Mechanical Cell Disruption Technologies for the Extraction of Dyes and Pigments from Microorganisms: A Review
Abstract
1. Introduction
2. Mechanical Cell Disruption
2.1. Bead Milling

2.1.1. Microalgae
2.1.2. Fungi
2.1.3. Bacteria
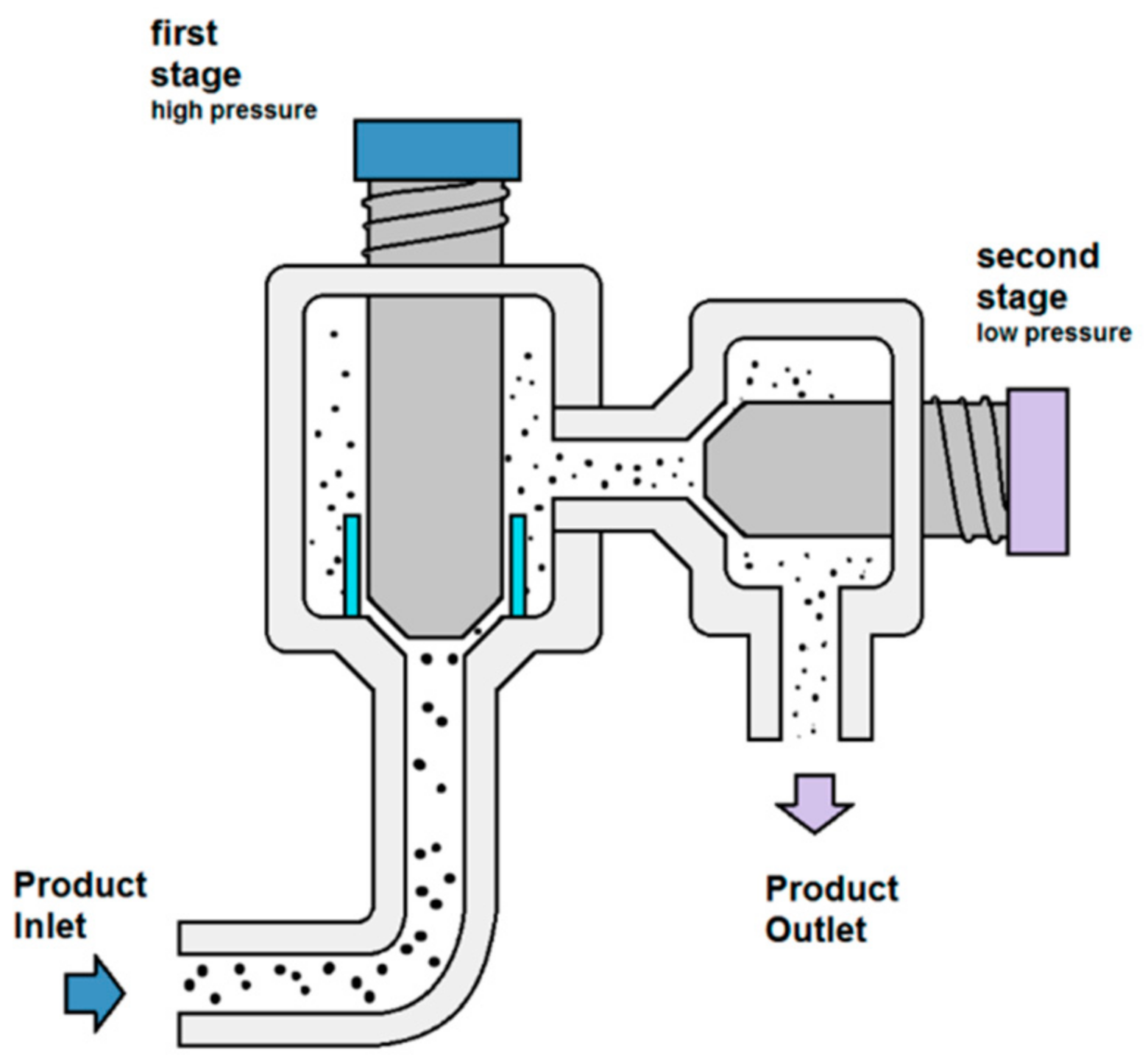
2.2. High Pressure Homogenization
2.2.1. Microalgae
2.2.2. Fungi
2.2.3. Bacteria
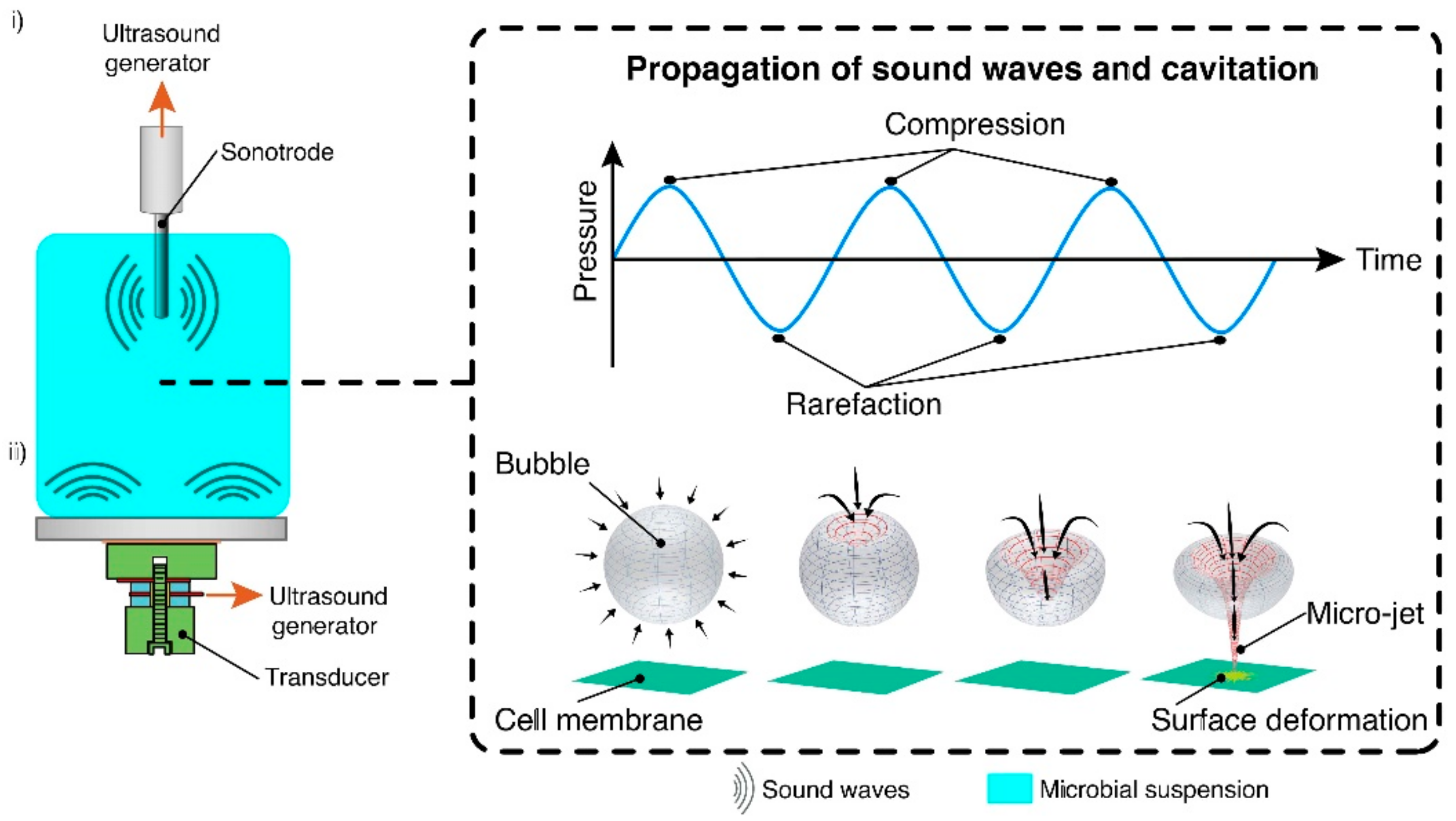
2.3. Ultrasonication
2.3.1. Microalgae
2.3.2. Fungi
2.3.3. Bacteria
3. Mechanical Cell Disruption Limitations
4. Conclusions
Funding
Conflicts of Interest
References
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed Electric Field-Assisted Extraction of Valuable Compounds from Microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef] [PubMed]
- Valduga, E.; Tatsch, P.O.; Tiggemann, L.; Treichel, H.; Toniazzo, G.; Zeni, J.; Di Luccio, M.; Fúrigo, A. Carotenoids Production: Microorganisms as Source of Natural Dyes. Quim. Nova 2009, 32, 2429–2436. [Google Scholar] [CrossRef]
- Aksu, Z.; Tuǧba Eren, A. Carotenoids Production by the Yeast Rhodotorula Mucilaginosa: Use of Agricultural Wastes as a Carbon Source. Process Biochem. 2005, 40, 2985–2991. [Google Scholar] [CrossRef]
- Whallans, R.C.M.; de Janaina, F.M.B. Optimization of Agroindustrial Medium for the Production of Carotenoids by Wild Yeast Sporidiobolus Pararoseus. Afr. J. Microbiol. Res. 2015, 9, 209–219. [Google Scholar] [CrossRef]
- Buzzini, P.; Martini, A. Production of Carotenoids by Strains of Rhodotorula Glutinis Cultured in Raw Materials of Agro-Industrial Origin. Bioresour. Technol. 2000, 71, 41–44. [Google Scholar] [CrossRef]
- Park, P.K.; Kim, E.Y.; Chu, K.H. Chemical Disruption of Yeast Cells for the Isolation of Carotenoid Pigments. Sep. Purif. Technol. 2007, 53, 148–152. [Google Scholar] [CrossRef]
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine Carotenoids: Biological Functions and Commercial Applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of Carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Choi, S.Y.; Son, S.; Mitchell, R.J. Combined Application of Bacterial Predation and Violacein to Kill Polymicrobial Pathogenic Communities. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Devi, P.R. Bacterial Pigments: Sustainable Compounds With Market Potential for Pharma and Food Industry. Front. Sustain. Food Syst. 2020, 4, 1–17. [Google Scholar] [CrossRef]
