Very High Gravity Bioethanol Revisited: Main Challenges and Advances
Abstract
1. Introduction
2. Main Challenges Arising from VHG Processes
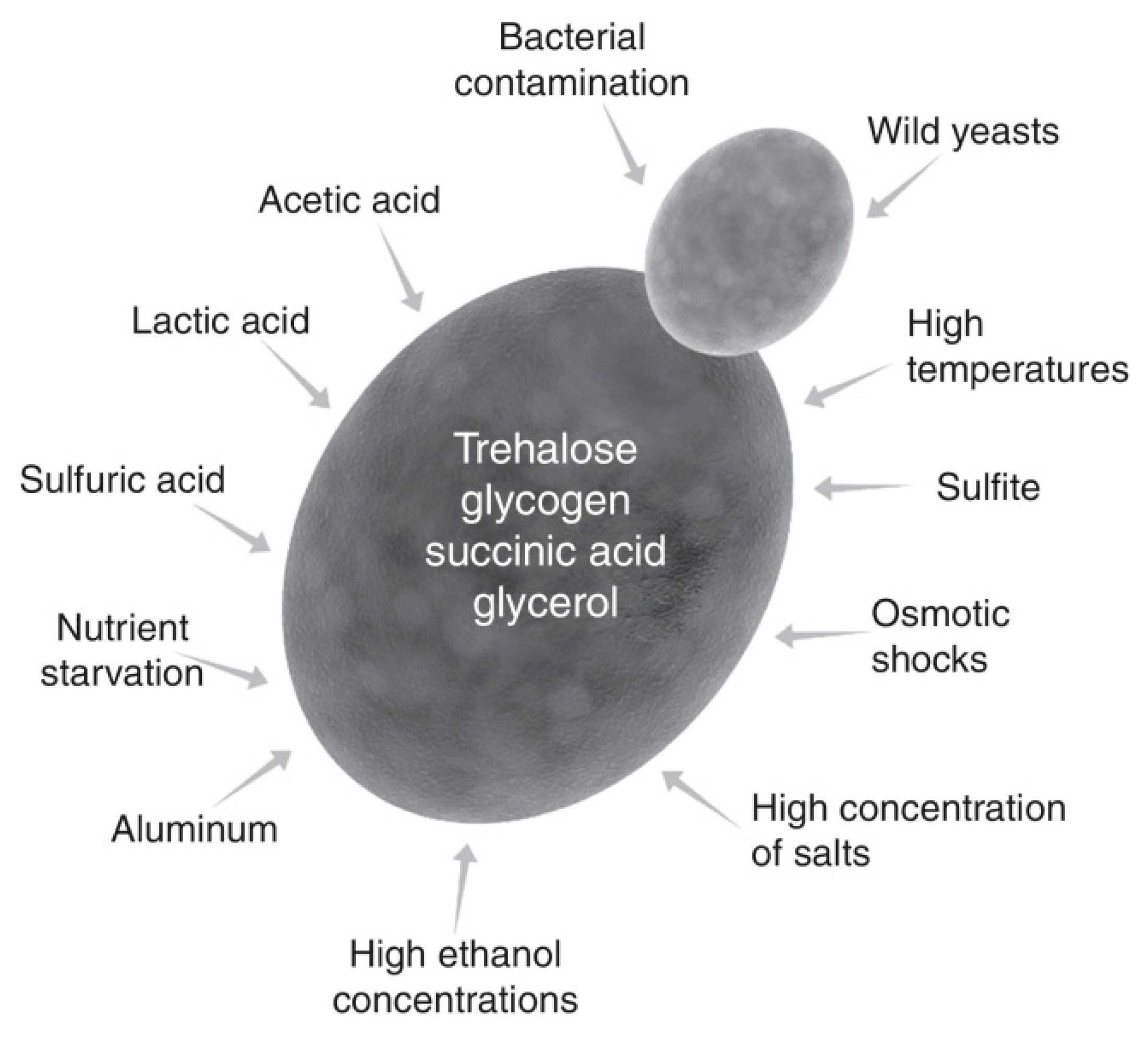
2.1. Physiological Stress
2.2. Robustness of the Fermentation Organism
| Strain | Substrate | Initial Substrate (g/L) | Temperature (°C) | Final Ethanol (g/L) | Reference |
|---|---|---|---|---|---|
| AT-3 | Glucose | 180 | 40 | 68.5 | [31] |
| SEMF1 | Sweet sorghum juice | 185 | 33 | 86.2 | [32] |
| NRRL Y-2034 | Sweet sorghum juice | 200 | 30 | 70.6 | [33] |
| DMKU 3-S087 | Molasses | 200 | 40 | 72.4 | [34] |
| KKU-VN8 | Sweet sorghum juice | 238 | 40 | 89.3 | [35] |
| CCTCC M206111 | Sweet potato | 240 | 30 | 128.5 | [36] |
| Y-904 | Sugarcane juice and molasses | 300 | 27 | 135.0 | [37] |
| PE-2 | Glucose | 323 | 30 | 149.0 | [38] |
| YF10-5 | Glucose | 350 | 30 | 115.0 | [39] |
| KL17 | Galactose and glucose | 500 | 30 | 96.9 | [40] |
| C10 | Sugar beet syrup | 270 | 30 | 116.0 | [41] |
2.3. Nutrients Depletion
3. Critical Factors for Improved VHG Fermentations
3.1. Temperature
3.2. Agitation and Aeration Conditions
3.3. Nutritional Supplementation
3.4. Operation Mode
3.4.1. Batch Operation
3.4.2. Fed-Batch
3.4.3. Continuous Regime
3.5. Cells Immobilization
3.6. Development of the Fermentation Organism
3.7. VHG Fermentation for Cellulosic Ethanol Production
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, F.W.; Anderson, W.A.; Moo-Young, M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Deesuth, A.; Laopaiboon, P.; Klanrit, P.; Laopaiboon, L. Improvement of ethanol production from sweet sorghum juice under high gravity and very high gravity conditions: Effect of nutrient supplementation and aeration. Ind. Crops Prod. 2015, 74, 95–102. [Google Scholar] [CrossRef]
- Arshad, M.; Hussain, T.; Iqbal, M.; Abbas, M. Enhanced ethanol production at commercial scale from molasses using high gravity technology by mutant S. cerevisiae. Braz. J. Microbiol. 2017, 48, 403–409. [Google Scholar] [CrossRef]
- Thomas, K.C.; Hynes, S.H.; Jones, A.M.; Ingledew, W.M. Production of fuel alcohol from wheat by VHG technology: Effect of sugar concentration and fermentation temperature. Appl. Biochem. Biotechnol. 1993, 43, 211–226. [Google Scholar] [CrossRef]
- Wang, F.Q.; Gao, C.J.; Yang, C.Y.; Xu, P. Optimization of an ethanol production medium in very high gravity fermentation. Biotechnol. Lett. 2007, 29, 233–236. [Google Scholar] [CrossRef]
- Maiorella, B.; Blanch, H.; Wilke, C.; Wyman, C.E. Economic evaluation of alternative ethanol fermentation processes. Biotechnol. Bioeng. 2009, 104, 419–443. [Google Scholar] [CrossRef]
- Wang, S.; Thomas, K.C.; Sosulski, K.; Ingledew, W.M. Grain pearling and very high gravity (VHG) fermentation technologies for fuel alcohol production from rye and triticale. Process Biochem. 1999, 34, 421–428. [Google Scholar] [CrossRef]
- Bai, F.W.; Chen, L.J.; Anderson, W.A.; Moo-Young, M. Parameter oscillations in very high gravity medium continuous ethanol fermentation and their attenuation on multi-stage packed column bioreactor system. Biotechnol. Bioeng. 2004, 88, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.W.; Chen, L.J.; Zhang, Z.; Anderson, W.A.; Moo-Young, M. Continuous ethanol production and evaluation of yeast cell lysis and viability loss under very high gravity medium conditions. J. Biotechnol. 2004, 110, 287–293. [Google Scholar] [CrossRef]
- Reis, V.R.; Bassi, A.P.G.; da Silva, J.C.G.; Ceccato-Antonini, S.R. Characteristics of Saccharomyces cerevisiae yeasts exhibiting rough colonies and pseudohyphal morphology with respect to alcoholic fermentation. Braz. J. Microbiol. 2013, 44, 1121–1131. [Google Scholar] [CrossRef]
- Lopes, M.L.; Paulillo, S.C.L.; Godoy, A.; Cherubin, R.A.; Lorenzi, M.S.; Giometti, F.H.C.; Bernardino, C.D.; Neto, H.B.A.; Amorim, H.V. Ethanol production in Brazil: A bridge between science and industry. Braz. J. Microbiol. 2016, 47, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.R.; Lawrence, F.M.; Leclaire, J.P.R.; Powell, C.D.; Smart, K.A. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol. Rev. 2007, 31, 535–569. [Google Scholar] [CrossRef] [PubMed]
