Increased Selectivity for Butanol in Clostridium Pasteurianum Fermentations via Butyric Acid Addition or Dual Feedstock Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Chemicals
2.3. Culturing and Fermentation Conditions
2.4. Fermentations with Butyric Acid Additizon
2.5. Statistical Methodology and Analysis
2.6. Substrate Screening and Scale-Up
2.7. Analytical Methods
3. Results and Discussion
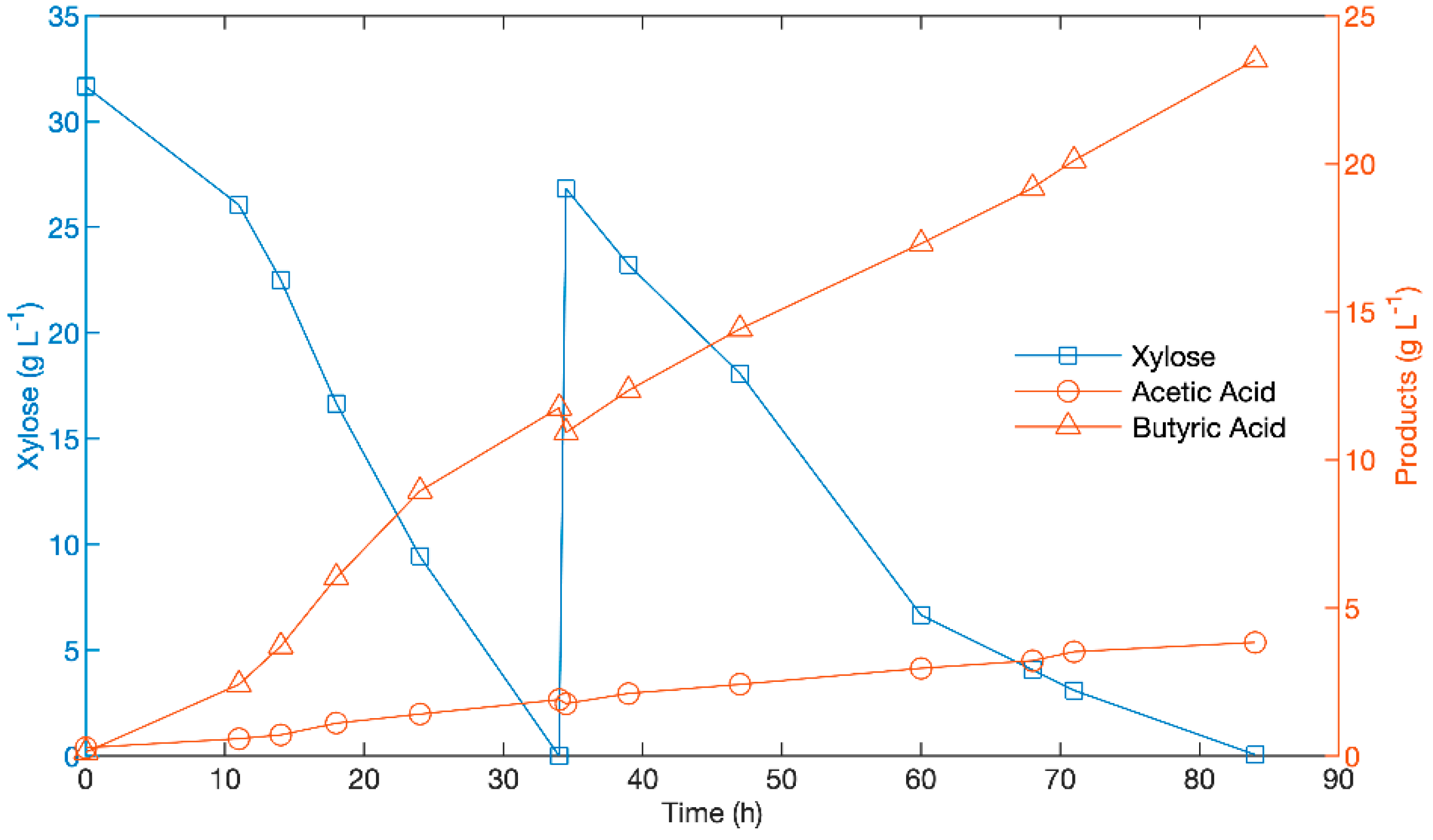
3.1. Generation of Butyric Acid Rich Process Fluid Using Xylose and C. tyrobutyricum
3.2. Effect of Butyric Acid Addition When Added to the Initial Media
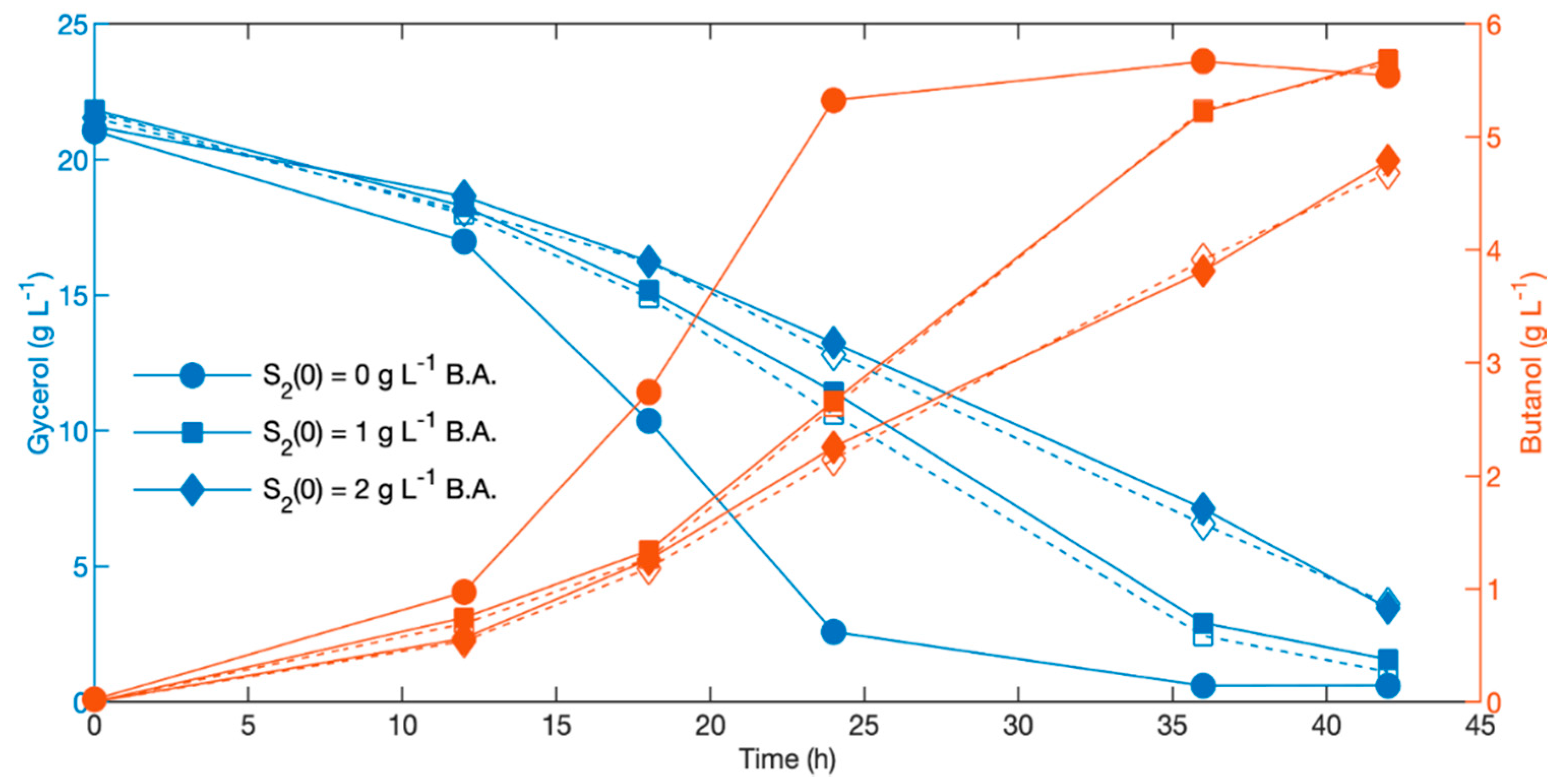
3.3. Effect of Delayed Addition of Butyric Acid to Fermentation
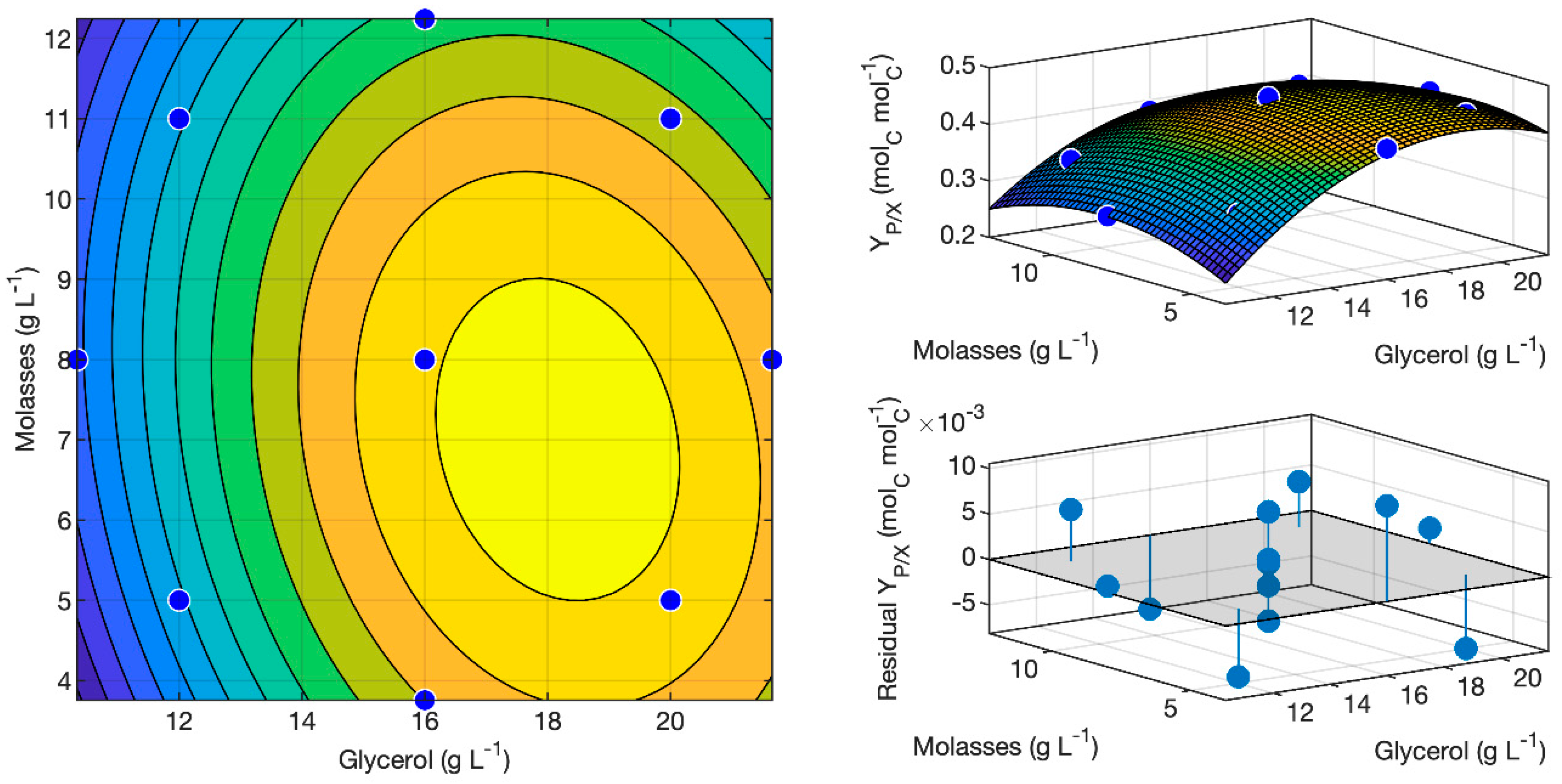
3.4. Optimization of Glycerol and Molasses Ratio for Butanol Selectivity
3.5. Model Validation
3.6. Testing Glycerol: Molasses Strategy at Reactor Scale with pH Control
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Jang, Y.-S.; Choi, S.J.; Im, J.A.; Song, H.; Cho, J.H.; Seung, D.Y.; Papoutsakis, E.T.; Bennett, G.N.; Lee, S.Y. Metabolic Engineering of Clostridium acetobutylicum ATCC 824 for Isopropanol-Butanol-Ethanol Fermentation. Appl. Environ. Microbiol. 2012, 78, 1416–1423. [Google Scholar] [CrossRef]