- Kholany, M.; Trébulle, P.; Martins, M.; Ventura, S.P.M.; Nicaud, J.M.; Coutinho, J.A.P. Extraction and Purification of Violacein from Yarrowia Lipolytica Cells Using Aqueous Solutions of Surfactants. J. Chem. Technol. Biotechnol. 2020, 95, 1126–1134. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from Microalgae: A Review of Recent Developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Nooralabettu, K.P. Cell Disruption Techniques. In Downstream Process Technology: A New Horizon In Biotechnology; PHI Leaning Private Limited: New Delhi, India, 2010; pp. 101–126. [Google Scholar]
- Montalescot, V.; Rinaldi, T.; Touchard, R.; Jubeau, S.; Frappart, M.; Jaouen, P.; Bourseau, P.; Marchal, L. Optimization of Bead Milling Parameters for the Cell Disruption of Microalgae: Process Modeling and Application to Porphyridium Cruentum and Nannochloropsis Oculata. Bioresour. Technol. 2015, 196, 339–346. [Google Scholar] [CrossRef]
- Postma, P.R.; Miron, T.L.; Olivieri, G.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M. Mild Disintegration of the Green Microalgae Chlorella Vulgaris Using Bead Milling. Bioresour. Technol. 2015, 184, 297–304. [Google Scholar] [CrossRef]
- Burmeister, C.F.; Kwade, A. Process Engineering with Planetary Ball Millss. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef]
- Koubaa, M.; Imatoukene, N.; Drévillon, L.; Vorobiev, E. Current Insights in Yeast Cell Disruption Technologies for Oil Recovery: A Review. Chem. Eng. Process. Process Intensif. 2020, 150, 107868. [Google Scholar] [CrossRef]
- Kermanshahi-Pour, A.; Zimmerman, J.B.; Anastas, P.T. Microalgae-Derived Chemicals: Opportunity for an Integrated Chemical Plant. In Natural and Artificial Photosynthesis; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2013; pp. 387–433. [Google Scholar]
- Burczyk, J.; Zych, M.; Ioannidis, N.E.; Kotzabasis, K. Polyamines in Cell Walls of Chlorococcalean Microalgae. Z. Nat. C 2014, 69, 75–80. [Google Scholar] [CrossRef]
- Alhattab, M.; Kermanshahi-Pour, A.; Brooks, M.S.-L. Microalgae Disruption Techniques for Product Recovery: Influence of Cell Wall Composition. J. Appl. Phycol. 2019, 31, 61–88. [Google Scholar] [CrossRef]
- Schüler, L.M.; Gangadhar, K.N.; Duarte, P.; Placines, C.; Molina-Márquez, A.M.; Léon-Bañares, R.; Sousa, V.S.; Varela, J.; Barreira, L. Improvement of Carotenoid Extraction from a Recently Isolated, Robust Microalga, Tetraselmis Sp. CTP4 (Chlorophyta). Bioprocess Biosyst. Eng. 2020, 43, 785–796. [Google Scholar] [CrossRef]
- Safi, C.; Camy, S.; Frances, C.; Varela, M.M.; Badia, E.C.; Pontalier, P.Y.; Vaca-Garcia, C. Extraction of lipids and pigments of Chlorella vulgaris by supercritical carbon dioxide: Influence of bead milling on extraction performance. J. Appl. Phycol. 2014, 26, 1711–1718. [Google Scholar] [CrossRef]
