Observations on the Malting of Ancient Wheats: Einkorn, Emmer and Spelt
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Grain Quality Tests
2.3. Micro-Malting
2.4. Determination of Malt Quality
2.5. Determination of α- and β-Amylase, Protease and Xylanase Activities in Germinating Grain and Finished Malt
2.5.1. Determination of Wort Arabinoxylans (AX)
2.5.2. Determination of Wort Phenolic Acids
2.6. Statistical Analysis
3. Results
3.1. Grain Quality
3.2. Water Uptake and Hydration
3.3. Malt Extract, Soluble Protein, S/T Protein, FAN, Wort Color and Turbidity
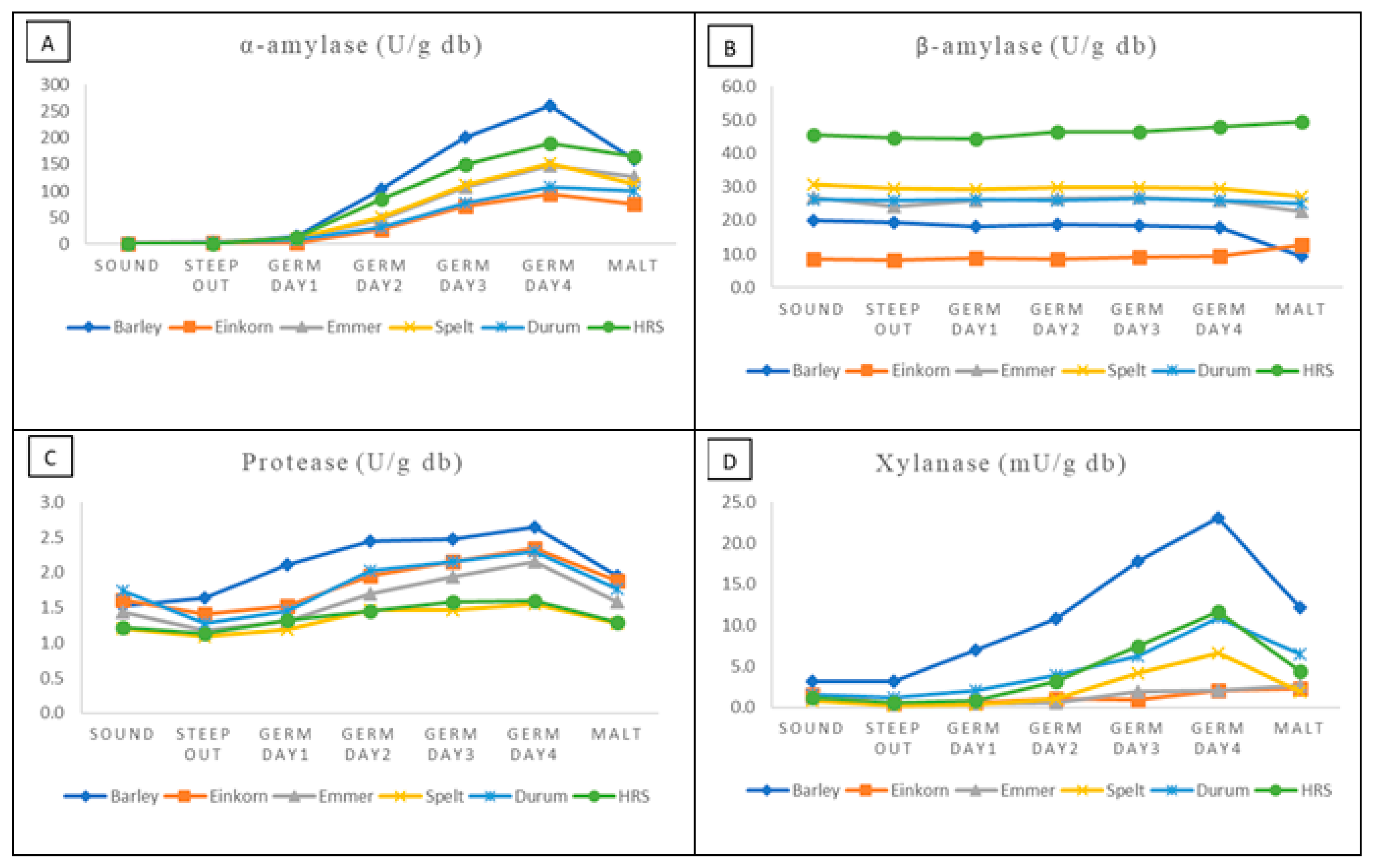
3.4. Malt Enzymes
3.5. Wort β-Glucan, Arabinoxylan and Viscosity
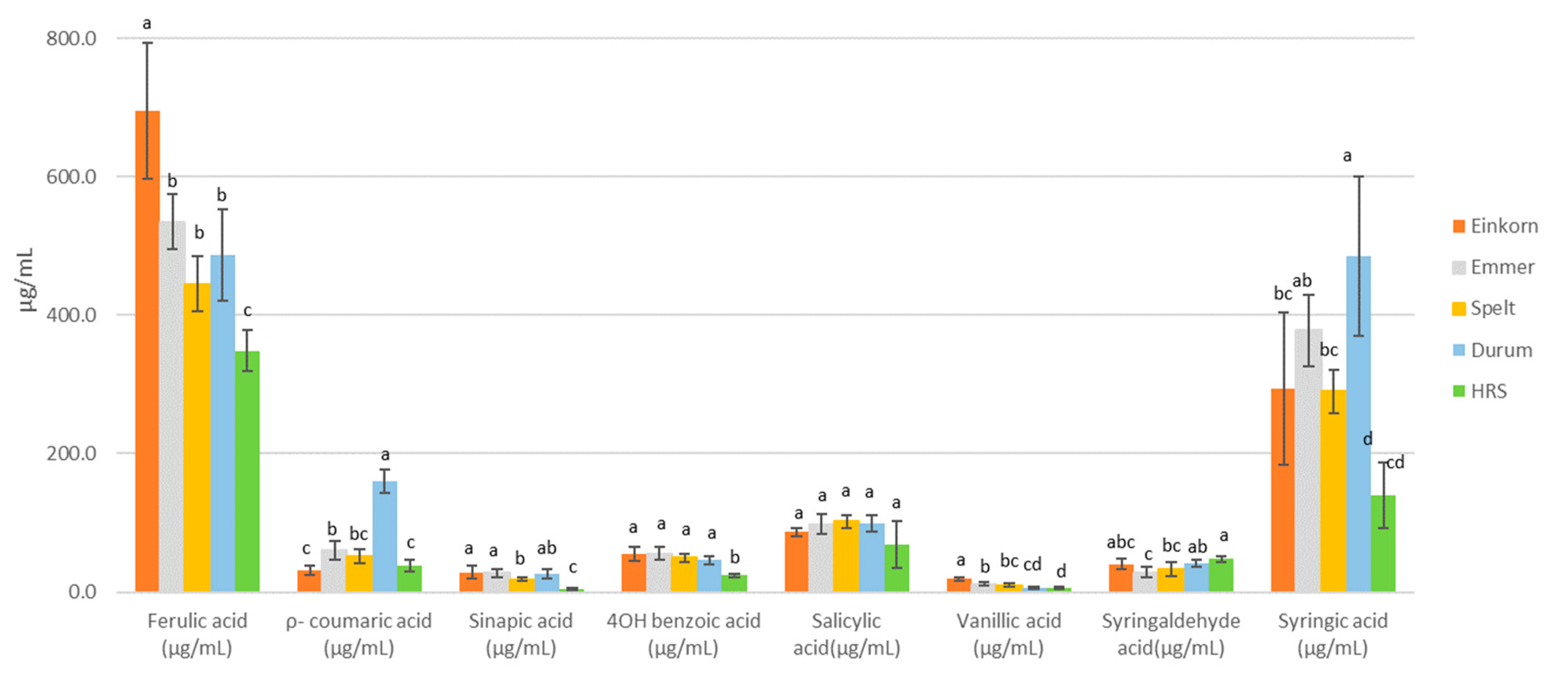
3.6. Wort Phenolic Acids
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hartmann, C.; Siegrist, M. Consumer perception and behaviour regarding sustainable protein consumption: A systematic review. Trends Food Sci. Technol. 2017, 61, 11–25. [Google Scholar] [CrossRef]
- MarketDataForecast. Ancient Grain Market Analysis by Applications (Bakery, Confectionary, Sports Nutrition, Infant Formula, Cereals, Frozen Food and Others), by Crop Type (Gluten Free Ancient Grains and Gluten Containing Ancient Grains) & by Region-Global Industry Size Share Growth Trends Analysis Forecast Report 2020–2025. 2020. Available online: https://www.marketdataforecast.com/market-reports/ancient-grain-market (accessed on 8 July 2020).
- Boukid, F.; Folloni, S.; Sforza, S.; Vittadini, E.; Prandi, B. Current Trends in Ancient Grains-Based Foodstuffs: Insights into Nutritional Aspects and Technological Applications. Compr. Rev. Food Sci. Food Saf. 2018, 17, 123–136. [Google Scholar] [CrossRef]
- Chiarello-Ebner, K. Why Ancient Grains are Sprouting Extra Sales at Retail. WholeFoods Magazine, April 2015. [Google Scholar]
- Shewry, P.R. Do ancient types of wheat have health benefits compared with modern bread wheat? J. Cereal Sci. 2018, 79, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, J.; Deal, K.R.; Luo, M.-C.; You, F.M.; von Borstel, K.; Dehghani, H. The Origin of Spelt and Free-Threshing Hexaploid Wheat. J. Hered. 2012, 103, 426–441. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S. Do “ancient” wheat species differ from modern bread wheat in their contents of bioactive components? J. Cereal Sci. 2015, 65, 236–243. [Google Scholar] [CrossRef]