- Lee, S.-M.; Cho, M.O.; Park, C.H.; Chung, Y.-C.; Kim, J.H.; Sang, B.-I.; Um, Y. Continuous Butanol Production Using Suspended and Immobilized Clostridium beijerinckii NCIMB 8052 with Supplementary Butyrate. Energy Fuels 2008, 22, 3459–3464. [Google Scholar] [CrossRef]
- Jones, D.T.; Woods, D.R. Acetone-butanol fermentation revisited. Microbiol. Rev. 1986, 50, 484–524. [Google Scholar] [CrossRef] [PubMed]
- Sarchami, T.; Johnson, E.; Rehmann, L. Optimization of fermentation condition favoring butanol production from glycerol by Clostridium pasteurianum DSM 525. Bioresour. Technol. 2016, 208, 73–80. [Google Scholar] [CrossRef]
- Gallardo, R.; Alves, M.; Rodrigues, L.R. Modulation of crude glycerol fermentation by Clostridium pasteurianum DSM 525 towards the production of butanol. Biomass Bioenergy 2014, 71, 134–143. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, Z.; Pan, H.; Zhao, L.; Xu, C.; Yu, X. Feasibility of butanol production from wheat starch wastewater by Clostridium acetobutylicum. Energy 2018, 154, 240–248. [Google Scholar] [CrossRef]
