Evaluation of Basidiomycetes Wild Strains Grown in Agro-Industrial Residues for Their Anti-Tyrosinase and Antioxidant Potential and for the Production of Biocatalysts
Abstract
1. Introduction
2. Results and Discussion
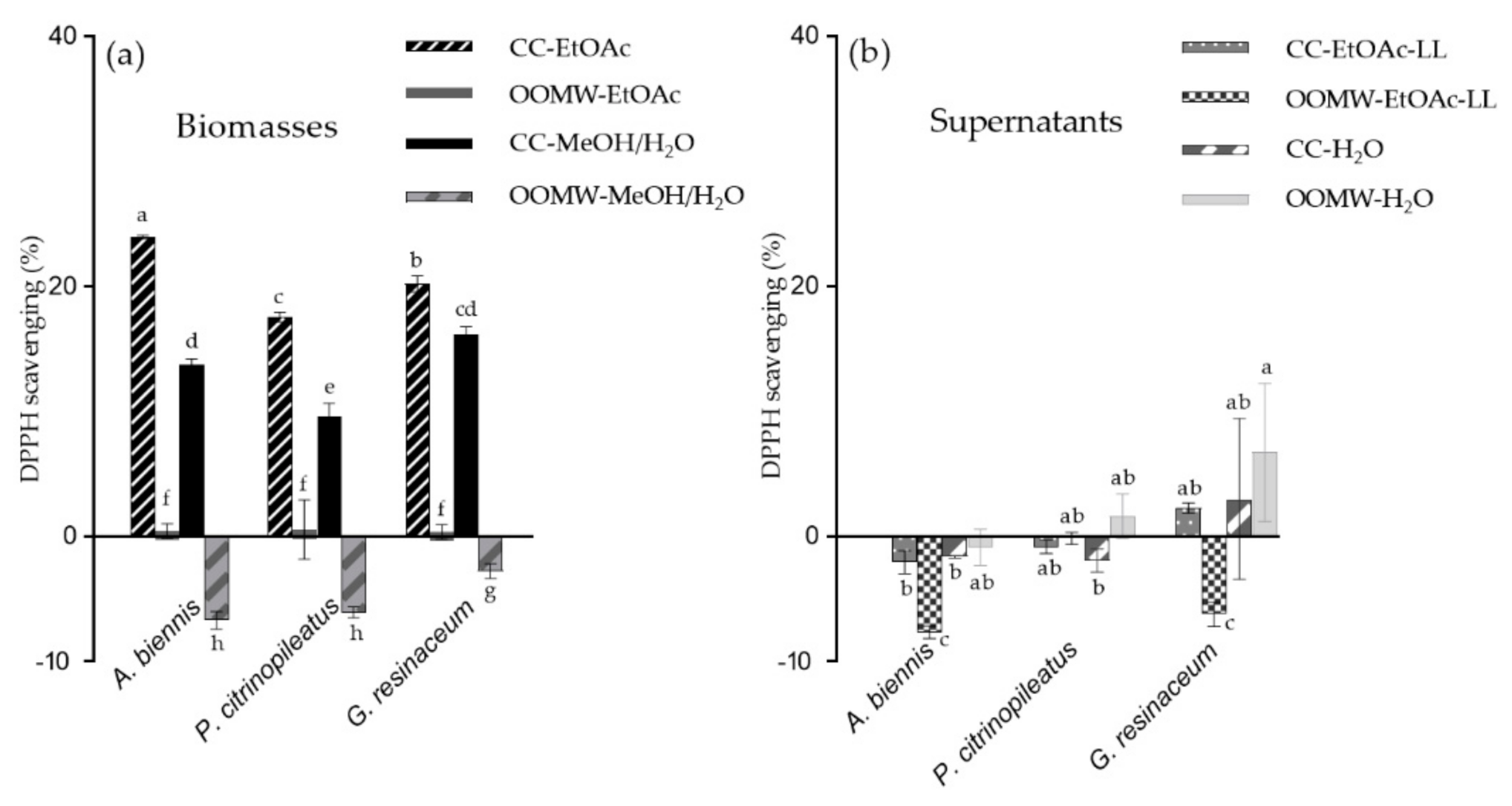
2.1. Anti-Oxidant and Anti-Tyrosinase Activity of Mushroom Extracts
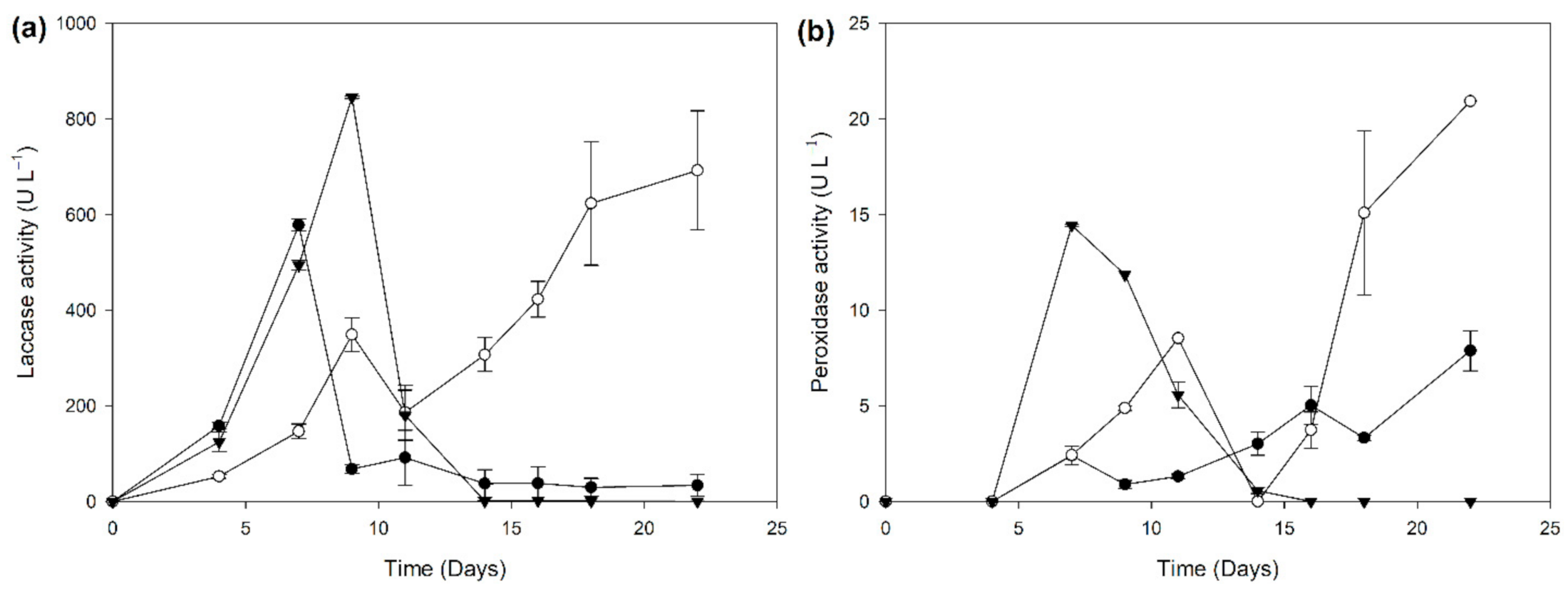
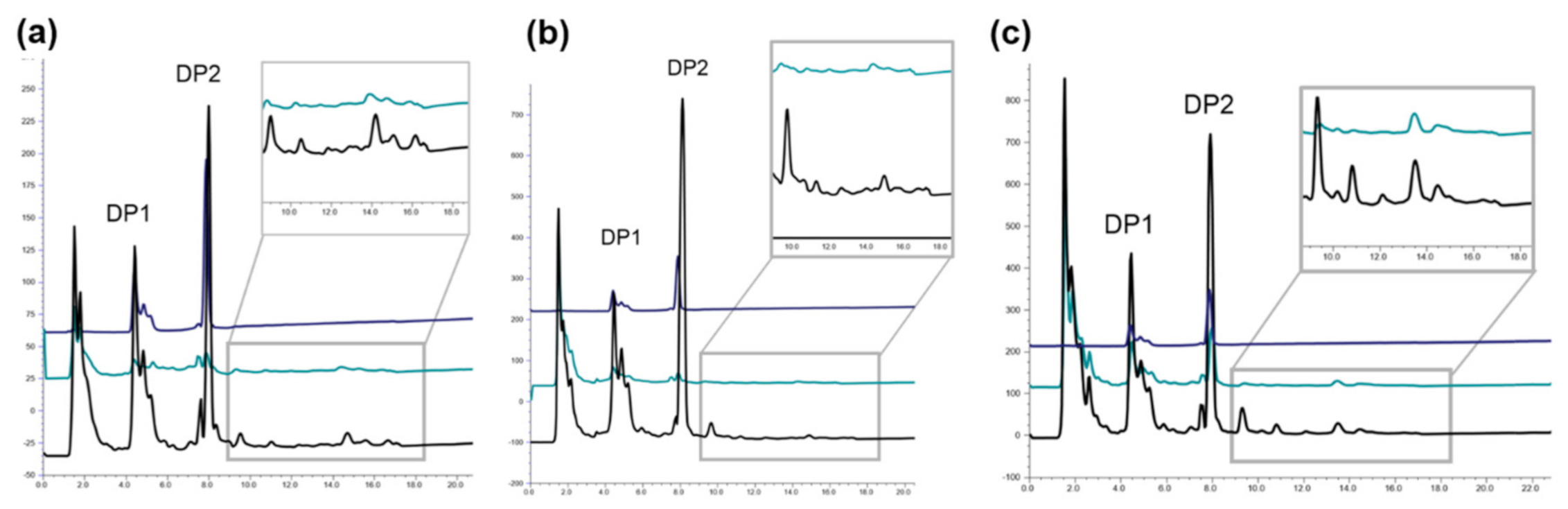
2.2. Biocatalyst Production in Different Induction Media
3. Methods
3.1. Microorganisms and Culture Procedures
3.2. Extraction of Fungal Cultures
3.3. Free Radical Scavenging (DPPH)
3.4. Tyrosinase Assay–Estimation of Skin Whitening Activity
3.5. Enzyme Assays
3.6. Determination of OMWW Total Phenolic Content and Decolorization
3.7. Bioinformatics Analyses
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elisashvili, V.; Penninckx, M.; Kachlishvili, E.; Tsiklauri, N.; Metreveli, E.; Kharziani, T.; Kvesitadze, G. Lentinus edodes and Pleurotus species lignocellulolytic enzymes activity in submerged and solid-state fermentation of lignocellulosic wastes of different composition. Bioresour. Technol. 2008, 99, 457–462. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Fernandes, M.; Milagres, A.M.F.; Roberto, I.C. Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer’s spent grain. Enzyme Microb. Technol. 2008, 43, 124–129. [Google Scholar] [CrossRef]
- Schilling, J.S.; Kaffenberger, J.T.; Held, B.W.; Ortiz, R.; Blanchette, R.A. Using Wood Rot Phenotypes to Illuminate the “Gray” Among Decomposer Fungi. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Dashtban, M.; Schraft, H.; Syed, T.A.; Qin, W. Fungal biodegradation and enzymatic modification of lignin. Int. J. Biochem. Mol. Biol. 2010, 1, 36–50. [Google Scholar] [PubMed]
- IOC, International Olive Council. 2021. Available online: https://www.internationaloliveoil.org/worlds-olive-oil-production-has-tripled/ (accessed on 10 December 2020).
- Koutrotsios, G.; Larou, E.; Mountzouris, K.C.; Zervakis, G.I. Detoxification of Olive Mill Wastewater and Bioconversion of Olive Crop Residues into High-Value-Added Biomass by the Choice Edible Mushroom Hericium erinaceus. Appl. Biochem. Biotechnol. 2016, 180, 195–209. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Koutrotsios, G.; Katsaris, P. Composted versus Raw Olive Mill Waste as Substrates for the Production of Medicinal Mushrooms: An Assessment of Selected Cultivation and Quality Parameters. BioMed Res. Int. 2013, 2013, 546830. [Google Scholar] [CrossRef]