- Cooper, R. Re-discovering ancient wheat varieties as functional foods. J. Tradit. Complement. Med. 2015, 5, 138–143. [Google Scholar] [CrossRef]
- Cubadda, R.; Marconi, E. Pseudocereals and Less Common Cereals: Grain Properties and Utilization Potential; Springer: Berlin, Germany, 2002. [Google Scholar]
- Fogarasi, A.; Kun, S.; Kiss, Z.; Vecseri-Hegyes, B. Einkorn (Triticum monococcum L.) in Organic Beer Production. In Proceedings of the Malting of Organic Einkorn; Conference: Third International Young Scientists Symposium for the Brewing, Distilling and Malting Sectors, Nottingham, UK, 23–25 October 2012. [Google Scholar]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, polyphenols and tannins: An overview. Plant Second. Metab. Occur. Struct. Role Hum. Diet 2006, 1, 1–24. [Google Scholar]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Innovative beer-brewing of typical, old and healthy wheat varieties to boost their spreading. bioRxiv 2017, 114157. [Google Scholar] [CrossRef]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010. [Google Scholar] [CrossRef]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef]
- Dinu, M.; Whittaker, A.; Pagliai, G.; Benedettelli, S.; Sofi, F. Ancient wheat species and human health: Biochemical and clinical implications. J. Nutr. Biochem. 2018, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.; Mathai, M.L.; Ashton, J.F.; Donkor, O.N.; Vasiljevic, T.; Mamilla, R.; Stojanovska, L. Effects of malted and non-malted whole-grain wheat on metabolic and inflammatory biomarkers in overweight/obese adults: A randomised crossover pilot study. Food Chem. 2016, 194, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Esposti, R.; Fastigi, M. The Irresistible Rise of the Craft-Brewing Sector in Italy: Can We Explain It? In Proceedings of the 4th AIEAA Conference “Innovation, Productivity and Growth: Towards Sustainable Agri-Food Production”, Ancona, Italy, 11–12 June 2015. [Google Scholar]
- Bond, J.; Capehart, T.; Allen, E.; Kim, G. Boutique Brews, Barley, and the Balance Sheet: Changes in Malt Barley Industrial Use Require an Updated Forecasting Approach; Economic Research Division, United Stated Department of Agriculture: Washington, DC, USA, 2015; pp. 18–23.
- Ratebeer.com. Available online: https://www.ratebeer.com (accessed on 20 October 2020).
