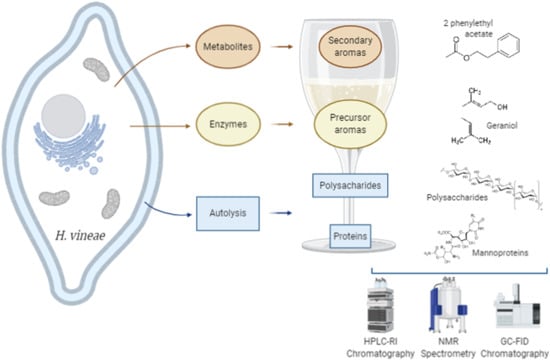
Impact of Hanseniaspora Vineae in Alcoholic Fermentation and Ageing on Lees of High-Quality White Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Species Used in Alcoholic Fermentation
2.2. Alcoholic Fermentation Conditions
2.3. Yeast Species Used in Ageing on Lees
2.4. Ageing on Lees Conditions
2.5. Basic Oenological Parameters Analysis
2.6. NMR Spectroscopy
- (a)
- Standard 1H-one-dimensional NMR experiment was carried out as step for calibration of the water-to-ethanol multi-presaturation module: with 4 transients of 32,768 complex points, having recycling delays of 5 s and with acquisition times of 1700 milliseconds, produced an experimental time of 26 s. No apodization function was applied during Fourier Transform.
- (b)
- {1Hwater_presat NMR}: 1D single pulse NOESY experiment with a homemade shaped-pulse water-to-ethanol presaturation during both the relaxation delay (5 s) and mixing times (100 milliseconds), with a 8.18 × 10−4 W power irradiation level for the solvent signals’ elimination, centering the transmitter frequency at 4.7 ppm and shifting the decoupler frequency between 3.55 ppm (CH2-ethanol) and 1.08 ppm (CH3-ethanol) for accurate multi-presaturation of all signals [16,17] were acquired for each sample as follows: a total of 128 transients were collected into 32,768 complex data points, with a spectral width of 9615.4 Hz and acquisition times of 1700 ms, produce experimental times of 10′58′’.
- (c)
- NMR post-processing was carried out as follows: ppm calibration and manual phase corrections were conducted with the use of Bruker TopSpin 4.0.8 software. Global and soft baseline corrections, least-squares NMR alignments, variable size bucketing and data matrix normalization were carried out with NMRProcFlow [18]. Scaling and statistical analysis workflow for obtaining the Principal Component Analysis to determine relationships between H. vineae and S. cerevisiae wine samples, from the constant sum normalized NMR data matrix, were developed with the BioStatFlow 2.9.2 software. Identified metabolites were quantified (Table 1) through qNMR methods [19,20] routinely used in oenology [21,22].