- Hou, X.; From, N.; Angelidaki, I.; Huijgen, W.J.J.; Bjerre, A.-B. Butanol fermentation of the brown seaweed Laminaria digitata by Clostridium beijerinckii DSM-6422. Bioresour. Technol. 2017, 238, 16–21. [Google Scholar] [CrossRef]
- Johnson, E.; Rehmann, L. The role of 1,3-propanediol production in fermentation of glycerol by Clostridium pasteurianum. Bioresour. Technol. 2016, 209, 1–7. [Google Scholar] [CrossRef]
- Sabra, W.; Groeger, C.; Sharma, P.N.; Zeng, A.P. Improved n-butanol production by a non-acetone producing Clostridium pasteurianum DSMZ 525 in mixed substrate fermentation. Appl. Microbiol. Biotechnol. 2014, 98, 4267–4276. [Google Scholar] [CrossRef]
- Biebl, H. Fermentation of glycerol by Clostridium pasteurianum—Batch and continuous culture studies. J. Ind. Microbiol. Biotechnol. 2001, 27, 18–26. [Google Scholar] [CrossRef]
- Gallazzi, A.; Branska, B.; Marinelli, F.; Patakova, P. Continuous production of n-butanol by Clostridium pasteurianum DSM 525 using suspended and surface-immobilized cells. J. Biotechnol. 2015, 216, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Regestein, L.; Doerr, E.W.; Staaden, A.; Rehmann, L. Impact of butyric acid on butanol formation by Clostridium pasteurianum. Bioresour. Technol. 2015, 196, 153–159. [Google Scholar] [CrossRef]