- Fogarasi, A.L.; Kun, S.; Tanko, G.; Stefanovits-Banyai, E.; Hegyesne-Vecseri, B. A comparative assessment of antioxidant properties, total phenolic content of einkorn, wheat, barley and their malts. Food Chem. 2015, 167, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sachambula, L.; Hartman, I.; Psota, V. Einkorn Wheat Malting Quality. Kvas. Prum. 2015, 61, 320–325. [Google Scholar] [CrossRef]
- Krahl, M.; Zarnkow, M.; Back, W.; Becker, T. Determination of the Influence of Malting Parameters on the Water-Extractable Arabinoxylan Content of Wheat (Triticum Aestivum), Rye (Secale cereale), and Spelt Wheat (Triticum aestivum spp. spelta). J. Am. Soc. Brew. Chem. 2010, 68, 34–40. [Google Scholar] [CrossRef]
- Bestmalz.de. Available online: https://bestmalz.de/ (accessed on 20 October 2020).
- Weyermann.de. Available online: https://www.weyermann.de/ (accessed on 20 October 2020).
- Dietrich, O.; Heun, M.; Notroff, J.; Schmidt, K.; Zarnkow, M. The role of cult and feasting in the emergence of Neolithic communities. New evidence from Göbekli Tepe, south-eastern Turkey. Antiquity 2012, 86, 674–695. [Google Scholar] [CrossRef]
- Damerow, P. Sumerian beer: The origins of brewing technology in ancient Mesopotamia. Cuneif. Digit. Libr. J. 2012, 2, 1–20. Available online: http://www.cdli.ucla.edu/pubs/cdlj/2012/cdlj2012_002.html (accessed on 20 October 2020).
- Samuel, D. Archaeology of ancient Egyptian beer. J. Am. Soc. Brew. Chem. 1996, 54, 3–12. [Google Scholar] [CrossRef]
- Larson, G. How wheat came to Britain. Sci. Am. Assoc. Adv. Sci. 2015, 347, 945–946. [Google Scholar] [CrossRef]
- Nelson, M. The Barbarian’s Beverage: A History of Beer in Ancient Europe; Routledge: Windsor, UK, 2005. [Google Scholar]
- Stika, H.-P. Early Iron Age and Late Mediaeval malt finds from Germany—Attempts at reconstruction of early Celtic brewing and the taste of Celtic beer. Archaeol. Anthropol. Sci. 2011, 3, 41–48. [Google Scholar] [CrossRef]
- Hornsey, I.S. A History of Beer and Brewing; The Royal Society of Chemistry: Cambridge, UK, 2003. [Google Scholar]
- Benincasa, P.; Galieni, A.; Manetta, A.C.; Pace, R.; Guiducci, M.; Pisante, M.; Stagnari, F. Phenolic compounds in grains, sprouts and wheatgrass of hulled and non-hulled wheat species. J. Sci. Food Agric. 2015, 95, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, P.; Tosti, G.; Farneselli, M.; Maranghi, S.; Bravi, E.; Marconi, O.; Falcinelli, B.; Guiducci, M. Phenolic content and antioxidant activity of einkorn and emmer sprouts and wheatgrass obtained under different radiation wavelengths. Ann. Agric. Sci. 2020. [Google Scholar] [CrossRef]
- Falcinelli, B.; Benincasa, P.; Calzuola, I.; Gigliarelli, L.; Lutts, S.; Marsili, V. Phenolic content and antioxidant activity in raw and denatured aqueous extracts from sprouts and wheatgrass of einkorn and emmer obtained under salinity. Molecules 2017, 22, 2132. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.; Marconi, O.; Perretti, G.; Sensidoni, M.; Fantozzi, P. Investigation of the Suitability of Hulled Wheats for Malting and Brewing. J. Am. Soc. Brew. Chem. 2011, 69, 116–120. [Google Scholar] [CrossRef]
- Muñoz-Insa, A.; Selciano, H.; Zarnkow, M.; Becker, T.; Gastl, M. Malting process optimization of spelt (Triticum spelta L.) for the brewing process. LWT Food Sci. Technol. 2013, 50, 99–109. [Google Scholar] [CrossRef]
- Briggs, D.E. Malts and Malting; Springer Science & Business Media: Padstow, Cornwall, 1998. [Google Scholar]
- Marconi, O.; Mayer, H.; Chiacchieroni, F.; Ricci, E.; Perretti, G.; Fantozzi, P. The Influence of Glumes on Malting and Brewing of Hulled Wheats. J. Am. Soc. Brew. Chem. 2013, 71, 41–48. [Google Scholar] [CrossRef]
- Doehlert, D.C.; McMullen, M.S. Optimizing conditions for experimental oat dehulling. Cereal Chem. 2001, 78, 675–679. [Google Scholar] [CrossRef]
- American Society of Brewing Chemists. Methods of Analysis, 14th ed.; American Society of Brewing Chemists: St Paul, MN, USA, 2009. [Google Scholar]
- Tacke, B.K.; Casper, H.H. Determination of deoxynivalenol in wheat, barley, and malt by column cleanup and gas chromatography with electron capture detection. J. AOAC Int. 1996, 79, 472–475. [Google Scholar] [CrossRef]
- Turner, H.M.; Elmore, L.; Walling, J.; Lachowiec, J.; Mangel, D.; Fischer, A.; Sherman, J. Effect of Steeping Regime on Barley Malt Quality and Its Impacts on Breeding Program Selection. J. Am. Soc. Brew. Chem. 2019, 77, 267–281. [Google Scholar] [CrossRef]
- Banasik, O.J.; Myhre, D.; Harris, R.H. A micro-malting method for nursery samples 1. Apparatus and development of the method. Brewers Digest 1956, 31, 50–55. [Google Scholar]
- Mallett, J. Malt: A Practical Guide from Field to Brewhouse; Brewers Publications: Boulder, CO, USA, 2014. [Google Scholar]
- Karababa, E.; Schwarz, P.B.; Horsley, R.D. Effect of Kiln Schedule on Micromalt Quality Parameters. J. Am. Soc. Brew. Chem. 1993, 51, 163–167. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Z.; Barr, J.; Gillespie, J.; Simsek, S.; Horsley, R.; Schwarz, P. Micro-Malting for the Quality Evaluation of Rye (Secale cereale) Genotypes. Fermentation 2018, 4, 50. [Google Scholar] [CrossRef]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Dona, J. Comparative Study on Hulled Wheats: Kernel, Flour, Dough Quality and Dietary Fiber Variation; North Dakota State University: Fargo, ND, USA, 2020. [Google Scholar]
- Kleijer, G.; Levy, L.; Schwaerzei, R.; Fossat, D.; Brabant, C. Relationship between test weight and several quality parameters in wheat. Rev. Suisse Agric. 2007, 39, 305–309. [Google Scholar]
- Baker, B. Dehulling Ancient Grains: Economic Considerations and Equipment; eOrganic, Oregon State University: Corvallis, OR, USA, 2018. [Google Scholar]
- AACC International. AACC International Approved Methods of Analysis. Available online: http://www.aaccnet.org/ApprovedMethods/ (accessed on 9 July 2020).