- Musieba, F.; Okoth, S.; Mibey, R.K.; Wanjiku, S.; Moraa, K. Proximate composition, amino acids and vitamins profile of Pleurotus citrinopileatus singer: An indigenous mushroom in Kenya. Am. J. Food Technol. 2013, 8, 200–206. [Google Scholar] [CrossRef]
- Rodrigues, D.M.F.; Freitas, A.C.; Rocha-Santos, T.A.P.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.P.; Duarte, A.C. Chemical composition and nutritive value of Pleurotus citrinopileatus var cornucopiae, P. eryngii, P. salmoneo stramineus, Pholiota nameko and Hericium erinaceus. J. Food Sci. Technol. 2015, 52, 6927–6939. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.; Calhelha, R.; Alves, M.; Barros, L.; Barreiro, M.; González-Paramás, A.; Ferreira, I. Development of Mushroom-Based Cosmeceutical Formulations with Anti-Inflammatory, Anti-Tyrosinase, Antioxidant, and Antibacterial Properties. Molecules 2016, 21, 1372. [Google Scholar] [CrossRef]
- Fusco, D.; Colloca, G.; Lo Monaco, M.R.; Cesari, M. Effects of antioxidant supplementation on the aging process. Clin. Interv. Aging 2007, 2, 377–387. [Google Scholar]
- Peyrat, L.-A.; Tsafantakis, N.; Georgousaki, K.; Ouazzani, J.; Genilloud, O.; Trougakos, I.P.; Fokialakis, N. Terrestrial Microorganisms: Cell Factories of Bioactive Molecules with Skin Protecting Applications. Molecules 2019, 24, 1836. [Google Scholar] [CrossRef]
- Chang, T.-S. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Georgousaki, K.; Tsafantakis, N.; Gumeni, S.; Gonzalez, I.; Mackenzie, T.A.; Reyes, F.; Lambert, C.; Trougakos, I.P.; Genilloud, O.; Fokialakis, N. Screening for tyrosinase inhibitors from actinomycetes; identification of trichostatin derivatives from Streptomyces sp. CA-129531 and scale up production in bioreactor. Bioorg. Med. Chem. Lett. 2020, 30, 126952. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Yoon, K.N.; Lee, K.R.; Kim, H.Y.; Shin, P.G.; Cheong, J.C.; Yoo, Y.B.; Shim, M.J.; Lee, M.W.; Lee, T.S. Assessment of antioxidant and phenolic compound concentrations as well as xanthine oxidase and tyrosinase inhibitory properties of different extracts of Pleurotus citrinopileatus fruiting bodies. Mycobiology 2011, 39, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.H.; Zeng, C.L.; Yi, C.C.; Juang, L.L.; Ker, S.C.; Min, Y.L.; Jinn, C.W. Antihyperlipidemic and antioxidant effects of extracts from Pleurotus citrinopileatus. J. Agric. Food Chem. 2006, 54, 2103–2110. [Google Scholar] [CrossRef]