- Dabrock, B.; Bahl, H.; Gottschalk, G. Parameters Affecting Solvent Production by Clostridium pasteurianum. Appl. Environ. Microbiol. 1992, 58, 1233–1239. [Google Scholar] [CrossRef]
- Moon, C.; Hwan Lee, C.; Sang, B.-I.; Um, Y. Optimization of medium compositions favoring butanol and 1,3-propanediol production from glycerol by Clostridium pasteurianum. Bioresour. Technol. 2011, 102, 10561–10568. [Google Scholar] [CrossRef]
- Kao, W.-C.; Lin, D.-S.; Cheng, C.-L.; Chen, B.-Y.; Lin, C.-Y. Enhancing butanol production with Clostridium pasteurianum CH4 using sequential glucose-glycerol addition and simultaneous dual-substrate cultivation strategies. Bioresour. Technol. 2013, 135, 324–330. [Google Scholar] [CrossRef]
- Sabra, W.; Wang, W.; Surandram, S.; Groeger, C.; Zeng, A.-P. Fermentation of mixed substrates by Clostridium pasteurianum and its physiological, metabolic and proteomic characterizations. Microb. Cell Fact. 2016, 15, 114. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, S.-T. Effect of pH on metabolic pathway shift in fermentation of xylose by Clostridium tyrobutyricum. J. Biotechnol. 2004, 110, 143–157. [Google Scholar] [CrossRef]