- Jin, Z.; Gillespie, J.; Barr, J.; Wiersma, J.J.; Sorrells, M.E.; Zwinger, S.; Gross, T.; Cumming, J.; Bergstrom, G.C.; Brueggeman, R.; et al. Malting of Fusarium Head Blight-Infected Rye (Secale cereale): Growth of Fusarium graminearum, Trichothecene Production, and the Impact on Malt Quality. Toxins 2018, 10, 369. [Google Scholar] [CrossRef]
- Schwarz, P.B.; Horsley, R.D.; Steffenson, B.J.; Salas, B.; Barr, J.M. Quality Risks Associated with the Utilization of Fusarium Head Blight Infected Malting Barley. J. Am. Soc. Brew. Chem. 2006, 64, 1–7. [Google Scholar] [CrossRef]
- Gibson, T.S.; Solah, V.; Holmes, M.G.; Taylor, H.R. Diastatic power in malted barley: Contributions of malt parameters to its development and the potential of barley grain beta-amylase to predict malt diastatic power. J. Inst. Brew. 1995, 101, 277–280. [Google Scholar] [CrossRef]
- Sadosky, P.; Schwarz, P.B.; Horsley, R.D. Effect of arabinoxylans, β-glucans, and dextrins on the viscosity and membrane filterability of a beer model solution. J. Am. Soc. Brew. Chem. 2002, 60, 153–162. [Google Scholar] [CrossRef]
- Rosicka-Kaczmarek, J.; Komisarczyk, A.; Nebesny, E.; Makowski, B. The influence of arabinoxylans on the quality of grain industry products. Eur. Food Res. Technol. 2016, 242, 295–303. [Google Scholar] [CrossRef]
- De Clerck, J. A Textbook of Brewing; Chapman & Hall Ltd.: London, UK, 1958; Volume 1, pp. 361–426. [Google Scholar]
- Bamforth, C.; Russell, I.; Stewart, G. Beer: A Quality Perspective; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Coghe, S.; Benoot, K.; Delvaux, F.; Vanderhaegen, B.; Delvaux, F.R. Ferulic Acid Release and 4-Vinylguaiacol Formation during Brewing and Fermentation: Indications for Feruloyl Esterase Activity in Saccharomyces cerevisiae. J. Agric. Food Chem. 2004, 52, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Wannenmacher, J.; Gastl, M.; Becker, T. Phenolic Substances in Beer: Structural Diversity, Reactive Potential and Relevance for Brewing Process and Beer Quality. Compr. Rev. Food Sci. Food Saf. 2018, 17, 953–988. [Google Scholar] [CrossRef]
- Ji, F.; Wu, J.; Zhao, H.; Xu, J.; Shi, J. Relationship of deoxynivalenol content in grain, chaff, and straw with Fusarium head blight severity in wheat varieties with various levels of resistance. Toxins 2015, 7, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhou, B.; Gillespie, J.; Gross, T.; Barr, J.; Simsek, S.; Brueggeman, R.; Schwarz, P. Production of deoxynivalenol (DON) and DON-3-glucoside during the malting of Fusarium infected hard red spring wheat. Food Control 2018, 85, 6–10. [Google Scholar] [CrossRef]
- Marconi, E.; Cubadda, R. Emmer Wheat. In Specialty Grains for Food and Feed; Abdelaal, E., Woods, P., Eds.; AACC: St Paul, MN, USA, 2005; Chapter 4; pp. 63–108. [Google Scholar]
- Abdel-Aal, E.S.M.; Hucl, P. Spelt: A Specialty Wheat for Emerging Food Uses. In Speciality Grains for Food and Feed; Abdelaal, E., Woods, P., Eds.; AACC: St Paul, MN, USA, 2005; Chapter 5; pp. 109–142. [Google Scholar]
- Hidalgo, A.; Brandolini, A. Nutritional properties of einkorn wheat (Triticum monococcum L.). J. Sci. Food Agric. 2014, 94, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.E. Accelerating Malting: A Review of Some Lessons of the Past from the United Kingdom. J. Am. Soc. Brew. Chem. 1987, 45, 1–6. [Google Scholar] [CrossRef]
- Wang, T.; He, F.; Chen, G. Improving bioaccessibility and bioavailability of phenolic compounds in cereal grains through processing technologies: A concise review. J. Funct. Foods 2014, 7, 101–111. [Google Scholar] [CrossRef]