- Ragunathan, R.; Swaminathan, K. Nutritional status of Pleurotus spp. grown on various agro-wastes. Food Chem. 2003, 80, 371–375. [Google Scholar] [CrossRef]
- Freitas, A.C.; Antunes, M.B.; Rodrigues, D.; Sousa, S.; Amorim, M.; Barroso, M.F.; Carvalho, A.; Ferrador, S.M.; Gomes, A.M. Use of coffee by-products for the cultivation of Pleurotus citrinopileatus and Pleurotus salmoneo-stramineus and its impact on biological properties of extracts thereof. Int. J. Food Sci. Technol. 2018, 53, 1914–1924. [Google Scholar] [CrossRef]
- Grandes-Blanco, A.I.; Antonio-Flores, A.L.; García-Barrientos, R.; Díaz-Godínez, G.; Sánchez-Minutti, L. Agro-food waste employed to design and develop culture media for fungal growth. J. Environ. Biol. 2020, 41, 195–201. [Google Scholar] [CrossRef]
- Younis, A.M.; Abdel-Aziz, M.M.; Yosri, M. Evaluation of Some Biological Applications of Pleurotus citrinopileatus and Boletus edulis Fruiting Bodies. Curr. Pharm. Biotechnol. 2019, 20, 1309–1320. [Google Scholar] [CrossRef]
- Acosta-Urdapilleta, M.L.; Villegas, E.; Estrada-Torres, A.; Téllez-Téllez, M.; Díaz-Godínez, G. Antioxidant activity and proximal chemical composition of fruiting bodies of mushroom, Pleurotus spp. produced on wheat straw y. J. Environ. Biol. 2020, 41, 1075–1081. [Google Scholar] [CrossRef]
- Krakowska, A.; Zięba, P.; Włodarczyk, A.; Kała, K.; Sułkowska-Ziaja, K.; Bernaś, E.; Sękara, A.; Ostachowicz, B.; Muszyńska, B. Selected edible medicinal mushrooms from Pleurotus genus as an answer for human civilization diseases. Food Chem. 2020, 327, 127084. [Google Scholar] [CrossRef]
- Debnath, S.; Chandra Upadhyay, R.; Das, P.; Saha, A.K. ANTIOXIDANT ACTIVITIES OF METHANOLIC EXTRACTS FROM TEN PLEUROTUS SPECIES. Int. Res. J. Pharm. 2017. [Google Scholar] [CrossRef]
