Production of Renewable Lipids by the Diatom Amphora copulata
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Isolation, Identification, and Screening
2.2. Diatom Preculture
2.3. Growth and Lipid Production
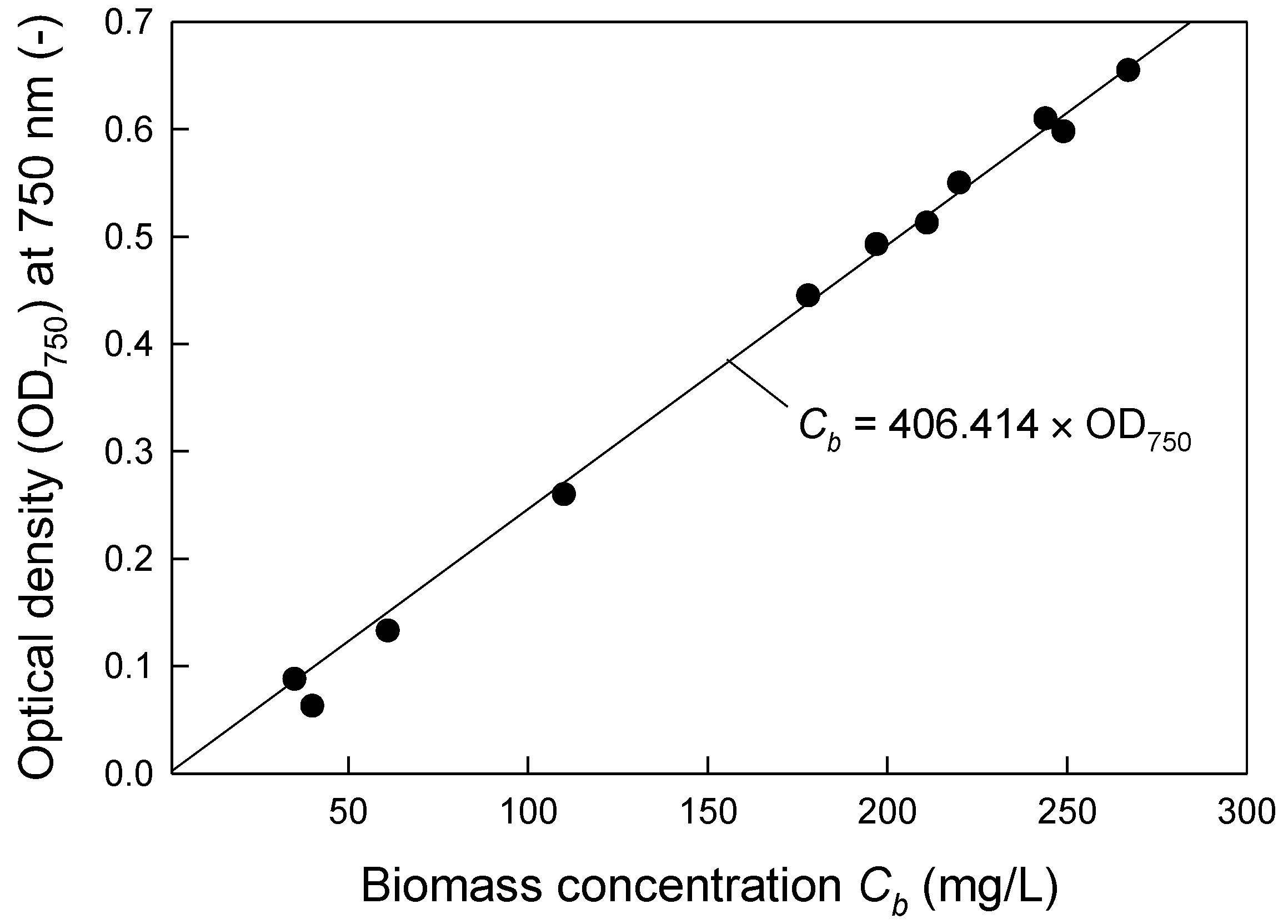
2.4. Determination of Dry Cell Mass Concentration and the Specific Growth Rate
2.5. Lipid Extraction and Quantification
2.6. Lipid Composition Analysis
3. Results and Discussion
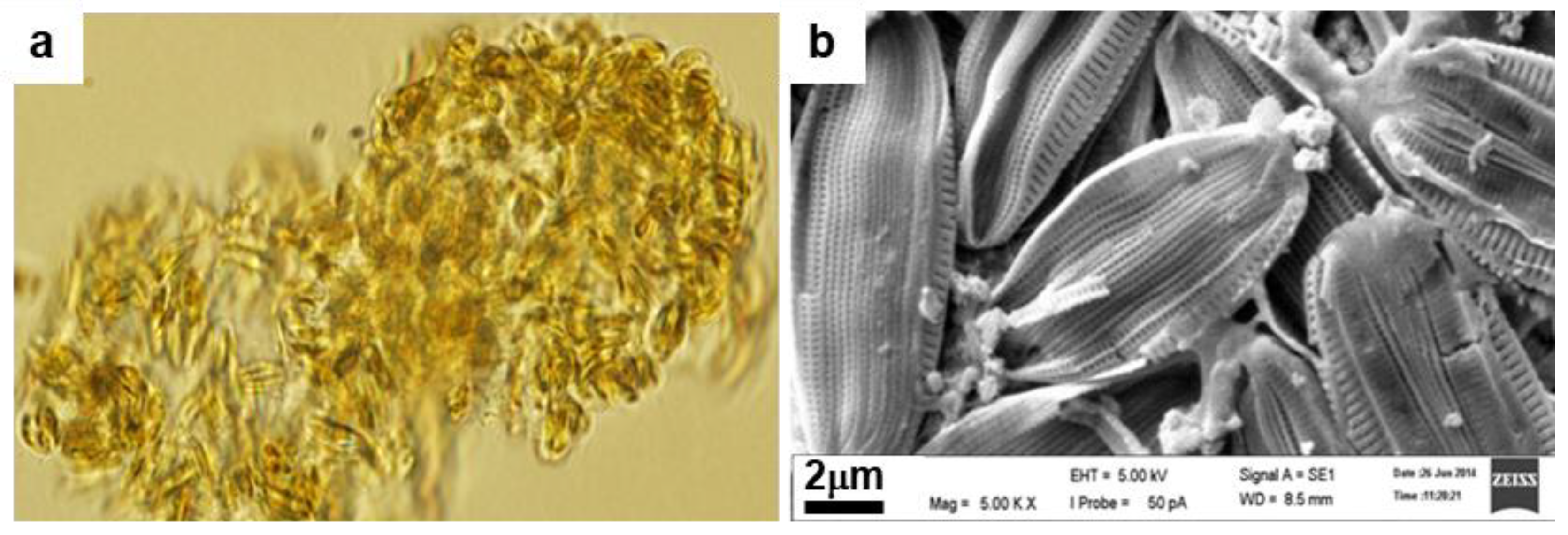
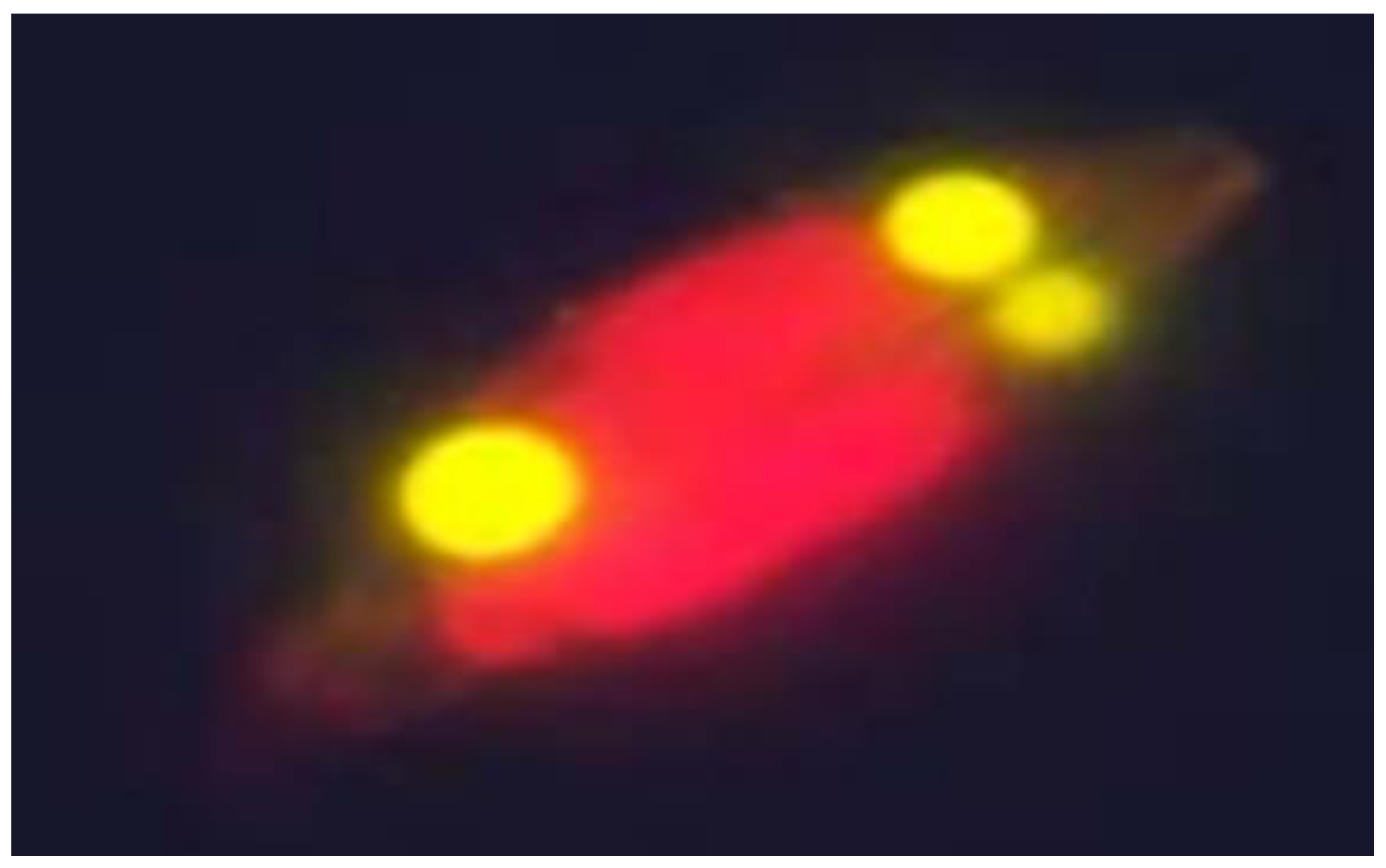
3.1. Identification of the Diatom
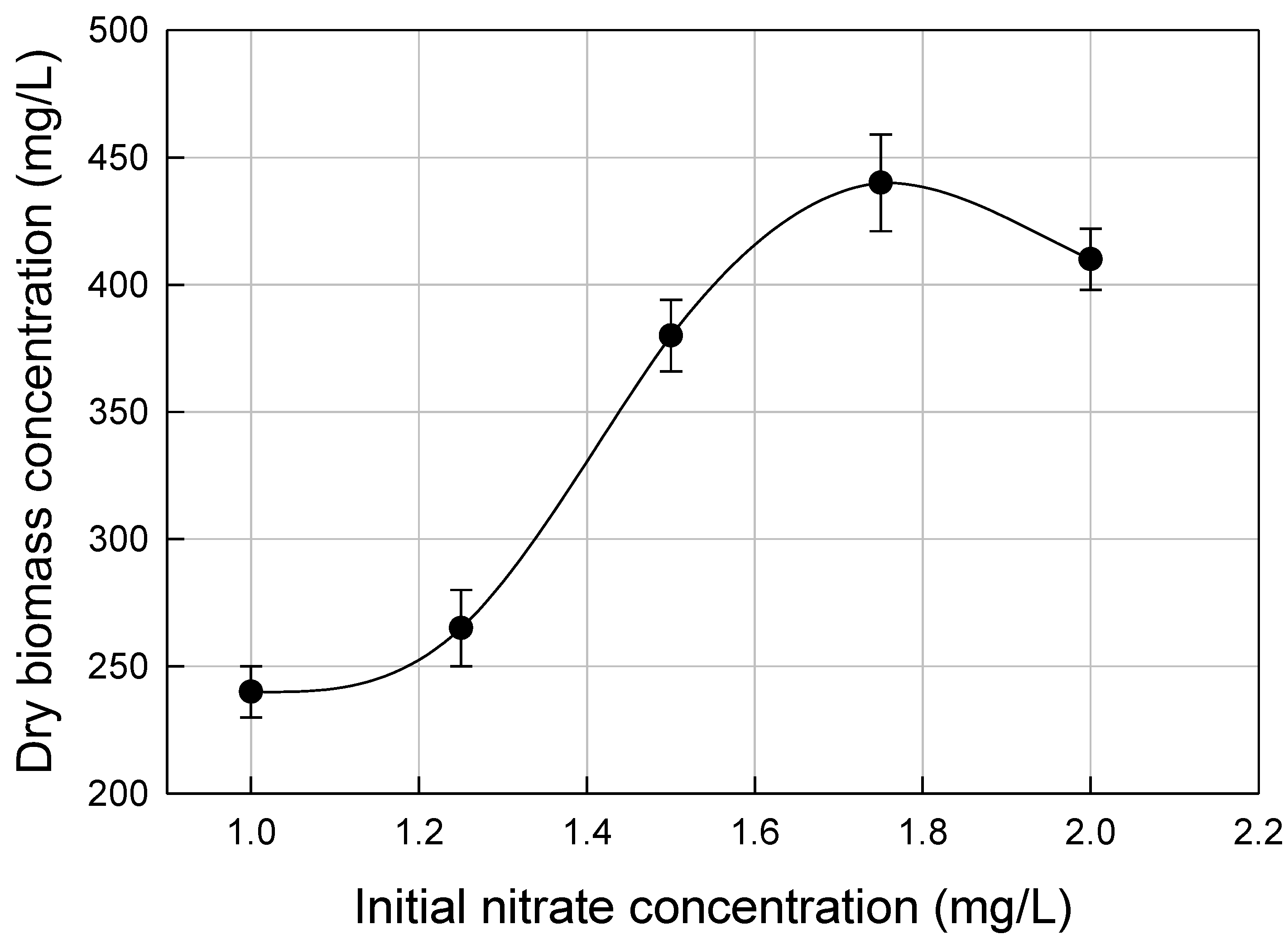
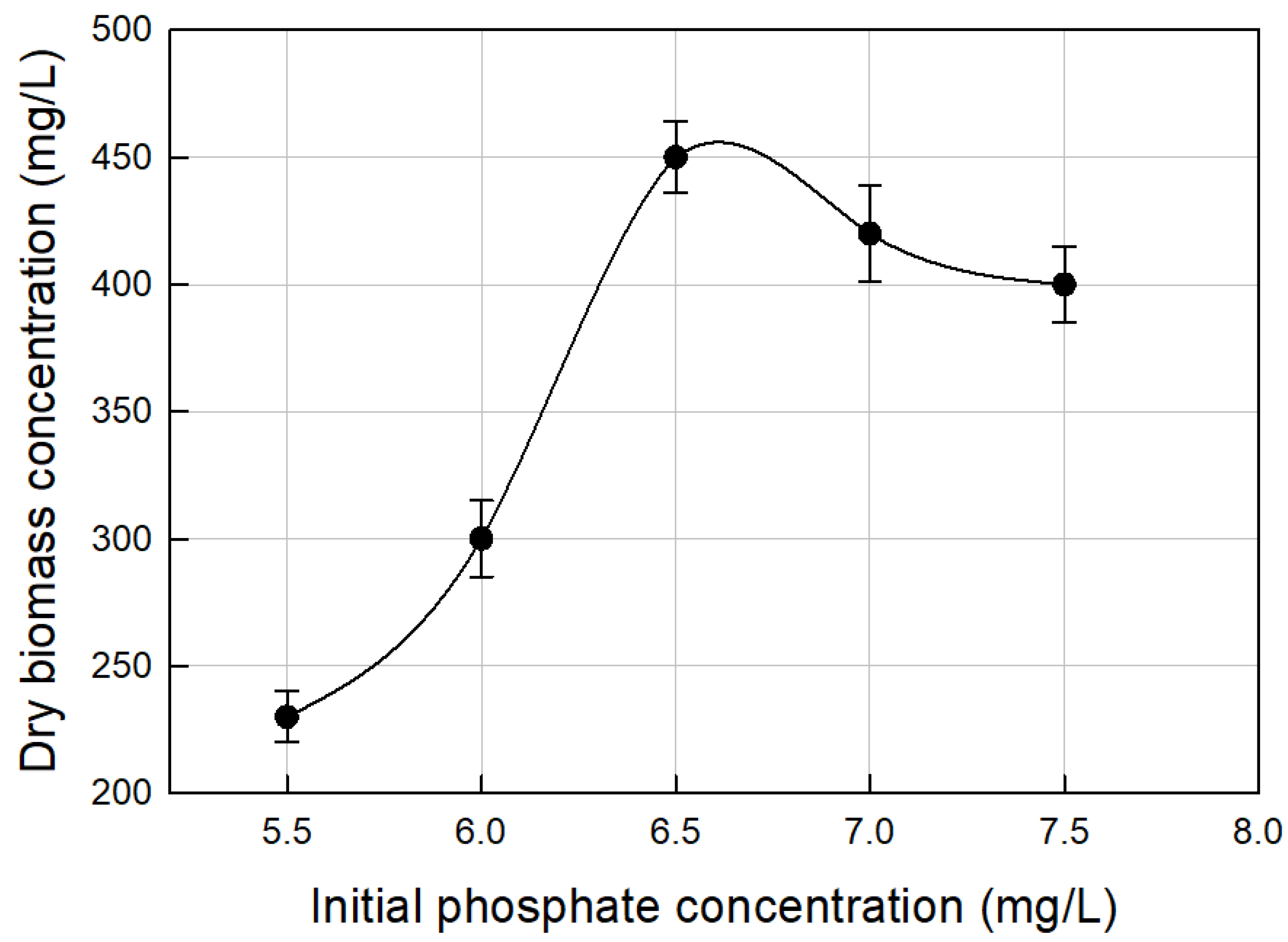
3.2. Nutrients Optimization
3.3. Biomass Growth in Optimal Medium
3.4. Lipid Content and Productivity
| Diatom | Lipids in Biomass (%, w/w) | Reference |
|---|---|---|
| Amphora angusta | 21.4 | [92] |
| Amphora arenaria | 24.7 | [92] |
| Amphora bigibba | 34.3–39.2 | [93] |
| Amphora coffeaeformis | 36.2 | [94] |
| Amphora copulata | 39.5 | This work |
| Amphora exigua | 33.2–45.0 | [93] |
| Amphora exigua | 18.1 | [95] |
| Amphora exigua | 32.1 | [92] |
| Amphora graeffii | 24.4 | [92] |
| Amphora subtropica | 19.0 | [91] |
| Amphora sp. | 19.3 | [95] |
| Caloneis platycephala | 38.4–42.0 | [93] |
| Chaetoceros muelleri | 31.0–35.2 | [93] |
| Cocconeis scutellum | 30.2–33.4 | [93] |
| Cyclotella cryptica | 19.8–28.3 | [73] |
| Cylindrotheca fusiformis | 23.9 | [56] |
| Cylindrotheca sp. | 31.3–35.0 | [93] |
| Gyrosigma sp. | 70.7 | [4] |
| Melosira nummuloides | 32.8–33.2 | [93] |
| Navicula lyra | 37.6–42.1 | [93] |
| Nitzschia grossestriata | 33.2–41.5 | [93] |
| Nitzschia panduriformis | 32.6–39.9 | [93] |
| Phaeodactylum tricornutum | 31.5 | [96] |
| Phaeodactylum tricornutum | 20.1 | [95] |
| Seminavis gracilenta | 36.0–43.0 | [93] |
| Skeletonema costatum | 36.9–41.4 | [93] |
| Alga | Biomass Productivity (mg L−1 d−1) | Lipid Productivity (mg L−1 d−1) | Reference |
|---|---|---|---|
| Amphora coffeaeformis1 | 43.0–71.0 | 7.6–21.9 | [94] 6 |
| Amphora copulata2 | 43.3 | 17.1 | This work 6 |
| Amphora subtropica1 | 100.0 | 19.0 | [91] 7 |
| Chlorella vulgaris3 (seawater) | 111.0 | 37.1 | [52] 7 |
| Chlorella vulgaris3 (freshwater) | 120.0 | 18.3 | [52] 7 |
| Choricystis minor3 | 351.0 | 82.0 | [53] 7 |
| Cylindrotheca fusiformis1 | 50.0 | 11.4 | [56] 7 |
| Gyrosigma sp. 1 | 34.1 | 24.1 | [4] 6 |
| Nannochloropsis gaditana4 | 150.0 | 30.1 | [100] 7 |
| Nannochloropsis oculata4 | ~110 | ~35 | [103] 6 |
| Nannochloropsis salina4 | 60.0 | 31.4 | [56] 7 |
| Neochloris sp. 5 | 139.0 | 32.1 | [52] 7 |
| Phaeodactylum tricornutum1 | 340.0 | 27.2–34.0 | [104] 6 |
| Pseudococcomyxa simplex5 | 127.0 | 22.9 | [52] 7 |
3.5. Issues of Optimality
3.6. Fatty Acid Profile of the Diatom Lipids
| Fatty Acid (as Methyl Ester) | Amount (%, w/w) | |
|---|---|---|
| A. Copulata | Palm Oil 2 | |
| Myristic acid (C14:0) | 6.8 | 0.5 |
| Palmitic acid (C16:0) | 31.9 | 40.5 |
| Palmitoleic acid (C16:1) | 39.8 | 0.1 |
| Stearic acid (C18:0) | 4.5 | 5.2 |
| Oleic acid (C18:1) | 4.7 | 43.5 |
| Linoleic acid (C18:2) | 2.5 | 9.3 |
| Linolenic acid (C18:3) | 0.6 | 0.2 |
| Arachidonic acid (C20:4) | 3.9 | 0.0 |
| Eicosapentaenoic acid (C20:5) | 3.6 | 0.0 |
| Tetracosanoic acid (C24:0) | 1.7 | 0.0 |
| Total saturated fatty acids | 44.9 | 46.6 |
| Total unsaturated fatty acids | 55.1 | 53.4 |
| Total monounsaturated fatty acids | 44.5 | 43.6 |
| Total polyunsaturated fatty acids | 10.6 | 9.8 |