- Rosa-Sibakov, N.; Poutanen, K.; Micard, V. How does wheat grain, bran and aleurone structure impact their nutritional and technological properties? Trends Food Sci. Technol. 2015, 41, 118–134. [Google Scholar] [CrossRef]
- Kroon, P.A.; Faulds, C.B.; Ryden, P.; Robertson, J.A.; Williamson, G. Release of Covalently Bound Ferulic Acid from Fiber in the Human Colon. J. Agric. Food Chem. 1997, 45, 661–667. [Google Scholar] [CrossRef]
- Zhao, Z.; Egashira, Y.; Sanada, H. Digestion and Absorption of Ferulic Acid Sugar Esters in Rat Gastrointestinal Tract. J. Agric. Food Chem. 2003, 51, 5534–5539. [Google Scholar] [CrossRef]

| Grain | Cultivar | 1000 Kernel Weight (g) | Protein (%) | Falling Number (s) | Grain DON (μg/g) | Malt DON(μg/g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | Average | Value | Average | Value | Average | Value | Average | Value | Average | ||
| Einkorn | TM23 | 30.2 | 29.5 ± 0.3 c | 14.0 | 13.3 ± 0.3 bc | 385 | 383.3 ± 6.1 bc | 0.03 | 0.03 ± 0.0 a | 1.0 | 0.6 ± 0.3 ab |
| WB Alpine | 29.3 | 12.7 | 393 | 0.03 | 0.2 | ||||||
| PI 538722 | 29.0 | 13.2 | 372 | 0.03 | ne | ||||||
| Emmer | Vernal | 34.8 | 34.0 ± 0.6 b | 12.5 | 12.0 ± 0.25 d | 467 | 475.8 ± 5.9 a | 0.10 | 0.04 ± 0.03 a | ne | 1.2 ± 0.5 a |
| Lucile | 35.3 | 12.3 | 485 | 0.05 | 2.2 | ||||||
| ND Common | 32.8 | 11.7 | 464 | 0.0 | 1.3 | ||||||
| Yaroslav | 33.2 | 11.4 | 487 | 0.0 | 0.1 | ||||||
| Spelt | CDC Zorba | 36.0 | 38.6 ± 2.1 a | 12.1 | 12.5 ± 0.3 cd | 270 | 363.0 ± 50.8 c | 0.0 | 0.02 ± 0.02 a | 0.0 | 0.0 ± 0.0 b |
| 94-288 | 37.1 | 12.4 | 445 | 0.0 | 0.0 | ||||||
| SK3P | 42.7 | 13.0 | 374 | 0.05 | 0.0 | ||||||
| Durum | Mountrail | ne | 38.6 ± 0.76 a | ne | 14.1 ± 0.1 b | 402 | 426.4 ± 8.2 ab | 0.08 | 0.09 ± 0.05 a | ne | 1.3 ± 0.07 a |
| ND Riverland | 38.5 | 14.2 | 421 | 0.05 | 1.3 | ||||||
| Joppa | 37.0 | 13.8 | 429 | 0.0 | 1.1 | ||||||
| Divide | 40.4 | 14.2 | 453 | 0.0 | 1.4 | ||||||
| VTPeak | ne | ne | 427 | 0.3 | ne | ||||||
| HRS | Barlow | 34.2 | 35.3 ± 0.7 b | 15.3 | 16.0 ± 0.4 a | 387 | 416.8 ± 18.8 bc | 0.0 | 0.0 ± 0.0 a | 0.0 | 0.08 ± 0.02 b |
| Glenn | 34.8 | 16.4 | 379 | 0.0 | 0.1 | ||||||
| Linkert | 37.9 | 16.1 | 434 | 0.0 | 0.1 | ||||||
| SY Ingmar | 34.0 | 17.3 | 482 | 0.0 | 0.1 | ||||||
| TCG Spitfire | 35.4 | 14.8 | 402 | 0.0 | ne | ||||||
| Barley | CDC Meredith | 43.6 | nc | 14.3 | nc | ne | nc | 0.0 | nc | 0.0 | nc |
| KWS Fantex | 43.9 | 9.4 | ne | 0.0 | 0.0 | ||||||
| Grain | Cultivar | Germinative Energy (%) | Hydration Index (%) | Steeping Time (h to 45% Moisture) | Malt Loss (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||||
| Einkorn | TM23 | 89.3 a | 93.0 bc | 93.0 bc | 76 a | 97 a | 100 a | 56.2 b | 10.6 a |
| WB Alpine | 93.8 a | 98.8 bc | 98.8 bc | 63 a | 100 a | 100 a | 42.5 b | 10.7 a | |
| Emmer | Vernal | 89.3 a | 92.3 c | 92.8 cd | 38 b | 81 b | 97 a | 74.7 a | ne |
| Lucile | 87.8 a | 92.3 c | 92.5 cd | 34 b | 69 b | 95 a | 65.7 a | 11.1 a | |
| ND Common | 89.8 a | 94.3 c | 94.3 cd | 44 b | 75 b | 99 a | 67.5 a | 12.8 a | |
| Yaroslav | 82.5 a | 93.0 c | 93.3 cd | 37 b | 79 b | 99 a | 55.0 a | 10.9 a | |
| Spelt | CDC Zorba | 88.7 a | 98.0 ab | 97.7 ab | 39 bc | 78 b | 94 a | 47.6 ab | 10.4 b |
| 94-288 | 94.8 a | 98.0 ab | 98.5 ab | 33 bc | 71 b | 94 a | 60.2 ab | 7.8 b | |
| SK3P | 82.5 a | 97.3 ab | 98.3 ab | 25 bc | 62 b | 90 a | 61.1 ab | 7.5 b | |
| Durum | Mountrail | 55.8 b | 86.0 d | 88.3 d | ne | ne | ne | ne | ne |
| ND Riverland | 42.8 b | 89.8 d | 92.0 d | 35 c | 71 bc | 98 ab | 67.0 a | 9.1 b | |
| Joppa | 45.8 b | 90.8 d | 93.0 d | 23 c | 53 bc | 86 ab | 63.8 a | 8.3 b | |
| Divide | 60.8 b | 89.5 d | 91.0 d | 16 c | 53 bc | 84 ab | 56.8 a | 8.1 b | |
| VTPeak | 57.0 b | 89.3 d | 90.0 d | ne | ne | ne | ne | ne | |
| HRS | Barlow | 89.7 a | 99.7 a | 99.7 a | 13 d | 47 c | 88 b | 63.6 a | 8.1 b |