- Mira, N.P.; Palma, M.; Guerreiro, J.F.; Sá-Correia, I. Genome-wide identification of Saccharomyces cerevisiae genes required for tolerance to acetic acid. Microb. Cell Fact. 2010, 9, 1–13. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Mira, N.P.; Sá-Correia, I. A genome-wide perspective on the response and tolerance to food-relevant stresses in Saccharomyces cerevisiae. Curr. Opin. Biotechnol. 2011, 22, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Estruch, F. Stress-controlled transcription factor, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol. Rev. 2000, 24, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 2002, 66, 300–372. [Google Scholar] [CrossRef] [PubMed]
- Hounsa, C.G.; Brandt, E.V.; Trevelein, J.; Hohmann, S.; Prior, B.A. Role of trehalose in survival of Saccharomyces cerevisiae under osmotic stress. Microbiology 1998, 144, 671–680. [Google Scholar] [CrossRef]
- Panek, A.C.; Vânia, J.J.M.; Paschoalin, M.F.; Panek, D. Regulation of trehalose metabolism in Saccharomyces cerevisiae mutants during temperature shifts. Biochimie 1990, 72, 77–79. [Google Scholar] [CrossRef]
- D’Amore, T.; Crumplen, R.; Stewart, G.G. The involvement of trehalose in yeast stress tolerance. J. Ind. Microbiol. 1991, 7, 191–195. [Google Scholar] [CrossRef]
- Stanley, D.; Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010, 109, 13–24. [Google Scholar] [CrossRef]
- Casey, G.P.; Ingledew, W.M. Ethanol tolerance in yeasts. Crit. Rev. Microbiol. 1986, 13, 219–280. [Google Scholar] [CrossRef]
- Hu, X.H.; Wang, M.H.; Tan, T.; Li, J.R.; Yang, H.; Leach, L.; Zhang, R.M.; Luo, Z.W. Genetic dissection of ethanol tolerance in the budding yeast Saccharomyces cerevisiae. Genetics 2007, 175, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Prasad, R. Relationship between ethanol tolerance and fatty acyl composition of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 1989, 30, 294–298. [Google Scholar] [CrossRef]
- Piper, P.W.; Talreja, K.; Panaretou, B.; Moradas-Ferreira, P.; Byrne, K.; Praekelt, U.M.; Meacock, P.; Récnacq, M.; Boucherie, H. Induction of major heat-shock proteins of Saccharomyces cerevisiae, including plasma membrane Hsp30, by ethanol levels above a critical threshold. Microbiology 1994, 140 Pt 11, 3031–3038. [Google Scholar] [CrossRef]
- Thomas, K.C.; Ingledew, W.M. Production of 21% (v/v) ethanol by fermentation of very high gravity (VHG) wheat mashes. J. Ind. Microbiol. 1992, 10, 61–68. [Google Scholar] [CrossRef]
- Pereira, F.B.; Guimarães, P.M.R.; Teixeira, J.A.; Domingues, L. Robust industrial Saccharomyces cerevisiae strains for very high gravity bio-ethanol fermentations. J. Biosci. Bioeng. 2011, 112, 130–136. [Google Scholar] [CrossRef]
- Paulillo, S.C.L.; Yokoya, F.; Basso, L.C. Mobilization of endogenous glycogen and trehalose of industrial yeasts. Braz. J. Microbiol. 2003, 34, 249–254. [Google Scholar] [CrossRef]
- François, J.M.; Walther, T.; Parrou, J.L. Genetics and Regulation of Glycogen and Trehalose Metabolism in Saccharomyces cerevisiae. In Microbial Stress Tolerance for Biofuels—Microbiology Monographs; Liu, Z., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 22, pp. 29–55. [Google Scholar]
- Lip, K.; García-Ríos, E.; Costa, C.E.; Guillamón, J.M.; Domingues, L.; Teixeira, J.; van Gulik, W.M. Selection and subsequent physiological characterization of industrial Saccharomyces cerevisiae strains during continuous growth at sub- and- supra optimal temperatures. Biotechnol. Rep. 2020, 26, e00462. [Google Scholar] [CrossRef]
- Pinheiro, T.; Lip, K.; García-Ríos, E.; Querol, A.; Teixeira, J.; van Gulik, W.; Guillamón, J.M.; Domingues, L. Differential proteomic analysis by SWATH-MS unravels the most dominant mechanisms underlying yeast adaptation to non-optimal temperatures under anaerobic conditions. Sci. Rep. 2020, 10, 22329. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, A.; Assadi, M.M.; Asadirad, M.H.A.; Karizi, S.Z. Bio-ethanol production by a novel autochthonous thermo-tolerant yeast isolated from wastewater. J. Environ. Health Sci. Eng. 2014, 12, 1–6. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Liang, X. Enhanced ethanol production from stalk juice of sweet sorghum by response surface methodology. Afr. J. Biotechnol. 2012, 11, 6117–6122. [Google Scholar]
- Phutela, U.G.; Kaur, J. Process optimization for ethanol production from sweet sorghum juice using Saccharomyces cerevisiae strain NRRL Y-2034 by response surface methodology. Sugar Tech. 2014, 16, 411–421. [Google Scholar] [CrossRef]