- Gomes, M.; Pennacchi, C.; Rodríguez-fernández, D.E.; Maranho, L.T.; Marc, I.; Alves, L.; Master, P.; Cnrs, U.P.R.; Svs, P. A Comparison of Cell Disruption Procedures for the Recovery of Intracellular Carotenoids from Sporobolomyces Ruberrimus H110. Int. J. Appl. Biol. Pharm. Technol. 2014, 6, 136–144. [Google Scholar]
- Tong, Y.; Zhou, J.; Zhang, L.; Xu, P. Engineering Oleaginous Yeast Yarrowia Lipolytica for Violacein Production: Extraction, Quantitative Measurement and Culture Optimization. bioRxiv 2019, 1, 1–19. [Google Scholar] [CrossRef]
- Liu, D.; Ding, L.; Sun, J.; Boussetta, N.; Vorobiev, E. Yeast Cell Disruption Strategies for Recovery of Intracellular Bio-Active Compounds—A Review. Innov. Food Sci. Emerg. Technol. 2016, 36, 181–192. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, J.; Zhu, Y.; Ding, X.; Xu, P. Engineering Yarrowia Lipolytica as a Chassis for de Novo Synthesis of Five Aromatic-Derived Natural Products and Chemicals. ACS Synth. Biol. 2020, 9, 2096–2106. [Google Scholar] [CrossRef]
- Zhu, Q.; Jackson, E.N. Metabolic Engineering of Yarrowia Lipolytica for Industrial Applications. Curr. Opin. Biotechnol. 2015, 36, 65–72. [Google Scholar] [CrossRef]
- Jacobsen, I.H.; Ledesma-Amaro, R.; Martinez, J.L. Recombinant β-Carotene Production by Yarrowia Lipolytica—Assessing the Potential of Micro-Scale Fermentation Analysis in Cell Factory Design and Bioreaction Optimization. Front. Bioeng. Biotechnol. 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Desouza, M.A.; Schroeder, W.A.; Kollman, S.R.; May, C.A. Carotenoid Biosynthesis. Patent Number WO 02/079395 A2, 10 October 2002. [Google Scholar]
- Flieger, K.; Knabe, N.; Toepel, J. Development of an Improved Carotenoid Extraction Method to Characterize the Carotenoid Composition under Oxidative Stress and Cold Temperature in the Rock Inhabiting Fungus Knufia Petricola A95. J. Fungi 2018, 4, 124. [Google Scholar] [CrossRef]
- Larrosa, A.P.Q.; Camara, Á.S.; Moura, J.M.; Pinto, L.A.A. Spirulina Sp. Biomass Dried/Disrupted by Different Methods and Their Application in Biofilms Production. Food Sci. Biotechnol. 2018, 27, 1659–1665. [Google Scholar] [CrossRef]
- Bux, F.; Ramesh, D. Lipid Identification and Extraction Techniques. Biotechnol. Appl. Microalgae 2013, 89–98. [Google Scholar] [CrossRef]
- Pott, R.W.M. The Release of the Blue Biological Pigment C-Phycocyanin through Calcium-Aided Cytolysis of Live Spirulina sp. Coloration Technol. 2019, 135, 17–21. [Google Scholar] [CrossRef]