| Glenn | 90.7 a | 99.3 a | 99.3 a | 10 d | 47 c | 81 b | 65.5 a | 8.5 b | |
| Linkert | 71.7 a | 99.0 a | 99.3 a | 13 d | 55 c | 84 b | 64.7 a | 9.2 b | |
| SY Ingmar | 91.3 a | 99.7 a | 99.7 a | 3 d | 27 c | 61 b | 71.7 a | 8.8 b | |
| Barley | CDC Meredith | ne | ne | ne | 20 | 61 | 90 | 43.9 | 7.9 |
| KWS Fantex | ne | ne | ne | 42 | 87 | 100 | 27.1 | 9.2 | |
| Sample | Variety | Extract (% Malt, db) | Soluble Protein (%) | S/T (%) | FAN (mg/L) | Color (SRM) | Turbidity (NTU) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | Average | Value | Average | Value | Average | Value | Average | Value | Average | Value | Average | ||
| Wheat Malt Samples | |||||||||||||
| Einkorn | TM23 | 84.0 | 84.8 ± 0.8 a | 7.2 | 7.1 ± 0.1 a | 51.4 | 52.9 ± 1.6 a | 166.1 | 180.2 ± 14 a | 4.2 | 3.9 ± 0.2 a | 28.9 | 23.0 ± 5.9 a |
| WB Alpine | 85.7 | 6.9 | 54.5 | 194.3 | 3.7 | 17.1 | |||||||
| Emmer | Lucile | 84.0 | 84.6 ± 0.4 a | 6.3 | 6.2 ± 0.1 ab | 51.6 | 52.2 ± 0.5 a | 169.1 | 162.9 ± 5.1 a | 2.3 | 2.0 ± 0.4 b | 10.4 | 10.9 ± 1.7 bc |
| ND Common | 84.5 | 6.2 | 53.2 | 167.0 | 2.4 | 14.0 | |||||||
| Yaroslav | 85.3 | 5.9 | 51.9 | 152.7 | 1.2 | 8.3 | |||||||
| Spelt | CDC Zorba | 83.8 | 82.4 ± 0.7 b | 6.5 | 5.7 ± 0.3 b | 53.3 | 46.1 ± 3.7 ab | 167.0 | 151.2 ± 8.7 ab | 1.8 | 1.7 ± 0.07 b | 13.5 | 12.3 ± 0.6 bc |
| 94-288 | 82.4 | 5.4 | 43.5 | 136.9 | 1.6 | 12.0 | |||||||
| SK3P | 81.1 | 5.4 | 41.4 | 149.9 | 1.8 | 11.3 | |||||||
| Durum | ND Riverland | 81.3 | 82.1 ± 0.4 b | 4.5 | 4.3 ± 0.1 c | 41.3 | 40.7 ± 0.7 bc | 128.2 | 127.4 ± 3.4 b | 1.7 | 1.7 ± 0.05 b | 13.1 | 14.9 ± 1.6 b |
| Joppa | 82.5 | 4.1 | 39.3 | 121.0 | 1.7 | 13.6 | |||||||
| Divide | 82.6 | 4.3 | 41.6 | 132.9 | 1.6 | 18.0 | |||||||
| HRS | Barlow | 78.9 | 77.1 ± 0.6 c | 6.4 | 5.7 ± 0.3 b | 42.0 | 35.2 ± 2.5 c | 189.0 | 174.3 ± 10.3 a | 1.9 | 1.67 ± 0.1 b | 7.4 | 7.7 ± 0.6 c |
| Glenn | 76.9 | 5.8 | 35.4 | 179.0 | 1.4 | 9.4 | |||||||
| Linkert | 75.9 | 4.9 | 30.3 | 144.0 | 1.7 | 8.0 | |||||||
| Sy Ingmar | 76.6 | 5.7 | 33.0 | 185.0 | 1.7 | 6.3 | |||||||
| Control Malt Samples | |||||||||||||
| Commercial wheat | 86.0 | nc | 5.7 | nc | ne | nc | 181.8 | nc | 2.2 | nc | 12.9 | nc | |
| CDC Meredith Barley Micro-Malt | 78.9 | 5.7 | 39.9 | 267.2 | 2.6 | 9.6 | |||||||
| KWS Fantex Barley Micro-Malt | 81.6 | 4.9 | 52.6 | 237.2 | 2.9 | 20.9 | |||||||
| Commercial Barley (A) | 82.5 | 4.7 | ne | 211.6 | 1.9 | 9.0 | |||||||
| Commercial Barley (B) | 82.4 | 5.0 | ne | 226.8 | 2.0 | 5.6 | |||||||
| Malt Extract | Protein | DP | α-Amylase | Sol Protein | S/T | FAN | Viscosity | Color | β-Glucan | Protease | Xylanase | α-Amylase | β-Amylase | AX HMW | Ferulic Acid | Total PA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Malt Extract | 1 | −0.781 | −0.649 | 0.251 | 0.330 | 0.920 | −0.075 | 0.221 | 0.438 | 0.130 | 0.545 | −0.343 | −0.629 | −0.903 | −0.163 | 0.737 | 0.749 |
| Protein | 1 | 0.613 | 0.261 | 0.299 | −0.541 | 0.594 | 0.064 | 0.015 | −0.228 | −0.511 | −0.024 | 0.609 | 0.769 | 0.359 | −0.440 | −0.483 | |
| DP | 1 | 0.160 | −0.061 | −0.499 | 0.317 | −0.323 | −0.523 | −0.045 | −0.693 | 0.155 | 0.779 | 0.893 | 0.078 | −0.615 | −0.631 | ||
| α-Amylase | 1 | 0.817 | 0.542 | 0.791 | 0.183 | 0.347 | 0.091 | 0.008 | −0.486 | 0.346 | 0.008 | 0.342 | 0.121 | 0.093 | |||
| Sol Protein | 1 | 0.635 | 0.822 | 0.355 | 0.647 | 0.045 | 0.015 | −0.523 | 0.043 | −0.205 | 0.276 | 0.388 | 0.357 | ||||
| S/T | 1 | 0.253 | 0.236 | 0.523 | 0.223 | 0.414 | −0.468 | −0.412 | −0.774 | −0.047 | 0.680 | 0.686 | |||||
| FAN | 1 | 0.171 | 0.408 | −0.122 | −0.169 | −0.281 | 0.451 | 0.243 | 0.345 | −0.022 | −0.065 | ||||||
| Viscosity | 1 | 0.472 | 0.041 | 0.080 | −0.625 | −0.388 | −0.295 | 0.723 | 0.348 | 0.344 | |||||||