2.7. Volatile Compounds from the Alcoholic Fermentation Analysis
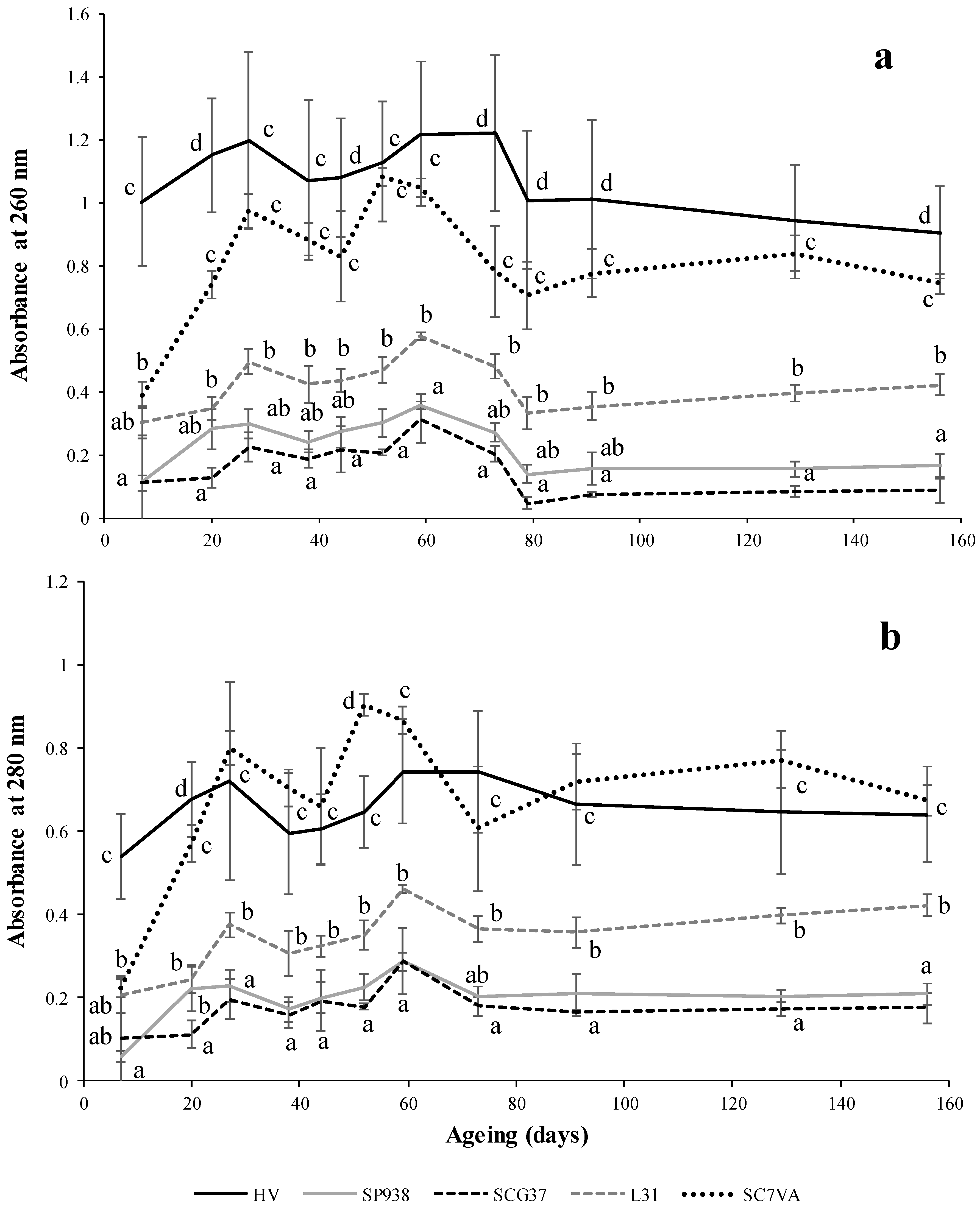
2.8. Proteins and Nucleic Acids Estimation by Absorbance at 260 and 280 nm
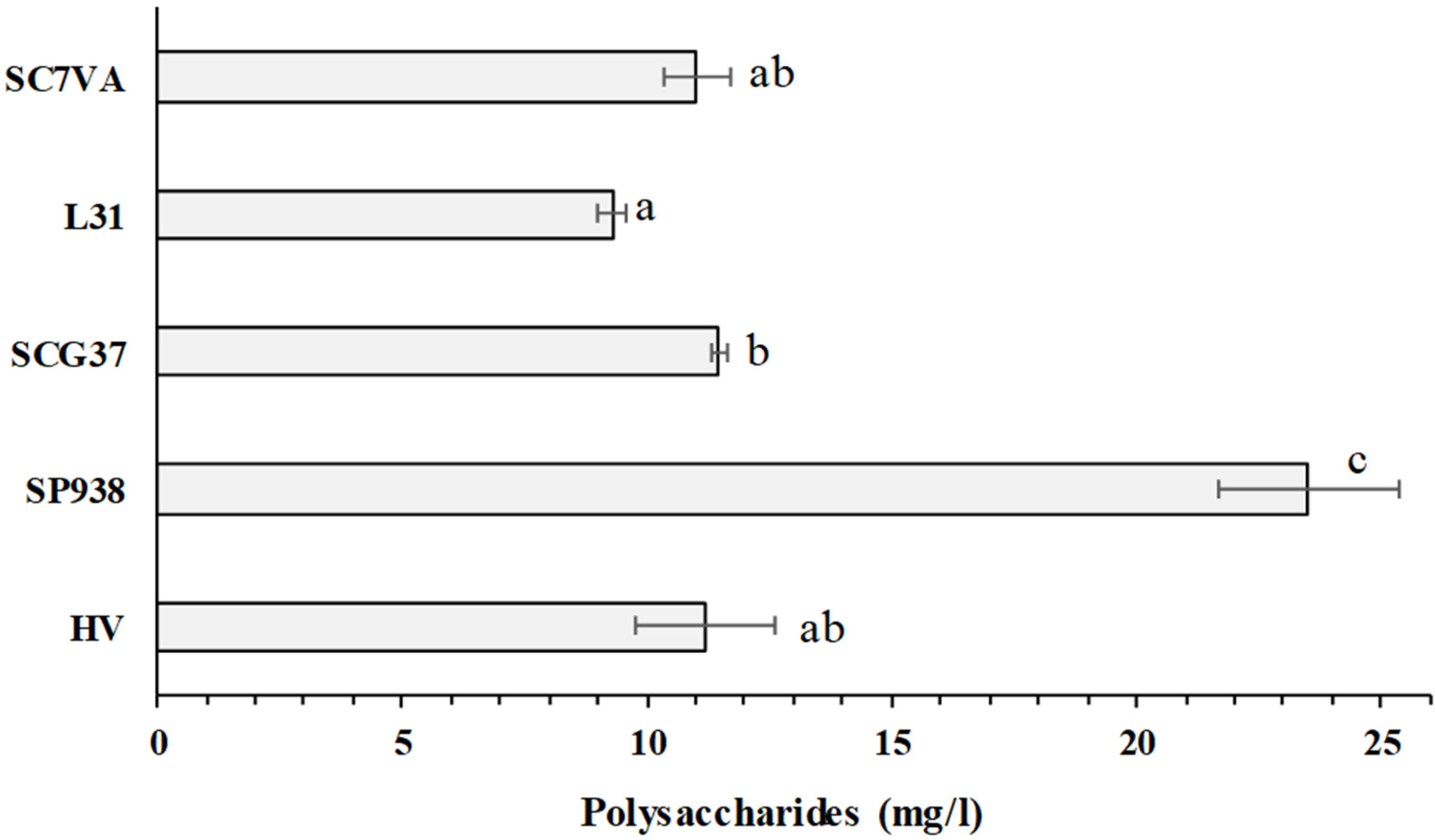
2.9. Polysaccharides Analysis (HPLC-RI)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Basic Oenological Parameters
3.2. Volatile Compounds from the Alcoholic Fermentation
3.3. Intracellular Components and Polysaccharides Content Measured in the Ageing on Lees
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Van der Westhuizen, T.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co-fermented with commercial non-Saccharomyces inocula. Aust. J. Grape Wine Res. 2018, 24, 166–180. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Morata, A.; Loira, I.; Bañuelos, M.A.; Escott, C.; Benito, S.; González Chamorro, C.; Suárez-Lepe, J.A. Use of non-Saccharomyces in single-culture, mixed and sequential fermentation to improve red wine quality. Eur. Food Res. Technol. 2017, 243, 2175–2185. [Google Scholar] [CrossRef]
- Martin, V.; Valera, M.; Medina, K.; Boido, E.; Carrau, F. Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef]
- Lleixà, J.; Martín, V.; Giorello, F.; Portillo, M.C.; Carrau, F.; Beltran, G.; Mas, A. Analysis of the NCR Mechanisms in Hanseniaspora vineae and Saccharomyces cerevisiae During Winemaking. Front. Genet. 2019, 9, 747. [Google Scholar] [CrossRef]
- Martin, V.; Giorello, F.; Fariña, L.; Minteguiaga, M.; Salzman, V.; Boido, E.; Aguilar, P.; Gaggero, C.; Dellacassa, E.S.; Mas, A.; et al. De novo Synthesis of Benzenoid Compounds by the yeast Hanseniaspora vineae Increases Flavor Diversity of Wines. J. Agric. Food Chem. 2016, 64, 4574–4583. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; del Fresno, J.M.; González, C.; Suárez-Lepe, J.A. Contribution of Non-Saccharomyces Yeasts to Wine Freshness. A Review. Biomolecules 2019, 10, 34. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Genovés, S.; Vallés, S.; Manzanares, P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, B.; Joseph, L.; Waterhouse, A.L. Effects of initial oxygenation on chemical and aromatic composition of wine in mixed starters of Hanseniaspora vineae and Saccharomyces cerevisiae. Food Microbiol. 2020, 90, 103460. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; de Castro, M.D.L. Role of lees in wine production: A review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef]
- Palomero, F.; Morata, A.; Benito, S.; Calderón, F.; Suárez-Lepe, J.A. New genera of yeasts for over-lees aging of red wine. Food Chem. 2009, 112, 432–441. [Google Scholar] [CrossRef]
- Del Fresno, J.; Morata, A.; Escott, C.; Loira, I.; Cuerda, R.; Suárez-Lepe, J. Sonication of Yeast Biomasses to Improve the Ageing on Lees Technique in Red Wines. Molecules 2019, 24, 635. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Palomero, F.; Loira, I.; Suárez-Lepe, J.A. New Trends in Aging on Lees. In Red Wine Technology; Elsevier: New York, NY, USA, 2019; pp. 163–176. ISBN 9780128144008. [Google Scholar]
- Giovani, G.; Rosi, I.; Bertuccioli, M. Quantification and characterization of cell wall polysaccharides released by non-Saccharomyces yeast strains during alcoholic fermentation. Int. J. Food Microbiol. 2012, 160, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Herbert-Pucheta, J.E.; Mejía-Fonseca, I.; Zepeda-Vallejo, L.G.; Milmo-Brittingham, D.; Maya, G.P. The “Wine-T1” NMR experiment for novel wine-metabolome fingerprinting with nuclear-spin relaxation. BIO Web Conf. 2019, 12, 02029. [Google Scholar] [CrossRef][Green Version]
- Herbert-Pucheta, J.E.; Padilla-Maya, G.; Milmo-Brittinham, D.; Lojero, D.; Gilmore, A.M.; Raventós-Llopart, L.; Hernández-Pulido, K.E.; Zepeda-Vallejo, L.G. Multivariate spectroscopy for targeting phenolic choreography in wine with A-TEEMTM and NMR crosscheck non-targeted metabolomics. BIO Web Conf. 2019, 15, 02006. [Google Scholar] [CrossRef]