- Mishra, K.K.; Pal, R.S.; Arunkumar, R.; Chandrashekara, C.; Jain, S.K.; Bhatt, J.C. Antioxidant properties of different edible mushroom species and increased bioconversion efficiency of Pleurotus eryngii using locally available casing materials. Food Chem. 2013, 138, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Macáková, K.; Opletal, L.; Polášek, M.; Samková, V. Free-radical scavenging activity of some European polyporales. Nat. Prod. Commun. 2010, 5, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Aggelis, G.; Ehaliotis, C.; Nerud, F.; Stoychev, I.; Lyberatos, G.; Zervakis, G. Evaluation of white-rot fungi for detoxification and decolorization of effluents from the green olive debittering process. Appl. Microbiol. Biotechnol. 2002, 59, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Koutrotsios, G.; Zervakis, G.I. Comparative examination of the olive mill wastewater biodegradation process by various wood-rot macrofungi. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Gunes, E.; Uysal, A.; Ceylan, R.; Uysal, S.; Gungor, H.; Aktumsek, A. Two Ganoderma species: Profiling of phenolic compounds by HPLC-DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer’s disease and skin disorders. Food Funct. 2015, 6, 2794–2802. [Google Scholar] [CrossRef]
- Peng, X.R.; Liu, J.Q.; Han, Z.H.; Yuan, X.X.; Luo, H.R.; Qiu, M.H. Protective effects of triterpenoids from Ganoderma resinaceum on H2O2-induced toxicity in HepG2 cells. Food Chem. 2013, 141, 920–926. [Google Scholar] [CrossRef]
- Al-Fatimi, M.; Wurster, M.; Schröder, G.; Lindequist, U. Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J. Ethnopharmacol. 2007, 111, 657–666. [Google Scholar] [CrossRef]
- Saltarelli, R.; Ceccaroli, P.; Buffalini, M.; Vallorani, L.; Casadei, L.; Zambonelli, A.; Iotti, M.; Badalyan, S.; Stocchi, V. Biochemical Characterization and Antioxidant and Antiproliferative Activities of Different Ganoderma Collections. J. Mol. Microbiol. Biotechnol. 2015, 25, 16–25. [Google Scholar] [CrossRef] [PubMed]
- El-Katony, T.M.; El-Dein, M.M.N.; El-Fallal, A.A.; Ibrahim, N.G. Effect of the taxonomic group of fungi and type of substrate on the antioxidant activity of a solid-state fermentation system. Int. Microbiol. 2019, 22, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Zaher, E.H.F. Biodegradation of reactive dyes using soil fungal isolates and Ganoderma resinaceum. Ann. Microbiol. 2010, 60, 269–278. [Google Scholar] [CrossRef]
- Castanera, R.; Pérez, G.; Omarini, A.; Alfaro, M.; Pisabarro, A.G.; Faraco, V.; Amore, A.; Ramírez, L. Transcriptional and Enzymatic Profiling of Pleurotus ostreatus Laccase Genes in Submerged and Solid-State Fermentation Cultures. Appl. Environ. Microbiol. 2012, 78, 4037–4045. [Google Scholar] [CrossRef]