- Pattanakittivorakul, S.; Lertwattanasakul, N.; Yamada, M.; Limtong, S. Selection of thermotolerant Saccharomyces cerevisiae for high temperature ethanol production from molasses and increasing ethanol production by strain improvement. Antonie Leeuwenhoek 2019, 112, 975–990. [Google Scholar] [CrossRef]
- Techaparin, A.; Thanonkeo, P.; Klanrit, P. High-temperature ethanol production using thermotolerant yeast newly isolated from Greater Mekong Subregion. Braz. J. Microbiol. 2017, 48, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, H.; Gan, M.; Jin, Y.; Gao, X.; Chen, Q.; Guan, J.; Wang, Z. Application of simultaneous saccharification and fermentation (SSF) from viscosity reducing of raw sweet potato for bioethanol production at laboratory, pilot and industrial scales. Bioresour. Technol. 2011, 102, 4573–4579. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.L.; de Resende, M.M.; Ribeiro, E.J. Improvement of ethanol production in fed-batch fermentation using a mixture of sugarcane juice and molasse under very high-gravity conditions. Bioprocess Biosyst. Eng. 2021, 44, 617–625. [Google Scholar] [CrossRef]
- Gomes, D.G.; Guimarães, P.M.R.; Pereira, F.B.; Teixeira, J.A.; Domingues, L. Plasmid-mediate transfer of FLO1 into industrial Saccharomyces cerevisiae PE-2 strain creates a strain useful for repeat-batch fermentations involving flocculation–sedimentation. Bioresour. Technol. 2012, 108, 162–168. [Google Scholar] [CrossRef]
- Zhang, Q.; Jin, Y.-L.; Fang, Y.; Zhao, H. Adaptive evolution and selection of stress-resistant Saccharomyces cerevisiae for very high gravity bioethanol fermentation. Electron. J. Biotechnol. 2019, 41, 88–94. [Google Scholar] [CrossRef]
- Kim, J.H.; Ryu, J.; Huh, I.Y. Ethanol production from galactose by a newly isolated Saccharomyces cerevisiae KL17. Bioprocess Biosyst. Eng. 2014, 37, 1871–1878. [Google Scholar] [CrossRef]
- Joannis-Cassan, C.; Riess, J.; Jolibert, F.; Taillandier, P. Optimization of very high gravity fermentation process for ethanol production from industrial sugar beet syrup. Biomass Bioenergy 2014, 70, 165–173. [Google Scholar] [CrossRef]
- Bafrncová, P.; šmogrovičová, D.; Sláviková, I.; Pátková, J.; Dömény, Z. Improvement of very high gravity ethanol fermentation by media supplementation using Sacchromyces serevisiae. Biotechnol. Lett. 1999, 21, 337–341. [Google Scholar] [CrossRef]
- Laopaiboon, L.; Nuanpeng, S.; Srinophakun, P.; Klanrit, P.; Laopaiboon, P. Ethanol production from sweet sorghum juice using very high gravity technology: Effects of carbon and nitrogen supplementations. Bioresour. Technol. 2009, 100, 4176–4182. [Google Scholar] [CrossRef]
- Khongsay, N.; Laopaiboon, L.; Jaisil, P.; Laopaiboon, P. Optimization of agitation and aeration for very high gravity ethanol fermentation from sweet sorghum juice by Saccharomyces cerevisiae using an orthogonal array design. Energies 2012, 5, 561–576. [Google Scholar] [CrossRef]
- Reddy, L.V.A.; Reddy, O.V.S. Improvement of ethanol production in very high gravity fermentation by horse gram (Dolichos biflorus) flour supplementation. Lett. Appl. Microbiol. 2005, 41, 440–444. [Google Scholar] [CrossRef]
- Reddy, L.V.A.; Reddy, O.V.S. Rapid and enhanced production of ethanol in very high gravity (VHG) sugar fermentations by Saccharomyces cerevisiae: Role of finger millet (Eleusine coracana L.) flour. Process Biochem. 2006, 41, 726–729. [Google Scholar] [CrossRef]
- Pereira, F.B.; Guimarães, P.M.R.; Teixeira, J.A.; Domingues, L. Optimization of low-cost medium for very high gravity ethanol fermentations by Saccharomyces cerevisiae using statistical experimental designs. Bioresour. Technol. 2010, 101, 7856–7863. [Google Scholar] [CrossRef]
- Kelbert, M.; Romaní, A.; Coelho, E.; Pereira, F.B.; Teixeira, J.A.; Domingues, L. Lignocellulosic bioethanol production with revalorization of low-cost agroindustrial by-products as nutritional supplements. Ind. Crops Prod. 2015, 64, 16–24. [Google Scholar] [CrossRef]
- Jones, A.M.; Ingledew, W.M. Fuel alcohol production: Optimization of temperature for efficient very-high-gravity fermentation. Appl. Microbiol. Biotechnol. 1994, 60, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Sridee, W.; Laopaiboon, L.; Jaisil, P.; Laopaiboon, P. The use of dried spent yeast as a low-cost nitrogen supplement in ethanol fermentation from sweet sorghum juice under very high gravity conditions. Electron. J. Biotechnol. 2011, 14, 1–15. [Google Scholar]