- Liu, X.; Yang, S.-T. Kinetics of butyric acid fermentation of glucose and xylose by Clostridium tyrobutyricum wild type and mutant. Process Biochem. 2006, 41, 801–808. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Liang, S.; Wang, X.; Cen, P.; Xu, Z. Production of Butyric Acid from Glucose and Xylose with Immobilized Cells of Clostridium tyrobutyricum in a Fibrous-Bed Bioreactor. Appl. Biochem. Biotechnol. 2010, 160, 350–359. [Google Scholar] [CrossRef]
- Fu, H.; Yang, S.-T.; Wang, M.; Wang, J.; Tang, I.-C. Butyric acid production from lignocellulosic biomass hydrolysates by engineered Clostridium tyrobutyricum overexpressing xylose catabolism genes for glucose and xylose co-utilization. Bioresour. Technol. 2017, 234, 389–396. [Google Scholar] [CrossRef]
- Munch, G.; Schulte, A.; Mann, M.; Dinger, R.; Regestein, L.; Rehmann, L.; Büchs, J. Online measurement of CO2 and total gas production in parallel anaerobic shake flask cultivations. Biochem. Eng. J. 2020, 153, 107418. [Google Scholar] [CrossRef]
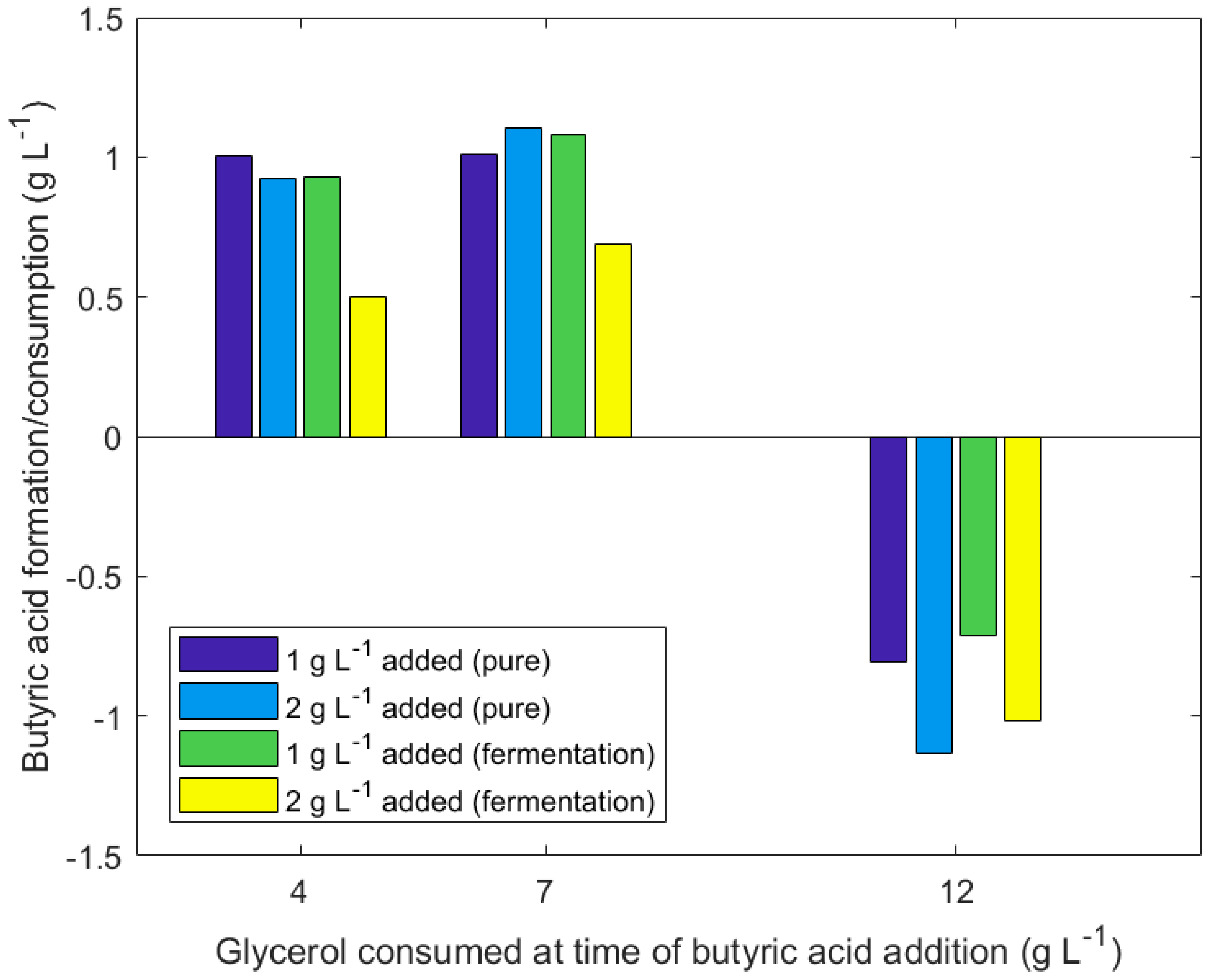
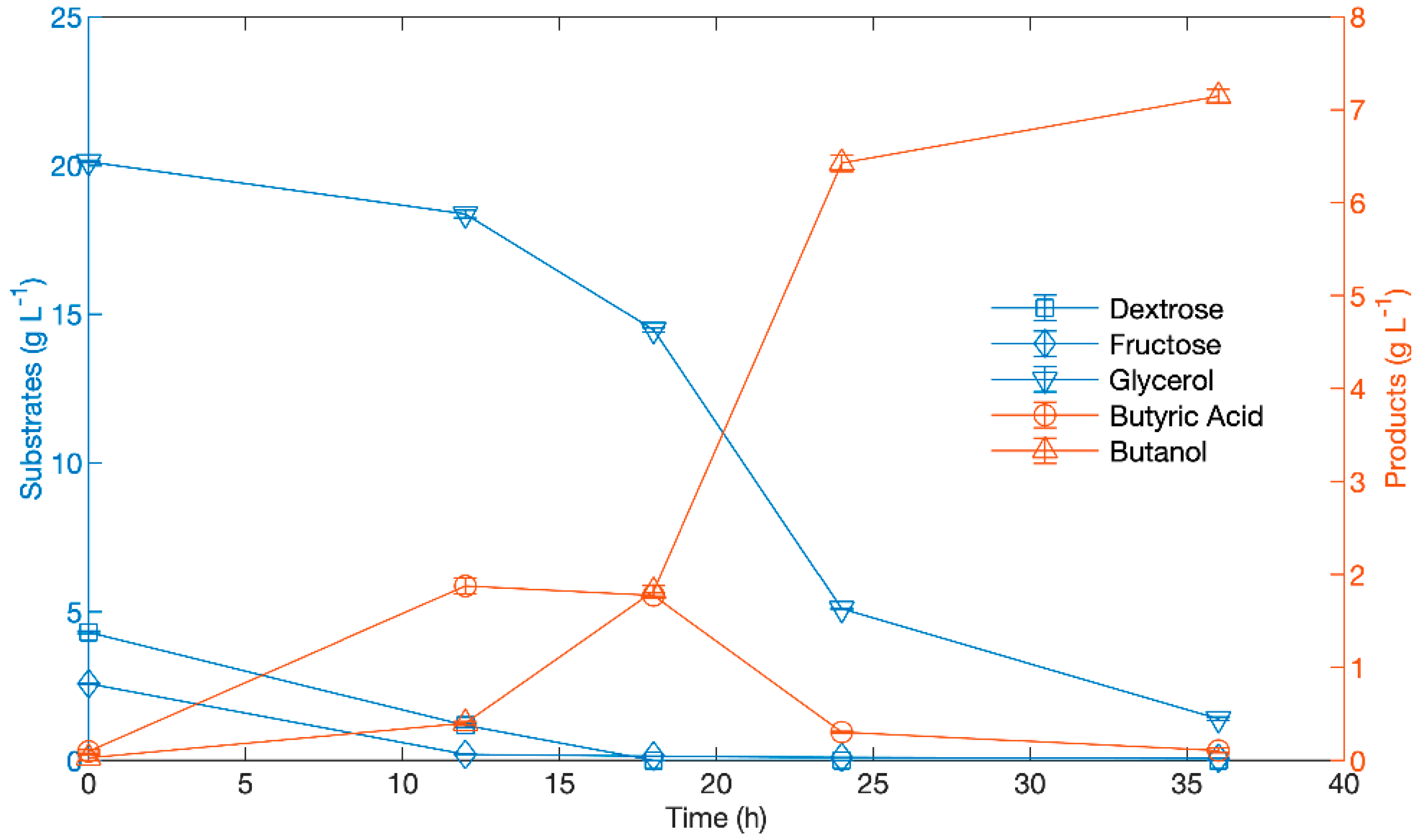
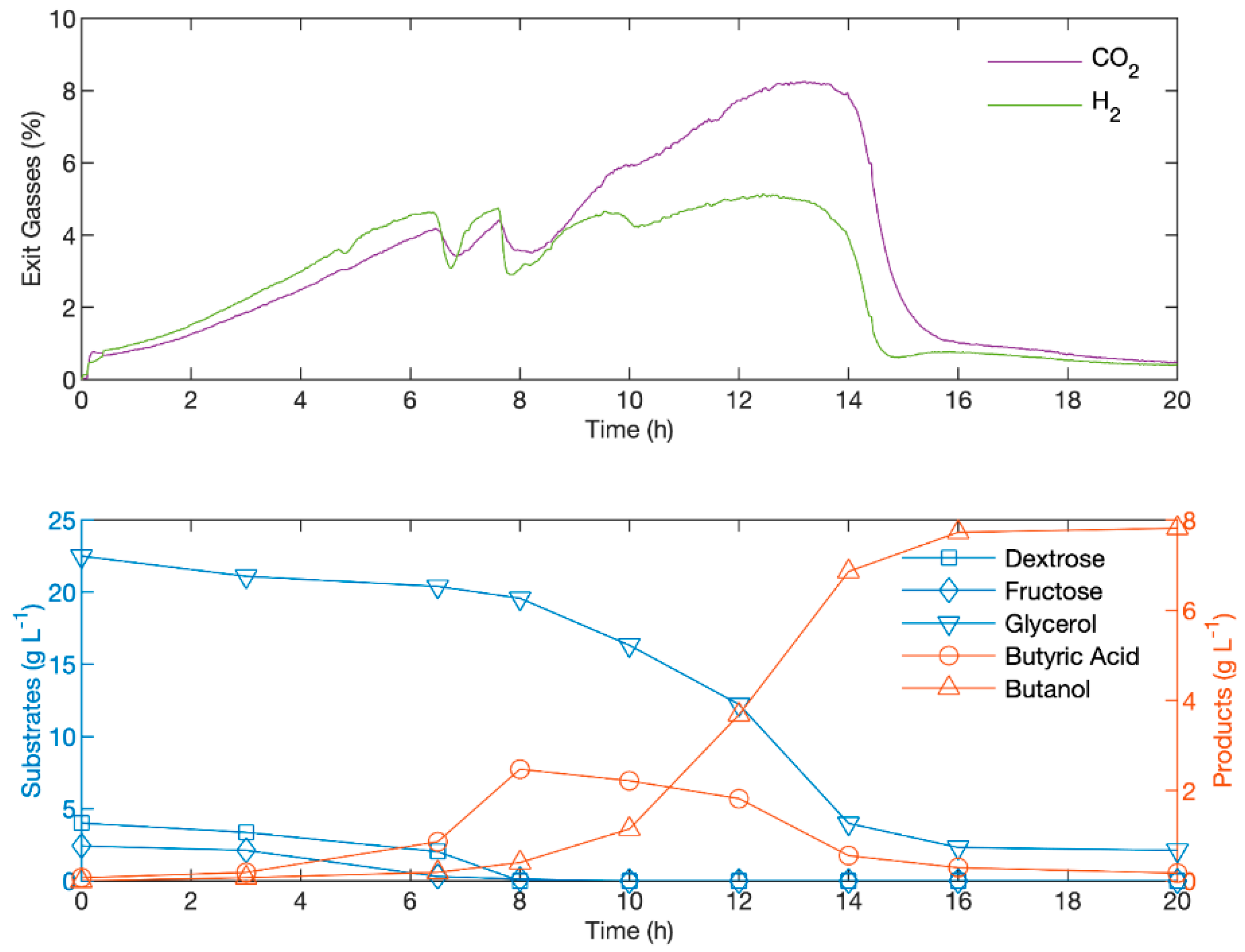