- Villares, A.; Moreau, C.; Bennati-Granier, C.; Garajova, S.; Foucat, L.; Falourd, X.; Saake, B.; Berrin, J.G.; Cathala, B. Lytic polysaccharide monooxygenases disrupt the cellulose fibers structure. Sci. Rep. 2017, 7, 40262. [Google Scholar] [CrossRef]
- Fernández-Fueyo, E.; Ruiz-Dueñas, F.J.; López-Lucendo, M.F.; Pérez-Boada, M.; Rencoret, J.; Gutiérrez, A.; Pisabarro, A.G.; Ramírez, L.; Martínez, A.T. A secretomic view of woody and nonwoody lignocellulose degradation by Pleurotus ostreatus. Biotechnol. Biofuels 2016, 9. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, J.; Ma, F.; Tang, C.; Tang, Q.; Zhang, X. Investigation of lignocellulolytic enzymes during different growth phases of Ganoderma lucidum strain G0119 using genomic, transcriptomic and secretomic analyses. PLoS ONE 2018, 13. [Google Scholar] [CrossRef]
- Ntougias, S.; Gaitis, F.; Katsaris, P.; Skoulika, S.; Iliopoulos, N.; Zervakis, G.I. The effects of olives harvest period and production year on olive mill wastewater properties: Evaluation of Pleurotus strains as bioindicators of the effluent’s toxicity. Chemosphere 2013, 92, 399–405. [Google Scholar] [CrossRef]
- Lee, S.E.; Hwang, H.J.; Ha, J.S.; Jeong, H.S.; Kim, J.H. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003, 73, 167–179. [Google Scholar] [CrossRef]
- Chaita, E.; Lambrinidis, G.; Cheimonidi, C.; Agalou, A.; Beis, D.; Trougakos, I.; Mikros, E.; Skaltsounis, A.-L.; Aligiannis, N. Anti-Melanogenic Properties of Greek Plants. A Novel Depigmenting Agent from Morus alba Wood. Molecules 2017, 22, 514. [Google Scholar] [CrossRef]
- Zerva, A.; Zervakis, G.I.; Christakopoulos, P.; Topakas, E. Degradation of olive mill wastewater by the induced extracellular ligninolytic enzymes of two wood-rot fungi. J. Environ. Manag. 2017, 203, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Zerva, A.; Savvides, A.L.; Katsifas, E.A.; Karagouni, A.D.; Hatzinikolaou, D.G. Evaluation of Paecilomyces variotii potential in bioethanol production from lignocellulose through consolidated bioprocessing. Bioresour technol 2014, 162, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Zerva, A.; Pentari, C.; Grisel, S.; Berrin, J.-G.; Topakas, E. A new synergistic relationship between xylan-active LPMO and xylobiohydrolase to tackle recalcitrant xylan. Biotechnol. Biofuels 2020, 13, 142. [Google Scholar] [CrossRef]