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yi, Z.; Xu, M.; Di, X.; Brynjolfsson, S.; Fu, W. Exploring valuable lipids in diatoms. Front. Mar. Sci. 2017, 4, 17. [Google Scholar] [CrossRef]
- Aratboni, H.A.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Fact. 2019, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Fuels from microalgae. Biofuels 2010, 1, 233–235. [Google Scholar] [CrossRef]
- Govindan, N.; Maniam, G.P.; Yusoff, M.M.; Rahim, M.H.A.; Chatsungnoen, T.; Ramaraj, R.; Chisti, Y. Statistical optimization of lipid production by the diatom Gyrosigma sp. grown in industrial wastewater. J. Appl. Phycol. 2020, 32, 375–387. [Google Scholar] [CrossRef]
- Levitan, O.; Dinamarca, J.; Hochman, G.; Falkowski, P.G. Diatoms: A fossil fuel of the future. Trends Biotechnol. 2014, 32, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Rosly, N.F.; Abdul Razak, R.A.; Kuppusamy, P.; Yusoff, M.M.Y.; Govindan, N. Induction of bioactive compound composition from marine microalgae (Lyngbya sp.) by using different stress condition. J. Coast. Life Med. 2013, 1, 205–209. [Google Scholar]
- Unpaprom, Y.; Tipnee, S.; Ramraj, R. Biodiesel from green alga Scenedesmus acuminatus. Int. J. Sust. Green Energ. 2015, 4, 1–6. [Google Scholar]
- Zhukova, N.V.; Aizdaicher, N.A. Fatty acid composition of 15 species of marine microalgae. Phytochemistry 1995, 39, 351–356. [Google Scholar] [CrossRef]
- Palanisamy, K.M.; Paramasivam, P.; Maniam, G.P.; Rahim, M.H.A.; Govindan, N.; Chisti, Y. Production of lipids by Chaetoceros affinis in media based on palm oil mill effluent. J. Biotechnol. 2021, 327, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, G.; Blanco, S.; Bueno, G. (Eds.) Modern Trends in Diatom Identification: Fundamentals and Applications; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Leterme, S.C. The oil production capacity of diatoms. Ann. Aquacult. Res. 2015, 2, 1007. [Google Scholar]
- Tomas, C.R. (Ed.) Identifying Marine Diatoms and Dinoflagellates; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Tuchman, N.C.; Schollett, M.A.; Rier, S.T.; Geddes, P. Differential heterotrophic utilization of organic compounds by diatoms and bacteria under light and dark conditions. Hydrobiologia 2006, 561, 167–177. [Google Scholar] [CrossRef]
- Cerón García, M.C.; García Camacho, F.; Sánchez Mirón, A.; Fernández Sevilla, J.M.; Chisti, Y.; Molina Grima, E. Mixotrophic production of marine microalga Phaeodactylum tricornutum on various carbon sources. J. Microbiol. Biotechnol. 2006, 16, 689–694. [Google Scholar]
- White, A.W. Growth of two facultatively heterotrophic marine centric diatoms. J. Phycol. 1974, 10, 292–300. [Google Scholar]
- Chairatana, C.; Powtongsook, S.; Piyatiratitivorakul, S. Growth of a diatom Amphora delicatissima in dark heterotrophic culture. Songklanakarin J. Sci. Technol. 2003, 25, 205–212. [Google Scholar]
- Chansang, H.; Cooksey, K.E. The glucose transport system of Amphora coeffeaeformis (Bacillariophyceae). J. Phycol. 1977, 13, 51–57. [Google Scholar] [CrossRef]
- Cooksey, K.E.; Chansang, H. Isolation and physiological studies of three isolates of Amphora (Bacillariopyceae). J. Phycol. 1976, 12, 455–460. [Google Scholar] [CrossRef]
- Lewin, J.C.; Lewin, R.A. Auxotrophy and heterotrophy in marine littoral diatoms. Can. J. Microbiol. 1960, 6, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Neilson, A.H.; Lewin, R.A. The uptake and utilization of organic carbon by algae: An essay in comparative biochemistry. Phycologia 1974, 13, 227–264. [Google Scholar] [CrossRef]