- D’Hondt, E.; Martín-Juárez, J.; Bolado, S.; Kasperoviciene, J.; Koreiviene, J.; Sulcius, S.; Elst, K.; Bastiaens, L. Cell Disruption Technologies. Microalgae-Based Biofuels Bioprod. Feedstock Cultiv. End-Prod. 2017, 133–154. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G.; Lenza, E.; Maresca, P. Main Factors Regulating Microbial Inactivation by High-Pressure Homogenization: Operating Parameters and Scale of Operation. Chem. Eng. Sci. 2009, 64, 520–532. [Google Scholar] [CrossRef]
- Kleinig, A.R.; Middelberg, A.P.J. On the Mechanism of Microbial Cell Disruption in High-Pressure Homogenisation. Chem. Eng. Sci. 1998, 53, 891–898. [Google Scholar] [CrossRef]
- Diels, A.M.J.; Michiels, C.W. High-Pressure Homogenization as a Non-Thermal Technique for the Inactivation of Microorganisms. Crit. Rev. Microbiol. 2006, 32, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Middelberg, A.P.J. Microbial Cell Disruption by High-Pressure Homogenization. In Downstream Processing of Proteins; Desai, M.A., Ed.; Humana Press: Totowa, NJ, USA, 2000; Volume 9, pp. 11–21. [Google Scholar]
- Hu, Y.; Bassi, A. Extraction of Biomolecules from Microalgae; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128185360. [Google Scholar]
- Comuzzo, P.; Calligaris, S. Potential Applications of High Pressure Homogenization in Winemaking: A Review. Beverages 2019, 5, 56. [Google Scholar] [CrossRef]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of Different Cell Disruption Process on Encysted Cells of Haematococcus Pluvialis. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- Grimi, N.; Dubois, A.; Marchal, L.; Jubeau, S.; Lebovka, N.I.; Vorobiev, E. Selective Extraction from Microalgae Nannochloropsis Sp. Using Different Methods of Cell Disruption. Bioresour. Technol. 2014, 153, 254–259. [Google Scholar] [CrossRef]
- Zhang, R.; Grimi, N.; Marchal, L.; Lebovka, N.; Vorobiev, E. Effect of Ultrasonication, High Pressure Homogenization and Their Combination on Efficiency of Extraction of Bio-Molecules from Microalgae Parachlorella Kessleri. Algal Res. 2019, 40. [Google Scholar] [CrossRef]
- Xie, Y.; Ho, S.H.; Chen, C.N.N.; Chen, C.Y.; Jing, K.; Ng, I.S.; Chen, J.; Chang, J.S.; Lu, Y. Disruption of Thermo-Tolerant Desmodesmus Sp. F51 in High Pressure Homogenization as a Prelude to Carotenoids Extraction. Biochem. Eng. J. 2016, 109, 243–251. [Google Scholar] [CrossRef]