- Jacob, D.; Deborde, C.; Lefevbre, M.; Maucourt, M.; Moing, A. NMRProcFlow: A graphical and interactive tool dedicated to 1D spectra processing for NMR-based metabolomics. Metabolomics 2017, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Cerceau, C.I.; Barbosa, L.C.A.; Filomeno, C.A.; Alvarenga, E.S.; Demuner, A.J.; Fidencio, P.H. An optimized and validated 1H NMR method for the quantification of α-pinene in essential soils. Talanta 2016, 150, 97–103. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, G.; Zhang, J.; Zhang, Y.; Li, J.; Xiong, C.; Zhang, Q.; Li, X.; Lai, X. Metabolic discrimination of sea buckthorn from different Hippophae species by 1H NMR based metabolomics. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Wider, G.; Dreier, L. Measuring protein concentrations by NMR spectroscopy. J. Am. Chem. Soc. 2006, 128, 2571–2576. [Google Scholar] [CrossRef]
- Godelman, R.; Fang, F.; Humpfer, E.; Schutz, B.; Bansbach, M.; Schafer, H.; Spraul, M. Targeted and nontargeted wine analysis by 1H NMR spectroscopy combined with multivariate statistical analysis. Differentiation of important parameters: Grape variety, geographical origin, year of vintage. J. Agric. Food Chem. 2013, 61, 5610–5619. [Google Scholar] [CrossRef] [PubMed]
- Abalos, D.; Vejarano, R.; Morata, A.; González, C.; Suárez-Lepe, J.A. The use of furfural as a metabolic inhibitor for reducing the alcohol content of model wines. Eur. Food Res. Technol. 2011, 232, 663–669. [Google Scholar] [CrossRef]
- Loira, I.; Vejarano, R.; Morata, A.; Ricardo-Da-Silva, J.M.; Laureano, O.; González, M.C.; Suárez-Lepe, J.A. Effect of Saccharomyces strains on the quality of red wines aged on lees. Food Chem. 2013, 139, 1044–1051. [Google Scholar] [CrossRef]
- Lleixà, J.; Martín, V.; Portillo, M.d.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of Fermentation and Wines Produced by Inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 338. [Google Scholar] [CrossRef]
- Martin, V.; Fariña, L.; Medina, K.; Boido, E.; Dellacassa, E.; Mas, A.; Carrau, F. Oenological attributes of the yeast Hanseniaspora vineae and its application for white and red winemaking. BIO Web Conf. 2019, 12, 02010. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Dellacassa, E.; Carrau, F. Non-Saccharomyces and Saccharomyces strains co-fermentation increases acetaldehyde accumulation: Effect on anthocyanin-derived pigments in Tannat red wines. Yeast 2016, 33, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Carrau, F.M.; Medina, K.; Farina, L.; Boido, E.; Henschke, P.A.; Dellacassa, E. Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: Effects of yeast assimilable nitrogen on two model strains. FEMS Yeast Res. 2008, 8, 1196–1207. [Google Scholar] [CrossRef]
- Giorello, F.; Valera, M.J.; Martin, V.; Parada, A.; Salzman, V.; Camesasca, L.; Fariña, L.; Boido, E.; Medina, K.; Dellacassa, E.; et al. Genomic and transcriptomic basis of Hanseniaspora vineae’s impact on flavor diversity and wine quality. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Zea, L.; Serratosa, M.P.; Mérida, J.; Moyano, L. Acetaldehyde as Key Compound for the Authenticity of Sherry Wines: A Study Covering 5 Decades. Compr. Rev. Food Sci. Food Saf. 2015, 14, 681–693. [Google Scholar] [CrossRef]
- Martínez-García, R.; García-Martínez, T.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J. Changes in sparkling wine aroma during the second fermentation under CO2 pressure in sealed bottle. Food Chem. 2017, 237, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Bartowsky, E.J.; Pretorius, I.S. Microbial formation and modification of flavor and off-flavor compounds in wine. In Biology of Microorganisms on Grapes, in Must and in Wine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 209–231. ISBN 9783540854623. [Google Scholar]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Vilanova, M.; Martínez, C. First study of determination of aromatic compounds of red wine from Vitis vinifera cv. Castañal grown in Galicia (NW Spain). Eur. Food Res. Technol. 2007, 224, 431–436. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Polo, M.C. Volatile and Aroma Compounds. In Wine Chemistry and Biochemistry; Springer: New York, NY, USA, 2008; p. 249. [Google Scholar]
- Peinado, R.A.; Moreno, J.; Medina, M.; Mauricio, J.C. Changes in volatile compounds and aromatic series in sherry wine with high gluconic acid levels subjected to aging by submerged flor yeast cultures. Biotechnol. Lett. 2004, 26, 757–762. [Google Scholar] [CrossRef]