- Rivkin, R.B.; Putt, M. Heterotrophy and photoheterotrophy by Antarctic microalgae: Light-dependent incorporation of amino acids and glucose. J. Phycol. 1987, 23, 442–452. [Google Scholar] [CrossRef]
- Furnas, M.J. In situ growth rates of marine phytoplankton: Approaches to measurement, community and species growth rates. J. Plank. Res. 1990, 12, 1117–1151. [Google Scholar] [CrossRef]
- Banerjee, A.; Sharma, R.; Chisti, Y.; Banerjee, U.C. Botryococcus braunii: A renewable source of hydrocarbons and other chemicals. Crit. Rev. Biotechnol. 2002, 22, 245–279. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A.; Moheimani, N.R. (Eds.) Algae for Biofuels and Energy; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Bux, F.; Chisti, Y. (Eds.) Algae Biotechnology: Products and Processes; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Potential applications of diatoms in medicinal and therapeutic approaches—A review. Indian J. Geo-Mar. Sci. 2017, 46, 663–667. [Google Scholar]
- Lebeau, T.; Robert, J.-M. Diatom cultivation and biotechnologically relevant products. Part II: Current and putative products. Appl. Microbiol. Biotechnol. 2003, 60, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Levine, I.A.; Fleurence, J. (Eds.) Microalgae in Health and Disease Prevention; Academic Press: London, UK, 2018. [Google Scholar]
- Pandey, A.; Chang, J.-S.; Soccol, C.R.; Lee, D.J.; Chisti, Y. (Eds.) Biofuels from Algae, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Posten, C.; Walter, C. (Eds.) Microalgal Biotechnology: Potential and Production; de Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Posten, C.; Walter, C. (Eds.) Microalgal Biotechnology: Integration and Economy; de Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Jayakumar, S.; Yusoff, M.M.; Rahim, M.H.A.; Maniam, G.P.; Govindan, N. The prospect of microalgal biodiesel using agro-industrial and industrial wastes in Malaysia. Renew. Sust. Energ. Rev. 2017, 72, 33–47. [Google Scholar] [CrossRef]
- Chisti, Y. Constraints to commercialization of algal fuels. J. Biotechnol. 2013, 167, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Raceways-based production of algal crude oil. Green 2013, 3, 197–216. [Google Scholar] [CrossRef]
- Chisti, Y. Microalgae biotechnology: A brief introduction. In Handbook of Microalgae-Based Processes and Products: Fundamentals and Advances in Energy, Food, Feed, Fertilizer, and Bioactive Compounds; Jacob-Lopes, E., Maroneze, M.M., Queiroz, M.I., Zepka, L.Q., Eds.; Academic Press: London, UK, 2020; pp. 3–23. [Google Scholar]
- Chisti, Y. Introduction to algal fuels. In Biofuels from Algae, 2nd ed.; Pandey, A., Chang, J.-S., Soccol, C.R., Lee, D.J., Chisti, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–31. [Google Scholar]
- Chisti, Y. The saga of algal energy. Biotechnol. Adv. 2018, 36, 1553. [Google Scholar] [CrossRef]
- Chisti, Y. Society and microalgae: Understanding the past and present. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: London, UK, 2018; pp. 11–21. [Google Scholar]
- Cheah, W.Y.; Show, P.L.; Juan, J.C.; Chang, J.-S.; Ling, T.C. Microalgae cultivation in palm oil mill effluent (POME) for lipid production and pollutants removal. Energ. Convers. Manag. 2018, 174, 430–438. [Google Scholar] [CrossRef]