- Zhang, H.; Yohe, T.; Huang, L.; Entwistle, S.; Wu, P.; Yang, Z.; Busk, P.; Xu, Y.; Yin, Y. dbCAN2: A meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2018, 46, W95–W101. [Google Scholar] [CrossRef] [PubMed]
- Mendiburu, F.D. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3–3. 2010. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 7 June 2020).



| Specific Activity | CaZy Families | No. of Predicted Sequences | ||
|---|---|---|---|---|
| A. biennis | P. citrinopileatus | |||
| Cellulases | Endoglucanases | GH 5, 7, 12, 45 | 14 | 17 |
| Cellobiohydrolases | GH 6, 7 | 4 | 10 | |
| β-glucosidases | GH 1, 3 | 2 | 5 | |
| Xylanases | Endoxylanases | GH 10, 11 | 6 | 15 |
| β-xylosidases | GH 3, 43 | 4 | 7 | |
| Oxidases | Laccases | AA 1 | 8 | 16 |
| Peroxidases | AA 2 | 12 | 11 | |
| Alcohol oxidases and cellobiose dehydrogenases | AA 3 | 3 | 16 | |
| Lytic polysaccharide monooxygenases | AA 9, 10, 11, 13, 14 | 12 | 28 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerva, A.; Tsafantakis, N.; Topakas, E. Evaluation of Basidiomycetes Wild Strains Grown in Agro-Industrial Residues for Their Anti-Tyrosinase and Antioxidant Potential and for the Production of Biocatalysts. Fermentation 2021, 7, 19. https://doi.org/10.3390/fermentation7010019
Zerva A, Tsafantakis N, Topakas E. Evaluation of Basidiomycetes Wild Strains Grown in Agro-Industrial Residues for Their Anti-Tyrosinase and Antioxidant Potential and for the Production of Biocatalysts. Fermentation. 2021; 7(1):19. https://doi.org/10.3390/fermentation7010019
Chicago/Turabian StyleZerva, Anastasia, Nikolaos Tsafantakis, and Evangelos Topakas. 2021. "Evaluation of Basidiomycetes Wild Strains Grown in Agro-Industrial Residues for Their Anti-Tyrosinase and Antioxidant Potential and for the Production of Biocatalysts" Fermentation 7, no. 1: 19. https://doi.org/10.3390/fermentation7010019
APA StyleZerva, A., Tsafantakis, N., & Topakas, E. (2021). Evaluation of Basidiomycetes Wild Strains Grown in Agro-Industrial Residues for Their Anti-Tyrosinase and Antioxidant Potential and for the Production of Biocatalysts. Fermentation, 7(1), 19. https://doi.org/10.3390/fermentation7010019