| Glycerol Consumed in Fermentation Prior to B.A. Addition (Starting Concentration of 20 g/L Glycerol) (g/L) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 7 | 12 | ||||||||||
| 1 g/L Pure B.A. added | 1 g/L ferm. B.A. added | 2 g/L Pure B.A. added | 2 g/L ferm. B.A. added | 1 g/L Pure B.A. added | 1 g/L ferm. B.A. added | 2 g/L Pure B.A. added | 2 g/L ferm. B.A. added | 1 g/L Pure B.A. added | 1 g/L ferm. B.A. added | 2 g/L Pure B.A. added | 2 g/L ferm. B.A. added | |
| B.A. Produced/Consumed (g/L) | 1.002 | 0.962 | 0.923 | 0.499 | 1.011 | 1.082 | 1.103 | 0.689 | −0.916 | −0.935 | −1.22 | −1.084 |
| Butanol Produced at end of fermentation (g/L) | 5.134 | 5.021 | 4.527 | 4.414 | 4.893 | 5.117 | 4.802 | 4.706 | 6.761 | 6.723 | 6.668 | 6.525 |
| Relative Carbon Yield (mols C/mols C) | 0.421 | 0.427 | 0.447 | 0.405 | 0.439 | 0.439 | 0.430 | 0.417 | 0.518 | 0.515 | 0.516 | 0.498 |
| Run | Glycerol | Molasses | Butanol | Relative Carbon Yield |
|---|---|---|---|---|
| 1 | 16 | 8 | 6.108 | 0.458 |
| 2 | 16 | 8 | 6.089 | 0.458 |
| 3 | 16 | 8 | 6.231 | 0.463 |
| 4 | 20 | 5 | 6.588 | 0.445 |
| 5 | 16 | 3.75736 | 5.107 | 0.429 |
| 6 | 12 | 11 | 4.227 | 0.342 |