- Palukurty, M.A.; Telgana, N.K.; Bora, H.S.R.; Mulampaka, S.N. Screening and optimization of metal ions to enhance ethanol production using statistical experimental designs. Afr. J. Microbiol. Res. 2008, 2, 87–94. [Google Scholar]
- Xue, C.; Zhao, X.Q.; Yuan, W.J.; Bai, F.W. Improving ethanol tolerance of a self-flocculating yeast by optimization of medium composition. World J. Microbiol. Biotechnol. 2008, 24, 2257–2261. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Bai, F.W. Yeast flocculation: New story in fuel ethanol production. Biotechnol. Adv. 2009, 27, 849–856. [Google Scholar] [CrossRef]
- Trofimova, Y.; Walker, G.; Rapoport, A. Anhydrobiosis in yeast: Influence of calcium and magnesium ions on yeast resistance to dehydration–rehydration. FEMS Microbiol. Lett. 2010, 308, 55–61. [Google Scholar] [CrossRef]
- Courchesne, W.E.; Vlasek, C.; Klukovich, R.; Coffee, S. Ethanol induces calcium influx via the Cch1-Mid1 transporter in Saccharomyces cerevisiae. Arch. Microbiol. 2011, 193, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.W.; Oliver, S.G. The effect of temperature on the ethanol tolerance of the yeast Saccharomyces uvarum. Biotechnol. Lett. 1982, 4, 269–273. [Google Scholar] [CrossRef]
- Patrascu, E.; Rapeanu, G.; Hopulele, T. Current approaches to efficient biotechnological production of ethanol. Innovative Rom. Food Biotechnol. 2009, 4, 1–11. [Google Scholar]
- Gao, C.; Fleet, G. The effects of temperature and pH on the ethanol tolerance of the wine yeasts, Saccharomyces cerevisiae, Candida stellata and Kloeckera apiculata. J. Appl. Bacteriol. 1988, 65, 405–409. [Google Scholar] [CrossRef]
- Pereira, F.B.; Gomes, D.G.; Guimarães, P.M.; Teixeira, J.A.; Domingues, L. Cell recycling during repeated very high gravity bio-ethanol fermentations using the industrial Saccharomyces cerevisiae strain PE-2. Biotechnol. Lett. 2012, 34, 45–53. [Google Scholar] [CrossRef]
- Laluce, C.; Tognolli, J.O.; de Oliveira, K.F.; Souza, C.S.; Morais, M.R. Optimization of temperature, sugar concentration, and inoculum size to maximize ethanol production without significant decrease in yeast cell viability. Appl. Microbiol. Biotechnol. 2009, 83, 627–637. [Google Scholar] [CrossRef]
- Limtong, S.; Sringiew, C.; Yongmanitchai, W. Production of fuel ethanol at high temperature from sugar cane juice by a newly isolated Kluyveromyces marxianus. Bioresour. Technol. 2007, 98, 3367–3374. [Google Scholar] [CrossRef]
- Malairuang, K.; Krajang, M.; Rotsattarat, R.; Chamsart, S. Intensive Multiple Sequential Batch Simultaneous Saccharification and Cultivation of Kluyveromyces marxianus SS106 Thermotolerant Yeast Strain for Single-Step Ethanol Fermentation from Raw Cassava Starch. Processes 2020, 8, 898. [Google Scholar] [CrossRef]
- Rivera, E.C.; Costa, A.C.; Atala, D.I.P.; Maugeri, F.; Maciel, M.R.W.; Filho, R.M. Evaluation of optimization techniques for parameter estimation: Application to ethanol fermentation considering the effect of temperature. Process Biochem. 2006, 41, 1682–1687. [Google Scholar] [CrossRef]
- You, K.M.; Rosenfield, C.L.; Knipple, D.C. Ethanol tolerance in the yeast Saccharomyces cerevisiae is dependent on cellular oleic acid content. Appl. Environ. Microbiol. 2003, 69, 1499–1503. [Google Scholar] [CrossRef]
- Visser, W.; Scheffers, W.A.; Batenburg-van der Vegte, W.H.; van Dijken, J.P. Oxygen requirements of yeasts. Appl. Environ. Microbiol. 1990, 56, 3785–3792. [Google Scholar] [CrossRef]
- Rosenfeld, E.; Beauvoit, B.; Blondin, B.; Salmon, J.M. Oxygen consumption by anaerobic Saccharomyces cerevisiae under enological conditions: Effect on fermentation kinetics. Appl. Environ. Microbiol. 2003, 69, 113–121. [Google Scholar] [CrossRef]
- Jouhten, P.; Rintala, E.; Huuskonen, A.; Tamminen, A.; Toivari, M.; Wiebe, M.; Ruohonen, L.; Penttilä, M.; Maaheimo, H. Oxygen dependence of metabolic fluxes and energy generation of Saccharomyces cerevisiae CEN.PK113-1A. BMC Syst. Biol. 2008, 2, 60. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chien, W.-S.; Duan, K.-J.; Chang, P.R. Effect of aeration timing and interval during very-high-gravity ethanol fermentation. Process Biochem. 2011, 46, 1025–1028. [Google Scholar] [CrossRef]