- Emparan, Q.; Jye, Y.S.; Danquah, M.K.; Harun, R. Cultivation of Nannochloropsis sp. microalgae in palm oil mill effluent (POME) media for phycoremediation and biomass production: Effect of microalgae cells with and without beads. J. Water Process Eng. 2020, 33, 101043. [Google Scholar] [CrossRef]
- Hariz, H.B.; Takriff, M.S.; Yasin, N.H.M.; Ba-Abbad, M.M.; Hakimi, N.I.N.M. Potential of the microalgae-based integrated wastewater treatment and CO2 fixation system to treat Palm Oil Mill Effluent (POME) by indigenous microalgae; Scenedesmus sp. and Chlorella sp. J. Water Process Eng. 2019, 32, 100907. [Google Scholar] [CrossRef]
- Madaki, Y.S.; Seng, L. Palm oil mill effluent (POME) from Malaysia palm oil mills: Waste or resource. Int. J. Sci. Environ. Technol. 2013, 2, 1138–1155. [Google Scholar]
- Rana, S.; Singh, L.; Wahid, Z.; Liu, H. A recent overview of palm oil mill effluent management via bioreactor configurations. Curr. Poll. Rep. 2017, 3, 254–267. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insight into the applications of palm oil mill effluent: A renewable utilization of the industrial agricultural waste. Renew. Sust. Energ. Rev. 2010, 14, 1445–1452. [Google Scholar] [CrossRef]
- Zulu, N.N.; Zienkiewicz, K.; Vollheyde, K.; Feussner, I. Current trends to comprehend lipid metabolism in diatoms. Prog. Lipid Res. 2018, 70, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.; Patil, M.D.; Soni, S.; Khobragade, T.P.; Chisti, Y.; Banerjee, U.C. Production of mycophenolic acid by Penicillium brevicompactum—A comparison of two methods of optimization. Biotechnol. Rep. 2016, 11, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.T.; Wangersky, P.J. Influence of phosphorus and silicon on lipid class production by the marine diatom Chaetoceros gracilis grown in turbidostat cage cultures. Mar. Ecol. Prog. Ser. 1991, 77, 39–47. [Google Scholar] [CrossRef]
- Hendey, N.I. An Introductory Account of the Smaller Algae of British Coastal Waters, Part V, Bacillariophyceae (Diatoms); HMSO: London, UK, 1964. [Google Scholar]
- Hustedt, F. Die Süsswasser-Flora Mitteleuropas: Heft 10: Bacillariophyta (Diatomeae), 2nd ed.; Verlag Von Gustav Fischer: Jena, Germany, 1930. [Google Scholar]
- Stepanek, J.; Kociolek, P. Amphora copulata. In Diatoms of North America: The Freshwater Flora of Waterbodies on the Atlantic Coastal Plain; Siver, P.A., Hamilton, P.B., Eds.; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2011. [Google Scholar]
- Wehr, J.D.; Sheath, R.G. Freshwater Algae of North America: Ecology and Classification; Academic Press: London, UK, 2003. [Google Scholar]
- Luangpipat, T.; Chisti, Y. Biomass and oil production by Chlorella vulgaris and four other microalgae—Effects of salinity and other factors. J. Biotechnol. 2017, 257, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca Sobczuk, T.; Chisti, Y. Potential fuel oils from the microalga Choricystis minor. J. Chem. Technol. Biotechnol. 2010, 85, 100–108. [Google Scholar] [CrossRef]
- Bouyam, S.; Choorit, W.; Sirisansaneeyakul, S.; Chisti, Y. Heterotrophic production of Chlorella sp. TISTR 8990—Biomass growth and composition under various production conditions. Biotechnol. Prog. 2017, 33, 1589–1600. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Chatsungnoen, T.; Chisti, Y. Oil production by six microalgae: Impact of flocculants and drying on oil recovery from the biomass. J. Appl. Phycol. 2016, 28, 2697–2705. [Google Scholar] [CrossRef]