- Murnane, S.S.; Lehocky, A.H.; Owens, P.D. Odor Thresholds for Chemicals with Established Occupational Health Standards, 2nd ed.; American Industrial Hygiene Association: Falls Church, VA, USA, 2013; ISBN 9781935082385. [Google Scholar]
- Sun, Q.; Gates, M.J.; Lavin, E.H.; Acree, T.E.; Sacks, G.L. Comparison of odor-active compounds in grapes and wines from Vitis vinifera and non-foxy American grape species. J. Agric. Food Chem. 2011, 59, 10657–10664. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, K.; Rönner, U.; Borch, E. Inactivation of Escherichia coli, Listeria innocua and Saccharomyces cerevisiae in relation to membrane permeabilization and subsequent leakage of intracellular compounds due to pulsed electric field processing. Int. J. Food Microbiol. 2005, 99, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of mannoproteins during Saccharomyces cerevisiae autolysis induced by pulsed electric field. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Bunduki, M.M.C.; Flanders, K.J.; Donnelly, C.W. Metabolic and structural sites of damage in heat- and sanitizer-lnjured populations of Listeria monocytogenes. J. Food Prot. 1995, 58, 410–415. [Google Scholar] [CrossRef]

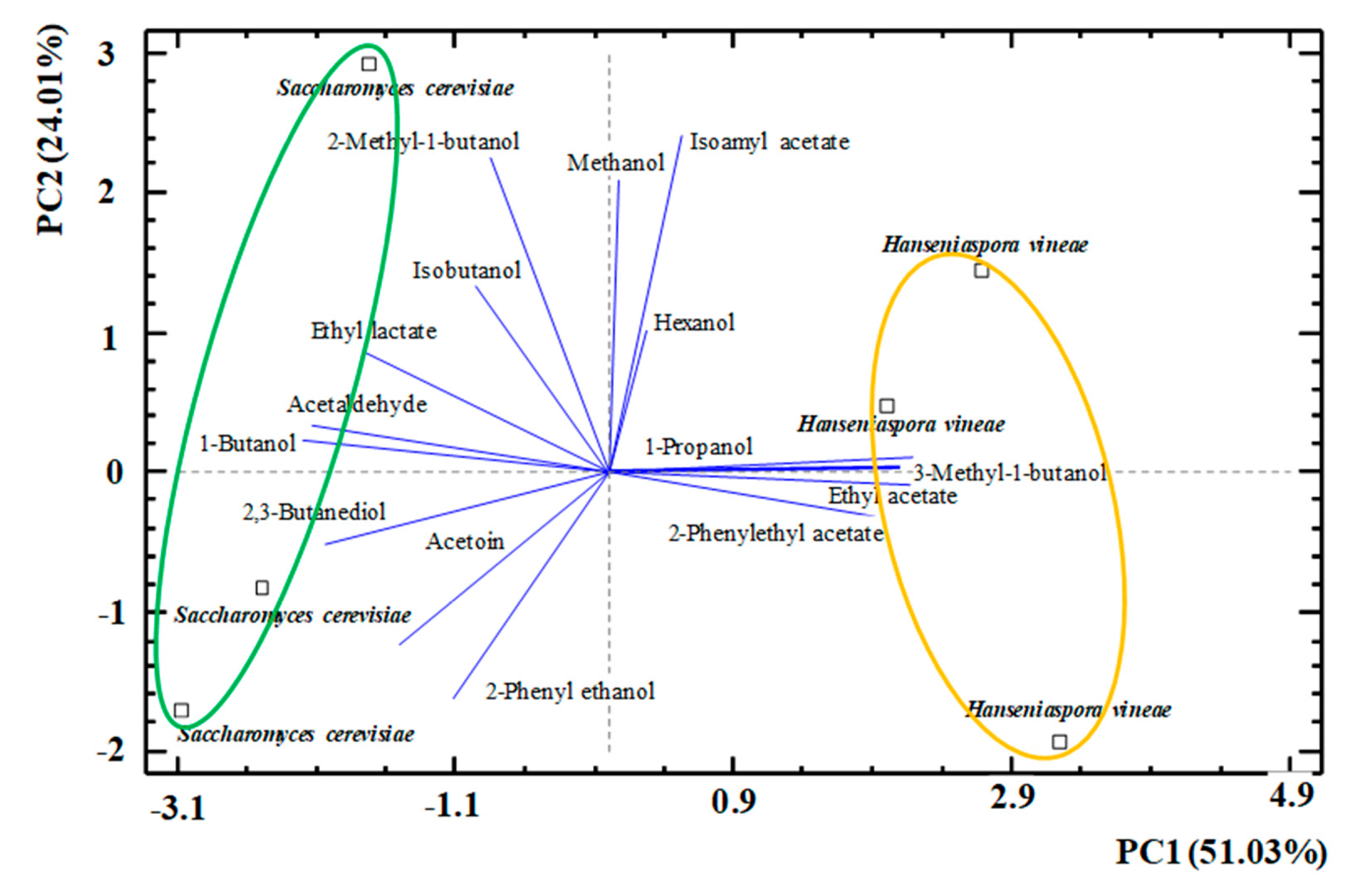
| mg/L | Fermentation with Saccharomyces cerevisiae | Fermentation with Hanseniaspora vineae |
|---|---|---|
| Furfural | 1.47 ± 1.14 a | 3.29 ± 2.82 a |
| Formiate | 2.44 ± 0.66 a | 3.05 ± 0.84 a |
| Shikimic | 1.54 ± 0.21 a | 1.85 ± 0.19 a |
| Fumaric | 0.58 ± 0.44 a | 0.53 ± 0.17 a |
| Sorbic | 1.63 ± 1.44 a | 2.73 ± 3.33 a |
| β-Glucose | 500.02 ± 58.39 b | 365.60 ± 37.23 a |
| Fructose | 695.11 ± 146.39 a | 803.69 ± 238.53 a |
| Citrate | 244.05 ± 25.82 a | 255.38 ± 7.52 a |
| Succinate | 291.47 ± 28.40 a | 233.36 ± 25.83 a |
| Glutamine | 54.03 ± 10.14 a | 59.20 ± 5.41 a |
| Acetate | 289.70 ± 18.64 a | 274.73 ± 22.25 a |
| Proline | 34.17 ± 7.66 a | 42.29 ± 6.35 a |
| γ-Aminobutyric acid | 67.68 ± 5.11 a | 73.61 ± 7.32 a |
| Arginine | 28.43 ± 11.10 a | 44.00 ± 26.46 a |