- Poonsrisawat, A.; Wanlapatit, S.; Wansuksri, R.; Piyachomkwan, K.; Paemanee, A.; Gamonpilas, C.; Eurwilaichitr, L.; Champreda, V. Synergistic effects of cell wall degrading enzymes on rheology of cassava root mash. Process Biochem. 2016, 51, 2104–2111. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.; Jin, Y.; Xue, H.; Guan, J.; Wang, Z.; Zhao, H. Energy-saving direct ethanol production from viscosity reduction mash of sweet potato at very high gravity (VHG). Fuel Process. Technol. 2010, 91, 1845–1850. [Google Scholar] [CrossRef]
- Cunha, J.T.; Romaní, A.; Inokuma, K.; Johansson, B.; Hasunuma, T.; Kondo, A.; Domingues, L. Consolidated bioprocessing of corn cob-derived hemicellulose: Engineered industrial Saccharomyces cerevisiae as efficient whole cell biocatalysts. Biotechnol. Biofuels 2020, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Kawa-Rygielska, J.; Pietrzak, W. Ethanol fermentation of very high gravity (VHG) maize mashes by Saccharomyces cerevisiae with spent brewer’s yeast supplementation. Biomass Bioenergy 2014, 60, 50–57. [Google Scholar] [CrossRef]
- Suwanapong, S.; Khongsay, N.; Laopaiboon, L.; Jaisil, P.; Laopaiboon, P. Dried Spent Yeast and Its Hydrolysate as Nitrogen Supplements for Single Batch and Repeated-Batch Ethanol Fermentation from Sweet Sorghum Juice. Energies 2013, 6, 1618–1631. [Google Scholar] [CrossRef]
- Appiah-Nkansah, N.B.; Zhang, K.; Rooney, W.; Wang, D. Ethanol production from mixtures of sweet sorghum juice and sorghum starch using very high gravity fermentation with urea supplementation. Ind. Crops Prod. 2018, 111, 247–253. [Google Scholar] [CrossRef]
- Yue, G.; Yu, J.; Zhang, X.; Tan, T. The influence of nitrogen sources on ethanol production by yeast from concentrated sweet sorghum juice. Biomass Bioenergy 2012, 39, 48–52. [Google Scholar] [CrossRef]
- Deesuth, O.; Laopaiboon, P.; Jaisil, P.; Laopaiboon, L. Optimization of Nitrogen and Metal Ions Supplementation for Very High Gravity Bioethanol Fermentation from Sweet Sorghum Juice Using an Orthogonal Array Design. Energies 2012, 5, 3178–3197. [Google Scholar] [CrossRef]
- Chan-u-tit, P.; Laopaiboon, L.; Jaisil, P.; Laopaiboon, P. High Level Ethanol Production by Nitrogen and Osmoprotectant Supplementation under Very High Gravity Fermentation Conditions. Energies 2013, 6, 884–899. [Google Scholar] [CrossRef]
- Phukoetphim, N.; Salakkam, A.; Laopaiboon, P.; Laopaiboon, L. Improvement of ethanol production from sweet sorghum juice under batch and fed-batch fermentations: Effects of sugar levels, nitrogen supplementation, and feeding regimes. Electron. J. Biotechnol. 2017, 26, 84–92. [Google Scholar] [CrossRef]
- Yang, H.; Zong, X.; Cui, C.; Mu, L.; Zhao, H. Peptide (Lys-Leu) and amino acids (Lys and Leu) supplementations improve physiological activity and fermentation performance of brewer’s yeast during very high-gravity (VHG) wort fermentation. Biotechnol. Appl. Biochem. 2018, 65, 630–638. [Google Scholar] [CrossRef]
- Hu, C.; Qin, Q.; Gao, P. Medium optimization for improved ethanol production in very high gravity fermentation. Chin. J. Chem. Eng. 2011, 19, 1017–1022. [Google Scholar] [CrossRef]
- Liu, C.G.; Wang, N.; Lin, Y.H.; Bai, F.W. Very high gravity ethanol fermentation by flocculating yeast under redox potential-controlled conditions. Biotechnol. Biofuels 2012, 5, 61. [Google Scholar] [CrossRef]
- Westman, J.O.; Franzén, C.J. Current progress in high cell density yeast bioprocesses for bioethanol production. Biotechnol. J. 2015, 10, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Vega, J.L.; Navarro, A.R.; Clausen, E.C.; Gaddy, J.L. Effects of inoculum size on ethanol inhibition modeling and other fermentation parameters. Biotechnol. Bioeng. 1987, 29, 633–638. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Gupta, S. 2.38—Fed-Batch Fermentation—Design Strategies. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Cambridge, MA, USA, 2011; pp. 515–526. [Google Scholar]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Chapter 2—Microbial growth kinetics. In Principles of Fermentation Technology, 3rd ed.; Stanbury, P.F., Whitaker, A., Hall, S.J., Eds.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 21–74. [Google Scholar]