- Chatsungnoen, T.; Chisti, Y. Optimization of oil extraction from Nannochloropsis salina biomass paste. Algal Res. 2016, 15, 100–109. [Google Scholar] [CrossRef]
- Schoeman, F.R.; Archibald, R.E.M. Observations on Amphora species (Bacillariophyceae) in the British Museum (Natural History) V. Some species from the subgenus Amphora. S. Afr. J. Bot. 1986, 52, 425–437. [Google Scholar] [CrossRef]
- Maeda, Y.; Nojima, D.; Yoshino, T.; Tanaka, T. Structure and properties of oil bodies in diatoms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160408. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, F.; del Puerto, L.; Venturini, N.; Pita, A.L.; Brugnoli, E.; Burone, L.; Muniz, P. Diatoms, protein and carbohydrate sediment content as proxies for coastal eutrophication in Montevideo, Rio de la Plata estuary, Uruguay. Braz. J. Oceanogr. 2011, 59, 293–310. [Google Scholar] [CrossRef]
- Lee, K.; Round, F.E. Studies on freshwater Amphora species. II. Amphora copulata (Kütz.) Schoeman & Archibald. Diatom Res. 1988, 3, 217–225. [Google Scholar]
- Novais, M.H.; Morais, M.M.; Rosado, J.; Dias, L.S.; Hoffmann, L.; Ector, L. Diatoms of temporary and permanent watercourses in Southern Europe (Portugal). River Res. Appl. 2014, 30, 1216–1232. [Google Scholar] [CrossRef]
- Lange, C.B.; Tiffany, M.A. The diatom flora of the Salton Sea, California. Hydrobiologia 2002, 473, 179–201. [Google Scholar] [CrossRef]
- Coetzer, G.C.; Toerien, D.F.; Schoeman, F.R. Silica, nitrogen and phosphorous requirements of some Southern African diatoms. J. Limnol. Soc. S. Afr. 1977, 3, 27–31. [Google Scholar]
- Geider, R.J.; La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef]
- Leonardos, N.; Geider, R.J. Responses of elemental and biochemical composition of Chaetoceros muelleri to growth under varying light and nitrate: Phosphate supply ratios and their influence on critical N: P. Limnol. Oceanogr. 2004, 49, 2105–2114. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Finenko, Z.Z.; Krupatkina-Akinina, D.K. Effect of inorganic phosphorous on the growth rate of diatoms. Mar. Biol. 1974, 26, 193–201. [Google Scholar] [CrossRef]
- Parslow, J.S.; Harrison, P.J.; Thompson, P.A. Saturated uptake kinetics: Transient response of the marine diatom Thalassiosira pseudonana to ammonium, nitrate, silicate or phosphate starvation. Mar. Biol. 1984, 83, 51–59. [Google Scholar] [CrossRef]
- Chtourou, H.; Dahmen, I.; Jebali, A.; Karray, F.; Hassairi, I.; Abdelkafi, S.; Ayadi, H.; Sayadi, S.; Dhouib, A. Characterization of Amphora sp., a newly isolated diatom wild strain, potentially usable for biodiesel production. Bioproc. Biosyst. Eng. 2015, 38, 1381–1392. [Google Scholar] [CrossRef]
- Collyer, D.M.; Fogg, G.E. Studies on fat accumulation by algae. J. Exp. Bot. 1955, 6, 256–275. [Google Scholar] [CrossRef]
- Roessler, P.G. Effects of silicon deficiency on lipid composition and metabolism in the diatom Cyclotella cryptica. J. Phycol. 1988, 24, 394–400. [Google Scholar] [CrossRef]
- Yang, Z.-K.; Niu, Y.-F.; Ma, Y.-H.; Xue, J.; Zhang, M.-H.; Yang, W.-D.; Liu, J.-S.; Lu, S.-H.; Guan, Y.; Li, H.-Y. Molecular and cellular mechanisms of neutral lipid accumulation in diatom following nitrogen deprivation. Biotechnol. Biofuels 2013, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Admiraal, W. Influence of light and temperature on the growth rate of estuarine benthic diatoms in culture. Mar. Biol. 1977, 39, 1–9. [Google Scholar] [CrossRef]