| Alanine | 119.82 ± 42.98 a | 150.20 ± 78.16 a |
| Lactic | 156.56 ± 31.04 a | 174.25 ± 44.30 a |
| Threonine | 188.38 ± 70.77 a | 230.49 ± 78.09 a |
| Valine | 52.72 ± 18.84 a | 37.63 ± 17.85 a |
| Isoleucine | 29.33 ± 9.08 a | 37.18 ± 7.66 a |
| Fermentation with Saccharomyces cerevisiae | Fermentation with Hanseniaspora vineae | |
|---|---|---|
| Ethanol (% v/v) | 11.93 ± 0.15 a | 11.90 ± 0.00 a |
| pH | 3.17 ± 0.03 a | 3.21 ± 0.02 a |
| Total Acidity (g/L) | 6.30 ± 0.10 b | 5.80 ± 0.17 a |
| Volatile Acidity (g/L) | 0.45 ± 0.07 a | 0.36 ± 0.02 a |
| Malic Acid (g/L) | 2.00 ± 0.10 a | 1.87 ± 0.06 a |
| Lactic Acid (g/L) | 0.10 ± 0.10 a | 0.00 ± 0.00 a |
| Gluc and Fruc (g/L) | 1.67 ± 0.60 a | 1.07 ± 0.38 a |
| Fermentation with Saccharomyces cerevisiae | Fermentation with Hanseniaspora vineae | OAV Saccharomyces cerevisiae | OAV Hanseniaspora vineae | Odor Character | Perception Threshold (mg/L) | |
|---|---|---|---|---|---|---|
| Acetaldehyde | 26.34 ± 2.19 b | 19.86 ± 2.29 a | 0.263 | 0.198 | pungent, fruity, suffocating, fresh, green 4 | 100–125 [31] |
| Methanol | 43.03 ± 2.76 a | 42.42 ± 1.56 a | 0.059 | 0.063 | pungent odor 6 | 668 [32] |
| 1-Propanol | 20.49 ± 1.72 a | 28.68 ± 0.52 b | 0.041 | 0.057 | alcohol, ripe fruit 7 | 500 1 [33] |
| Diacetyl | nd | nd | - | - | pleasant, buttery 4 | 0.2 [33] |
| Ethyl acetate | 45.99 ± 2.72 a | 79.26 ± 3.31 b | 3.832 | 6.605 | fruity, sweet, fingernail polish, etherous 4 | 12 2 [34] |
| 2-Butanol | nd | nd | - | - | medicinal, wine-like 7 | 150 2 [33] |
| Isobutanol | 23.80 ± 0.57 a | 22.64 ± 3.49 a | 0.595 | 0.566 | Coca 5 | 40 2 [33] |
| 1-Butanol | 3.97 ± 0.10 b | 0.00 ± 0.00 a | 0.026 | 0 | Medicinal 7 | 150 2 [33] |
| Acetoin | 5.66 ± 0.21 a | 5.50 ± 0.09 a | 0.037 | 0.036 | from buttery to plastic 6 | 150 1 [33] |
| 2-Methyl-1-butanol | 22.96 ± 0.90 a | 25.29 ± 0.15 b | 0.574 | 0.632 | pungent odor 6 | 40 1 [35] |
| 3-Methyl-1-butanol | 112.83 ± 17.57 a | 101.43 ± 17.53 a | 2.821 | 5.536 | pungent odor 6 | 40 1 [35] |
| isobutyl acetate | nd | nd | - | - | sweet, ester, medicinal 4 | 1.6 3 [33] |