- Safi, C.; Frances, C.; Ursu, A.V.; Laroche, C.; Pouzet, C.; Vaca-Garcia, C.; Pontalier, P.Y. Understanding the effect of cell disruption methods on the diffusion of chlorella vulgaris proteins and pigments in the aqueous phase. Algal Res. 2015, 8, 61–68. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, Y.; Du, C.; Lv, T.; Guo, Y.; Han, M.; Pi, F.; Zhang, W.; Qian, H. Study on the Wall-Breaking Method of Carotenoids Producing Yeast Sporidiobolus Pararoseus and the Antioxidant Effect of Four Carotenoids on SK-HEP-1 Cells. Prep. Biochem. Biotechnol. 2019, 49, 767–774. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Jáuregui, M.; Medina, E.; Jaime, C.; Cerezal, P. Rapid Green Extractions of C-Phycocyanin from Arthrospira Maxima for Functional Applications. Appl. Sci. 2019, 9, 1987. [Google Scholar] [CrossRef]
- Song, W.; Zhao, C.; Wang, S. A Large-Scale Preparation Method of High Purity c-Phycocyanin. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 293–297. [Google Scholar] [CrossRef]
- Jiang, D.; Wei, F.; Caim, Q.; Lan, L.; Ji, H.; Gao, A. High-Prodigiosin-Yield Serratia Marcescens Strain Sm-128 and Use Thereof. Patent Number CN102277323B, 6 February 2013. [Google Scholar]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable Production of Large Quantities of Defect-Free Few-Layer Graphene by Shear Exfoliation in Liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Koubaa, M.; Bals, O.; Vorobiev, E. Recent Insights in the Impact of Emerging Technologies on Lactic Acid Bacteria: A Review. Food Res. Int. 2020, 137, 109544. [Google Scholar] [CrossRef] [PubMed]
- Furuki, T.; Maeda, S.; Imajo, S.; Hiroi, T.; Amaya, T.; Hirokawa, T.; Ito, K.; Nozawa, H. Rapid and Selective Extraction of Phycocyanin From. J. Appl. Phycol. 2003, 15, 319–324. [Google Scholar] [CrossRef]
- Hadiyanto; Suttrisnorhadi; Sutanto, H.; Suzery, M. Phyocyanin Extraction from Microalgae Spirulina Platensis Assisted by Ultrasound Irradiation: Effect of Time and Temperature. Songklanakarin J. Sci. Technol. 2016, 38, 391–398. [Google Scholar]
- Herrero, M.; Martín-Álvarez, P.J.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Optimization of Accelerated Solvent Extraction of Antioxidants from Spirulina Platensis Microalga. Food Chem. 2005, 93, 417–423. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, H.Y. Effect of Ultrasonic Extraction on Production and Structural Changes of C-Phycocyanin from Marine Spirulina Maxima. Int. J. Mol. Sci. 2018, 19, 220. [Google Scholar] [CrossRef]
- Moraes, C.C.; Sala, L.; Cerveira, G.P.; Kalil, S.J. C-Phycocyanin Extraction from Spirulina Platensis Wet Biomass. Braz. J. Chem. Eng. 2011, 28, 45–49. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N]Nitrate in Biological Fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Dietz, B.M.; Kang, Y.-H.; Liu, G.; Eggler, A.L.; Yao, P.; Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R.; Mesecar, A.D.; van Breemen, R.B.; et al. Xanthohumol Isolated from Humulus Lupulus Inhibits Menadione-Induced DNA Damage through Induction of Quinone Reductase. Chem. Res. Toxicol. 