| 7 | 16 | 12.2426 | 6.211 | 0.389 |
| 8 | 16 | 8 | 6.122 | 0.457 |
| 9 | 21.6569 | 8 | 8.063 | 0.431 |
| 10 | 20 | 11 | 7.494 | 0.413 |
| 11 | 12 | 5 | 3.663 | 0.329 |
| 12 | 10.3431 | 8 | 3.537 | 0.296 |
| 13 | 16 | 8 | 6.101 | 0.453 |
| Source | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | Comment |
|---|---|---|---|---|---|---|
| Model | 0.0378 | 5 | 0.0050 | 202.58 | <0.0001 | significant |
| A-Glycerol | 0.0179 | 1 | 0.0110 | 478.55 | <0.0001 | significant |
| B-Molasses | 0.0007 | 1 | 0.0002 | 19.13 | 0.0033 | significant |
| AB | 0.0005 | 1 | 0.0004 | 13.57 | 0.0078 | significant |
| A2 | 0.0161 | 1 | 0.0111 | 432.32 | <0.0001 | significant |
| B2 | 0.0045 | 1 | 0.0037 | 120.30 | <0.0001 | significant |
| R-squared | 0.993 | |||||
| Adj-squared | 0.988 | |||||
| Adeq Precision | 39.3 |
| Predicted Mean | Observed Mean | 95% PI Low | 95% PI High | |
|---|---|---|---|---|
| Relative Carbon Yield | 0.460 | 0.459 | 0.449 | 0.471 |
| Mols Sugar Substrate Consumed | Mols Glycerol Consumed | Mols Butanol Produced | Relative Carbon Yield (mols C Consumed/mols C Butanol) | |
|---|---|---|---|---|
| Kao et al. [15] | 0.11 | 0.65 | 0.18 | 0.274 |