- Gilstad, M.; Sakshaug, E. Growth rates of ten diatom species from the Barents Sea at different irradiances and day lengths. Mar. Ecol. Prog. Ser. 1990, 64, 169–173. [Google Scholar] [CrossRef]
- Peltomaa, E.; Hällfors, H.; Taipale, S.J. Comparison of diatoms and dinoflagellates from different habitats as sources of PUFAs. Mar. Drugs 2019, 17, 233. [Google Scholar] [CrossRef] [PubMed]
- Montagnes, D.J.S.; Franklin, D.J. Effect of temperature on diatom volume, growth rate, and carbon and nitrogen content: Reconsidering some paradigms. Limnol. Oceanogr. 2001, 46, 2008–2018. [Google Scholar] [CrossRef]
- d’Ippolito, G.; Sardo, A.; Paris, D.; Vella, F.M.; Adelfi, M.G.; Botte, P.; Gallo, C.; Fontana, A. Potential of lipid metabolism in marine diatoms for biofuel production. Biotechnol. Biofuels 2015, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Werner, D. (Ed.) The Biology of Diatoms; Blackwell: Los Angeles, CA, USA, 1977. [Google Scholar]
- Brzezinski, M.A.; Villareal, T.A.; Lipschultz, F. Silica production and the contribution of diatoms to new and primary production in the central North Pacific. Mar. Ecol. Prog. Ser. 1998, 167, 89–104. [Google Scholar] [CrossRef]
- Conley, D.J.; Kilham, S.S. Differences in silica content between marine and freshwater diatoms. Limnol. Oceanogr. 1989, 34, 205–213. [Google Scholar] [CrossRef]
- Flynn, K.J.; Martin-Jézéquel, V. Modelling Si–N-limited growth of diatoms. J. Plank. Res. 2000, 22, 447–472. [Google Scholar] [CrossRef]
- Vrieling, E.G.; Poort, L.; Beelen, T.P.M.; Gieskes, W.W.C. Growth and silica content of the diatoms Thalassiosira weissflogii and Navicula salinarum at different salinities and enrichments with aluminium. Eur. J. Phycol. 1999, 34, 307–316. [Google Scholar] [CrossRef]
- Azam, F.; Hemmingsen, B.B.; Volcani, B.E. Role of silicon in diatom metabolism V. Silicic acid transport and metabolism in the heterotrophic diatom Nitzschia alba. Arch. Microbiol. 1974, 97, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Conway, H.L.; Harrison, P.J.; Davis, C.O. Marine diatoms grown in chemostats under silicate or ammonium limitation. II. Transient response of Skeletonema costatum to a single addition of the limiting nutrient. Mar. Biol. 1976, 35, 187–199. [Google Scholar] [CrossRef]
- Davis, C.O. Continuous culture of marine diatoms under silicate limitation. II. Effect of light intensity on growth and nutrient uptake of Skeletonema costatum. J. Phycol. 1976, 12, 291–300. [Google Scholar]
- Nelson, D.M.; Goering, J.J.; Kilham, S.S.; Guillard, R.R.L. Kinetics of silicic acid uptake and rates of silica dissolution in the marine diatom Thalassiosira pseudonana. J. Phycol. 1976, 12, 246–252. [Google Scholar] [CrossRef]
- Paasche, E. The influence of cell size on growth rate, silica content and some other properties of four marine diatom species. Nor. J. Bot. 1973, 20, 197–204. [Google Scholar]
- Hildebrand, M.; Lerch, S.J.L.; Shrestha, R.P. Understanding diatom cell wall silicification—Moving forward. Front. Mar. Sci. 2018, 5, 125. [Google Scholar] [CrossRef]
- BenMoussa-Dahmen, A.; Chtourou, H.; Rezgui, F.; Sayadi, S.; Dhouib, A. Salinity stress increases lipid, secondary metabolites and enzyme activity in Amphora subtropica and Dunaliella sp. for biodiesel production. Bioresour. Technol. 2016, 218, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Liebezeit, G. Biochemical characterisation and fatty acid profiles of 25 benthic marine diatoms isolated from the Solthörn tidal flat (southern North Sea). J. Appl. Phycol. 2013, 25, 453–465. [Google Scholar] [CrossRef]