- Rolland, F.; Winderickx, J.; Thevelein, J.M. Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res. 2002, 2, 183–201. [Google Scholar] [CrossRef]
- Gomar-Alba, M.; Morcillo-Parra, M.Á.; Olmo, M.L. Response of yeast cells to high glucose involves molecular and physiological differences when compared to other osmostress conditions. FEMS Yeast Res. 2015, 15, fov039. [Google Scholar] [CrossRef] [PubMed]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Principles of Fermentation Technology; Butterworth-Heinemann: Oxford, UK, 1995. [Google Scholar]
- Laopaiboon, L.; Thanonkeo, P.; Jaisil, P.; Laopaiboon, P. Ethanol production from sweet sorghum juice in batch and fed-batch fermentations by Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 1497–1501. [Google Scholar] [CrossRef]
- Cruz, L.M.; Resende, M.M.; Ribeiro, E.J. Evaluation of process conditions in the performance of yeast on alcoholic fermentation. Chem. Eng. Commun. 2018, 205, 846–855. [Google Scholar] [CrossRef]
- Puligundla, P.; Smogrovicova, D.; Mok, C.; Obulam, V.S.R. A review of recent advances in high gravity ethanol fermentation. Renew. Energy 2019, 133, 1366–1379. [Google Scholar] [CrossRef]
- Bayrock, D.P.; Ingledew, W.M. Application of multistage continuous fermentation for production of fuel alcohol by very-high-gravity fermentation technology. J. Ind. Microbiol. Biotechnol. 2001, 27, 87–93. [Google Scholar] [CrossRef]
- Mishra, A.; Sharma, A.K.; Sharma, S.; Bagai, R.; Mathur, A.S.; Gupta, R.P.; Tuli, D.K. Lignocellulosic ethanol production employing immobilized Saccharomyces cerevisiae in packed bed reactor. Renew. Energy 2016, 98, 57–63. [Google Scholar] [CrossRef]
- Soares, E.V. Flocculation in Saccharomyces cerevisiae: A review. J. Appl. Microbiol. 2010, 110, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kourkoutas, Y.; Bekatorou, A.; Banat, I.M.; Marchant, R.; Koutinas, A. Immobilization technologies and support materials suitable in alcohol beverages production: A review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
- Brandao, A.C.T.; de Resende, M.M.; Ribeiro, E.J. Alcoholic fermentation with high sugar and cell concentrations at moderate temperatures using flocculant yeasts. Korean J. Chem. Eng. 2020, 37, 1717–1725. [Google Scholar] [CrossRef]
- Domingues, L.; Dantas, M.M.; Lima, N.; Teixeira, J.A. Continuous ethanol fermentation of lactose by a recombinant flocculating Saccharomyces cerevisiae strain. Biotechnol. Bioeng. 1999, 64, 692–697. [Google Scholar] [CrossRef]
- Domingues, L.; Lima, N.; Teixeira, J.A. Alcohol production from cheese whey permeate using genetically modified flocculent yeast cells. Biotechnol. Bioeng. 2001, 72, 507–514. [Google Scholar] [CrossRef]
- Domingues, L.; Lima, N.; Teixeira, J.A. Aspergillus niger β-galactosidase production by yeast in a continuous high cell density reactor. Process Biochem. 2005, 40, 1151–1154. [Google Scholar] [CrossRef]
- Klein, J.; Maia, J.; Vicente, A.A.; Domingues, L.; Teixeira, J.A.; Jurascík, M. Relationships between hydrodynamics and rheology of flocculating yeast suspensions in a high-cell-density airlift bioreactor. Biotechnol. Bioeng. 2005, 89, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Andrietta, S.R.; Seckelberg, C.; Andrietta, M.G.S. Study of flocculent yeast performance in tower reactors for bioethanol production in a continuous fermentation process with no cell recycling. Bioresour. Technol. 2008, 99, 3002–3008. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, T.F.; Júnior, W.G.M.; Guidini, C.Z.; Marquez, L.D.S.; Cardoso, V.L.; Resende, M.M.; Ribeiro, E.J. Alcoholic fermentation with self-flocculating yeast in a tower upflow reactor. Chem. Eng. Technol. 2015, 38, 345–354. [Google Scholar] [CrossRef]
- Viegas, M.C.; Andrietta, M.G.S.; Andrietta, S.R. Use of tower reactors for continuous ethanol production. Braz. J. Chem. Eng. 2002, 2, 167–173. [Google Scholar] [CrossRef]
- Landaeta, R.; Aroca, G.; Acevedo, F.; Teixeira, J.A.; Mussatto, S.I. Adaptation of a flocculent Saccharomyces cerevisiae strain to lignocellulosic inhibitors by cell recycle batch fermentation. Appl. Energy 2013, 102, 124–130. [Google Scholar] [CrossRef]
- Domingues, L.; Lima, N.; Teixeira, J.A. Contamination of a high-cell-density continuous bioreactor. Biotechnol. Bioeng. 2000, 68, 584–587. [Google Scholar] [CrossRef][Green Version]