| Color | 1 | −0.258 | 0.482 | −0.164 | −0.538 | −0.545 | 0.377 | 0.785 | 0.768 | ||||||||
| β-glucan | 1 | −0.125 | −0.346 | 0.025 | −0.104 | 0.043 | −0.164 | −0.103 | |||||||||
| Protease | 1 | 0.036 | −0.527 | −0.627 | 0.008 | 0.620 | 0.626 | ||||||||||
| Xylanase | 1 | 0.046 | 0.256 | −0.296 | −0.175 | −0.165 | |||||||||||
| α-amylase | 1 | 0.822 | −0.027 | −0.802 | −0.820 | ||||||||||||
| β-amylase | 1 | 0.147 | −0.775 | −0.793 | |||||||||||||
| AX HMW | 1 | 0.095 | 0.101 | ||||||||||||||
| Ferulic acid | 1 | 0.996 | |||||||||||||||
| Total PA | 1 |
| Sample | α-Amylase (DU) (Official ASBC Method) | α-Amylase (U/g db) (Test Kit) | DP (ASBC) (Official ASBC Method) | DP/N | β-Amylase (U/g db) (Test Kit) | Protease (U/g db) (Test Kit) | Xylanase (mU/g db) (Test Kit) |
|---|---|---|---|---|---|---|---|
| Wheat Malt Samples | |||||||
| Einkorn | 49.5 ± 3.1 a | 75.3 ± 15.3 c | 116.0 ± 4.0 c | 8.7 ± 0.7 b | 12.7 ± 0.0 c | 1.8 ± 0.05 a | 2.3 ± 1.1 bc |
| Emmer | 46.6 ± 2.7 a | 126.3 ± 9.2 b | 168.1 ± 7.2 ab | 14.2 ± 0.5 a | 22.6 ± 0.5 bc | 1.6 ± 0.1 a | 2.7 ± 1.3 bc |
| Spelt | 43.3 ± 5.8 ab | 112.7 ± 9.5 b | 167.3 ± 10.9 ab | 13.4 ± 1.1 a | 27.2 ± 1.4 b | 1.3 ± 0.03 b | 1.9 ± 0.2 c |
| Durum | 35.0 ± 0.9 b | 99.8 ± 6.8 bc | 145.2 ± 12.6 bc | 13.7 ± 1.3 a | 25.0 ± 2.1 b | 1.8 ± 0.2 a | 6.4 ± 0.4 a |
| HRS | 44.6 ± 2.6 ab | 164.7 ± 10.1 a | 206.8 ± 18.7 a | 12.7 ± 0.8 a | 49.4 ± 4.4 a | 1.3 ± 0.0 b | 4.4 ± 0.8 ab |
| Control Malt Samples | |||||||
| Commercial Wheat | 44.5 | 107.0 | 192.2 | ne | 27.2 | 1.3 | 1.1 |
| CDC Meredith Barley Micro-Malt | 65.4 | 177.7 | 137.6 | 9.63 | 16.1 | 1.6 | 17.1 |
| KWS Fantex Barley Micro-Malt | 66.4 | 139.1 | 44.2 | 4.70 | 3.0 | 2.3 | 7.2 |
| Commercial Barley (A) | 59.9 | 154.9 | 132.8 | ne | 12.0 | 1.5 | 5.5 |
| Commercial Barley (B) | 55.0 | 124.2 | 148.1 | ne | 13.7 | 1.7 | 3.5 |
| Sample | Variety | β-glucan (mg/L) | Total AX (g/L) | A/X (in Total AX) | HMW AX (g/L) | A/X (in HMWAX) | Viscosity (mPa.s] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | Average | Value | Average | Value | Average | Value | Average | Value | Average | Value | Average | ||
| Wheat Malt Samples | |||||||||||||
| Einkorn | TM23 | 26.3 | 25.4 ± 0.9 b | 2.18 | 2.6 ± 0.3 a | 1.03 | 1.0 ± 0.03 c | 1.76 | 2.1 ± 0.3 a | 1.06 | 1.0 ± 0.04 c | 1.8 | 1.95 ± 0.1 a |
| WB Alpine | 24.5 | 2.95 | 0.97 | 2.53 | 0.97 | 2.1 | |||||||
| Emmer | Lucile | 28.4 | 28.6 ± 0.1 b | 1.13 | 1.3 ± 0.06 c | 1.45 | 1.4 ± 0.0 a | 1.08 | 1.2 ± 0.1 c | 1.40 | 1.4 ± 0.24 a | 1.5 | 1.6 ± 0.03 c |
| ND Common | 28.8 | 1.3 | 1.43 | 1.47 | 1.41 | 1.6 | |||||||
| Yaroslav | 28.6 | 1.34 | 1.45 | 1.04 | 1.48 | 1.6 | |||||||
| Spelt | CDC Zorba | 43.4 | 37.5 ± 3.5 a | 1.98 | 2.2 ± 0.1 ab | 0.96 | 1.0 ± 0.0 c | 1.97 | 1.8 ± 0.2 ab | 1.04 | 1.0 ± 0.02 c | 1.7 | 1.8 ± 0.1 ab |
| 94-288 | 31.2 | 2.32 | 0.95 | 2.07 | 0.99 | 2.0 | |||||||
| SK3P | 37.9 | 2.29 | 0.98 | 1.38 | 0.97 | 1.8 | |||||||
| Durum | ND Riverland | 36.5 | 28.6 ± 4.0 b | 1.82 | 1.7 ± 0.3 bc | 1.11 | 1.2 ± 0.05 b | 1.79 | 1.4 ± 0.2 bc | 1.16 | 1.2 ± 0.04 b | 1.6 | 1.6 ± 0.03 c |
| Joppa | 23.5 | 1.14 | 1.27 | 1.12 | 1.28 | 1.5 | |||||||
| Divide | 25.8 | 2.01 | 1.20 | 1.42 | 1.17 | 1.6 | |||||||
| HRS | Barlow | 27.0 | 26.8 ± 1.6 b | 1.85 | 2.2 ± 0.2 ab | 1.00 | 1.0 ± 0.02 c | 1.58 | 1.8 ± 0.1 ab | 1.00 | 1.0 ± 0.0 c | 1.7 | 1.7 ± 0.03 bc |
| Glenn | 31.0 | 2.03 | 0.95 | 1.63 | 1.01 | 1.6 | |||||||
| Linkert | 25.0 | 2.54 | 1.03 | 2.00 | 1.00 | 1.7 | |||||||
| Sy Ingmar | 24.0 | 2.24 | 0.99 | 1.94 | 0.99 | 1.6 | |||||||
| Control Malt Samples | |||||||||||||
| Commercial Wheat | 43.8 | nc | 1.82 | nc | 0.90 | nc | 1.61 | nc | 0.96 | nc | 1.6 | nc | |