- Chen, Y.-C. The biomass and total lipid content and composition of twelve species of marine diatoms cultured under various environments. Food Chem. 2012, 131, 211–219. [Google Scholar] [CrossRef]
- Rajaram, M.G.; Nagaraj, S.; Manjunath, M.; Boopathy, A.B.; Kurinjimalar, C.; Rengasamy, R.; Jayakumar, T.; Sheu, J.-R.; Li, J.-Y. Biofuel and biochemical analysis of Amphora coffeaeformis RR03, a novel marine diatom, cultivated in an open raceway pond. Energies 2018, 11, 1341. [Google Scholar] [CrossRef]
- Orcutt, D.M.; Patterson, G.W. Sterol, fatty acid and elemental composition of diatoms grown in chemically defined media. Comp. Biochem. Physiol. B 1975, 50B, 579–583. [Google Scholar] [CrossRef]
- Lewin, J.; Hellebust, J.A. Heterotrophic nutrition of the marine pennate diatom, Cylindrotheca fusiformis. Can. J. Microbiol. 1970, 16, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Sayanova, O.; Mimouni, V.; Ulmann, L.; Morant-Manceau, A.; Pasquet, V.; Schoefs, B.; Napier, J.A. Modulation of lipid biosynthesis by stress in diatoms. Phil. Trans. Royal. Soc. B 2017, 372, 20160407. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Wang, J.-K.; Seibert, M. Prospects for commercial production of diatoms. Biotechnol. Biofuels 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Menegol, T.; Romero-Villegas, G.I.; López-Rodríguez, M.; Navarro-López, E.; López-Rosales, L.; Chisti, Y.; Cerón-García, M.C.; Molina-Grima, E. Mixotrophic production of polyunsaturated fatty acids and carotenoids by the microalga Nannochloropsis gaditana. J. Appl. Phycol. 2019, 31, 2823–2832. [Google Scholar] [CrossRef]
- Shene, C.; Chisti, Y.; Vergara, D.; Burgos, C.; Rubilar, M.; Bustamante, M. Production of eicosapentaenoic acid by Nannochloropsis oculata: Effects of carbon dioxide and glycerol. J. Biotechnol. 2016, 239, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Singhasuwan, S.; Choorit, W.; Sirisansaneeyakul, S.; Kokkaew, N.; Chisti, Y. Carbon-to-nitrogen ratio affects the biomass composition and the fatty acid profile of heterotrophically grown Chlorella sp. TISTR 8990 for biodiesel production. J. Biotechnol. 2015, 216, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Shene, C.; Chisti, Y.; Bustamante, M.; Rubilar, M. Effect of CO2 in the aeration gas on cultivation of the microalga Nannochloropsis oculata: Experimental study and mathematical modeling of CO2 assimilation. Algal Res. 2016, 13, 16–29. [Google Scholar] [CrossRef]
- Sánchez Mirón, A.; Cerón García, M.-C.; Contreras Gómez, A.; García Camacho, F.; Molina Grima, E.; Chisti, Y. Shear stress tolerance and biochemical characterization of Phaeodactylum tricornutum in quasi steady-state continuous culture in outdoor photobioreactors. Biochem. Eng. J. 2003, 16, 287–297. [Google Scholar] [CrossRef]
- Sompech, K.; Chisti, Y.; Srinophakun, T. Design of raceway ponds for producing microalgae. Biofuels 2012, 3, 387–397. [Google Scholar] [CrossRef]
- Chutmanop, J.; Chuichulcherm, S.; Chisti, Y.; Srinophakun, P. Protease production by Aspergillus oryzae in solid-state fermentation using agroindustrial substrates. J. Chem. Technol. Biotechnol. 2008, 83, 1012–1018. [Google Scholar] [CrossRef]
- Qureshi, A.S.; Khushk, I.; Ali, C.H.; Chisti, Y.; Ahmad, A.; Majeed, H. Coproduction of protease and amylase by thermophilic Bacillus sp. BBXS-2 using open solid-state fermentation of lignocellulosic biomass. Biocatal. Agric. Biotechnol. 2016, 8, 146–151. [Google Scholar] [CrossRef]