| Sabra et al. [9] | 0.28 | 0.54 | 0.28 | 0.344 |
| This work | 0 | 0.23 | 0.076 | 0.439 |
| This work | 0.04 | 0.23 | 0.11 | 0.480 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munch, G.; Mittler, J.; Rehmann, L. Increased Selectivity for Butanol in Clostridium Pasteurianum Fermentations via Butyric Acid Addition or Dual Feedstock Strategy. Fermentation 2020, 6, 67. https://doi.org/10.3390/fermentation6030067
Munch G, Mittler J, Rehmann L. Increased Selectivity for Butanol in Clostridium Pasteurianum Fermentations via Butyric Acid Addition or Dual Feedstock Strategy. Fermentation. 2020; 6(3):67. https://doi.org/10.3390/fermentation6030067
Chicago/Turabian StyleMunch, Garret, Justus Mittler, and Lars Rehmann. 2020. "Increased Selectivity for Butanol in Clostridium Pasteurianum Fermentations via Butyric Acid Addition or Dual Feedstock Strategy" Fermentation 6, no. 3: 67. https://doi.org/10.3390/fermentation6030067
APA StyleMunch, G., Mittler, J., & Rehmann, L. (2020). Increased Selectivity for Butanol in Clostridium Pasteurianum Fermentations via Butyric Acid Addition or Dual Feedstock Strategy. Fermentation, 6(3), 67. https://doi.org/10.3390/fermentation6030067






