melo Pereira, G.V.; Soccol, V.T.; Pandey, A.; Medeiros, A.B.; Andrade Lara, J.M.; Gollo, A.L.; Soccol, C.R. Isolation, selection and evaluation of yeasts for use in fermentation of coffee beans by the wet process. Int. J. Food Microbiol. 2014, 188, 60–66. [Google Scholar] [CrossRef] [PubMed]
- De Melo Pereira, G.V.; Neto, E.; Soccol, V.T.; Medeiros, A.B.P.; Woiciechowski, A.L.; Soccol, C.R. Conducting starter culture-controlled fermentations of coffee beans during on-farm wet processing: Growth, metabolic analyses and sensorial effects. Food Res. Int. 2015, 75, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Cox, J.; Zhao, J. Ecological diversity, evolution and metabolism of microbial communities in the wet fermentation of Australian coffee beans. Int. J. Food Microbiol. 2020, 321, 108544. [Google Scholar] [CrossRef]
- Pehl, C.; Pfeiffer, A.; Wendl, B.; Kaess, H. The effect of decaffeination of coffee on gastro-oesophageal reflux in patients with reflux disease. Aliment. Pharmacol. Ther. 1997, 11, 483–486. [Google Scholar] [CrossRef]
- Rubach, M.; Lang, R.; Skupin, C.; Hofmann, T.; Somoza, V. Activity-guided fractionation to characterize a coffee beverage that effectively down-regulates mechanisms of gastric acid secretion as compared to regular coffee. J. Agric. Food Chem. 2010, 58, 4153–4161. [Google Scholar] [CrossRef]
- Rubach, M.; Lang, R.; Seebach, E.; Somoza, M.M.; Hofmann, T.; Somoza, V. Multi-parametric approach to identify coffee components that regulate mechanisms of gastric acid secretion. Mol. Nutr. Food Res. 2012, 56, 325–335. [Google Scholar] [CrossRef]
- Tinoco, N.A.B.; Pacheco, S.; Godoy, R.L.O.; Bizzo, H.R.; de Aguiar, P.F.; Leite, S.G.F.; Rezende, C.M. Reduction of βN-alkanoyl-5-hydroxytryptamides and diterpenes by yeast supplementation to green coffee during wet processing. Food Res. Int. 2019, 115, 487–492. [Google Scholar] [CrossRef]
- Velmourougane, K.; Bhat, R.; Gopinandhan, T.N.; Panneerselvam, P. Impact of delay in processing on mold development, ochratoxin-A and cup quality in Arabica and Robusta coffee. World J. Microb. Biot. 2011, 27, 1809–1816. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Miguel, M.G.; Evangelista, S.R.; Machado Martins, P.M.; van Mullem, J.; Belizario, M.H.; Schwan, R.F. Behavior of yeast inoculated during semi-dry coffee fermentation and the effect on chemical and sensorial properties of the final beverage. Food Res. Int. 2017, 92, 26–32. [Google Scholar] [CrossRef]
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Liu, S.Q. Coffee flavour modification through controlled fermentations of green coffee beans by Saccharomyces cerevisiae and Pichia kluyveri: Part I. Effects from individual yeasts. Food Res. Int. 2020, 136, 109588. [Google Scholar] [CrossRef]
- Bressani, A.P.P.; Martinez, S.J.; Evangelista, S.R.; Ribeiro, D.D.; Schwan, R.F. Characteristics of fermented coffee inoculated with yeast starter cultures using different inoculation methods. LWT Food Sci. Technol. 2018, 92, 212–219. [Google Scholar] [CrossRef]
- Pereira, L.L.; Guarçoni, R.C.; Pinheiro, P.F.; Osório, V.M.; Pinheiro, C.A.; Moreira, T.R.; Ten Caten, C.S. New propositions about coffee wet processing: Chemical and sensory perspectives. Food Chem. 2020, 310, 125943. [Google Scholar] [CrossRef] [PubMed]
- Brando, C.H.J.; Brando, M.F. Methods of coffee fermentation and drying. In Cocoa and Coffee Fermentations; Schwan, R.F., Fleet, G.H., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 367–396. [Google Scholar]
- Da Mota, M.C.B.; Batista, N.N.; Rabelo, M.H.S.; Ribeiro, D.E.; Borém, F.M.; Schwan, R.F. Influence of fermentation conditions on the sensorial quality of coffee inoculated with yeast. Food Res. Int. 2020, 136, 109482. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, M.; Liu, F.; Zeng, S.; Hu, J. Identification of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one as a strong antioxidant in glucose-histidine Maillard reaction products. Food Res. Int. 2013, 51, 397–403. [Google Scholar] [CrossRef]
- Cutzach, I.; Chatonnet, P.; Dubourdieu, D. Study of the formation mechanisms of some volatile compounds during the aging of sweet fortified wines. J. Agric. Food Chem. 1999, 47, 2837–2846. [Google Scholar] [CrossRef]
- Moreira, R.F.A.; Trugo, L.C.; Maria, C.A.B. Volatile components in roasted coffee. Part II. Aliphatic, alicyclic and aromatic compounds. Quím. Nova 2000, 23, 195–201. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Tech. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Mussatto, S.J.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, composition and application of coffee and its industrial residues. Food Bioprocess. Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- Hughes, S.R.; López-Núñez, J.C.; Jones, M.A.; Moser, B.R.; Cox, E.J.; Lindquist, M.; Galindo-Leva, L.A.; Riaño-Herrera, N.M.; Rodriguez-Valencia, N.; Gast, F.; et al. Sustainable conversion of coffee and other crop wastes to biofuels and bioproducts using coupled biochemical and thermochemical processes in a multi-stage biorefinery concept. Appl. Microbiol. Biotechnol. 2014, 98, 8413–8431. [Google Scholar] [CrossRef]