| CDC Meredith Barley Micro-Malt | 295.7 | 1.33 | 0.89 | 0.66 | 1.01 | 1.4 | |||||||
| KWS Fantex Barley Micro-Malt | 314.3 | 1.08 | 1.03 | 0.67 | 1.10 | 1.6 | |||||||
| Commercial Barley (A) | 104.9 | 1.17 | 1.00 | 1.01 | 0.91 | 1.5 | |||||||
| Commercial Barley (B) | 59.6 | 1.12 | 1.05 | 0.85 | 1.01 | 1.5 | |||||||
| Classes | Compounds (μg/mL) | Forms- | Wheat Malt Samples | Control Malt Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Einkorn | Emmer | Spelt | Durum | HRS | Wheat | CDC Meredith | KWS Fantex | Barley (A) | Barley (B) | |||
| Hydroxycinnamic acid | Ferulic acid | Free | 205.8 ± 2.5 a | 120.8 ± 17.0 bc | 73.4 ± 7.0 d | 153.0 ± 13.9 b | 106.7 ± 7.6 cd | 58.9 | 266.5 | 261.1 | 143.7 | 109.7 |
| Bound | 489.5 ± 71.8 a | 413.8 ± 16.7 ab | 371.8 ± 23.0 b | 333.6 ± 24.2 b | 241.9 ± 9.8 c | 344.2 | 456.0 | 364.8 | 412.1 | 308.0 | ||
| ρ-coumaric acid | Free | 3.5 ± 2.4 b | 25.4 ± 3.9 b | 17.9 ± 2.9 b | 108.6 ± 12.2 a | 16.8 ± 3.2 b | 12.6 | 64.8 | 54.8 | 76.2 | 48.1 | |
| Bound | 28.1 ± 2.5 bc | 34.4 ± 4.1 b | 34.4 ± 4.2 b | 51.2 ± 2.4 a | 21.2 ± 2.8 c | 24.1 | 31.4 | 37.9 | 41.4 | 26.6 | ||
| Sinapic acid | Free | 4.2 ± 0.9 ab | 2.4 ± 0.1 bc | 2.6 ± 0.6 b | 5.6 ± 1.1 a | 0.6 ± 0.3 c | 0.1 | 0.8 | 3.4 | 9.9 | 8.4 | |
| Bound | 24.6 ± 5.5 a | 25.0 ± 3.4 a | 15.5 ± 0.9 b | 20.8 ± 2.6 ab | 4.4 ± 0.9 c | 18.0 | 23.0 | 89.1 | 131.0 | 62.8 | ||
| Hydroxybenzoic acid | 4OH benzoic acid | Free | 34.3 ± 5.5 a | 35.4 ± 4.9 a | 30.7 ± 2.8 a | 28.6 ± 2.6 a | 15.0 ± 0.8 b | 32.7 | 4.7 | 5.9 | 3.8 | 3.3 |
| Bound | 20.8 ± 1.3 a | 20.6 ± 0.9 a | 18.8 ± 0.9 a | 17.7 ± 0.6 a | 9.0 ± 0.9 b | 15.5 | 15.7 | 9.4 | 8.7 | 13.3 | ||
| Salicylic acid | Free | 23.3 ± 0.7 a | 29.0 ± 2.6 a | 41.9 ± 10.6 a | 28.7 ± 2.8 a | 38.7 ± 6.75 a | 33.2 | 44.3 | 99.8 | 54.4 | 48.6 | |
| Bound | 63.4 ± 3.5 a | 69.1 ± 5.8 a | 60.5 ± 5.5 a | 71.1 ± 4.1 a | 29.6 ± 11.5 b | 65.8 | 14.4 | 25.1 | 22.9 | 58.7 | ||
| Vanillic acid | Free | 13.3 ± 0.9 a | 7.1 ± 0.9 b | 6.5 ± 0.7 bc | 4.7 ± 0.4 cd | 3.7 ± 0.3 d | 4.4 | 22.6 | 23.5 | 24.4 | 16.9 | |
| Bound | 5.8 ± 1.1 a | 4.7 ± 0.9 a | 3.7 ± 1.0 ab | 1.8 ± 0.8 b | 2.3 ± 0.25 b | 2.8 | 37.6 | 33.2 | 41.5 | 31.1 | ||
| Syringaldehyde acid | Free | 10.1 ± 2.1 b | 7.2 ± 0.8 b | 3.3 ± 0.1 c | 8.1 ± 0.2 b | 14.7 ± 0.9 a | 4.7 | 1.9 | 3.1 | 3.0 | 2.9 | |
| Bound | 30.3 ± 3.0 ab | 21.2 ± 3.8 b | 29.6 ± 5.8 ab | 33.3 ± 2.9 a | 33.0 ± 1.5 a | 26.9 | 1.2 | 2.3 | 0.9 | 0.8 | ||
| Syringic acid | Free | 8.7 ± 5.4 c | 28.6 ± 3.2 ab | 21.3 ± 3.4 b | 34.3 ± 3.1 a | 10.2 ± 0.9 c | 5.2 | 1.8 | 2.2 | 2.5 | 1.0 | |
| Bound | 284.9 ± 72.1 b | 349.2 ± 26.9 ab | 269.0 ± 15.9 b | 450.6 ± 62.9 a | 129.2 ± 23.3 c | 164.6 | 6.9 | 5.7 | 9.8 | 6.2 | ||
| Sum of analyzed Phenolic acids | 1251 ± 1.4 ab | 1194 ± 41.6 b | 1000 ± 34.6 c | 1352 ± 52.5 a | 677 ± 33 d | 814.0 | 994.0 | 1021.0 | 986.0 | 746.0 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, A.; Simsek, S.; Schwarz, P.B. Observations on the Malting of Ancient Wheats: Einkorn, Emmer and Spelt. Fermentation 2020, 6, 125. https://doi.org/10.3390/fermentation6040125
Fujita A, Simsek S, Schwarz PB. Observations on the Malting of Ancient Wheats: Einkorn, Emmer and Spelt. Fermentation. 2020; 6(4):125. https://doi.org/10.3390/fermentation6040125
Chicago/Turabian StyleFujita, Alice, Senay Simsek, and Paul B. Schwarz. 2020. "Observations on the Malting of Ancient Wheats: Einkorn, Emmer and Spelt" Fermentation 6, no. 4: 125. https://doi.org/10.3390/fermentation6040125
APA StyleFujita, A., Simsek, S., & Schwarz, P. B. (2020). Observations on the Malting of Ancient Wheats: Einkorn, Emmer and Spelt. Fermentation, 6(4), 125. https://doi.org/10.3390/fermentation6040125