- Patil, M.D.; Dev, M.J.; Tangadpalliwar, S.; Patel, G.; Garg, P.; Chisti, Y.; Banerjee, U.C. Ultrasonic disruption of Pseudomonas putida for the release of arginine deiminase: Kinetics and predictive models. Bioresour. Technol. 2017, 233, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.D.; Shinde, K.D.; Patel, G.; Chisti, Y.; Banerjee, U.C. Use of response surface method for maximizing the production of arginine deiminase by Pseudomonas putida. Biotechnol. Rep. 2016, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Belarbi, E.H.; Molina, E.; Chisti, Y. A process for high yield and scaleable recovery of high purity eicosapentaenoic acid esters from microalgae and fish oil. Enzym. Microb. Technol. 2000, 26, 516–529. [Google Scholar] [CrossRef]
- Kates, M.; Volcani, B.E. Lipid components of diatoms. Biochim. Biophys. Acta 1966, 116, 264–278. [Google Scholar] [CrossRef]
- Opute, F.I. Lipid and fatty-acid composition of diatoms. J. Exp. Bot. 1974, 25, 823–835. [Google Scholar] [CrossRef]
- Montoya, C.; Cochard, B.; Flori, A.; Cros, D.; Lopes, R.; Cuellar, T.; Espeout, S.; Syaputra, I.; Villeneuve, P.; Pina, M.; et al. Genetic architecture of palm oil fatty acid composition in cultivated oil palm (Elaeis guineensis Jacq.) compared to its wild relative E. oleifera (H.B.K) Cortés. PLoS ONE 2014, 9, e95412. [Google Scholar] [CrossRef]
- Yongmanitchai, W.; Ward, O.P. Growth of and omega-3 fatty acid production by Phaeodactylum tricornutum under different culture conditions. Appl. Environ. Microbiol. 1991, 57, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Zahan, K.A.; Kano, M. Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef]
- Richard, T.; Chisti, Y.; Somerville, C.R.; Blanch, H.; Babcock, B.; Gheewala, S.H.; Zilberman, D. The food versus fuel debate. Biofuels 2012, 3, 635–648. [Google Scholar]
- Talebi, A.F.; Tabatabaei, M.; Chisti, Y. BiodieselAnalyzer: A user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res. J. 2014, 2, 55–57. [Google Scholar] [CrossRef]
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Value |
|---|---|
| pH | 7.3 |
| Chemical oxygen demand (COD, mg L−1) | 1480 ± 19 |
| Total suspended solids (TSS, mg L−1) | 71 ± 7 |
| Ammonium (mg L−1) | 11.1 ± 0.5 |
| Phosphate (mg L−1) | 5.5 ± 0.2 |
| Nitrite (mg L−1) | 1.3 ± 0.3 |
| Nitrate (mg L−1) | 3.1 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindan, N.; Maniam, G.P.; Ab. Rahim, M.H.; Sulaiman, A.Z.; Ajit, A.; Chatsungnoen, T.; Chisti, Y. Production of Renewable Lipids by the Diatom Amphora copulata. Fermentation 2021, 7, 37. https://doi.org/10.3390/fermentation7010037
Govindan N, Maniam GP, Ab. Rahim MH, Sulaiman AZ, Ajit A, Chatsungnoen T, Chisti Y. Production of Renewable Lipids by the Diatom Amphora copulata. Fermentation. 2021; 7(1):37. https://doi.org/10.3390/fermentation7010037
Chicago/Turabian StyleGovindan, Natanamurugaraj, Gaanty Pragas Maniam, Mohd Hasbi Ab. Rahim, Ahmad Ziad Sulaiman, Azilah Ajit, Tawan Chatsungnoen, and Yusuf Chisti. 2021. "Production of Renewable Lipids by the Diatom Amphora copulata" Fermentation 7, no. 1: 37. https://doi.org/10.3390/fermentation7010037
APA StyleGovindan, N., Maniam, G. P., Ab. Rahim, M. H., Sulaiman, A. Z., Ajit, A., Chatsungnoen, T., & Chisti, Y. (2021). Production of Renewable Lipids by the Diatom Amphora copulata. Fermentation, 7(1), 37. https://doi.org/10.3390/fermentation7010037