- Choi, I.S.; Wi, S.G.; Kim, S.B.; Bae, H.J. Conversion of coffee residue waste into bioethanol with using popping pretreatment. Bioresour. Technol. 2012, 125, 132–137. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.S.; Durand, N.; Lacour, S.; Belleville, M.P.; Perez, A.; Loiseau, G.; Dornier, M. Solid-state fermentation as a sustainable method for coffee pulp treatment and production of an extract rich in chlorogenic acids. Food Bioprod. Process. 2019, 115, 175–184. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Brand, D.; Mohan, R.; Roussos, S. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem. Eng. J. 2000, 6, 153–162. [Google Scholar] [CrossRef]
- Kim, K.; Tsao, R.; Yang, R.; Cui, S. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Solhy, A.; Clark, J.H.; Koutinas, A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Nguyen, Q.A.; Cho, E.; Trinh, L.T.P.; Jeong, J.S.; Bae, H.J. Development of an integrated process to produce d-mannose and bioethanol from coffee residue waste. Bioresour. Technol. 2017, 244 Pt 1, 1039–1048. [Google Scholar] [CrossRef]
- Hughes, S.R.; Sterner, D.E.; Bischoff, K.M.; Hector, R.E.; Dowd, P.F.; Qureshi, N.; Bang, S.S.; Grynaviski, N.; Chakrabarty, T.; Johnson, E.T.; et al. Three-plasmid SUMO yeast vector system for automated high-level functional expression of value-added co-products in a Saccharomyces cerevisiae strain engineered for xylose utilization. Plasmid 2009, 61, 22–38. [Google Scholar] [CrossRef]
- Hughes, S.R.; Cox, E.J.; Bang, S.S.; López-Núñez, J.C.; Saha, B.C.; Qureshi, N.; Gibbons, W.R.; Fry, M.R.; Moser, B.R.; Bischoff, K.M.; et al. Process for assembly and transformation into saccharomyces cerevisiae of a synthetic yeast artificial chromosome containing a multigene cassette to express enzymes that enhance xylose utilization designed for an automated platform. J. Lab. Autom. 2015, 20, 621–635. [Google Scholar] [CrossRef][Green Version]
- Rocha, M.V.; de Matos, L.J.; Lima, L.P.; Figueiredo, P.M.; Lucena, I.L.; Fernandes, F.A.; Gonçalves, L.R. Ultrasound-assisted production of biodiesel and ethanol from spent coffee grounds. Bioresour. Technol. 2014, 167, 343–348. [Google Scholar] [CrossRef]
- Gouvea, B.M.; Torres, C.; Franca, A.S.; Oliveira, L.S.; Oliveira, E.S. Feasibility of ethanol production from coffee husks. Biotechnol. Lett. 2009, 31, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.E.; Yi, H.; Jeon, Y.J. Sequential co-production of biodiesel and bioethanol with spent coffee grounds. Bioresour. Technol. 2013, 136, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Sarangapani, C.; Jaiswal, S.; Cullen, P.J.; Jaiswal, A.K. Ferric chloride assisted plasma pretreatment of lignocellulose. Bioresour. Technol. 2017, 243, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Harsono, S.S.; Fauzi, M.; Purwono, G.S.; Soemarno, D. Second generation bioethanol from Arabica coffee waste processing at small holder plantation in Ijen Plateau region of East Java. Procedia Chem. 2015, 14, 408–413. [Google Scholar] [CrossRef]
- Lee, J.W.; In, J.H.; Park, J.B.; Shin, J.; Park, J.H.; Sung, B.H.; Sohn, J.H.; Seo, J.H.; Park, J.B.; Kim, S.R.; et al. Co-expression of two heterologous lactate dehydrogenases genes in Kluyveromyces marxianus for l-lactic acid production. J. Biotechnol. 2017, 241, 81–86. [Google Scholar] [CrossRef]
- Kim, J.W.; Jang, J.H.; Yeo, H.J.; Seol, J.; Kim, S.R.; Jung, Y.H. Lactic acid production from a whole slurry of acid-pretreated spent coffee grounds by engineered Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2019, 189, 206–216. [Google Scholar] [CrossRef]
- Menezes, E.G.; do Carmo, J.R.; Menezes, A.G.; Alves, J.G.; Pimenta, C.J.; Queiroz, F. Use of different extracts of coffee pulp for the production of bioethanol. Appl. Biochem. Biotechnol. 2013, 169, 673–687. [Google Scholar] [CrossRef]
- Bonilla-Hermosa, V.A.; Duarte, W.F.; Schwan, R.F. Utilization of coffee by-products obtained from semi-washed process for production of value-added compounds. Bioresour. Technol. 2014, 166, 142–150. [Google Scholar] [CrossRef]
- Moreira, M.D.; Melo, M.M.; Coimbra, J.M.; Reis, K.C.D.; Schwan, R.F.; Silva, C.F. Solid coffee waste as alternative to produce carotenoids with antioxidant and antimicrobial activities. Waste Manag. 2018, 82, 93–99. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).