2005, 18, 1296–1305. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Rech, R.; Marczak, L.D.F.; Mercali, G.D. Ultrasound as an Alternative Technology to Extract Carotenoids and Lipids from Heterochlorella Luteoviridis. Bioresour. Technol. 2017, 224, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Press: Washington, DC, USA, 2001; ISBN 1578810728. [Google Scholar]
- Jaeschke, D.P.; Menegol, T.; Rech, R.; Mercali, G.D.; Marczak, L.D.F. Carotenoid and Lipid Extraction from Heterochlorella Luteoviridis Using Moderate Electric Field and Ethanol. Process Biochem. 2016, 51, 1636–1643. [Google Scholar] [CrossRef]
- Parniakov, O.; Apicella, E.; Koubaa, M.; Barba, F.J.; Grimi, N.; Lebovka, N.; Pataro, G.; Ferrari, G.; Vorobiev, E. Ultrasound-Assisted Green Solvent Extraction of High-Added Value Compounds from Microalgae Nannochloropsis spp. Bioresour. Technol. 2015, 198, 262–267. [Google Scholar] [CrossRef]
- Hasan, M.; Azhar, M.; Nangia, H.; Bhatt, P.C.; Panda, B.P. Influence of High-Pressure Homogenization, Ultrasonication, and Supercritical Fluid on Free Astaxanthin Extraction from β-Glucanase-Treated Phaffia Rhodozyma Cells. Prep. Biochem. Biotechnol. 2016, 46, 116–122. [Google Scholar] [CrossRef]
- Urnau, L.; Colet, R.; Soares, V.F.; Franceschi, E.; Valduga, E.; Steffens, C. Extraction of Carotenoids from Xanthophyllomyces Dendrorhous Using Ultrasound-Assisted and Chemical Cell Disruption Methods. Can. J. Chem. Eng. 2018, 96, 1377–1381. [Google Scholar] [CrossRef]
- Gogate, P.R.; Nadar, S.G. Ultrasound-Assisted Intensification of Extraction of Astaxanthin from Phaffia Rhodozyma. Indian Chem. Eng. 2015, 57, 240–255. [Google Scholar] [CrossRef]
- Michelon, M.; de Matos de Borba, T.; da Silva Rafael, R.; Burkert, C.A.V.; de Medeiros Burkert, J.F. Extraction of Carotenoids from Phaffia Rhodozyma: A Comparison between Different Techniques of Cell Disruption. Food Sci. Biotechnol. 2012, 21, 1–8. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Aguilar, D.E.; Álvarez, I.; Raso, J. Organic-Solvent-Free Extraction of Carotenoids from Yeast Rhodotorula Glutinis by Application of Ultrasound under Pressure. Ultrason. Sonochem. 2020, 61, 104833. [Google Scholar] [CrossRef]
- Huryn, D.M.; Wipf, P. Natural Product Chemistry and Cancer Drug Discovery, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123965219. [Google Scholar]