- Li, F.; Zhao, X.Q.; Ge, X.M.; Bai, F.W. An innovative consecutive batch fermentation process for very high gravity ethanol fermentation with self-flocculating yeast. Appl. Microbiol. Biotechnol. 2009, 84, 1079–1086. [Google Scholar] [CrossRef]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Faik, A.A.M.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar]
- Margaritis, A.; Merchant, F.J.A. The technology of anaerobic yeast growth. In Yeast Biotechnology; Berry, D.R., Russell, B.I., Stewart, G.G., Eds.; Springer: Dordrecht, The Netherlands, 1987; pp. 37–53. [Google Scholar]
- Chibata, I.; Wingard, L.B. Immobilized Microbial Cells: Applied Biochemistry and Bioengineering, 4th ed.; Academic Press: Oxford, UK, 1983; pp. 203–215. [Google Scholar]
- Ylitervo, P.; Franzen, C.J.; Taherzadeh, M.J. Ethanol production at elevated temperatures using encapsulation of yeast. J. Biotechnol. 2011, 156, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, G.; Li, Y. Immobilization of Saccharomyces cerevisiae alcohol dehydrogenase on hybrid alginate-chitosan beads. Int. J. Biol. Macromol. 2010, 47, 21–26. [Google Scholar] [CrossRef]
- Bangrak, P.; Limtong, S.; Phisalaphong, M. Continuous ethanol production using immobilized yeast cells entrapped in loofa-reinforced alginate carriers. Braz. J. Microbiol. 2011, 42, 676–684. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Tan, T. A novel immobilization method of Saccharomyces cerevisiae to sorghum bagasse for ethanol production. J. Biotechnol. 2007, 129, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Stolarzewicz, I.; Bialecka-Florjanczyk, E.; Majewska, E.; Krzyczkowska, J. Immobilization of yeast on polymeric supports. Chem. Biochem. Eng. Q. 2011, 25, 135–144. [Google Scholar]
- Pereira, F.B.; Guimarães, P.M.R.; Gomes, D.G.; Mira, N.P.; Teixeira, M.C.; Sá-Correia, I.; Domingues, L. Identification of candidate genes for yeast engineering to improve bioethanol production in very high gravity and lignocellulosic biomass industrial fermentations. Biotechnol. Biofuels 2011, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-Q.; Hou, X.-Y.; Hao, A.-L.; Wang, P.-F.; Fu, X.-M.; Lv, A.; Dong, J. Truncation of CYR1 promoter in industrial ethanol yeasts for improved ethanol yield in high temperature condition. Process Biochem. 2018, 65, 37–45. [Google Scholar] [CrossRef]
- Tao, X.; Zheng, D.; Liu, T.; Wang, P.; Zhao, W.; Zhu, M.; Jiang, X.; Zhao, Y.; Wu, X. A novel strategy to construct yeast Saccharomyces cerevisiae strains for very high gravity fermentation. PLoS ONE 2012, 7, e31235. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Ding, W.T.; Zhang, G.C.; Wang, J.Y. Improving ethanol fermentation performance of Saccharomyces cerevisiae in very high-gravity fermentation through chemical mutagenesis and meiotic recombination. Appl. Microbiol. Biotechnol. 2011, 91, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, S.; Wang, W.T.; Wang, K.T.; Jia, X. Very high gravity ethanol and fatty acid production of Zymomonas mobilis without amino acid and vitamin. J. Ind. Microbiol. Biotechnol. 2016, 43, 861–871. [Google Scholar] [CrossRef]
- Guo, Z.P.; Qiu, C.Y.; Zhang, L.; Ding, Z.Y.; Wang, Z.X.; Shi, G.Y. Expression of aspartic protease from Neurospora crassa in industrial ethanol-producing yeast and its application in ethanol production. Enzyme Microb. Technol. 2011, 48, 148–154. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Gomes, D.G.; Rodrigues, A.C.; Domingues, L.; Gama, F.M. Cellulase recycling in biorefineries—Is it possible? Appl. Microbiol. Biotechnol. 2015, 99, 4131–4143. [Google Scholar] [CrossRef]
- Gomes, D.G.; Domingues, L.; Gama, F.M. Valorizing recycled paper sludge by a bioethanol production process with cellulase recycling. Bioresour. Technol. 2016, 216, 637–644. [Google Scholar] [CrossRef]
- Koppram, R.; Tomás-Pejó, E.; Xiros, C.; Olsson, L. Lignocellulosic ethanol production at high-gravity: Challenges and perspectives. Trends Biotechnol. 2014, 32, 46–53. [Google Scholar] [CrossRef]
- Almeida, J.R.M.; Bertilsson, M.; Gorwa-Grauslund, M.F.; Gorsich, S.; Lidén, G. Metabolic effects of furaldehydes and impacts on biotechnological process. Appl. Microbiol. Biotechnol. 2009, 82, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Q.; Cai, L.-Y.; Ma, Y.-L. Study on inhibitors from acid pretreatment of corn stalk on ethanol fermentation by alcohol yeast. RSC Adv. 2020, 10, 38409–38415. [Google Scholar] [CrossRef]