| Ethyl butyrate | nd | nd | - | - | strawberry, apple, banana 7 | 0.02 1 [35] |
| Ethyl lactate | 7.97 ± 0.13 a | 7.15 ± 0.81 a | 0.569 | 0.511 | Floral 5 | 14 1 [35] |
| 2.3-Butanediol | 892.38 ± 97.06 b | 643.99 ± 28.48 a | 5.94 | 4.293 | creamy, buttery 8 | 150 1 [36] |
| 3-ethoxy-1-propanol | nd | nd | - | - | - | 0.1 1 [37] |
| Isoamyl acetate | 3.63 ± 0.44 a | 3.71 ± 0.22 a | 121 | 123.66 | banana, fresh 4 | 0.03 2 [33] |
| Hexanol | 4.64 ± 0.05 a | 4.75 ± 0.32 a | 1.16 | 1.187 | green 5 | 4 1 [33] |
| 2-Phenyl ethanol | 9.67 ± 0.33 a | 9.52 ± 0.30 a | 0.976 | 0.976 | rose talc, honey 8 | 10 [32] |
| 2-Phenylethyl acetate | 6.33 ± 0.45 a | 7.96 ± 0.34 b | 25.32 | 31.84 | Flowery 7 | 0.25 1 [33] |
| Higher Alcohols | 189.76 ± 18.63 a | 187.55 ± 21.03 a | ||||
| Esters | 55.96 ± 2.73 a | 90.93 ± 3.13 b | ||||
| Total Volatiles | 1.229.71 ± 77.42 b | 1.002.14 ± 33.29 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Fresno, J.M.; Escott, C.; Loira, I.; Herbert-Pucheta, J.E.; Schneider, R.; Carrau, F.; Cuerda, R.; Morata, A. Impact of Hanseniaspora Vineae in Alcoholic Fermentation and Ageing on Lees of High-Quality White Wine. Fermentation 2020, 6, 66. https://doi.org/10.3390/fermentation6030066
Del Fresno JM, Escott C, Loira I, Herbert-Pucheta JE, Schneider R, Carrau F, Cuerda R, Morata A. Impact of Hanseniaspora Vineae in Alcoholic Fermentation and Ageing on Lees of High-Quality White Wine. Fermentation. 2020; 6(3):66. https://doi.org/10.3390/fermentation6030066
Chicago/Turabian StyleDel Fresno, Juan Manuel, Carlos Escott, Iris Loira, José Enrique Herbert-Pucheta, Rémi Schneider, Francisco Carrau, Rafael Cuerda, and Antonio Morata. 2020. "Impact of Hanseniaspora Vineae in Alcoholic Fermentation and Ageing on Lees of High-Quality White Wine" Fermentation 6, no. 3: 66. https://doi.org/10.3390/fermentation6030066
APA StyleDel Fresno, J. M., Escott, C., Loira, I., Herbert-Pucheta, J. E., Schneider, R., Carrau, F., Cuerda, R., & Morata, A. (2020). Impact of Hanseniaspora Vineae in Alcoholic Fermentation and Ageing on Lees of High-Quality White Wine. Fermentation, 6(3), 66. https://doi.org/10.3390/fermentation6030066