- Khanam, B.; Chandra, R. Comparative Analysis of Prodigiosin Isolated from Endophyte Serratia Marcescens. Lett. Appl. Microbiol. 2018, 66, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Vali Aftari, R.; Rezaei, K.; Mortazavi, A.; Bandani, A.R. The Optimized Concentration and Purity of Spirulina PlatensisC-Phycocyanin: A Comparative Study on Microwave-Assisted and Ultrasound-Assisted Extraction Methods. J. Food Process. Preserv. 2015, 39, 3080–3091. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Paciulli, M.; Abbaspourrad, A. Extraction of Phycocyanin—A Natural Blue Colorant from Dried Spirulina Biomass: Influence of Processing Parameters and Extraction Techniques. J. Food Sci. 2020, 85, 727–735. [Google Scholar] [CrossRef]
- Sun, S.-Q.; Wang, Y.-J.; Xu, W.; Zhu, C.-J.; Liu, X.-X. Optimizing Ultrasound-Assisted Extraction of Prodigiosin by Response Surface Methodology. Prep. Biochem. Biotechnol. 2015, 45, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, L.; Xue, Y.; Zhang, C.; Xing, X.H.; Lou, K.; Zhang, Z.; Li, Y.; Zhang, G.; Bi, J.; et al. Production of Violet Pigment by a Newly Isolated Psychrotrophic Bacterium from a Glacier in Xinjiang, China. Biochem. Eng. J. 2009, 43, 135–141. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Evaluating Microalgal Cell Disruption upon Ultra High Pressure Homogenization. Algal Res. 2019, 42, 101616. [Google Scholar] [CrossRef]
| Cell Disruption Technique | Advantages | Drawbacks |
|---|---|---|
| Bead milling |
|
|
| High-pressure homogenization |
|
|
| Ultrasonication |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemer, G.; Louka, N.; Vorobiev, E.; Salameh, D.; Nicaud, J.-M.; Maroun, R.G.; Koubaa, M. Mechanical Cell Disruption Technologies for the Extraction of Dyes and Pigments from Microorganisms: A Review. Fermentation 2021, 7, 36. https://doi.org/10.3390/fermentation7010036
Nemer G, Louka N, Vorobiev E, Salameh D, Nicaud J-M, Maroun RG, Koubaa M. Mechanical Cell Disruption Technologies for the Extraction of Dyes and Pigments from Microorganisms: A Review. Fermentation. 2021; 7(1):36. https://doi.org/10.3390/fermentation7010036
Chicago/Turabian StyleNemer, Georgio, Nicolas Louka, Eugène Vorobiev, Dominique Salameh, Jean-Marc Nicaud, Richard G. Maroun, and Mohamed Koubaa. 2021. "Mechanical Cell Disruption Technologies for the Extraction of Dyes and Pigments from Microorganisms: A Review" Fermentation 7, no. 1: 36. https://doi.org/10.3390/fermentation7010036
APA StyleNemer, G., Louka, N., Vorobiev, E., Salameh, D., Nicaud, J.-M., Maroun, R. G., & Koubaa, M. (2021). Mechanical Cell Disruption Technologies for the Extraction of Dyes and Pigments from Microorganisms: A Review. Fermentation, 7(1), 36. https://doi.org/10.3390/fermentation7010036