- Gu, H.; Zhu, Y.; Peng, Y.; Liang, X.; Liu, X.; Shao, L.; Xu, Y.; Xu, Z.; Liu, R.; Li, J. Physiological mechanism of improved tolerance of Saccharomyces cerevisiae to lignin-derived phenolic acids in lignocellulosic ethanol fermentation by short-term adaptation. Biotechnol. Biofuels 2019, 12, 268. [Google Scholar] [CrossRef]
- Gomes, D.G.; Gama, F.M.; Domingues, L. Determinants on an efficient cellulase recycling process for the production of bioethanol from recycled paper sludge under high solid loadings. Biotechnol. Biofuels 2018, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Alkasrawi, M.; Rudolf, A.; Lidén, G.; Zacchi, G. Influence of strain and cultivation procedure on the performance of simultaneous saccharification and fermentation of steam pretreated spruce. Enzyme Microb. Technol. 2006, 38, 279–286. [Google Scholar] [CrossRef]
- Hawkins, G.M.; Doran-Peterson, J. A strain of Saccharomyces cerevisiae evolved for fermentation of lignocellulosic biomass displays improved growth and fermentative ability in high solids concentrations and in the presence of inhibitory compounds. Biotechnol. Biofuels 2011, 4, 49. [Google Scholar] [CrossRef]
- Rudolf, A.; Alkasrawi, M.; Zacchi, G.; Lidén, G. A comparison between batch and fed-batch simultaneous saccharification and fermentation of steam pretreated spruce. Enzyme Microb. Technol. 2005, 37, 195–204. [Google Scholar] [CrossRef]
- Rosgaard, L.; Andric, P.; Dam-Johansen, K.; Pedersen, S.; Meyer, A.S. Effects of substrate loading on enzymatic hydrolysis and viscosity of pretreated barley straw. Appl. Biochem. Biotechnol. 2007, 143, 27–40. [Google Scholar] [CrossRef]
- Sotaniemi, V.-H.; Taskila, S.; Ojamo, H.; Tanskanen, J. Controlled feeding of lignocellulosic substrate enhances the performance of fed-batch enzymatic hydrolysis in a stirred tank reactor. Biomass Bioenergy 2016, 91, 271–277. [Google Scholar] [CrossRef]
| Process Element | Challenge | Research Efforts |
|---|---|---|
| Fermentation organism | Stress induced by high ethanol concentrations | New high ethanol-tolerance organisms |
| Optimization of process conditions (e.g., temperature) | ||
| Alternative process configurations (e.g., cells immobilization) | ||
| Optimization of cells pitching strategy | ||
| Stress induced by high sugar concentrations | ||
| New high osmolites-tolerance organisms | ||
Alternative process configurations
| ||
| Nutrients starvation (especially nitrogen) | New low-cost nutrient sources | |
| Optimization of media composition | ||
| Cost of cells | Cells immobilization | |
| Process productivity | ||
| Sugar feedstock | Competition with food crops | New feedstocks not competing with food crops and/or with an inferior cost |
| Feedstock price | ||
| Sugar Yields | Plant development for high sugar contents | |
| Highly viscous materials | Process adjuvants (e.g., cell wall degrading enzymes) | |
| Optimization of mixing/improved mixing alternatives | ||
| Process | Mass transferlimitations | |
| Oxygen limitation | Optimization of oxygen supply/new strategies for oxygen supply | |
| Contamination risk | More robust organisms towards microbial contamination | |
| More thermotolerant organisms |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, D.; Cruz, M.; de Resende, M.; Ribeiro, E.; Teixeira, J.; Domingues, L. Very High Gravity Bioethanol Revisited: Main Challenges and Advances. Fermentation 2021, 7, 38. https://doi.org/10.3390/fermentation7010038
Gomes D, Cruz M, de Resende M, Ribeiro E, Teixeira J, Domingues L. Very High Gravity Bioethanol Revisited: Main Challenges and Advances. Fermentation. 2021; 7(1):38. https://doi.org/10.3390/fermentation7010038
Chicago/Turabian StyleGomes, Daniel, Mariana Cruz, Miriam de Resende, Eloízio Ribeiro, José Teixeira, and Lucília Domingues. 2021. "Very High Gravity Bioethanol Revisited: Main Challenges and Advances" Fermentation 7, no. 1: 38. https://doi.org/10.3390/fermentation7010038
APA StyleGomes, D., Cruz, M., de Resende, M., Ribeiro, E., Teixeira, J., & Domingues, L. (2021). Very High Gravity Bioethanol Revisited: Main Challenges and Advances. Fermentation, 7(1), 38. https://doi.org/10.3390/fermentation7010038







